Oxidative Stress Evaluation in Ischemia Reperfusion Models: Characteristics, Limits and Perspectives
Abstract
1. Introduction
2. Biochemical Aspects of Oxidative Stress
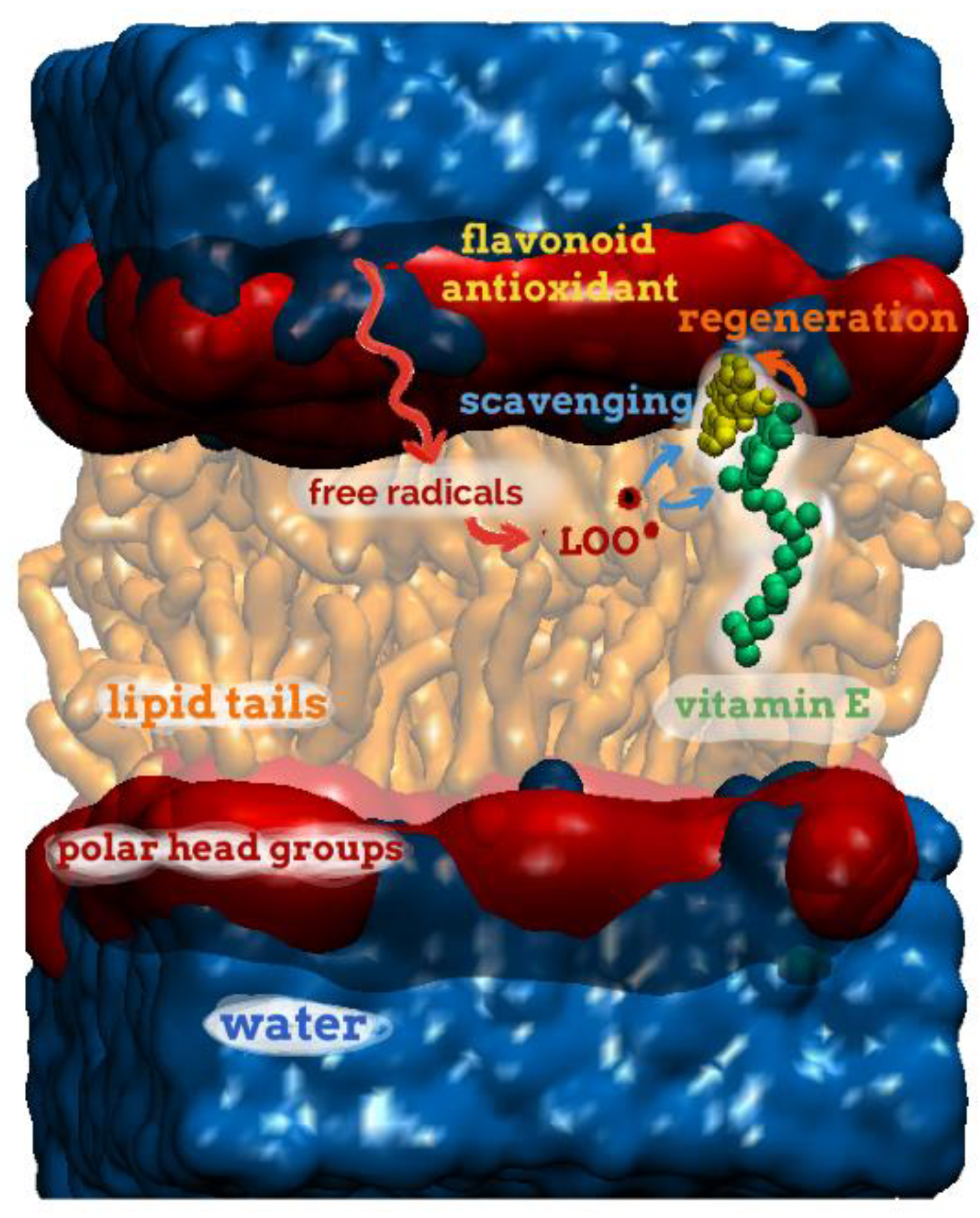
2.1. Definition of Oxidative Stress
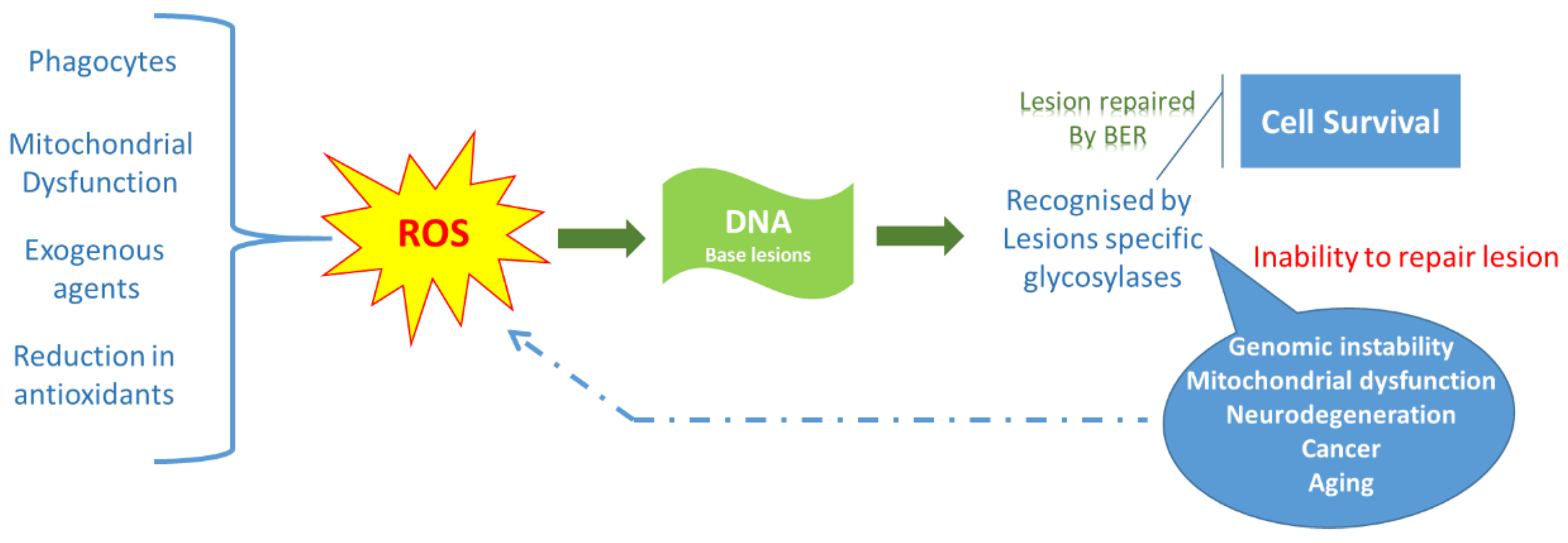
2.2. Reactive Oxygen Species
2.3. Sources of Reactive Oxygen Species
2.3.1. The Mitochondrial Respiratory Chain
2.3.2. NADPH Oxidase
2.3.3. The Xanthine Oxidase (XO) Pathway
2.3.4. Nitric Oxide Synthetases (NOS)
2.4. Antioxidant Factors
2.4.1. The Non-Enzymatic Antioxidant System
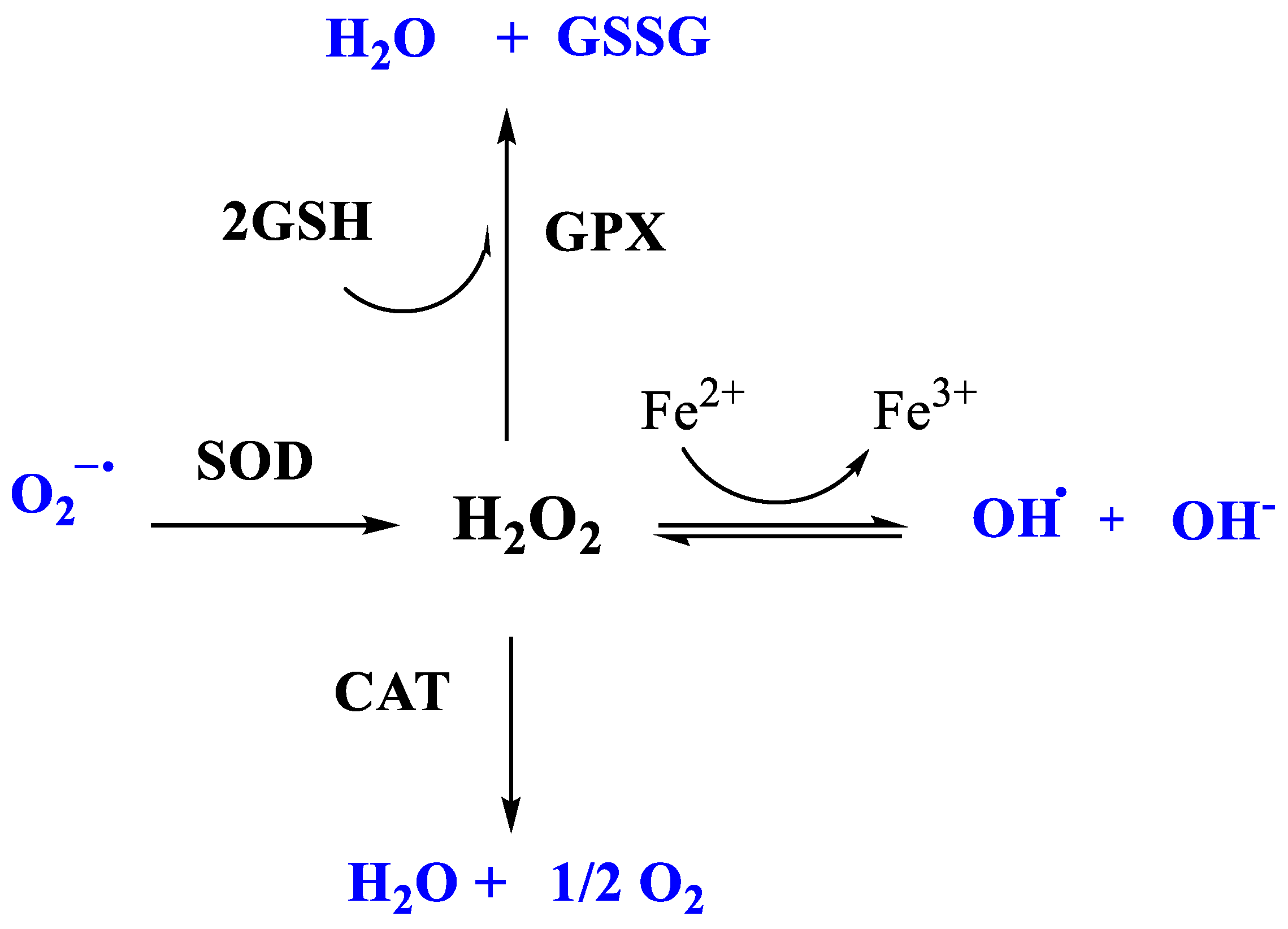
2.4.2. The Enzymatic-Protein Antioxidant System
- Superoxide Dismutase (SOD)
- b.
- Glutathione peroxidase (GPx)
- c.
- Catalase (CAT)
2.5. Measurement of Oxidative Stress
2.6. Preliminary Concepts for Oxidative Stress Models
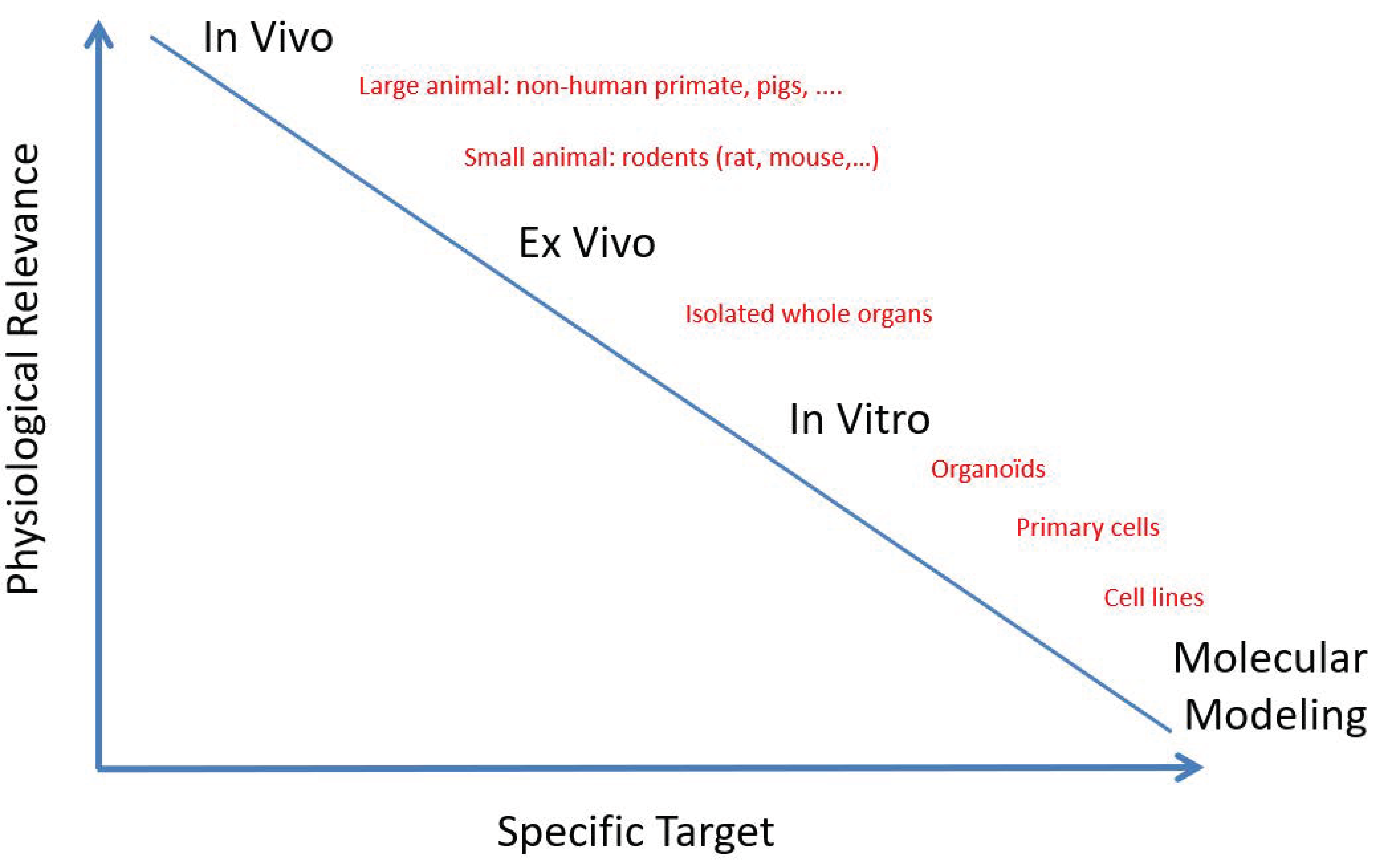
3. Models of Oxidative Stress
3.1. Animal Models of Oxidative Stress
- -
- Oxidative injuries specific to selected organs. For instance, in the eurotoxin 6-OHDA animal model, the compound is infused within the brain’s ventricular system. This induces depletion of the striatal dopamine, which in turn fosters the production of ROS, injuring neurons [36]. To target the gut, pure ethanol can be used to induce mucosal damage, fostering superoxide anion formation, lipid peroxidation, extracellular matrix degradation, and mitochondrial damage [37].
- -
- OS as a component of diabetes. It can be explored in alloxan-treated rodents. Alloxan reacts with disulfide bounds, of which the regulation involves the generation of H2O2. This also produces dialuric acid, further reacting with alloxan to generate ROS and cell death [38]. Other models include Streptozotocin-treated animals, specifically targeting β cells, inducing the depletion of cellular NAD+ and ATP, and promoting xanthine oxidase activation and ROS production.
- -
- Systemic OS. This can be obtained with tert-Butyl hydroperoxide (tBuOOH) [39]. Other approaches use hyperlipidemia, highlighted by an unhealthy diet with high fat (butter, cholesterol, etc.) and subsequent increased plasmatic total and LDL cholesterol levels. Our team determined that LDL oxidation could play a major role in IRI development. Indeed, a high level of LDL oxidation in a large animal model of kidney transplantation was shown to promote severe chronic injury, evidenced through interstitial fibrosis development [40], likely involving OxLDL-induced maladapted vascular repair [41].
3.2. Cellular Models of Oxidative Stress
Hypoxia-Reoxygenation Models
3.3. Ex Vivo Models
4. New Concepts in Oxidative Stress
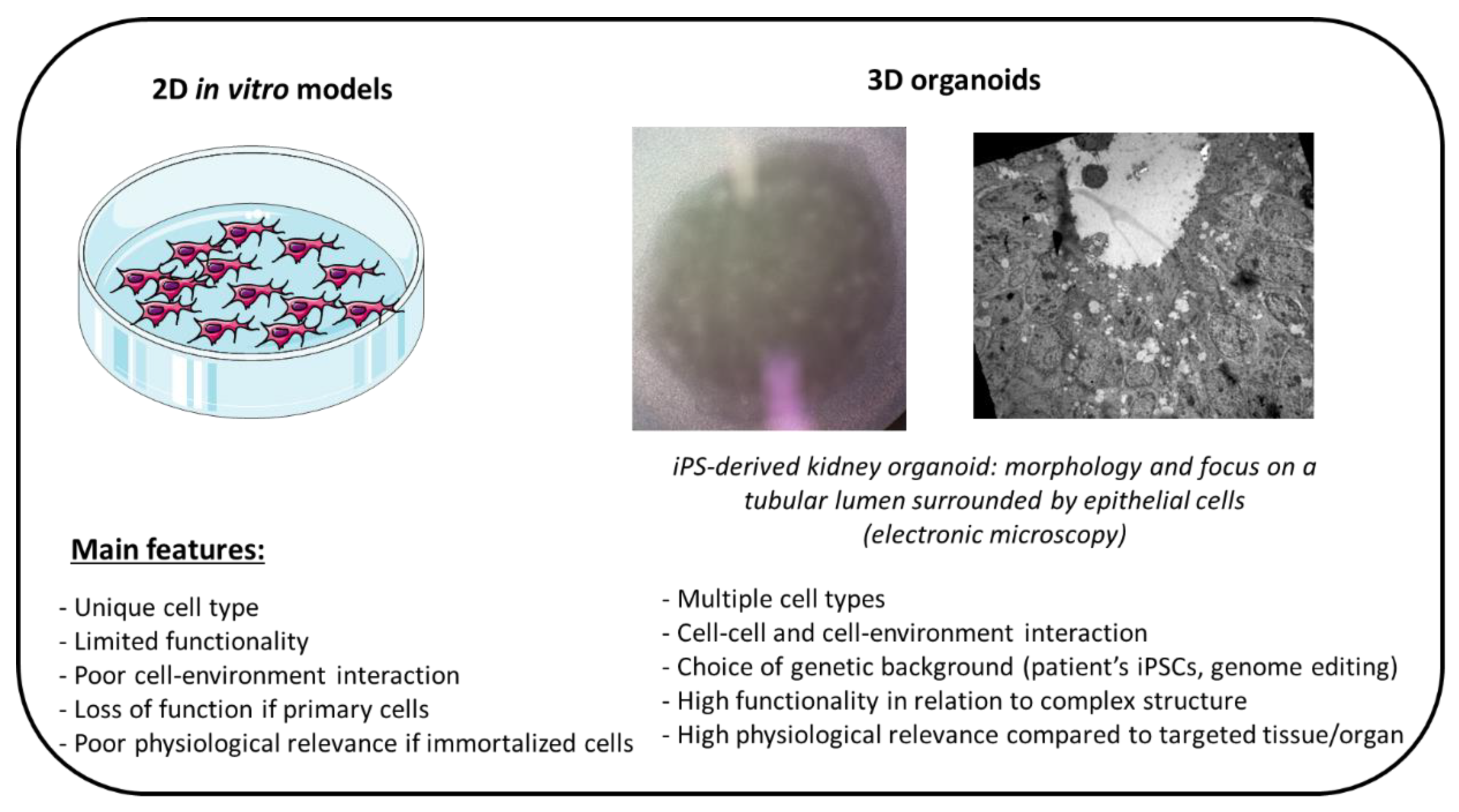
4.1. Cellular Bioengineering
4.2. Molecular Modelling
4.3. Mathematical Modeling at the Cell Level
4.3.1. Overview of Cell Redox Complexity Warranting Mathematical Modeling Combined with Quantitative Approaches.
4.3.2. A Brief Survey of Mathematical Modelling and Simulation of Cell Redox Biology
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen Peroxide as a Central Redox Signaling Molecule in Physiological Oxidative Stress: Oxidative Eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Cortese-Krott, M.M.; Koning, A.; Kuhnle, G.G.C.; Nagy, P.; Bianco, C.L.; Pasch, A.; Wink, D.A.; Fukuto, J.M.; Jackson, A.A.; Van Goor, H.; et al. The Reactive Species Interactome: Evolutionary Emergence, Biological Significance, and Opportunities for Redox Metabolomics and Personalized Medicine. Antioxid. Redox Signal. 2017, 27, 684–712. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.L.; Gruszczyk, A.V.; Beach, T.E.; Murphy, M.P.; Saeb-Parsy, K. Mitochondrial Mechanisms and Therapeutics in Ischaemia Reperfusion Injury. Pediatr. Nephrol. 2019, 34, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Pell, V.R.; Chouchani, E.T.; Frezza, C.; Murphy, M.P.; Krieg, T. Succinate Metabolism: A New Therapeutic Target for Myocardial Reperfusion Injury. Cardiovasc. Res. 2016, 111, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Pell, V.R.; Chouchani, E.T.; Murphy, M.P.; Brookes, P.S.; Krieg, T. Moving Forwards by Blocking Back-Flow: The Yin and Yang of MI Therapy. Circ. Res. 2016, 118, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijević, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic Accumulation of Succinate Controls Reperfusion Injury through Mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Zuk, A.; Bonventre, J.V. Acute Kidney Injury. Annu. Rev. Med. 2016, 67, 293–307. [Google Scholar] [CrossRef]
- Hsu, R.K.; Hsu, C.-Y. The Role of Acute Kidney Injury in Chronic Kidney Disease. Semin. Nephrol. 2016, 36, 283–292. [Google Scholar] [CrossRef]
- Bon, D.; Chatauret, N.; Giraud, S.; Thuillier, R.; Favreau, F.; Hauet, T. New Strategies to Optimize Kidney Recovery and Preservation in Transplantation. Nat. Rev. 2012, 8, 339–347. [Google Scholar] [CrossRef]
- Favreau, F.; Petit-Paris, I.; Hauet, T.; Dutheil, D.; Papet, Y.; Mauco, G.; Tallineau, C. Cyclooxygenase 1-Dependent Production of F2-Isoprostane and Changes in Redox Status during Warm Renal Ischemia-Reperfusion. Free Radic. Biol. Med. 2004, 36, 1034–1042. [Google Scholar] [CrossRef]
- Cadenas, E.; Davies, K.J. Mitochondrial Free Radical Generation, Oxidative Stress, and Aging. Free Radic. Biol. Med. 2000, 29, 222–230. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fiskum, G.; Schubert, D. Generation of Reactive Oxygen Species by the Mitochondrial Electron Transport Chain. J. Neurochem. 2002, 80, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Vazquez, E.J.; Moghaddas, S.; Hoppel, C.L.; Lesnefsky, E.J. Production of Reactive Oxygen Species by Mitochondria: Central Role of Complex III. J. Biol. Chem. 2003, 278, 36027–36031. [Google Scholar] [CrossRef] [PubMed]
- Turrens, J.F. Mitochondrial Formation of Reactive Oxygen Species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Griendling, K.K.; Sorescu, D.; Ushio-Fukai, M. NAD(P)H Oxidase: Role in Cardiovascular Biology and Disease. Circ. Res. 2000, 86, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Brandes, R.P.; Weissmann, N.; Schröder, K. NADPH Oxidases in Cardiovascular Disease. Free Radic. Biol. Med. 2010, 49, 687–706. [Google Scholar] [CrossRef]
- Octavia, Y.; Brunner-La Rocca, H.P.; Moens, A.L. NADPH Oxidase-Dependent Oxidative Stress in the Failing Heart: From Pathogenic Roles to Therapeutic Approach. Free Radic. Biol. Med. 2012, 52, 291–297. [Google Scholar] [CrossRef]
- Chambers, D.E.; Parks, D.A.; Patterson, G.; Roy, R.; McCord, J.M.; Yoshida, S.; Parmley, L.F.; Downey, J.M. Xanthine Oxidase as a Source of Free Radical Damage in Myocardial Ischemia. J. Mol. Cell. Cardiol. 1985, 17, 145–152. [Google Scholar] [CrossRef]
- McCord, J.M.; Roy, R.S.; Schaffer, S.W. Free Radicals and Myocardial Ischemia. The Role of Xanthine Oxidase. Adv. Myocardiol. 1985, 5, 183–189. [Google Scholar] [PubMed]
- Granger, D.N.; Kvietys, P.R. Reperfusion Injury and Reactive Oxygen Species: The Evolution of a Concept. Redox Biol. 2015, 6, 524–551. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Antioxidant Defence Mechanisms: From the Beginning to the End (of the Beginning). Free Radic. Res. 1999, 31, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Curin, Y.; Andriantsitohaina, R. Polyphenols as Potential Therapeutical Agents against Cardiovascular Diseases. Pharmacol. Rep. 2005, 57, 97–107. [Google Scholar] [PubMed]
- Alechinsky, L.; Favreau, F.; Cechova, P.; Inal, S.; Faye, P.-A.; Ory, C.; Thuillier, R.; Barrou, B.; Trouillas, P.; Guillard, J.; et al. Tannic Acid Improves Renal Function Recovery after Renal Warm Ischemia-Reperfusion in a Rat Model. Biomolecules 2020, 10, 439. [Google Scholar] [CrossRef]
- Soussi, D.; Danion, J.; Baulier, E.; Favreau, F.; Sauvageon, Y.; Bossard, V.; Matillon, X.; Turpin, F.; Belgsir, E.M.; Thuillier, R.; et al. Vectisol Formulation Enhances Solubility of Resveratrol and Brings Its Benefits to Kidney Transplantation in a Preclinical Porcine Model. Int. J. Mol. Sci. 2019, 20, 2268. [Google Scholar] [CrossRef] [PubMed]
- Caillaud, M.; Chantemargue, B.; Richard, L.; Vignaud, L.; Favreau, F.; Faye, P.-A.; Vignoles, P.; Sturtz, F.; Trouillas, P.; Vallat, J.-M.; et al. Local Low Dose Curcumin Treatment Improves Functional Recovery and Remyelination in a Rat Model of Sciatic Nerve Crush through Inhibition of Oxidative Stress. Neuropharmacology 2018, 139, 98–116. [Google Scholar] [CrossRef] [PubMed]
- Melov, S. Animal Models of Oxidative Stress, Aging, and Therapeutic Antioxidant Interventions. Int. J. Biochem. Cell Biol. 2002, 34, 1395–1400. [Google Scholar] [CrossRef]
- Held, J.M. Redox Systems Biology: Harnessing the Sentinels of the Cysteine Redoxome. Antioxid. Redox Signal. 2020, 32, 659–676. [Google Scholar] [CrossRef]
- Pillay, C.S.; Hofmeyr, J.-H.; Mashamaite, L.N.; Rohwer, J.M. From Top-down to Bottom-up: Computational Modeling Approaches for Cellular Redoxin Networks. Antioxid. Redox Signal. 2013, 18, 2075–2086. [Google Scholar] [CrossRef] [PubMed]
- Brandes, N.; Schmitt, S.; Jakob, U. Thiol-Based Redox Switches in Eukaryotic Proteins. Antioxid. Redox Signal. 2009, 11, 997–1014. [Google Scholar] [CrossRef]
- Tebay, L.E.; Robertson, H.; Durant, S.T.; Vitale, S.R.; Penning, T.M.; Dinkova-Kostova, A.T.; Hayes, J.D. Mechanisms of Activation of the Transcription Factor Nrf2 by Redox Stressors, Nutrient Cues, and Energy Status and the Pathways through Which It Attenuates Degenerative Disease. Free Radic. Biol. Med. 2015, 88, 108–146. [Google Scholar] [CrossRef] [PubMed]
- Kurutas, E.B. The Importance of Antioxidants Which Play the Role in Cellular Response against Oxidative/Nitrosative Stress: Current State. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Free Radicals and Antioxidants: Updating a Personal View. Nutr. Rev. 2012, 70, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Glutathione and Its Role in Cellular Functions. Free Radic. Biol. Med. 1999, 27, 916–921. [Google Scholar] [CrossRef]
- Tanaka, K.; Ogawa, N.; Asanuma, M. Molecular Basis of 6-Hydroxydopamine-Induced Caspase Activations Due to Increases in Oxidative Stress in the Mouse Striatum. Neurosci. Lett. 2006, 410, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Stalin, S.; Das, N.; Choudhury, S.T.; Ghosh, S.; Swarnakar, S. The Use of Nano-Quercetin to Arrest Mitochondrial Damage and MMP-9 Upregulation during Prevention of Gastric Inflammation Induced by Ethanol in Rat. Biomaterials 2012, 33, 2991–3001. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.-Y.; Jackson, G.M.; Jiao, X.; Chen, J.; Wu, J.-Z.; Huang, X.-S. Protection against Oxidative Stress in Diabetic Rats by Wheat Bran Feruloyl Oligosaccharides. J. Agric. Food Chem. 2007, 55, 3191–3195. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Hwang, J.-W.; Sung, S.-H.; Jeon, Y.-J.; Jeong, J.-H.; Jeon, B.-T.; Moon, S.-H.; Park, P.-J. Antioxidant Activity and Protective Effect of Extract of Celosia Cristata L. Flower on Tert-Butyl Hydroperoxide-Induced Oxidative Hepatotoxicity. Food Chem. 2015, 168, 572–579. [Google Scholar] [CrossRef]
- Chatauret, N.; Favreau, F.; Giraud, S.; Thierry, A.; Rossard, L.; Le Pape, S.; Lerman, L.O.; Hauet, T. Diet-Induced Increase in Plasma Oxidized LDL Promotes Early Fibrosis in a Renal Porcine Auto-Transplantation Model. J. Transl. Med. 2014, 12, 76. [Google Scholar] [CrossRef]
- Kerforne, T.; Favreau, F.; Khalifeh, T.; Maiga, S.; Allain, G.; Thierry, A.; Dierick, M.; Baulier, E.; Steichen, C.; Hauet, T. Hypercholesterolemia-Induced Increase in Plasma Oxidized LDL Abrogated pro Angiogenic Response in Kidney Grafts. J. Transl. Med. 2019, 17, 26. [Google Scholar] [CrossRef] [PubMed]
- Melis, N.; Thuillier, R.; Steichen, C.; Giraud, S.; Sauvageon, Y.; Kaminski, J.; Pelé, T.; Badet, L.; Richer, J.P.; Barrera-Chimal, J.; et al. Emerging Therapeutic Strategies for Transplantation-Induced Acute Kidney Injury: Protecting the Organelles and the Vascular Bed. Expert Opin. Ther. Targets 2019, 23, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Giraud, S.; Favreau, F.; Chatauret, N.; Thuillier, R.; Maiga, S.; Hauet, T. Contribution of Large Pig for Renal Ischemia-Reperfusion and Transplantation Studies: The Preclinical Model. J. Biomed. Biotechnol. 2011, 2011, 532127. [Google Scholar] [CrossRef] [PubMed]
- Pavlacky, J.; Polak, J. Technical Feasibility and Physiological Relevance of Hypoxic Cell Culture Models. Front. Endocrinol. 2020, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Schroecksnadel, S.; Gostner, J.; Zaknun, C.; Schennach, H.; Uberall, F.; Fuchs, D. Comparison of in Vitro Tests for Antioxidant and Immunomodulatory Capacities of Compounds. Phytomedicine 2014, 21, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Dufour, J.M. Cell Lines: Valuable Tools or Useless Artifacts. Spermatogenesis 2012, 2, 1–5. [Google Scholar] [CrossRef]
- Loi, M.; Trazzi, S.; Fuchs, C.; Galvani, G.; Medici, G.; Gennaccaro, L.; Tassinari, M.; Ciani, E. Increased DNA Damage and Apoptosis in CDKL5-Deficient Neurons. Mol. Neurobiol. 2020, 57, 2244–2262. [Google Scholar] [CrossRef]
- Li, C.; Jackson, R.M. Reactive Species Mechanisms of Cellular Hypoxia-Reoxygenation Injury. Am. J. Physiol. Cell Physiol. 2002, 282, C227–C241. [Google Scholar] [CrossRef] [PubMed]
- Plateel, M.; Dehouck, M.P.; Torpier, G.; Cecchelli, R.; Teissier, E. Hypoxia Increases the Susceptibility to Oxidant Stress and the Permeability of the Blood-Brain Barrier Endothelial Cell Monolayer. J. Neurochem. 1995, 65, 2138–2145. [Google Scholar] [CrossRef]
- Giraud, S.; Steichen, C.; Couturier, P.; Tillet, S.; Mallet, V.; Coudroy, R.; Goujon, J.-M.; Hannaert, P.; Hauet, T. Influence of Hypoxic Preservation Temperature on Endothelial Cells and Kidney Integrity. Biomed. Res. Int. 2019, 2019, 8572138. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Sánchez, J.; Chánez-Cárdenas, M.E. The Use of Cobalt Chloride as a Chemical Hypoxia Model. J. Appl. Toxicol. 2019, 39, 556–570. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, F.; Kong, X.; Gao, X.; Gu, Y.; Zhang, J. Oscillatory Shear Stress Induces Oxidative Stress via TLR4 Activation in Endothelial Cells. Mediat. Inflamm. 2019, 2019, 7162976. [Google Scholar] [CrossRef]
- Szczesny, S.E. Ex Vivo Models of Musculoskeletal Tissues. Connect. Tissue Res. 2020, 61, 245–247. [Google Scholar] [CrossRef]
- Giraud, S.; Thuillier, R.; Cau, J.; Hauet, T. In Vitro/Ex Vivo Models for the Study of Ischemia Reperfusion Injury during Kidney Perfusion. Int. J. Mol. Sci. 2020, 21, 8156. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, J.; Xia, T.C.; Xu, R.; He, X.; Xia, Y. Preservation Solutions for Kidney Transplantation: History, Advances and Mechanisms. Cell Transpl. 2019, 28, 1472–1489. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Hockemeyer, D.; Jaenisch, R. Induced Pluripotent Stem Cells Meet Genome Editing. Cell Stem Cell 2016, 18, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable Dual-RNA-Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Steichen, C.; Giraud, S.; Hauet, T. Combining Kidney Organoids and Genome Editing Technologies for a Better Understanding of Physiopathological Mechanisms of Renal Diseases: State of the Art. Front. Med. 2020, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Vergara, M.N.; Flores-Bellver, M.; Aparicio-Domingo, S.; McNally, M.; Wahlin, K.J.; Saxena, M.T.; Mumm, J.S.; Canto-Soler, M.V. Three-Dimensional Automated Reporter Quantification (3D-ARQ) Technology Enables Quantitative Screening in Retinal Organoids. Development 2017, 144, 3698–3705. [Google Scholar] [CrossRef] [PubMed]
- Njoroge, R.N.; Vatapalli, R.J.; Abdulkadir, S.A. Organoids Increase the Predictive Value of in Vitro Cancer Chemoprevention Studies for in Vivo Outcome. Front. Oncol. 2019, 9, 77. [Google Scholar] [CrossRef] [PubMed]
- Hartman, K.G.; Bortner, J.D.; Falk, G.W.; Yu, J.; Martín, M.G.; Rustgi, A.K.; Lynch, J.P. Modeling Inflammation and Oxidative Stress in Gastrointestinal Disease Development Using Novel Organotypic Culture Systems. Stem Cell Res. Ther. 2013, 4, S5. [Google Scholar] [CrossRef]
- Kalabis, J.; Wong, G.S.; Vega, M.E.; Natsuizaka, M.; Robertson, E.S.; Herlyn, M.; Nakagawa, H.; Rustgi, A.K. Isolation and Characterization of Mouse and Human Esophageal Epithelial Cells in 3D Organotypic Culture. Nat. Protoc. 2012, 7, 235–246. [Google Scholar] [CrossRef]
- Hale, L.J.; Howden, S.E.; Phipson, B.; Lonsdale, A.; Er, P.X.; Ghobrial, I.; Hosawi, S.; Wilson, S.; Lawlor, K.T.; Khan, S.; et al. 3D Organoid-Derived Human Glomeruli for Personalised Podocyte Disease Modelling and Drug Screening. Nat. Commun. 2018, 9, 5167. [Google Scholar] [CrossRef] [PubMed]
- Dey, M.; Ozbolat, I.T. 3D Bioprinting of Cells, Tissues and Organs. Sci. Rep. 2020, 10, 14023. [Google Scholar] [CrossRef]
- Datta, P.; Dey, M.; Ataie, Z.; Unutmaz, D.; Ozbolat, I.T. 3D Bioprinting for Reconstituting the Cancer Microenvironment. NPJ Precis. Oncol. 2020, 4, 18. [Google Scholar] [CrossRef]
- Fabre, G.; Bayach, I.; Berka, K.; Paloncýová, M.; Starok, M.; Rossi, C.; Duroux, J.-L.; Otyepka, M.; Trouillas, P. Synergism of Antioxidant Action of Vitamins E, C and Quercetin Is Related to Formation of Molecular Associations in Biomembranes. Chem. Commun. 2015, 51, 7713–7716. [Google Scholar] [CrossRef] [PubMed]
- Leopoldini, M.; Russo, N.; Toscano, M. The Molecular Basis of Working Mechanism of Natural Polyphenolic Antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Dangles, O.; Dufour, C.; Tonnelé, C.; Trouillas, P. The Physical Chemistry of Polyphenols: Insights into the Activity of Polyphenols in Humans at the Molecular Level. In Recent Advances in Polyphenol Research; Yoshida, K., Cheynier, V., Quideau, S., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2016; pp. 1–35. ISBN 978-1-118-88330-3. [Google Scholar]
- Trouillas, P.; Marsal, P.; Svobodová, A.; Vostálová, J.; Gazák, R.; Hrbác, J.; Sedmera, P.; Kren, V.; Lazzaroni, R.; Duroux, J.-L.; et al. Mechanism of the Antioxidant Action of Silybin and 2,3-Dehydrosilybin Flavonolignans: A Joint Experimental and Theoretical Study. J. Phys. Chem. A 2008, 112, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Trouillas, P.; Marsal, P.; Siri, D.; Lazzaroni, R.; Duroux, J.-L. A DFT Study of the Reactivity of OH Groups in Quercetin and Taxifolin Antioxidants: The Specificity of the 3-OH Site. Food Chem. 2006, 97, 679–688. [Google Scholar] [CrossRef]
- Richa, K.; Karmaker, R.; Ao, T.; Longkumer, N.; Singha, B.; Sinha, U.B. Rationale for Antioxidant Interaction Studies of 4-Bromo-1-Isothiocyanato-2-Methylbenzene—An Experimental and Computational Investigation. Chem. Phys. Lett. 2020, 753, 137611. [Google Scholar] [CrossRef]
- Lauberte, L.; Fabre, G.; Ponomarenko, J.; Dizhbite, T.; Evtuguin, D.V.; Telysheva, G.; Trouillas, P. Lignin Modification Supported by DFT-Based Theoretical Study as a Way to Produce Competitive Natural Antioxidants. Molecules 2019, 24, 1794. [Google Scholar] [CrossRef]
- Vacek, J.; Zatloukalová, M.; Desmier, T.; Nezhodová, V.; Hrbáč, J.; Kubala, M.; Křen, V.; Ulrichová, J.; Trouillas, P. Antioxidant, Metal-Binding and DNA-Damaging Properties of Flavonolignans: A Joint Experimental and Computational Highlight Based on 7-O-Galloylsilybin. Chem. Biol. Interact. 2013, 205, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Reza Nazifi, S.M.; Asgharshamsi, M.H.; Dehkordi, M.M.; Zborowski, K.K. Antioxidant Properties of Aloe Vera Components: A DFT Theoretical Evaluation. Free Radic. Res. 2019, 53, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Lingwood, M.; Hammond, J.R.; Hrovat, D.A.; Mayer, J.M.; Borden, W.T. MPW1K Performs Much Better than B3LYP in DFT Calculations on Reactions That Proceed by Proton-Coupled Electron Transfer (PCET). J. Chem. Theory Comput. 2006, 2, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Chiodo, S.G.; Leopoldini, M.; Russo, N.; Toscano, M. The Inactivation of Lipid Peroxide Radical by Quercetin. A Theoretical Insight. Phys. Chem. Chem. Phys. 2010, 12, 7662–7670. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, F.; Lemaur, V.; Cornil, J.; Lazzaroni, R.; Duroux, J.-L.; Olivier, Y.; Trouillas, P. Free Radical Scavenging by Natural Polyphenols: Atom versus Electron Transfer. J. Phys. Chem. A 2013, 117, 2082–2092. [Google Scholar] [CrossRef] [PubMed]
- Anouar, E.H.; Raweh, S.; Bayach, I.; Taha, M.; Baharudin, M.S.; Di Meo, F.; Hasan, M.H.; Adam, A.; Ismail, N.H.; Weber, J.-F.F.; et al. Antioxidant Properties of Phenolic Schiff Bases: Structure-Activity Relationship and Mechanism of Action. J. Comput. Aided Mol. Des. 2013, 27, 951–964. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, N.; Nakao, Y.; Sato, H.; Sakaki, S. Theoretical Study of Dioxygen Binding Process in Iron(III) Catechol Dioxygenase: “Oxygen Activation” vs “Substrate Activation”. J. Phys. Chem. B 2009, 113, 4826–4836. [Google Scholar] [CrossRef] [PubMed]
- Furia, E.; Marino, T.; Russo, N. Insights into the Coordination Mode of Quercetin with the Al(III) Ion from a Combined Experimental and Theoretical Study. Dalton Trans. 2014, 43, 7269–7274. [Google Scholar] [CrossRef]
- Kaviani, S.; Izadyar, M.; Housaindokht, M.R. A DFT Study on the Metal Ion Selectivity of Deferiprone Complexes. Comput. Biol. Chem. 2020, 86, 107267. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, F.; Fabre, G.; Berka, K.; Ossman, T.; Chantemargue, B.; Paloncýová, M.; Marquet, P.; Otyepka, M.; Trouillas, P. In Silico Pharmacology: Drug Membrane Partitioning and Crossing. Pharmacol. Res. 2016, 111, 471–486. [Google Scholar] [CrossRef] [PubMed]
- Ossman, T.; Fabre, G.; Trouillas, P. Interaction of Wine Anthocyanin Derivatives with Lipid Bilayer Membranes. Comput. Theor. Chem. 2016, 1077, 80–86. [Google Scholar] [CrossRef]
- Pyszková, M.; Biler, M.; Biedermann, D.; Valentová, K.; Kuzma, M.; Vrba, J.; Ulrichová, J.; Sokolová, R.; Mojović, M.; Popović-Bijelić, A.; et al. Flavonolignan 2,3-Dehydroderivatives: Preparation, Antiradical and Cytoprotective Activity. Free Radic. Biol. Med. 2016, 90, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Socrier, L.; Rosselin, M.; Gomez Giraldo, A.M.; Chantemargue, B.; Di Meo, F.; Trouillas, P.; Durand, G.; Morandat, S. Nitrone-Trolox Conjugate as an Inhibitor of Lipid Oxidation: Towards Synergistic Antioxidant Effects. Biochim. Biophys. Acta Biomembr. 2019, 1861, 1489–1501. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zhang, G.; Liao, Y.; Pan, J.; Gong, D. Dietary Flavonoids as Xanthine Oxidase Inhibitors: Structure-Affinity and Structure-Activity Relationships. J. Agric. Food Chem. 2015, 63, 7784–7794. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Huang, L.; Sun, C.; Zhao, D.; Tang, H. Studies on the Structure-Activity Relationship and Interaction Mechanism of Flavonoids and Xanthine Oxidase through Enzyme Kinetics, Spectroscopy Methods and Molecular Simulations. Food Chem. 2020, 323, 126807. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.; Xiang, H.; Lei, H.; Zhang, D.; Qiu, Y.; Xu, L. Inhibition and Molecular Mechanism of Diosmetin against Xanthine Oxidase by Multiple Spectroscopies and Molecular Docking. New J. Chem. 2020, 44, 6799–6809. [Google Scholar] [CrossRef]
- Tang, H.; Zhao, D. Investigation of the Interaction between Salvianolic Acid C and Xanthine Oxidase: Insights from Experimental Studies Merging with Molecular Docking Methods. Bioorg. Chem. 2019, 88, 102981. [Google Scholar] [CrossRef]
- Malik, N.; Dhiman, P.; Khatkar, A. In Silico Design and Synthesis of Targeted Curcumin Derivatives as Xanthine Oxidase Inhibitors. Curr. Drug Targets 2019, 20, 593–603. [Google Scholar] [CrossRef]
- Zeng, N.; Zhang, G.; Hu, X.; Pan, J.; Gong, D. Mechanism of Fisetin Suppressing Superoxide Anion and Xanthine Oxidase Activity. J. Funct. Foods 2019, 58, 1–10. [Google Scholar] [CrossRef]
- Santolini, J.; Wootton, S.A.; Jackson, A.A.; Feelisch, M. The Redox Architecture of Physiological Function. Curr. Opin. Physiol. 2019, 9, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-S.; Oldham, W.M.; Maron, B.A.; Loscalzo, J. Systems Biology Approaches to Redox Metabolism in Stress and Disease States. Antioxid. Redox Signal. 2018, 29, 953–972. [Google Scholar] [CrossRef] [PubMed]
- Buettner, G.R.; Wagner, B.A.; Rodgers, V.G.J. Quantitative Redox Biology: An Approach to Understand the Role of Reactive Species in Defining the Cellular Redox Environment. Cell Biochem. Biophys. 2013, 67, 477–483. [Google Scholar] [CrossRef]
- Tomar, N.; Sadri, S.; Cowley, A.W.; Yang, C.; Quryshi, N.; Pannala, V.R.; Audi, S.H.; Dash, R.K. A Thermodynamically-Constrained Mathematical Model for the Kinetics and Regulation of NADPH Oxidase 2 Complex-Mediated Electron Transfer and Superoxide Production. Free Radic. Biol. Med. 2019, 134, 581–597. [Google Scholar] [CrossRef]
- Collin, F. Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 2407. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Wang, R.-S.; Handy, D.E.; Loscalzo, J. NAD(H) and NADP(H) Redox Couples and Cellular Energy Metabolism. Antioxid. Redox Signal. 2018, 28, 251–272. [Google Scholar] [CrossRef]
- Selvaggio, G.; Coelho, P.M.B.M.; Salvador, A. Mapping the Phenotypic Repertoire of the Cytoplasmic 2-Cys Peroxiredoxin—Thioredoxin System. 1. Understanding Commonalities and Differences among Cell Types. Redox Biol. 2018, 15, 297–315. [Google Scholar] [CrossRef] [PubMed]
- Caravaca, M.; Sanchez-Andrada, P.; Soto-Meca, A. SimKinet: A Free Educational Tool Based on an Electrical Analogy to Solve Chemical Kinetic Equations. PLoS ONE 2019, 14, e0213302. [Google Scholar] [CrossRef] [PubMed]
- Benfeitas, R.; Selvaggio, G.; Antunes, F.; Coelho, P.M.B.M.; Salvador, A. Hydrogen Peroxide Metabolism and Sensing in Human Erythrocytes: A Validated Kinetic Model and Reappraisal of the Role of Peroxiredoxin II. Free Radic. Biol. Med. 2014, 74, 35–49. [Google Scholar] [CrossRef]
- An, G. In Silico Experiments of Existing and Hypothetical Cytokine-Directed Clinical Trials Using Agent-Based Modeling. Crit. Care Med. 2004, 32, 2050–2060. [Google Scholar] [CrossRef]
- Park, J.; Lee, J.; Choi, C. Mitochondrial Network Determines Intracellular ROS Dynamics and Sensitivity to Oxidative Stress through Switching Inter-Mitochondrial Messengers. PLoS ONE 2011, 6, e23211. [Google Scholar] [CrossRef]
- Abou-Jaoudé, W.; Traynard, P.; Monteiro, P.T.; Saez-Rodriguez, J.; Helikar, T.; Thieffry, D.; Chaouiya, C. Logical Modeling and Dynamical Analysis of Cellular Networks. Front. Genet. 2016, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Antunes, F.; Salvador, A.; Marinho, H.S.; Alves, R.; Pinto, R.E. Lipid Peroxidation in Mitochondrial Inner Membranes. I. An Integrative Kinetic Model. Free Radic. Biol. Med. 1996, 21, 917–943. [Google Scholar] [CrossRef]
- Noble, D. From the Hodgkin-Huxley Axon to the Virtual Heart. J. Physiol. 2007, 580, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Kembro, J.M.; Aon, M.A.; Winslow, R.L.; O’Rourke, B.; Cortassa, S. Integrating Mitochondrial Energetics, Redox and ROS Metabolic Networks: A Two-Compartment Model. Biophys. J. 2013, 104, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Selivanov, V.A.; Cascante, M.; Friedman, M.; Schumaker, M.F.; Trucco, M.; Votyakova, T.V. Multistationary and Oscillatory Modes of Free Radicals Generation by the Mitochondrial Respiratory Chain Revealed by a Bifurcation Analysis. PLoS Comput. Biol. 2012, 8, e1002700. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Selivanov, V.A.; Votyakova, T.V.; Pivtoraiko, V.N.; Zeak, J.; Sukhomlin, T.; Trucco, M.; Roca, J.; Cascante, M. Reactive Oxygen Species Production by Forward and Reverse Electron Fluxes in the Mitochondrial Respiratory Chain. PLoS Comput. Biol. 2011, 7, e1001115. [Google Scholar] [CrossRef] [PubMed]
- Altıntaş, A.; Davidsen, K.; Garde, C.; Mortensen, U.H.; Brasen, J.C.; Sams, T.; Workman, C.T. High-Resolution Kinetics and Modeling of Hydrogen Peroxide Degradation in Live Cells. Free Radic. Biol. Med. 2016, 101, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Schittenhelm, D.; Neuss-Radu, M.; Verma, N.; Pink, M.; Schmitz-Spanke, S. ROS and Pentose Phosphate Pathway: Mathematical Modelling of the Metabolic Regulation in Response to Xenobiotic-Induced Oxidative Stress and the Proposed Impact of the Gluconate Shunt. Free Radic. Res. 2019, 53, 979–992. [Google Scholar] [CrossRef]
- Korla, K. Reactive Oxygen Species and Energy Machinery: An Integrated Dynamic Model. J. Biomol. Struct. Dyn. 2016, 34, 1625–1640. [Google Scholar] [CrossRef] [PubMed]
- Marinho, H.S.; Real, C.; Cyrne, L.; Soares, H.; Antunes, F. Hydrogen Peroxide Sensing, Signaling and Regulation of Transcription Factors. Redox Biol. 2014, 2, 535–562. [Google Scholar] [CrossRef]
- Kinoshita, A.; Nakayama, Y.; Kitayama, T.; Tomita, M. Simulation Study of Methemoglobin Reduction in Erythrocytes. Differential Contributions of Two Pathways to Tolerance to Oxidative Stress. FEBS J. 2007, 274, 1449–1458. [Google Scholar] [CrossRef]
- Komalapriya, C.; Kaloriti, D.; Tillmann, A.T.; Yin, Z.; Herrero-de-Dios, C.; Jacobsen, M.D.; Belmonte, R.C.; Cameron, G.; Haynes, K.; Grebogi, C.; et al. Integrative Model of Oxidative Stress Adaptation in the Fungal Pathogen Candida Albicans. PLoS ONE 2015, 10, e0137750. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.C.; Edwards, A.; Layton, A.T. Impact of Nitric-Oxide-Mediated Vasodilation and Oxidative Stress on Renal Medullary Oxygenation: A Modeling Study. Am. J. Physiol. Renal Physiol. 2016, 310, F237–F247. [Google Scholar] [CrossRef] [PubMed]
- Mapuskar, K.A.; Wen, H.; Holanda, D.G.; Rastogi, P.; Steinbach, E.; Han, R.; Coleman, M.C.; Attanasio, M.; Riley, D.P.; Spitz, D.R.; et al. Persistent Increase in Mitochondrial Superoxide Mediates Cisplatin-Induced Chronic Kidney Disease. Redox Biol. 2019, 20, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Schleicher, J.; Dahmen, U. Computational Modeling of Oxidative Stress in Fatty Livers Elucidates the Underlying Mechanism of the Increased Susceptibility to Ischemia/Reperfusion Injury. Comput. Struct. Biotechnol. J. 2018, 16, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Benfeitas, R.; Uhlen, M.; Nielsen, J.; Mardinoglu, A. New Challenges to Study Heterogeneity in Cancer Redox Metabolism. Front. Cell Dev. Biol. 2017, 5, 65. [Google Scholar] [CrossRef]
| Name | Type | Selected Comments/Examples | |
|---|---|---|---|
| Endogenous | GSH | H | Major cell ChAO (1–10 mM concentration) |
| α-Lipoic acid | H, L |
ROS scavenging Transition metal chelation | |
| CoQ | L |
Inhibits lipid peroxidation Stabilizes ETC | |
| Bilirubin | H |
From heme degradation Potent against peroxyl radicals | |
| Uric acid | H |
From purine metabolism 2/3rd of plasma ROS scavenging | |
| Melanins |
Family of pigment (photoprotective AO) Eyes, skin | ||
| Melatonin |
“Sleep hormone” (pineal gland) Inhibits lipid peroxidation. Increase AO enzymes In mitochondria: increases ETC and reduces electron leakage | ||
| Exogenous | Vit C * | H | L-Ascorbate Very low standard 1st reduction (−282 mV) |
| Vit A * | L |
Retinol, retinoic acid Membrane–bound. Inhibits lipid peroxidation (Scavenge peroxyl radicals, LOO°). | |
| Vit E * | L |
α
-tocopherol Powerful membrane-bound AO Inhibits lipid peroxidation. Regenerated by ascorbic acid or CoQ. | |
| Carotenoids | L |
Plant origin (e.g., Lycopene). Inhibits lipid peroxidation. (scavenge peroxyl radicals, LOO°) | |
| Polyphenols | H, L |
Plant origin Flavonoids (e.g., Quercitin), Anthocyanins Strong inhibitors of lipid peroxidation | |
|
Oligo-elements (Zn, Se) | Na |
Competes with Fe and Cu (reduce OH° from H2O2) Protects SH groups from oxidation. Reduces the activities of iNOS and NADPH oxidase. Inhibits lipid peroxidation. |
| Name | Target, Mechanism | Comment, Examples | |
|---|---|---|---|
| SOD | Superoxide dismutase | O2°− → H2O2, O2 | Considered “1st line” AO enzyme. SOD1, CuZnSOD (cytosol) SOD2, MnSOD (mitochondria) |
| CAT | Catalase | H2O2 → H2O, O2 | Mostly in peroxisome |
| GPx | Glutathione peroxidase | Peroxides: H2O2, ROOH | 2 forms: Se-dpdt and Se-indepdt. GPx-1 (cytosol, mitochondria) GPx-3 (extracellular) |
| Trx | Thioredoxin | Reduce other proteins by cysteine thiol-disulfide exchange | Maintains/regulates the reduced state of many redox proteins. Trx1 (cytosol), Trx2 (mitochondria) |
| TrxR | Trx reductase | Reduce Trx | Only enzymes able to reduce Trx. NADPH e- transferred via TrxR to Trx active site |
| Prx | Peroxiredoxin | H2O2 reduction to H2O | Regenerated by Trx. Prxd1 (cytosol, nucleus), Prxd3 (mitochondria) |
| Ferritin | Ferritin | Iron-binding (limits Fe(II)) | Intracellular. Stores iron Reduces OH°-producing (Fe(II)-dependent) |
| Alb | Albumin | Met and Cys residues (account for 40–80% of AO activity of HSA) | Alb: 20–25% of plasma ROS-scavenging capacities |
| Models | Species | Interests | Limits |
|---|---|---|---|
| Animal | Mouse Rat Pig Non-human primates Others |
-Integrative models -Mimic human pathophysiology -Mimic potential severity of diseases -Allow longer follow-up -Systemic and remote effects -Availability of genetically modified models -Required by regulatory authorities before starting clinical studies -Availability of biological materials |
-Variability, inconsistency -Low reproducibility -Possible high mortality rate -Low survival rate in early phase -Few or no efficiency markers (no cell specific markers) -Expensive and delicate maintenance -Housing structure required -Ethical aspects -Strain creation may be difficult and expensive |
| Cells | Rat Mouse Human Others |
-Cell of human origin -Results often generalizable -Cell immortalization -Cryopreservation -Preservation of phenotypic characteristics (primary cultures but low level of division) related to cell-specific function -Economic and possible infinite growth -Possibility to modify the genetic background (using genome editing) -Controlled conditions and easy maintenance -Good reproducibility -Overcomes ethical aspects -Large volume of data | -Tedious to harvest (primary cultures) -Loss of specific function during expansion for primary cells -Poor biological relevance for immortalized cells -Cross-contamination -Difficulty in optimizing cross-talk, cell-matrix and cell-to-cell interaction -No microenvironment and immune influence |
| Formalism | Principle | Entities Addressed | Software or Environment | Pros and Cons |
|---|---|---|---|---|
| Equation-based modeling (EBM) | (1) Equations driving the system are written: (i) Kinetics(reaction rates) (ii) Dynamics (ODE’s, PDE’s) (iii) Mass conservation (2) Boundary conditions are set (3) Numerical integration is performed allowing to monitor model variables | Concentration of species One single compartment, or several communicating compartments Best adapted to chemical biochemical reaction networks where properties and kinetic parameters are established | Cell-Designer COPASI Berleley–Madonna Simulink (Matlab) (see also FEM software *) | Very mature methodology: Quantitative, accurate, straightforward Numerous, powerful and versatile software Provides steady-state and quantitative dynamic information Requires (numerous) kinetic parameters Parameters can diverge from in situ (spatially organized situations, crowding…) Assumes spatial homogeneity in each compartment |
| Agent-based modeling (ABM) | Represents discrete entities (agents) Each agent defined by its own variables, functions, and interactions with other agents and the environment | Cells and different cell types simultaneously Cell compartments Molecules Cellular and/or molecular environment Best adapted to multiple, discrete interacting molecular and/or cellular systems | NetLogo Repast Swarm MASON | By nature, assumes discreteness, hetero- geneity and compart- ments (closer to biology) Requires much less parameter values than EBM Mature methodology Relatively straightforward, with an intuitive GUI (NetLogo); otherwise requires programming skills (JAVA, C++, Python) Qualitative dynamic properties Non-deterministic (requires repeated runs and statistical analysis) |
| Logic-based modeling (LBM) | Interactions are cast in a network, in which nodes represent abstractions of biological com-ponents (level of activity, concentration) Can be boolean (binary) or multivalued Transition between states calculated from logical rules (e.g., “if A & B, then C”) | Can be -molecules, -cells, -pathophysiological phenotypes Best adapted to complex signaling and transduction pathways, and gene expression networks | GINSim GNA CellNetAnalyzer (see CoLoMoTo) | Requires much less parametric values than EBM Software still “rare” and usually not user-friendly (but very active community, see CoLoMoTo) Qualitative dynamic properties Complex exploitation and analysis Dynamic transition scheme must be chosen: synchronous/deterministic vs. asynchronous/non- deterministic) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chazelas, P.; Steichen, C.; Favreau, F.; Trouillas, P.; Hannaert, P.; Thuillier, R.; Giraud, S.; Hauet, T.; Guillard, J. Oxidative Stress Evaluation in Ischemia Reperfusion Models: Characteristics, Limits and Perspectives. Int. J. Mol. Sci. 2021, 22, 2366. https://doi.org/10.3390/ijms22052366
Chazelas P, Steichen C, Favreau F, Trouillas P, Hannaert P, Thuillier R, Giraud S, Hauet T, Guillard J. Oxidative Stress Evaluation in Ischemia Reperfusion Models: Characteristics, Limits and Perspectives. International Journal of Molecular Sciences. 2021; 22(5):2366. https://doi.org/10.3390/ijms22052366
Chicago/Turabian StyleChazelas, Pauline, Clara Steichen, Frédéric Favreau, Patrick Trouillas, Patrick Hannaert, Raphaël Thuillier, Sébastien Giraud, Thierry Hauet, and Jérôme Guillard. 2021. "Oxidative Stress Evaluation in Ischemia Reperfusion Models: Characteristics, Limits and Perspectives" International Journal of Molecular Sciences 22, no. 5: 2366. https://doi.org/10.3390/ijms22052366
APA StyleChazelas, P., Steichen, C., Favreau, F., Trouillas, P., Hannaert, P., Thuillier, R., Giraud, S., Hauet, T., & Guillard, J. (2021). Oxidative Stress Evaluation in Ischemia Reperfusion Models: Characteristics, Limits and Perspectives. International Journal of Molecular Sciences, 22(5), 2366. https://doi.org/10.3390/ijms22052366







