Abstract
To discover new compounds with broad spectrum and high activity, we designed a series of novel benzamides containing 1,2,4-oxadiazole moiety by bioisosterism, and 28 benzamides derivatives with antifungal activity were synthesized. These compounds were evaluated against four fungi: Botrytis cinereal, FusaHum graminearum, Marssonina mali, and Thanatephorus cucumeris. The results indicated that most of the compounds displayed good fungicidal activities, especially against Botrytis cinereal. For example, 10a (84.4%), 10d (83.6%), 10e (83.3%), 10f (83.1%), 10i (83.3%), and 10l (83.6%) were better than pyraclostrobin (81.4%) at 100 mg/L. In addition, the acute toxicity of 10f to zebrafish embryo was 20.58 mg/L, which was classified as a low-toxicity compound.
1. Introduction
Chemical pesticides play a vital role in solving food problems. However, as people’s environmental awareness gradually deepens, high-efficiency, low-toxicity, and environmentally friendly pesticides have become an inevitable trend in the creation of new pesticides [1,2,3]. There is no doubt that heterocyclic structures are important feature in synthetic pesticides for their high-efficiency, various biological activities, and diversity of possible substituents [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25]. 1,2,4-Oxadiazole heterocycle, as an important kind of the five-membered oxygen-nitrogen heterocycle, has exhibited a wide range of biological activities in the field of pesticides, such as insecticidal [26,27,28], antifungal [29,30], and herbicidal activities [31]. It has often been introduced as a synergist into the structure of pesticides in order to improve the biological activities of the compounds. In addition, the 1,2,4-oxadiazole heterocycle, as a bio-isostere of the amide bond, has better hydrolytic and metabolic properties.
Diamide insecticides have attracted a lot of attention due to their novel mechanism of action, high efficiency, and low toxicity [32,33,34,35,36,37,38,39,40,41,42]. Following Bayer’s report on the first diamide insecticide flubendiamide, the use of chlorantraniliprole, cyantraniliprole, cyclaniliprole, (Figure 1) and other products was launched successively. However, while this type of insecticide showed excellent insecticidal effects, it gradually exposed potential risks to environmental non-target organisms [43,44]. Broflanilide is a meta-diamide insecticide developed by Mitsui Chemicals and co-developed with BASF SE. Because of its novel mechanism of action, this product was expected to become a blockbuster product.
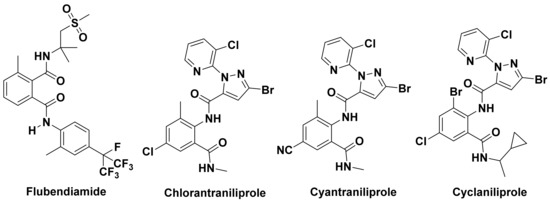
Figure 1.
Chemical structures of flubendiamide, chlorantraniliprole, cyantraniliprole, and cyclaniliprole.
In view of these facts mentioned above, broflanilide was employed as the lead compound in this study. According to the principle of bioisosterism [45,46], we searched for the amide group of broflanilide in the 1,2,4-oxadiazole ring, replaced the benzene ring with a pyridine structure containing a thioether derivative, and designed (Figure 2) and synthesized (Scheme 1) a series of novel benzamides substituted with 1,2,4-oxadiazole. These new compounds were confirmed by 1H NMR, 13C NMR, and HRMS, and their insecticidal activities, fungicidal activities, and toxicity test of zebrafish embryo were studied.
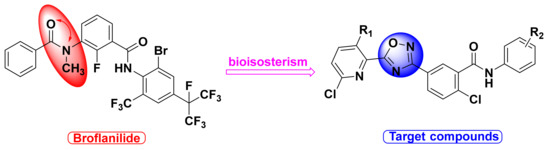
Figure 2.
Design strategy of target compounds.
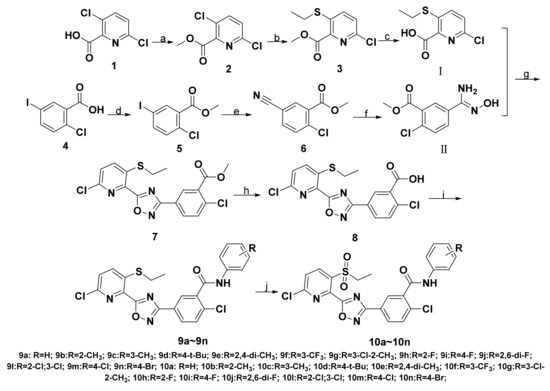
Scheme 1.
Synthetic route of target compounds. Reagents and conditions: (a) DMS, K2CO3, acetone, reflux; (b) CH3CH2SH, KTB, DMF, 0–5 °C, rt; (c) THF, OH-, reflux; (d) CH3OH, H+, reflux; (e) CuCN, l-proline, DMF, 100 °C; (f) NH2OH•HCl, CH3CH2OH, rt; (g) SOCl2, reflux; Et3N, toluene, reflux; (h) THF, OH-, reflux; (i) EDCI, Et3N, CH2Cl2, rt; (j) mCPBA, CH2Cl2, rt.
2. Results and Discussion
2.1. Synthesis of Target Compounds
The synthetic pathways to target compounds 9 and 10 are shown in Scheme 1. Intermediate I was prepared from 3,6-dichloropyridinecarboxylic acid 1 as a starting material via methylation, thioetherification, and hydrolysis reactions. During the thioetherification reaction, the newly prepared potassium ethanethiolate should be slowly added to the DMF solution of methyl 3,6-dichloropicolinate, and the temperature should be controlled at 0–5 °C to avoid the by-products (Scheme 2). As in our previous procedures [47], intermediate Ⅱ was easily obtained. Methyl 2-chloro-5-(5-(6-chloro-3-(ethylthio) pyridin-2-yl)-1,2,4-oxadiazol-3-yl) benzoate 7 was synthesized by cyclization reaction from imtermediate Ⅱ and 6-chloro-3-(ethylthio)picolinoyl chloride that had been synthesized from intermediate Ⅰ. Then, the compound 7 was hydrolyzed and spliced with substituted anilines to give a series of target compounds 9a–9n. Finally, compound 9 was oxidized with mCPBA at room temperature to give target compound 10, which avoided the impurities of pyridine-N-oxide. The characterization data for all synthesized compounds are provided in the supporting information file (Figures S1–S39).
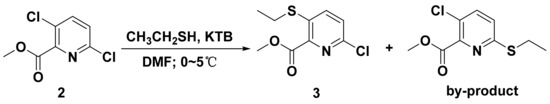
Scheme 2.
Synthesis of compound 3 via thioetherification.
2.2. Spectrum Analysis of Target Compounds
All the target compounds were confirmed by 1H NMR, 13C NMR, and HRMS. In the 1H NMR spectra of 10a, the -NH proton signals were found at δ 10.70 ppm. The signals of CH of the benzene and pyridine rings were assigned at 8.61–7.13 ppm. The signals at δ 3.78 ppm and 1.26 ppm were -CH2 and -CH3 peak, respectively. In the 13C-NMR spectra of compound 10a, the appearances of signals at 166.81 and 163.79 ppm were assigned to the carbons of the 1,2,4-oxadiazole ring. Finally, all of the novel benzamides substituted with 1,2,4-oxadiazole exhibited a strong [M + H]+ peak in positive ion high-resolution electrospray mass spectra (HR-ESI-MS) analysis.
2.3. Biological Activities of Target Compounds
In Table 1, The target compounds had weak death rates against Mythimna sepatara (5–40%) at 500 mg/L, which were lower than the control drug broflanilide (100%). In addition, all of the target compounds had good fungicidal activities at 100 mg/L. Overall, the target compounds had better inhibitory activities against Botrytis cinereal than Fusarium graminearum, Marssonina mali, and Thanatephorus cucumeris. In particular, the inhibitory activity of compounds 10a (84.4%), 10d (83.6%), 10e (83.3%), 10f (83.1%), 10i (83.3%), and 10l (83.6%) were better than pyraclostrobin (81.4%), and 10b (80.8%), 10g (81.1%), 10h (81.4%), and 10k (81.9%) were comparable to pyraclostrobin. At the same time, compounds 9e (57.7%), 9f (63.5%), 9g (65.9%), 9h (57.1%), and 9k (61.2%) also showed moderate activities. For Fusarium graminearum and Marssonina mali, compounds 9a–9n displayed weak inhibition, both of which were less than 25%. Some of compound 10 had good activities (46.4–54.4%) but were inferior to pyraclostrobin. For Thanatephorus cucumeris, compound 9 had no inhibitory activity, and some of compound 10 exhibited moderate inhibitory activities (37.5–50.3%). From Table 2, we can see that compound 10f had good inhibitory activity against Botrytis cinereal with EC50 of 14.44 μg/mL.

Table 1.
Insecticidal and fungicidal activities of title compounds 9 and 10.

Table 2.
EC50 of compounds 10f to Botrytis cinereal.
According to the insecticidal activity result, we speculated that the amide bridge bond played a key role in maintaining insecticidal activity, and it was likely that it would interact with the receptor through its hydrogen bond. Searching the amide bridge bond in 1,2,4-oxadiazole, we found that it lacked the corresponding hydrogen bond, and the group bulk increased, which resulted in the blocking of the binding of the compounds to the receptor and did not show a good insecticidal activity. It could be seen from Table 1 that the fungicidal activities of compound 10 was significantly higher than compound 9, indicating that the structure containing ethylsulfonyl was beneficial in increasing the activity. The SAR of compound 10 in terms of fungicidal activities (Table 1) was that when there was no substituent on the benzene ring, the activity against Botrytis cinereal was superior to other compounds. In addition, by comparing the control efficacy of compounds 10k, 10l, 10m, 10c, and 10n, we found that they showed that the para-position of aniline-containing substituents was not conducive to improving the activity.
2.4. Toxicity to Zebrafish Embryo
According to the fungicidal activity result, we selected compounds 9f and 10f with better activity to study the lethal and teratogenic effects exposure on zebrafish embryos from 6 to 96 hpf (Figure 3). When the 9f concentration exceeded 2 mg/L, the mortality rate increased sharply. At 10 mg/L, the mortality rate reached as high as 90%. The resulting LC50 value for compound 9f was 5.26 mg/L (Figure 3A). Similarly, the mortality rate of 10f showed concentration-dependent curves (Figure 3) with a LC50 value of 20.58 mg/L (Figure 3B). Moreover, 9f and 10f produced similar teratogenic and decreased hatching effects on zebrafish embryos at 72 hpf (Figure 3C–F). At 72 hpf, the hatching rates of the compounds 9f and 10f under 10 mg/L exposure were about 43% and 82%, respectively. In addition, the malformation rate of 9f was significantly higher than 10f at the same concentration.
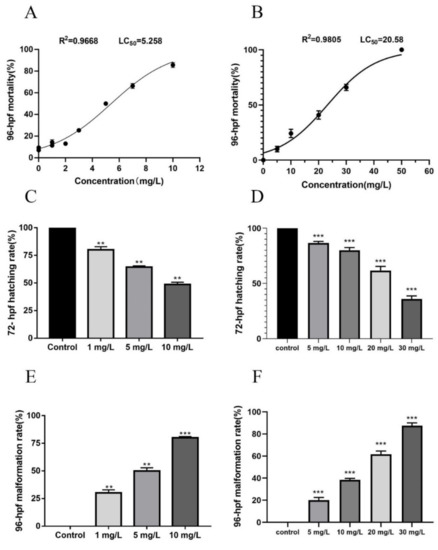
Figure 3.
Zebrafish embryo mortality, hatching rate, and malformation rates after exposure to compounds 9f (A,C,E)and 10f (B,D,F). Note: “*” represents significant differences at ** p ≤ 0.01 and *** p ≤ 0.001 by one-way ANOVA followed by Dunnett’s test.
As the time and concentration increased, zebrafish embryos showed obvious developmental delay. At 76–96 hpf, a series of malformations appeared, such as delayed yolk absorption, pericardial cyst, lack of melanin, yolk sac, and bent spine (Figure 4). Among them, yolk cyst was the most obvious. By comparing the lethal and teratogenic effects of 9f and 10f exposure on zebrafish embryos, we were able to find that the toxicity of 9f to zebrafish embryos was higher than that of 10f. Thus, we speculated that the structure containing ethylsulfonyl was beneficial to reduce the toxicity to zebrafish embryos.
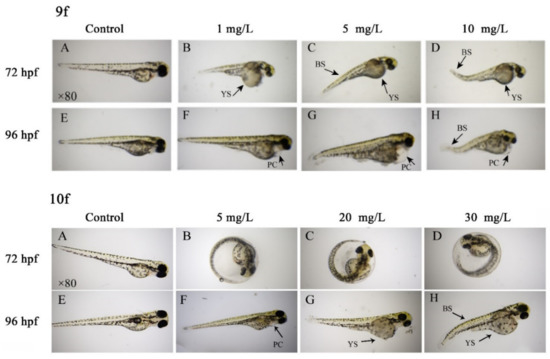
Figure 4.
Zebrafish embryo malformation after exposure to compounds 9f and 10f. Note: PC, pericardial cyst; YS, yolk sac; BS, bent spine.
3. Experimental Section
3.1. General Information
Melting points were determined using an X-4 apparatus (Taike, Beijing, China) and the thermometer was uncorrected. 1H NMR and 13C NMR spectra were measured on BRUKER Avance 500 MHz spectrometer (Bruker 500 MHz, Fallanden, Switzerland) using CDCl3 or DMSO as the solvent. High-resolution electrospray mass spectra (HR-ESI–MS) were determined using an UPLC H CLASS/QTOF G2 XS mass spectrometer (Waters, Milford, CT, USA). All the reagents were analytical grade or synthesized in our laboratory.
Ethics statement: The Institutional Animal Care and Use Committee (IACUC) at Wenzhou Medical University (SYXK 2019-0009, April 4, 2019 to April 4, 2024) approved our study plan for proper use of zebrafish. All studies were carried out in strict accordance with the guidelines of the IACUC. All dissection was performed on ice, and all efforts were made to minimize suffering.
3.2. Synthesis
3.2.1. Synthesis of Intermediate Ⅰ
Methyl 3,6-dichloropicolinate (2): To a stirred solution of 3,6-dichloropicolinic acid (1) (0.10 mmol) in acetone (10 mL), we added DMS (1.3 g, 0.01 mol) and K2CO3 (1.7 g). After stirring at 40 °C for 8 h, the mixture was cooled to room temperature and poured into water, the precipitation was filtered and dried to give 1.8 g light yellow solid. Yield: 90.1%, m.p. 54–55 °C ([48], 53–54 °C); 1H NMR (500 MHz, Chloroform-d) δ 7.77 (d, J = 8.5 Hz, 1H), 7.42 (d, J = 8.5 Hz, 1H), 4.00 (s, 3H).
Methyl 6-chloro-3-(ethylthio) picolinate (3): KTB (1.4 g), DMF (30 mL), and ethyl mercaptan (2.5 g, 0.04 mol) were added to a three-necked flask and reacted at 0 °C for about 1 h to give CH3CH2SK. The CH3CH2SK was dropped into a stirred solution of compound 2 (3.7 g) in DMF (20 mL) at 0 °C. After stirring at room temperature for 2 h, the mixture was quenched with water and extracted by EtOAc (100 mL). The extraction was dried over anhydrous MgSO4 and filtered. The filtration was concentrated and separated by column chromatography to give 3.1 g yellow solid. Yield: 74.5%, m.p. 128–129 °C; 1H NMR (500 MHz, Chloroform-d) δ 7.66 (d, J = 8.5 Hz, 1H), 7.41 (d, J = 8.5 Hz, 1H), 4.00 (s, 3H), 2.95 (q, J = 7.5 Hz, 2H), 1.39 (t, J = 7.5 Hz, 3H).
Intermediate Ⅰ: 30% NaOH (5 mL) was added to a solution of compound 3 (3.3 g) in THF. The mixture was then refluxed for 3 h. Afterwards, the mixture was cooled to room temperature and poured into water. Then, we adjusted the pH to 2–3, and 2.8 g white solid precipitate was obtained. Yield: 92.1%, m.p. 119–120 °C; 1H NMR (500 MHz, Chloroform-d) δ 10.76 (s, 1H), 7.71 (d, J = 8.5 Hz, 1H), 7.49 (d, J = 8.5 Hz, 1H), 2.97 (q, J = 7.5 Hz, 2H), 1.44 (t, 3H).
3.2.2. Synthesis of Intermediate Ⅱ
The synthesis of intermediate Ⅱ refers to our previous work.
3.2.3. Methyl 2-Chloro-5-(5-(6-Chloro-3-(Ethylthio) Pyridin-2-yl)-1,2,4-Oxadiazol-3-yl) Benzoate (7)
The intermediate Ⅰ (1.1 g, 5 mmol) and SOCl2 (20 mL) were added to a 100 mL flask and the mixture was refluxed for 6 h. Then, SOCl2 was removed under reduced pressure to give 6-chloro-3-(ethylthio)picolinoyl chloride.
To a solution of intermediate Ⅱ (1.1 g, 5.5 mmol) and triethylamine (1.2 g, 12 mmol) in toluene (100 mL), we added the prepared 6-chloro-3-(ethylthio)picolinoyl chloride dropwise at 0 °C for 1 h. The mixture was then refluxed for 2 h. Afterwards, the mixture was cooled to room temperature and washed by saturated sodium chloride solution (100 mL × 3). The organic layer was dried by Na2SO4 and removed to give yellow solid (1.4 g). Yield: 62.2%, m.p. 155–157 °C; 1H NMR (500 MHz, Chloroform-d) δ 8.63 (d, J = 2.0 Hz, 1H), 8.35–8.20 (m, 1H), 7.89 (d, J = 8.5 Hz, 1H), 7.66 (d, J = 8.0 Hz, 1H), 7.54 (d, J = 8.5 Hz, 1H), 3.87 (s, 3H), 3.17 (q, J = 7.0 Hz, 2H), 1.32 (t, J = 7.5 Hz, 3H); HRMS calcd for C17H14Cl2N3O3S [M + H]+ 410.0127, found 410.0126.
3.2.4. 2-Chloro-5-(5-(6-Chloro-3-(Ethylthio) Pyridin-2-yl)-1,2,4-Oxadiazol-3-yl) Benzoic Acid (8)
We added 30% NaOH (5 mL) to a solution of compound 7 (0.8 g, 2.0 mmol) in THF. The mixture was then refluxed for 2 h. Afterwards, the mixture was cooled to room temperature and the solvent was removed. Then, we adjusted the pH to 2–3, and white solid precipitate was obtained (0.7 g). Yield: 90.8%, m.p. 225–227 °C; 1H NMR (500 MHz, Chloroform-d) δ 13.47 (s, 1H), 8.55 (d, J = 2.0 Hz, 1H), 8.36 (d, J = 8.5 Hz, 1H), 8.26–8.19 (m, 1H), 7.91 (d, J = 8.5 Hz, 1H), 7.80 (d, J = 8.0 Hz, 1H), 3.16 (q, J = 7.5 Hz, 2H), 1.30 (t, J = 7.0 Hz, 3H); HRMS calcd for C16H12Cl2N3O3S [M + H]+ 395.9971, found 395.9973.
3.2.5. Synthesis of Target Compound 9
To a solution of compound 8 (1.0 g, 2.0 mmol), EDCI (0.1 g) and triethylamine (0.2 g) in DCM (100 mL), we added the substituted aniline (3.0 mmol) at 0 °C, and the mixture was stirred for 8 h to give compound 9 by the method of column chromatography separation.
2-Chloro-5-(5-(6-chloro-3-(ethylthio)pyridin-2-yl)-1,2,4-oxadiazol-3-yl)-N-phenylbenzamide (9a): White solid, yield 73.4%, m.p. 243–245 °C; 1H NMR (500 MHz, DMSO-d6) 1H NMR (500 MHz, Chloroform-d) δ 10.70 (s, 1H), 8.23–8.18 (m, 2H), 8.13 (d, J = 9.0 Hz, 1H), 7.88–7.84 (m, 1H), 7.79 (d, J = 8.5 Hz, 1H), 7.73 (d, J = 8.0 Hz, 2H), 7.38 (t, J = 8.0 Hz, 2H), 7.14 (t, J = 7.0 Hz, 1H), 3.15 (q, J = 7.5 Hz, 2H), 1.30 (t, J = 7.0 Hz, 3H); 13C NMR (500 MHz, DMSO-d6) δ 172.43, 167.11, 164.10, 145.88, 138.71, 138.64, 138.22, 138.13, 137.88, 135.87, 133.66, 131.22, 129.60, 129.01, 127.81, 127.29, 124.30, 119.89, 25.53, 13.36; HRMS calcd for C22H17Cl2N4O2S [M + H]+ 471.0444, found 471.0447.
2-Chloro-5-(5-(6-chloro-3-(ethylthio)pyridin-2-yl)-1,2,4-oxadiazol-3-yl)-N-(o-tolyl)benzamide (9b): White solid, yield 77.4%, m.p. 230–232 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.19 (s, 1H), 8.26 (d, J = 2.0 Hz, 1H), 8.20 (dd, J = 8.5, 2.0 Hz, 1H), 8.14 (d, J = 9.0 Hz, 1H), 7.86 (d, J = 8.5 Hz, 1H), 7.80 (d, J = 8.5 Hz, 1H), 7.49 (d, J = 7.5 Hz, 1H), 7.29 (d, J = 7.5 Hz, 1H), 7.25 (t, J = 6.5 Hz, 1H), 7.19 (t, J = 7.5 Hz, 1H), 3.17 (q, J = 7.0 Hz, 2H), 2.30 (s, 3H), 1.32 (t, J = 7.5 Hz, 3H); 13C NMR (500 MHz, DMSO-d6) 13C NMR (126 MHz, DMSO-d6) δ 172.45, 166.07, 163.92, 145.90, 138.67, 137.96, 137.61, 136.24, 133.69, 133.53, 133.38, 132.91, 131.23, 130.94, 129.56, 129.38, 127.83, 127.29, 125.25, 119.91, 25.55, 20.64, 13.38; HRMS calcd for C23H19Cl2N4O2S [M + H]+ 485.0600, found 485.0600.
2-Chloro-5-(5-(6-chloro-3-(ethylthio)pyridin-2-yl)-1,2,4-oxadiazol-3-yl)-N-(p-tolyl)benzamide (9c): White solid, yield 74.3%, m.p. 251–253 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.61 (s, 1H), 8.19 (m, 2H), 8.13 (d, J = 9.0 Hz, 1H), 7.85 (d, J = 8.5 Hz, 1H), 7.79 (d, J = 9.0 Hz, 1H), 7.61 (d, J = 8.0 Hz, 2H), 7.18 (d, J = 8.0 Hz, 2H), 3.16 (d, J = 7.5 Hz, 2H), 2.29 (s, 3H), 1.30 (t, J = 7.5 Hz, 3H); 13C NMR (500 MHz, DMSO-d6) δ 172.46, 167.15, 163.96, 145.90, 138.63, 138.29, 137.98, 137.57, 136.26, 133.71, 133.41, 131.25, 129.58, 129.41, 127.85, 127.32, 125.02, 119.93, 25.56, 20.67, 13.39; HRMS calcd for C23H19Cl2N4O2S [M + H]+ 485.0600, found 485.0606.
N-(4-(tert-Butyl) phenyl)-2-chloro-5-(5-(6-chloro-3-(ethylthio) pyridin-2-yl)-1,2,4-oxadiazol-3-yl) benzamide (9d): White solid, yield 78.4%,. m.p. 243–244 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.63 (s, 1H), 8.23–8.17 (m, 2H), 8.14 (d, J = 9.0 Hz, 1H), 7.85 (d, J = 8.0 Hz, 1H), 7.79 (d, J = 8.5 Hz, 1H), 7.64 (d, J = 8.5 Hz, 2H), 7.39 (d, J = 8.5 Hz, 2H), 3.16 (q, J = 7.5 Hz, 2H), 1.34–1.26 (m, 12H); 13C NMR (500 MHz, DMSO-d6) δ 172.46, 167.40, 167.19, 138.54, 138.11, 135.64, 133.72, 133.30, 133.04, 133.00, 131.21, 127.88, 127.53, 126.84, 126.17, 124.98, 25.64, 20.70, 18.01, 13.34; HRMS calcd for C26H25Cl2N4O2S [M + H]+ 527.1070, found 527.1071.
2-Chloro-5-(5-(6-chloro-3-(ethylthio)pyridin-2-yl)-1,2,4-oxadiazol-3-yl)-N-(2,4-dimethylphenyl)benzamide (9e): Grey solid, yield 69.2%, m.p. 255–256 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.10 (s, 1H), 8.24 (d, J = 2.0 Hz, 1H), 8.19 (dd, J = 8.0, 2.0 Hz, 1H), 8.14 (d, J = 8.5 Hz, 1H), 7.83 (dd, J = 24.0, 8.5 Hz, 2H), 7.34 (d, J = 8.0 Hz, 1H), 7.10 (s, 1H), 7.05 (d, J = 8.0 Hz, 1H), 3.17 (q, J = 7.0 Hz, 2H), 2.28 (d, J = 6.0 Hz, 6H), 1.32 (t, J = 7.5 Hz, 3H); 13C NMR (500 MHz, DMSO-d6) δ 172.46, 167.40, 167.19, 138.54, 138.11, 135.64, 133.72, 133.30, 133.04, 131.21, 127.88, 127.53, 126.84, 126.17, 25.64, 20.70, 18.01, 13.34; HRMS calcd for C24H21Cl2N4O2S [M + H]+ 499.0757, found 499.0763.
2-Chloro-5-(5-(6-chloro-3-(ethylthio)pyridin-2-yl)-1,2,4-oxadiazol-3-yl)-N-(3-(trifluoromethyl)phenyl)benzamide (9f): White solid, yield 77.8%, m.p. 211–214 °C; 1H NMR (500 MHz, DMSO-d6) δ 11.06 (s, 1H), 8.27 (d, J = 2.0 Hz, 1H), 8.25–8.21 (m, 2H), 8.14 (d, J = 9.0 Hz, 1H), 7.92 (d, J = 8.0 Hz, 1H), 7.89 (d, J = 8.5 Hz, 1H), 7.80 (d, J = 9.0 Hz, 1H), 7.64 (t, J = 8.0 Hz, 1H), 7.51 (d, J = 8.0 Hz, 1H), 3.16 (q, J = 7.5 Hz, 2H), 1.30 (t, J = 7.0 Hz, 3H); 13C NMR (500 MHz, DMSO-d6) δ 172.48, 167.10, 164.59, 145.91, 139.49, 138.68, 138.63, 138.59, 138.28, 137.58, 137.35, 133.70, 131.35, 131.29, 130.37, 127.88, 127.81, 125.11, 123.53, 120.68, 116.00, 25.57, 13.36; HRMS calcd for C23H16Cl2F3N4O2S [M + H]+ 539.0318, found 539.0322.
2-Chloro-N-(3-chloro-2-methylphenyl)-5-(5-(6-chloro-3-(ethylthio) pyridin-2-yl)-1,2,4-oxadiazol-3-yl) benzamide (9g): Yellow solid, yield 79.7%. m.p. 225–226 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.44 (s, 1H), 8.29 (d, J = 1.5 Hz, 1H), 8.21 (dd, J = 8.0, 1.5 Hz, 1H), 8.14 (d, J = 9.0 Hz, 1H), 7.87 (d, J = 8.0 Hz, 1H), 7.80 (d, J = 9.0 Hz, 1H), 7.47 (d, J = 8.0 Hz, 1H), 7.39 (d, J = 8.0 Hz, 1H), 7.29 (t, J = 8.0 Hz, 1H), 3.16 (q, J = 7.5 Hz, 2H), 2.35 (s, 3H), 1.32 (t, J = 7.5 Hz, 3H); 13C NMR (500 MHz, DMSO-d6) δ 172.39, 167.05, 164.53, 145.85, 138.65, 138.24, 137.61, 137.10, 134.00, 133.62, 131.53, 131.19, 129.61, 127.77, 127.40, 127.18, 127.14, 127.08, 125.45, 124.97, 25.54, 15.30, 13.26; HRMS calcd for C23H18Cl3N4O2S [M + H]+ 519.0211, found 519.0211.
2-Chloro-5-(5-(6-chloro-3-(ethylthio)pyridin-2-yl)-1,2,4-oxadiazol-3-yl)-N-(2-fluorophenyl)benzamide (9h): White solid, yield 66.5%, m.p. 213–217 °C; 1H NMR (500 MHz, DMSO-d6) 1H NMR (500 MHz, Chloroform-d) δ 10.56 (s, 1H), 8.21 (m, 2H), 8.14 (d, J = 8.5 Hz, 1H), 7.91–7.82 (m, 2H), 7.80 (d, J = 8.5 Hz, 1H), 7.36–7.21 (m, 3H), 3.16 (q, J = 7.5 Hz, 2H), 1.31 (t, J = 7.0 Hz, 3H); 13C NMR (500 MHz, DMSO-d6) 13C NMR (126 MHz, DMSO-d6) δ 172.42, 164.54, 158.63, 145.88, 143.26, 139.54, 138.66, 138.23, 137.37, 136.91, 132.59, 131.18, 129.68, 127.80, 127.49, 125.78, 125.19, 124.60 (d, J = 10 Hz), 123.80, 25.55, 13.31; HRMS calcd for C22H16Cl2FN4O2S [M + H]+ 489.0350, found 489.0355.
2-Chloro-5-(5-(6-chloro-3-(ethylthio)pyridin-2-yl)-1,2,4-oxadiazol-3-yl)-N-(4-fluorophenyl)benzamide (9i): Yellow solid, yield 61.6%, m.p. 268–270 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.76 (s, 1H), 8.24–8.18 (m, 2H), 8.13 (d, J = 9.0 Hz, 1H), 7.89–7.83 (m, 1H), 7.82–7.72 (m, 3H), 7.22 (t, J = 9.0 Hz, 2H), 3.16 (q, J = 7.5 Hz, 2H), 1.30 (t, J = 7.5 Hz, 3H); 13C NMR (500 MHz, DMSO-d6) δ 172.41, 167.06, 163.96, 145.85, 138.61, 138.19, 137.70, 137.57, 133.64, 131.20, 129.64, 127.77, 127.28, 124.99, 121.74, 121.68, 115.67, 115.49, 25.50, 13.33; HRMS calcd for C22H16Cl2FN4O2S [M + H]+ 489.0350, found 489.0350.
2-Chloro-5-(5-(6-chloro-3-(ethylthio)pyridin-2-yl)-1,2,4-oxadiazol-3-yl)-N-(2,6-difluorophenyl)benzamide (9j): Grey solid, yield 57.8%, m.p. 258–261 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.57 (s, 1H), 8.27–8.18 (m, 2H), 8.14 (d, J = 8.5 Hz, 1H), 7.90–7.76 (m, 3H), 7.39 (t, J = 8.5 Hz, 1H), 7.16 (t, J = 8.5 Hz, 1H), 3.16 (q, J = 7.5 Hz, 2H), 1.31 (t, J = 7.5 Hz, 3H); 13C NMR (500 MHz, DMSO-d6) δ 172.46, 167.78, 167.11, 158.28, 152.27, 146.15, 145.92, 138.69, 138.25, 137.62, 137.22, 133.78, 131.25, 129.81, 127.84, 127.51, 117.00, 116.01, 111.58, 25.59, 13.33; HRMS calcd for C22H15Cl2F2N4O2S [M + H]+ 507.0255, found 507.0258.
2-Chloro-5-(5-(6-chloro-3-(ethylthio)pyridin-2-yl)-1,2,4-oxadiazol-3-yl)-N-(2-chlorophenyl)benzamide (9k): White solid, yield 75.0%, m.p. 207–208 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.48 (s, 1H), 8.30 (s, 1H), 8.21 (d, J = 8.5 Hz, 1H), 8.14 (d, J = 8.5 Hz, 1H), 7.86 (d, J = 8.5 Hz, 1H), 7.80 (d, J = 8.5 Hz, 1H), 7.73 (d, J = 7.5 Hz, 1H), 7.58 (d, J = 8.0 Hz, 1H), 7.42 (t, J = 7.0 Hz, 1H), 7.32 (t, J = 7.5 Hz, 1H), 3.16 (q, J = 7.0 Hz, 2H), 1.32 (t, J = 7.5 Hz, 3H); 13C NMR (500 MHz, DMSO-d6) δ 172.45, 167.07, 164.37, 145.87, 140.12, 138.25, 137.55, 137.44, 133.65, 133.32, 131.24, 130.76, 129.93, 129.85, 127.78, 127.29, 125.06, 119.40, 119.32, 118.33, 25.54, 13.35; HRMS calcd for C22H16Cl3N4O2S [M + H]+ 505.0054, found 505.0055.
2-Chloro-5-(5-(6-chloro-3-(ethylthio)pyridin-2-yl)-1,2,4-oxadiazol-3-yl)-N-(3-chlorophenyl)benzamide (9l): White solid, yield 75.4%, m.p. 232–234 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.90 (s, 1H), 8.26–8.20 (m, 2H), 8.14 (d, J = 8.5 Hz, 1H), 7.94 (t, J = 1.5 Hz, 1H), 7.87 (d, J = 8.5 Hz, 1H), 7.80 (d, J = 9.0 Hz, 1H), 7.60 (d, J = 8.0 Hz, 1H), 7.42 (t, J = 8.0 Hz, 1H), 7.24–7.18 (m, 1H), 3.16 (q, J = 7.0 Hz, 2H), 1.30 (t, J = 7.0 Hz, 3H); 13C NMR (126 MHz, DMSO-d6) 13C NMR (126 MHz, Chloroform-d) δ 177.49, 172.13, 169.39, 150.90, 145.27, 143.38, 142.55, 138.73, 138.41, 136.35, 136.28, 135.80, 134.93, 132.82, 132.51, 130.17, 129.06, 124.45, 124.38, 123.35, 30.60, 18.41; HRMS calcd for C22H16Cl3N4O2S [M + H]+ 505.0054, found 505.0055.
2-Chloro-5-(5-(6-chloro-3-(ethylthio)pyridin-2-yl)-1,2,4-oxadiazol-3-yl)-N-(4-chlorophenyl)benzamide (9m): White solid, yield 77.3%, m.p. 242–243 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.84 (s, 1H), 8.24–8.18 (m, 2H), 8.11 (d, J = 9.0 Hz, 1H), 7.85 (d, J = 8.0 Hz, 1H), 7.77 (m, 3H), 7.43 (d, J = 9.0 Hz, 2H), 3.14 (q, J = 7.5 Hz, 2H), 1.29 (t, J = 7.5 Hz, 3H); 13C NMR (126 MHz, DMSO-d6) δ 172.42, 167.07, 164.17, 145.86, 138.55, 138.25, 137.66, 137.57, 137.52, 133.65, 131.22, 129.75, 128.92, 127.92, 127.78, 127.33, 125.04, 121.45, 25.54, 13.33; HRMS calcd for C22H16Cl3N4O2S [M + H]+ 505.0054, found 505.0055.
N-(4-Bromophenyl)-2-chloro-5-(5-(6-chloro-3-(ethylthio)pyridin-2-yl)-1,2,4-oxadiazol-3-yl)benzamide (9n): Yellow solid, yield 78.8%, m.p. 267–269 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.84 (s, 1H), 8.25–8.19 (m, 2H), 8.13 (d, J = 8.5 Hz, 1H), 7.87 (d, J = 8.0 Hz, 1H), 7.79 (d, J = 9.0 Hz, 1H), 7.72 (d, J = 9. Hz, 2H), 7.57 (d, J = 8.5 Hz, 2H), 3.16 (q, J = 7.5 Hz, 2H), 1.30 (t, J = 7.0 Hz, 3H); 13C NMR (126 MHz, DMSO-d6) δ 172.43, 167.08, 164.20, 145.88, 138.60, 138.24, 138.08, 137.57, 133.64, 131.84, 131.24, 129.77, 127.80, 127.33, 125.04, 121.83, 115.99, 25.55, 13.35; HRMS calcd for C22H16BrCl2N4O2S [M + H]+ 548.9549, found 548.9554.
3.2.6. Synthesis of Target Compound 10
To a stirred solution of compound 9 (1.0 mmol) in DCM (20 mL), we added mCPBA (0.5 g, 3.0 mmol). After stirring at room temperature for 3 h, the mixture was poured into water and the pH was adjusted to 7–8 with NaHCO3. The organic layer was dried by Na2SO4 and removed under reduced pressure to give compound 10.
2-Chloro-5-(5-(6-chloro-3-(ethylsulfonyl)pyridin-2-yl)-1,2,4-oxadiazol-3-yl)-N-phenylbenzamide (10a): Pink solid, yield 83.7%, m.p. 263–267 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.70 (s, 1H), 8.61 (d, J = 8.5 Hz, 1H), 8.23 (d, J = 2.5 Hz, 1H), 8.20 (m, 2H), 7.85 (d, J = 8.5 Hz, 1H), 7.74 (d, J = 8.0 Hz, 2H), 7.37 (t, J = 8.0 Hz, 2H), 7.13 (t, J = 7.5 Hz, 1H), 3.78 (q, J = 7.5 Hz, 2H), 1.26 (t, J = 7.5 Hz, 3H); 13C NMR (500 MHz, DMSO-d6) δ 171.62, 166.81, 163.79, 154.63, 142.95, 142.22, 138.64, 137.80, 135.62, 133.90, 131.16, 129.50, 128.82, 127.36, 124.27, 124.07, 119.71, 50.37, 6.64; HRMS calcd for C22H17Cl2N4O4S [M + H]+ 503.0342, found 503.0347.
2-Chloro-5-(5-(6-chloro-3-(ethylsulfonyl)pyridin-2-yl)-1,2,4-oxadiazol-3-yl)-N-(o-tolyl)benzamide (10b): White solid, yield 77.4%, m.p. 257–259 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.18 (s, 1H), 8.62 (d, J = 9.0 Hz, 1H), 8.26 (d, J = 2.0 Hz, 1H), 8.20 (d, J = 8.5 Hz, 2H), 7.85 (d, J = 8.5 Hz, 1H), 7.49 (d, J = 7.5 Hz, 1H), 7.31–7.20 (m, 2H), 7.18 (td, J = 7.5, 1.5 Hz, 1H), 3.79 (q, J = 7.5 Hz, 2H), 2.31 (s, 3H), 1.27 (t, J = 7.5 Hz, 3H); 13C NMR (500 MHz, DMSO-d6) δ 171.64, 166.85, 164.14, 154.64, 142.98, 142.23, 137.97, 135.64, 135.47, 133.89, 132.94, 131.14, 130.61, 130.44, 129.37, 128.79, 127.38, 126.07, 124.24, 50.37, 39.93, 17.90, 6.63; HRMS calcd for C23H19Cl2N4O4S [M + H]+ 517.0499, found 517.0500.
2-Chloro-5-(5-(6-chloro-3-(ethylsulfonyl)pyridin-2-yl)-1,2,4-oxadiazol-3-yl)-N-(p-tolyl)benzamide (10c): White solid, yield 84.3%, m.p. 260–261 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.59 (s, 1H), 8.61 (d, J = 8.5 Hz, 1H), 8.26–8.15 (m, 3H), 7.84 (d, J = 8.5 Hz, 1H), 7.61 (d, J = 8.5 Hz, 2H), 7.17 (d, J = 8.0 Hz, 2H), 3.77 (q, J = 7.5 Hz, 2H), 2.28 (s, 3H), 1.26 (t, J = 7.0 Hz, 3H); 13C NMR (500 MHz, DMSO-d6) 13C NMR (126 MHz, DMSO-d6) δ 171.61, 166.81, 163.60, 154.63, 142.94, 142.20, 137.85, 136.11, 135.60, 133.90, 133.14, 131.16, 129.45, 129.18, 128.78, 127.32, 124.22, 119.72, 50.36, 20.46, 6.61; HRMS calcd for C23H19Cl2N4O4S [M + H]+ 517.0499, found 517.0505.
N-(4-(tert-Butyl)phenyl)-2-chloro-5-(5-(6-chloro-3-(ethylsulfonyl)pyridin-2-yl)-1,2,4-oxadiazol-3-yl)benzamide (10d): Grey yield, yield 79.2%, m.p. 268–270 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.61 (s, 1H), 8.62 (d, J = 8.5 Hz, 1H), 8.24–8.12 (m, 3H), 7.85 (d, J = 9.0 Hz, 1H), 7.64 (d, J = 8.5 Hz, 2H), 7.39 (d, J = 8.5 Hz, 2H), 3.78 (q, J = 7.5 Hz, 2H), 1.33–1.27 (m, 12H); 13C NMR (500 MHz, DMSO-d6) δ 176.85, 172.05, 168.89, 159.88, 151.78, 148.21, 147.45, 143.08, 141.26, 140.83, 139.14, 136.39, 134.64, 134.06, 132.50, 130.66, 129.46, 124.77, 55.61, 39.28, 36.36, 11.87; HRMS calcd for C26H25Cl2N4O4S [M + H]+ 559.0968, found 559.0971.
2-Chloro-5-(5-(6-chloro-3-(ethylsulfonyl)pyridin-2-yl)-1,2,4-oxadiazol-3-yl)-N-(2,4-dimethylphenyl)benzamide (10e): White solid, yield 78.4%,. m.p. 246–248 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.09 (s, 1H), 8.61 (d, J = 8.5 Hz, 1H), 8.24 (d, J = 2.0 Hz, 1H), 8.22–8.17 (m, 2H), 7.84 (d, J = 8.5 Hz, 1H), 7.34 (d, J = 8.0 Hz, 1H), 7.08 (s, 1H), 7.04 (dd, J = 8.0, 2.0 Hz, 1H), 3.79 (q, J = 7.0 Hz, 2H), 2.27 (d, J = 7.5 Hz, 6H), 1.27 (t, J = 7.5 Hz, 3H); 13C NMR (500 MHz, DMSO-d6) δ 171.62, 166.84, 164.16, 154.64, 142.96, 142.21, 138.02, 135.62, 135.34, 133.87, 132.84, 132.81, 131.12, 130.92, 129.32, 128.78, 127.34, 126.57, 125.93, 124.20, 50.37, 20.49, 17.81, 6.62; HRMS calcd for C24H21Cl2N4O4S [M + H]+ 531.0655, found 531.0660.
2-Chloro-5-(5-(6-chloro-3-(ethylsulfonyl)pyridin-2-yl)-1,2,4-oxadiazol-3-yl)-N-(3-(trifluoromethyl)phenyl)benzamide (10f): Yellow solid, yield 76.8%, m.p. 214–217 °C; H NMR (500 MHz, DMSO-d6) δ 11.07 (s, 1H), 8.61 (d, J = 8.5 Hz, 1H), 8.30 (d, J = 2.0 Hz, 1H), 8.26–8.17 (m, 3H), 7.92 (d, J = 8.5 Hz, 1H), 7.87 (d, J = 8.5 Hz, 1H), 7.62 (t, J = 8.0 Hz, 1H), 7.50 (d, J = 7.5 Hz, 1H), 3.78 (q, J = 7.5 Hz, 2H), 1.26 (t, J = 7.0 Hz, 3H); 13C NMR (500 MHz, DMSO-d6) δ 171.64, 166.77, 164.23, 154.62, 142.95, 142.20, 139.37, 137.23, 135.61, 133.90, 131.23, 130.13, 129.80, 129.54 (d, J = 32.1 Hz), 128.79, 127.49, 125.10, 124.32, 123.30, 120.44 (d), 115.78 (d, J = 4.0 Hz), 50.37, 6.62; HRMS calcd for C23H16Cl2F3N4O4S [M + H]+ 571.0216, found 571.0222.
2-Chloro-N-(3-chloro-2-methylphenyl)-5-(5-(6-chloro-3-(ethylsulfonyl)pyridin-2-yl)-1,2,4-oxadiazol-3-yl)benzamide (10g): Yellow solid, yield 69.1%, m.p. 255–256 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.44 (s, 1H), 8.62 (d, J = 8.5 Hz, 1H), 8.29 (d, J = 2.0 Hz, 2H), 8.21 (m, 1H), 7.86 (d, J = 8.5 Hz, 1H), 7.46 (d, J = 8.0 Hz, 1H), 7.38 (d, J = 7.5 Hz, 1H), 7.28 (t, J = 8.0 Hz, 1H), 3.79 (q, J = 7.0 Hz, 2H), 2.34 (s, 3H), 1.27 (t, J = 7.5 Hz, 3H); 13C NMR (500 MHz, DMSO-d6) 13C NMR (126 MHz, DMSO-d6) δ 171.64, 166.81, 164.30, 154.63, 142.97, 142.21, 137.60, 137.00, 135.63, 133.88, 131.45, 131.17, 129.55, 128.81, 128.78, 127.40, 127.01, 126.95, 125.34, 124.28, 50.36, 15.19, 6.64; HRMS calcd for C23H18Cl3N4O4S [M + H]+ 551.0109, found 551.0108.
2-Chloro-5-(5-(6-chloro-3-(ethylsulfonyl)pyridin-2-yl)-1,2,4-oxadiazol-3-yl)-N-(2-fluorophenyl)benzamide (10h): Brown solid, yield 73.7%, m.p. 253–257 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.58 (s, 1H), 8.61 (d, J = 8.5 Hz, 1H), 8.27–8.11 (m, 3H), 7.94–7.81 (m, 2H), 7.38–7.17 (m, 3H), 3.78 (q, J = 7.0 Hz, 2H), 1.26 (t, J = 7.0 Hz, 3H); 13C NMR (500 MHz, DMSO-d6) 13C NMR (126 MHz, DMSO-d6) δ 171.62, 166.80, 166.07, 154.64, 142.94, 142.19, 137.26, 135.60, 133.97, 133.31, 132.69, 131.16, 130.64, 129.60, 128.77, 127.87, 127.51, 125.62, 124.45, 115.80 (d, J = 77.5 Hz), 50.37, 6.58; HRMS calcd for C22H16Cl2FN4O4S [M + H]+ 521.0248, found 521.0255.
2-Chloro-5-(5-(6-chloro-3-(ethylsulfonyl)pyridin-2-yl)-1,2,4-oxadiazol-3-yl)-N-(2-fluorophenyl)benzamide (10i): Yellow solid, yield 71.6%, m.p. 229–231 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.75 (s, 1H), 8.61 (d, J = 8.5 Hz, 1H), 8.24 (d, J = 2.0 Hz, 1H), 8.23–8.18 (m, 2H), 7.85 (d, J = 8.5 Hz, 1H), 7.78–7.71 (m, 2H), 7.22 (t, J = 9.0 Hz, 2H), 3.78 (q, J = 7.0 Hz, 2H), 1.26 (t, J = 7.5 Hz, 3H); 13C NMR (500 MHz, DMSO-d6) 13C NMR (126 MHz, DMSO-d6) δ 171.62, 166.79, 163.71, 157.51, 154.63, 142.94, 142.20, 137.61, 135.60, 134.97, 133.90, 131.18, 129.58, 128.78, 127.37, 124.27, 121.60, 115.43 (d, J = 88.0 Hz), 50.37, 6.62; HRMS calcd for C22H16Cl2FN4O4S [M + H]+ 521.0248, found 521.0251.
2-Chloro-5-(5-(6-chloro-3-(ethylsulfonyl)pyridin-2-yl)-1,2,4-oxadiazol-3-yl)-N-(2,6-difluorophenyl)benzamide (10j): Brown solid, yield 72.8%, m.p. 237–239 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.56 (s, 1H), 8.61 (d, J = 8.5 Hz, 1H), 8.25 (m, 1H), 8.21 (d, J = 8.5 Hz, 2H), 7.89–7.82 (m, 2H), 7.42–7.34 (m, 1H), 7.19–7.12 (m, 1H), 3.78 (q, J = 7.5 Hz, 2H), 1.27 (t, J = 7.5 Hz, 3H); 13C NMR (500 MHz, DMSO-d6) δ 171.63, 166.79, 164.35, 154.64, 142.94, 142.18, 137.08, 135.61, 133.97, 131.19, 129.68, 128.79, 127.52, 127.17 (d, J = 7.5 Hz), 124.17, 111.40 (d, J = 12.5 Hz), 111.22, 104.60, 104.40 (d, J = 9.0 Hz), 104.19, 50.36, 6.58; HRMS calcd for C22H15Cl2F2N4O4S [M + H]+ 539.0154, found 539.0159.
2-Chloro-5-(5-(6-chloro-3-(ethylsulfonyl)pyridin-2-yl)-1,2,4-oxadiazol-3-yl)-N-(2-chlorophenyl)benzamide (10k): White solid, yield 82.0%, m.p. 209–212 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.50 (s, 1H), 8.62 (d, J = 8.0 Hz, 1H), 8.34–8.14 (m, 3H), 7.93–7.82 (m, 1H), 7.73 (d, J = 7.0 Hz, 1H), 7.58 (d, J = 7.5 Hz, 1H), 7.48–7.38 (m, 1H), 7.36–7.29 (m, 1H), 3.86–3.73 (m, 2H), 1.28 (t, J = 7.0 Hz, 3H); 13C NMR (500 MHz, DMSO-d6) 13C NMR (126 MHz, DMSO-d6) δ 171.62, 166.79, 164.38, 154.66, 142.94, 142.18, 135.58, 134.07, 134.00, 131.23, 129.68, 129.63, 128.79, 128.77, 127.87, 127.84, 127.73, 127.59, 127.48, 124.15, 50.38, 6.61; HRMS calcd for C22H16Cl3N4O4S [M + H]+ 536.9952, found 536.9958.
2-Chloro-5-(5-(6-chloro-3-(ethylsulfonyl)pyridin-2-yl)-1,2,4-oxadiazol-3-yl)-N-(2-chlorophenyl)benzamide (10l): Yellow solid, yield 85.4%, m.p. 217–218 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.91 (s, 1H), 8.62 (d, J = 8.5 Hz, 1H), 8.31–8.17 (m, 3H), 7.97–7.84 (m, 2H), 7.60 (d, J = 7.5 Hz, 1H), 7.42 (t, J = 8.0 Hz, 1H), 7.21 (d, J = 7.5 Hz, 1H), 3.79 (q, J = 7.0 Hz, 2H), 1.26 (t, J = 7.0 Hz, 3H); 13C NMR (126 MHz, DMSO-d6) 13C NMR (126 MHz, DMSO-d6) δ 171.63, 166.77, 164.09, 154.64, 142.93, 142.18, 139.97, 137.32, 135.58, 133.87, 133.15, 131.23, 130.58, 129.75, 128.79, 127.40, 124.30, 123.85, 119.19, 118.15, 50.37, 6.62; HRMS calcd for C22H16Cl3N4O4S [M + H]+ 536.9952, found 536.9959.
2-Chloro-5-(5-(6-chloro-3-(ethylsulfonyl)pyridin-2-yl)-1,2,4-oxadiazol-3-yl)-N-(2-chlorophenyl)benzamide (10m): White solid, yield 84.3%, m.p. 233–236 °C; 1H NMR (500 MHz, DMSO-d6) 13C NMR (126 MHz, DMSO-d6) δ 171.63, 166.77, 164.09, 154.64, 142.93, 142.18, 139.97, 137.32, 135.58, 133.87, 133.15, 131.23, 130.58, 129.75, 128.79, 127.40, 124.30, 123.85, 119.19, 118.15, 50.37, 6.62; 13C NMR (126 MHz, DMSO-d6) 13C NMR (126 MHz, DMSO-d6) δ 171.62, 166.78, 163.88, 154.64, 142.94, 142.19, 137.53, 137.47, 135.59, 133.88, 131.20, 129.66, 128.78, 128.75, 127.74, 127.39, 124.28, 121.29, 50.37, 6.62; HRMS calcd for C22H16Cl3N4O4S [M + H]+ 536.9952, found 521.0251.
N-(4-Bromophenyl)-2-chloro-5-(5-(6-chloro-3-(ethylsulfonyl)pyridin-2-yl)-1,2,4-oxadiazol-3-yl)benzamide (10n): Yellow solid, yield 78.3%, m.p. 263–265 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.83 (s, 1H), 8.61 (d, J = 8.5 Hz, 1H), 8.33–8.11 (m, 3H), 7.85 (d, J = 8.0 Hz, 1H), 7.71 (d, J = 8.5 Hz, 2H), 7.56 (d, J = 8.5 Hz, 2H), 3.77 (q, J = 7.5 Hz, 2H), 1.26 (t, J = 7.5 Hz, 3H); 13C NMR (126 MHz, DMSO-d6) 13C NMR (126 MHz, DMSO-d6) δ 171.61, 166.77, 163.91, 154.65, 142.93, 142.18, 137.93, 137.44, 135.58, 133.87, 131.66, 131.21, 129.67, 128.78, 127.38, 124.27, 121.67, 115.81, 50.38, 6.61; HRMS calcd for C22H16BrCl2N4O4S [M + H]+ 580.9447, found 580.9449.
3.3. Biological Activity and Toxicity Determination
The insecticidal and fungicidal activities were investigated in the National Pesticide Engineering Research Centre, Nankai University, according to references [49,50], and the results of the activity test are shown in Table 1.
Through acute exposure, we assessed the toxicity of compounds 9f and 10f on zebrafish embryo. According to the preliminary exposure experiments, a series of gradient concentrations of compounds 9f and 10f were set on the basis of mortality rates in the range of 10–95%. LC50 values for zebrafish embryos exposed to compound 9f or 10f from 6 to 96 hpf: control (0 mg/L of 9f), 1, 5, 10 mg/L of 9f; control (0 mg/L of 10f), 5, 10 mg/L of 10f. The LC50 (median lethal concentration) values were computed by the Boltzmann equation [51,52]. The observational indexes included 96 hpf mortality rate, 72 hpf hatching rate, and 96 hpf malformation rate.
4. Conclusions
In conclusion, a series of novel benzamides containing 1,2,4-oxadiazole moiety were designed by bioisosterism and were synthesized easily via thioetherification, cyclization, aminolysis, and oxidation reactions. Their structures were confirmed by 1H NMR, 13C NMR, and HRMS. The bioassay results showed that some of the title compounds displayed excellent fungicidal activities against Botrytis cinereal at 100 mg/L. For example, 10a (84.4%), 10d (83.6%), 10e (83.3%), 10f (83.1%), 10i (83.3%), and 10l (83.6%) were better than the control fungicide pyraclostrobin (81.4%). In addition, the acute toxicity of 10f to zebrafish embryo was 20.58 mg/L, which was classified as a low toxicity compound. Therefore, these compounds could potentially be the lead compounds for further study.
Supplementary Materials
The following are available online at https://www.mdpi.com/1422-0067/22/5/2367/s1.
Author Contributions
S.Y., X.-Y.T., T.-Y.M., L.D., C.-L.R., and W.-Q.Z. carried out experimental work; S.Y. prepared the manuscript; C.-X.T. designed the material and supervised the project; and X.-H.L. and C.-X.T. revised the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Wynca Group and Siga Co. Ltd. R&D Program, grant numbers KYY-HX-20180412 and KYY-HX-20180737.
Data Availability Statement
Samples of the compounds are not available from the authors.
Acknowledgments
We acknowledge Hui-Li Wang for support with the toxicity determination.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
HRMS (High Resolution Mass Spectrometer), DMF (N,N-Dimethylformamide), mCPBA (3-Chloroperbenzoic acid), DMS (Dimethyl sulfate), rt (Room temperature), KTB (Potassium t-butoxide), THF (Tetrahydrofuran), EtOAc (Ethyl acetate), EDCI (1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride), SAR (Structure activity relationships).
References
- Verger, P.J.P.; Boobis, A.R. Reevaluate pesticides for food security and safety. Science 2013, 341, 717–718. [Google Scholar] [CrossRef]
- Wang, B.L.; Zhu, H.W.; Ma, Y.; Xiong, L.X.; Li, Y.Q.; Zhao, Y.; Zhang, J.F.; Chen, Y.W.; Zhou, S.; Li, Z.M. Synthesis, insecticidal activities, and SAR studies of novel pyridylpyrazole acid derivatives based on amide bridge modification of anthranilic diamide insecticides. J. Agric. Food Chem. 2013, 61, 5483–5493. [Google Scholar] [CrossRef] [PubMed]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Shi:, J.J.; Ren, G.H.; Wu, N.-J.; Weng, J.-Q.; Xu, T.-M.; Liu, X.-H.; Tan, C.-X. Design, synthesis and insecticidal activities of novel anthranilic diamides containing polyfluoroalkyl pyrazole moiety. Chin. Chem. Lett. 2017, 28, 1727–1730. [Google Scholar] [CrossRef]
- Alnufaie, R.; Alsup, N.; Whitt, J.; Chambers, A.S.; Gilmore, D.; Alam, M.A. Synthesis and Antimicrobial Studies of Coumarin-Substituted Pyrazole Derivatives as Potent Anti-Staphylococcus aureus Agents. Molecules 2020, 25, 2758. [Google Scholar] [CrossRef] [PubMed]
- Avenot, H.F.; Michailides, T.J. Progress in understanding molecular mechanisms and evolution of resistance to succinate dehydrogenase inhibiting (SDHI) fungicides in phytopathogenic fungi. Crop Prot. 2010, 29, 643–651. [Google Scholar] [CrossRef]
- Fuentes-Gutiérrez, A.; Curiel-Quesada, E.; Correa-Basurto, J.; Martínez-Muñoz, A.; Reyes-Arellano, A. N-Heterocycles Scaffolds as Quorum Sensing Inhibitors. Design, Synthesis, Biological and Docking Studies. Int. J. Mol. Sci. 2020, 21, 9512. [Google Scholar]
- Hu, Y.; Li, C.Y.; Wang, X.M.; Yang, Y.H.; Zhu, H.L. 1,3,4-Thiadiazole: Synthesis, reactions, and applications in medicinal, agricultural, and materials chemistry. Chem. Rev. 2014, 114, 5572–5610. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhao, B.; Fan, Z.J.; Yang, D.Y.; Zhang, N.L.; Wu, Q.F.; Yu, B.; Zhou, S.; Kalinina, T.A.; Belskaya, N.P. Discovery of novel thiazole carboxamides as antifungal succinate dehydrogenase inhibitors. J. Agric. Food Chem. 2019, 67, 1647–1655. [Google Scholar] [CrossRef]
- Duchowicz, P.R.; Vitale, M.G.; Castro, E.A.; Fernández, M.; Caballero, J. QSAR analysis for heterocyclic antifungals. Bioorg. Med. Chem. 2007, 15, 2680–2689. [Google Scholar] [CrossRef]
- Du, S.J.; Lu, H.Z.; Yang, D.Y.; Li, H.; Gu, X.L.; Wan, C.; Jia, C.M.; Wang, M.; Li, X.Y.; Qin, Z.H. Synthesis, antifungal activity and QSAR of some novel carboxylic acid amides. Molecules 2015, 20, 4071–4087. [Google Scholar] [CrossRef]
- Hou, Y.P.; Mao, X.W.; Wang, J.X.; Zhan, S.W.; Zhou, M.G. Sensitivity of Fusarium asiaticum to a novel succinate dehydrogenase inhibitor fungicide pydiflumetofen. Crop Prot. 2017, 96, 237–244. [Google Scholar] [CrossRef]
- Liu, X.H.; Qiao, L.; Zhai, Z.W.; Cai, P.P.; Cantrell, C.L.; Tan, C.X.; Weng, J.Q.; Han, L.; Wu, H.K. Novel 4-Pyrazole Carboxamide Derivatives Containing Flexible Chain Motif: Design, Synthesis and Antifungal Activity. Pest Manag. Sci. 2019, 75, 2892–2900. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Shao, W.B.; Zhu, J.J.; Long, Z.Q.; Liu, L.W.; Wang, P.Y.; Li, Z.; Yang, S. Novel 1, 3, 4-Oxadiazole-2-carbohydrazides as Prospective Agricultural Antifungal Agents Potentially Targeting Succinate Dehydrogenase. J. Agric. Food Chem. 2019, 67, 13892–13903. [Google Scholar] [CrossRef]
- Hua, X.W.; Liu, W.R.; Su, Y.Y.; Liu, X.H.; Liu, J.B.; Liu, N.N.; Wang, G.Q.; Jiao, X.Q.; Fan, X.Y.; Xue, C.M.; et al. Studies on the novel pyridine sulfide containing SDH based heterocyclic amide fungicide. Pest Manag. Sci. 2020, 76, 2368–2378. [Google Scholar] [CrossRef]
- Eckroat, T.J.; Manross, D.L.; Cowan, S.C. Merged Tacrine-Based, Multitarget-Directed Acetylcholinesterase Inhibitors 2015-Present: Synthesis and Biological Activity. Int. J. Mol. Sci. 2020, 21, 5696. [Google Scholar] [CrossRef]
- Zhang, A.G.; Zhou, J.Y.; Tao, K.; Hou, T.P.; Jin, H. Design, synthesis and antifungal evaluation of novel pyrazole carboxamides with diarylamines scaffold as potent succinate dehydrogenase inhibitors. Bioorg. Med. Chem. Lett. 2018, 28, 3042–3045. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Dai, F.Y.; Zhu, J.; Dong, K.K.; Wang, Y.L.; Chen, T. Synthesis and antibacterial activities of pleuromutilin derivatives with thiazole-5-carboxamide and thioether moiety. J. Chem. Res. 2011, 35, 313–316. [Google Scholar] [CrossRef]
- Fu, Q.; Cai, P.P.; Cheng, L.; Zhong, L.K.; Shen, Z.H.; Han, L.; Liu, X.H. Synthesis and herbicidal activity of novel pyrazole aromatic ketone analogs as HPPD inhibitor. Pest Manag. Sci. 2020, 76, 868–879. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Yu, W.; Min, L.J.; Wedge, D.E.; Weng, J.Q.; Wu, H.-K.; Cantrell, C.L.; Bajsa-Hirschel, J.; Hua, X.-W. Synthesis and pesticidal activities of quinoxalines. J. Agric. Food Chem. 2020, 68, 7324–7332. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, R.R.; Wu, H.K.; Xu, T.M.; Liu, X.H. The synthesis of 6-(tertbutyl)-8-fluoro-2,3-dimethylquinoline carbonate derivatives and their antifungal activity against Pyricularia oryzae. Front. Chem. Sci. Eng. 2019, 13, 369–376. [Google Scholar] [CrossRef]
- Yao, T.T.; Xiao, D.X.; Li, Z.S.; Cheng, J.L.; Fang, S.W.; Du, Y.J.; Zhao, J.-H.; Dong, X.W.; Zhu, G.N. Design, synthesis, and fungicidal evaluation of novel pyrazole-furan and pyrazole-pyrrole carboxamide as succinate dehydrogenase inhibitors. J. Agric. Food Chem. 2017, 65, 5397–5403. [Google Scholar] [CrossRef]
- Yao, T.T.; Fang, S.W.; Li, Z.S.; Xiao, D.X.; Cheng, J.L.; Ying, H.Z.; Du, Y.J.; Zhao, J.H.; Dong, X.W. Discovery of Novel Succinate Dehydrogenase Inhibitors by the Integration of in Silico Library Design and Pharmacophore Mapping. J. Agric. Food Chem. 2017, 65, 3204–3211. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.L.; Wang, H.X.; Liu, H.; Xiong, L.; Yang, N.; Zhang, Y.; Li, Z.M. Synthesis and structure-insectidical relationship of novel phenylpyrazole carboxylic acid dervatives containing fluorine moiety. Chin. Chem. Lett. 2020, 31, 739–745. [Google Scholar] [CrossRef]
- Xue, H.S.; Liu, A.P.; Liu, W.D.; Li, J.M.; Ren, Y.G.; Huang, L.; He, L.; Ou, X.M.; Ye, J.; Huang, M.Z. Syntheses and fungicidal activities of thiazole-5-carboxanilides bearing thioether group. Chem. Res. Chin. Univ. 2016, 32, 781–785. [Google Scholar] [CrossRef]
- King, W.F.; Wheeler, R.E. Substituted Oxadiazoles and Their Use as Corn Root Worm Insecticides. US Patent US4237121A, 2 December 1981. [Google Scholar]
- Liu, Q.; Zhu, R.; Gao, S.; Ma, S.H.; Tang, H.J.; Diao, Y.M.; Wang, H.L.; Zhu, H.J. Structure-based bioisosterism design, synthesis, insecticidal activity and structure-activity relationship (SAR) of anthranilic diamide analogues containing 1,2,4-oxadiazole rings. Pest Manage. Sci. 2017, 73, 917–924. [Google Scholar] [CrossRef]
- Haugwitz, R.D.; Martinez, A.J.; Venslavsky, J.; Angel, R.G.; Maurer, B.V.; Jacobs, G.A.; Narayanan, V.L.; Cruthers, L.R.; Szanto, J. Synyhesis and anthelmintic acyivities of novel isothiocyanatophenyl-1,2,4-oxadiazoles. J. Med. Chem. 1985, 28, 1234–1241. [Google Scholar] [CrossRef]
- Terteryan-Seiser, V.; Grammenos, W.; Wiebe, C.; Kretschmer, M.; Craig, I.R.; Escribano, C.A.; Marcus, F.; Tobias, M.; Palomar, M.A.Q.; Grote, T.; et al. Substituted Oxadiazoles for Combating Phytopathogenic Fungi. WO Patent WO2017178245A1, 19 October 2017. [Google Scholar]
- Iwata, J.; Nakamura, Y.; Hayashi, T.; Watanabe, S.; Sano, H. Oxadiazole Compound and Fungicide for Agricultural and Horticultural Use. WO Patent WO2019022061A1, 31 January 2019. [Google Scholar]
- Ryu, E.K.; Chung, K.H.; Lee, W.H.; Kim, J.N.; Hong, K.S. Herbicidal Quinolinyloxadiazoles. WO Patent WO9404530A1, 3 March 1994. [Google Scholar]
- Feng, M.L.; Li, Y.F.; Zhu, H.J.; Zhao, L.; Xi, B.B.; Ni, J.P. Synthesis, Insecticidal Activity, and Structure-Activity Relationship of Trifluoromethyl-Containing Phthalic Acid Diamide Structures. J. Agric. Food Chem. 2010, 58, 10999–11006. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, Y.; Wangyang, W.Z.; Liu, F.; Cui, Y.L.; Duan, Y.S.; Wang, M.; Liu, S.Z.; Rui, C.H. Design, Synthesis, and Insecticidal Activities of Phthalamides Containing a Hydrazone Substructure. J. Agric. Food Chem. 2010, 58, 6858–6863. [Google Scholar] [CrossRef]
- Lahm, G.P.; Stevenson, T.M.; Selby, T.P.; Freudenberger, J.H.; Cordova, D.; Flexner, L.; Bellin, C.A.; Dubas, C.M.; Smith, B.K.; Hughes, K.A.; et al. RynaxypyrTM: A new insecticidal anthranilic diamide that acts as a potent and selective ryanodine receptor activator. Bioorg. Med. Chem. Lett. 2007, 17, 6274–6279. [Google Scholar] [CrossRef]
- Hughes, K.A.; Lahm, G.P.; Selby, T.P.; Stevenson, T.M. Cyano Anthranilamide Insecticides. WO Patent WO2004067528A1, 21 January 2004. [Google Scholar]
- Clark, D.A.; Lahm, G.P.; Smith, B.K.; Barry, J.D.; Clagg, D.G. Synthesis of insecticidal fluorinated anthranilic diamides. Bioorg. Med. Chem. 2008, 16, 3163–3170. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Song, B.A.; Hu, D.Y.; Yue, M.; Yang, S. Design, synthesis and insecticidal activities of novel pyrazole amides containing hydrazone substructures. Pest Manag. Sci. 2012, 68, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.F.; Xu, J.Y.; Wang, B.L.; Li, Y.X.; Xiong, L.X.; Li, Y.Q.; Ma, Y.; Li, Z.M. Synthesis and insecticidal activities of novel anthranilic diamides containing acylthiourea and acylurea. J. Agric. Food Chem. 2012, 60, 7565–7572. [Google Scholar] [CrossRef]
- Chen, K.; Liu, Q.; Ni, J.P.; Zhu, H.J.; Li, Y.F.; Wang, Q. Synthesis: Insecticidal activities and structureactivity relationship studies of novel anthranilic diamides containing pyridylpyrazole-4-carboxamide. Pest Manag. Sci. 2015, 71, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.W.; Mao, W.T.; Fan, Z.J.; Ji, X.T. Novel anthranilic diamide insecticides: Design, synthesis, and insecticidal evaluation. Aust. J. Chem. 2014, 67, 1491–1503. [Google Scholar] [CrossRef]
- Zhou, S.; Jia, Z.H.; Xiong, L.X.; Yan, T.; Yang, N.; Wu, G.; Song, H.; Li, Z. Chiral dicarboxamide scaffolds containing a sulfiliminyl moiety as potential ryanodine receptor activators. J. Agric. Food Chem. 2014, 62, 6269–6277. [Google Scholar] [CrossRef]
- Zhou, S.; Gu, Y.C.; Liu, M.; Wu, C.; Zhou, S.; Zhao, Y.; Jia, Z.; Wang, B.; Xiong, L.; Yang, N.; et al. Insecticidal activities of chiral N-trifluoroacetyl sulfilimines as potential ryanodine receptor modulators. J. Agric. Food Chem. 2014, 62, 11054–11061. [Google Scholar] [CrossRef] [PubMed]
- Amarasekare, K.G.; Shearer, P.W. Comparing effects of insecticides on two green lacewings species Chrysoperla johnsoni and Chrysoperla carnea (Neuroptera: Chrysopidae). J. Econ. Entomol. 2013, 106, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.A.; Beers, E.H. Impacts of orchard pesticides on Galendromus occidentalis: Lethal and sublethal effects. Crop Prot. 2013, 56, 16–24. [Google Scholar]
- Kohara, Y.; Kubo, K.; Imamiya, E.; Wada, T.; Inada, Y.; Naka, T. Synthesis and angiotensin II receptor antagonistic activities of benzimidazole derivatives bearing acidic heterocycles as novel tetrazole bioisosteres. J. Med. Chem. 1996, 39, 5228–5235. [Google Scholar] [CrossRef]
- Tagad, H.D.; Hamada, Y.; Nguyen, J.T.; Hamada, T.; Abdel-Rahman, H.; Yamani, A.; Nagamine, A.; Ikari, H.; Igawa, N.; Hidaka, K.; et al. Design of pentapeptidic BACE1 inhibitors with carboxylic acid bioisosteres at P1’ and P4 positions. Bioorg. Med. Chem. 2010, 18, 3175–3186. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Tian, X.Y.; Liu, X.H.; Tan, C.X. Synthesis and Biological Activity of Benzamides Substituted with Pyridine-Linked 1,2,4-Oxadiazole. Molecules 2020. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Mal, D. Total synthesis of BE-23254, a chlorinated angucycline antibiotic. Tetrahedron Lett. 2005, 46, 5483–5486. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, H.W.; Shang, J.F.; Wang, B.L.; Li, Z.M. Synthesis and Biological Activities of Novel 3-(((3-Bromo1-(3-chloropyridin-2-yl)-1H-pyrazol-5-yl) methylene) amino)-substituted-benzo [d] [1–3] triazin-4(3H)-ones. Chin. J. Org. Chem. 2019, 39, 861–866. [Google Scholar] [CrossRef]
- Mu, J.X.; Shi, Y.X.; Yang, M.Y.; Sun, Z.H.; Liu, X.H.; Li, B.J.; Sun, N.B. Design, Synthesis, DFT Study and Antifungal Activity of Pyrazolecarboxamide Derivatives. Molecules 2016, 21, 68. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Yin, X.; Wang, H. Toxicity assessment of combined fluoroquinolone and tetracycline exposure in zebrafish (Danio rerio). Environ. Toxicol. Pharmacol. 2016, 31, 736–750. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Liu, M.; Liu, J.F.; Wang, X.; Wang, C.; Ai, W.; Chen, S.; Wang, H. Combined toxicity of triclosan 1,2,4-dichlorophenol and 2,4,6-trichlorophenol to zebrafish (Danio rerio). Environ. Toxicol. Pharmacol. 2018, 57, 9–18. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).