Derma-Hc, a New Developed Herbal Formula, Ameliorates Cutaneous Lichenification in Atopic Dermatitis
Abstract
1. Introduction
2. Results
2.1. Effect of Derma-Hc on Morphological Phenotype and Dermatitis Score
2.2. Effect of Derma-Hc on Scratching Behavior
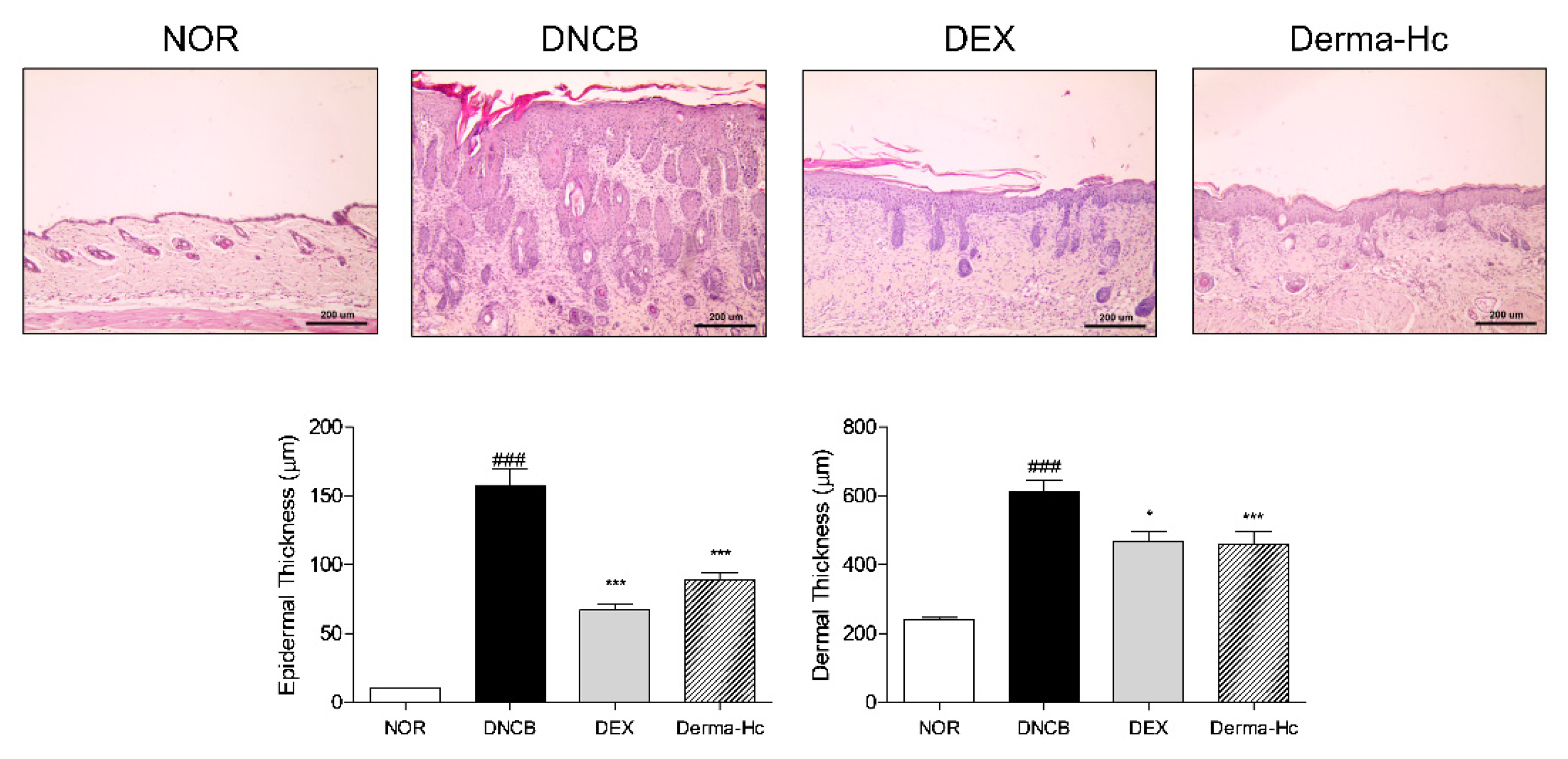
2.3. Effect of Derma-Hc on Skin Thickness
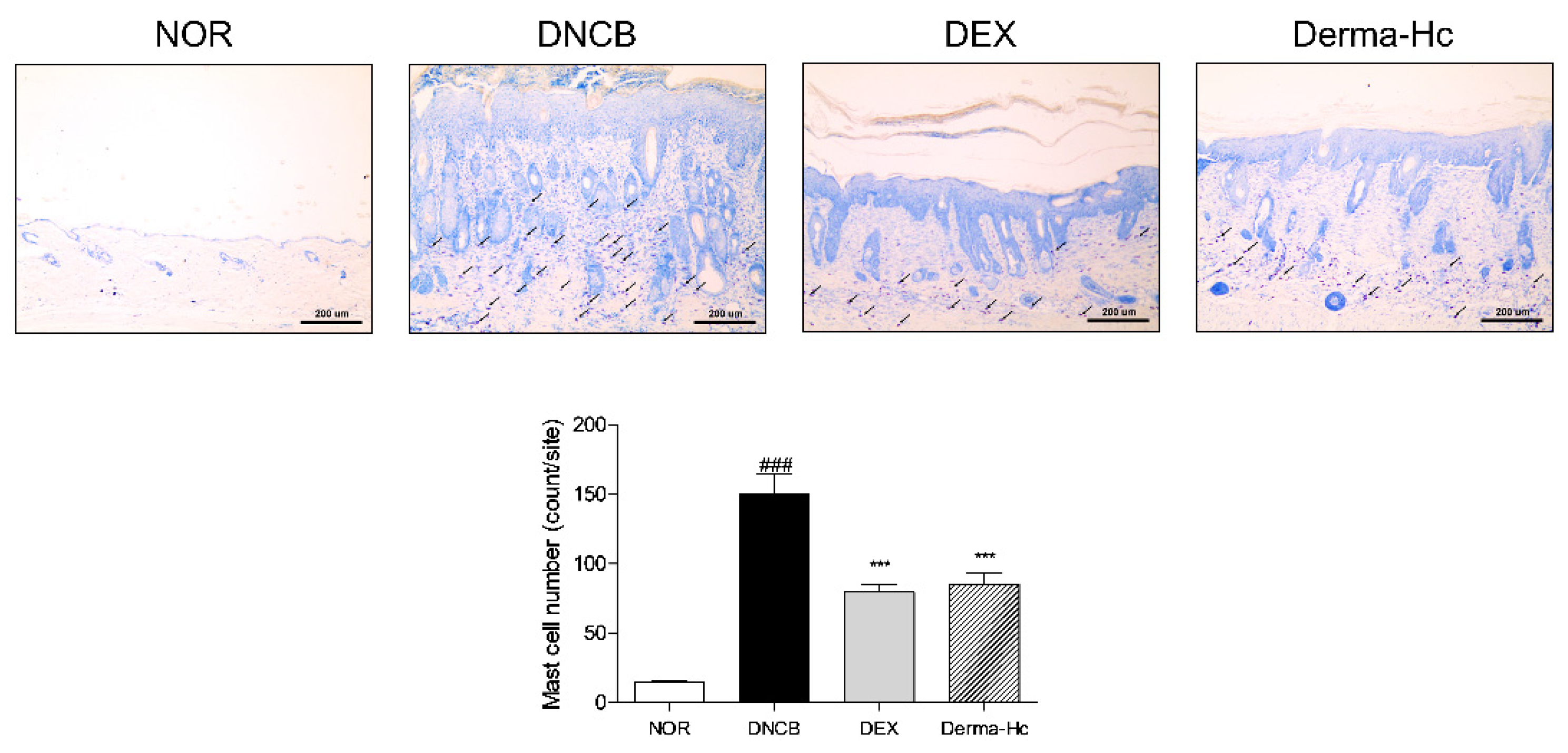
2.4. Effect of Derma-Hc on Mast Cell Infiltration
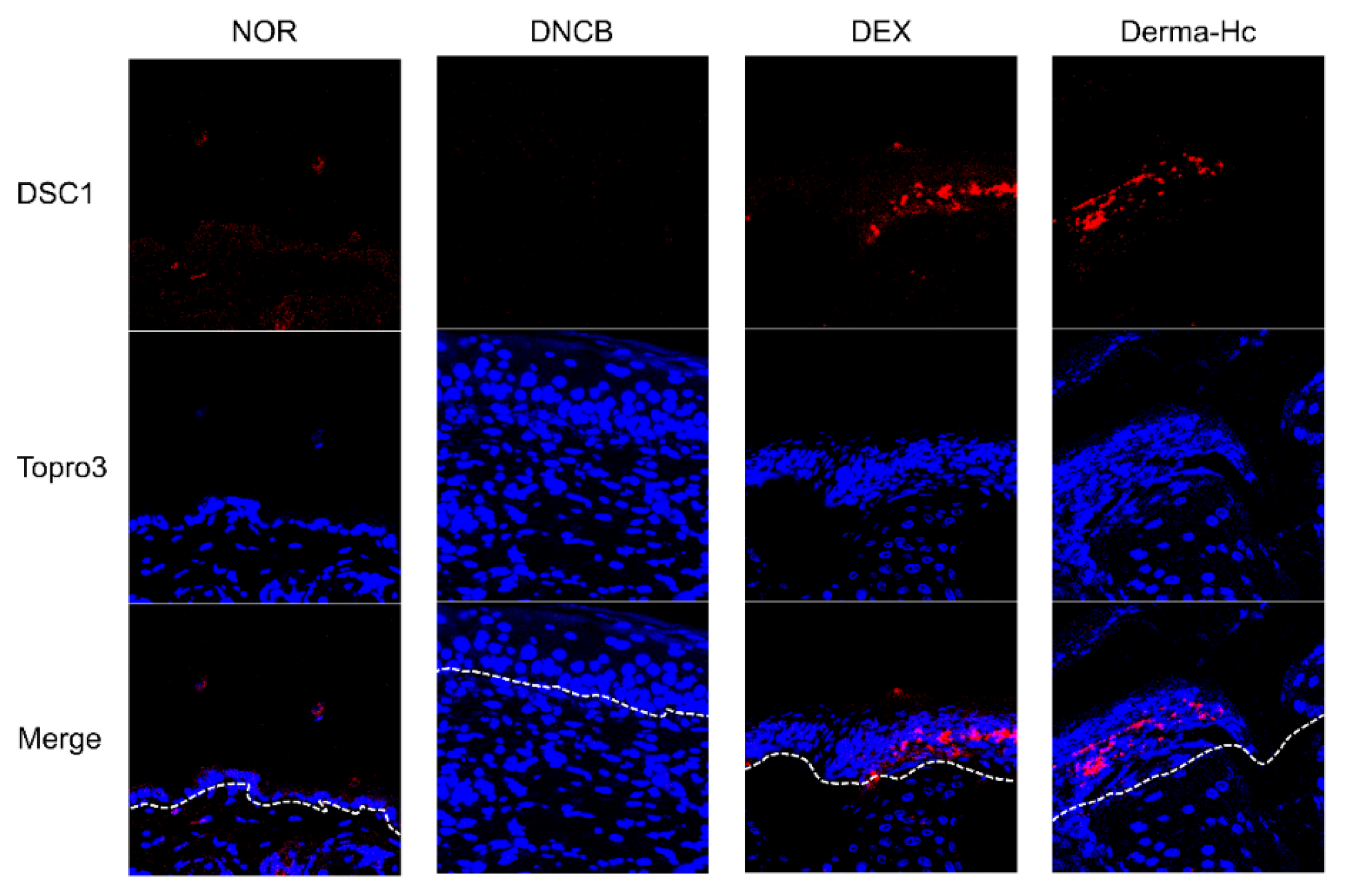
2.5. Effect of Derma-Hc on Expression Level of DSC1
2.6. Effect of Derma-Hc on Expression Level of FLG
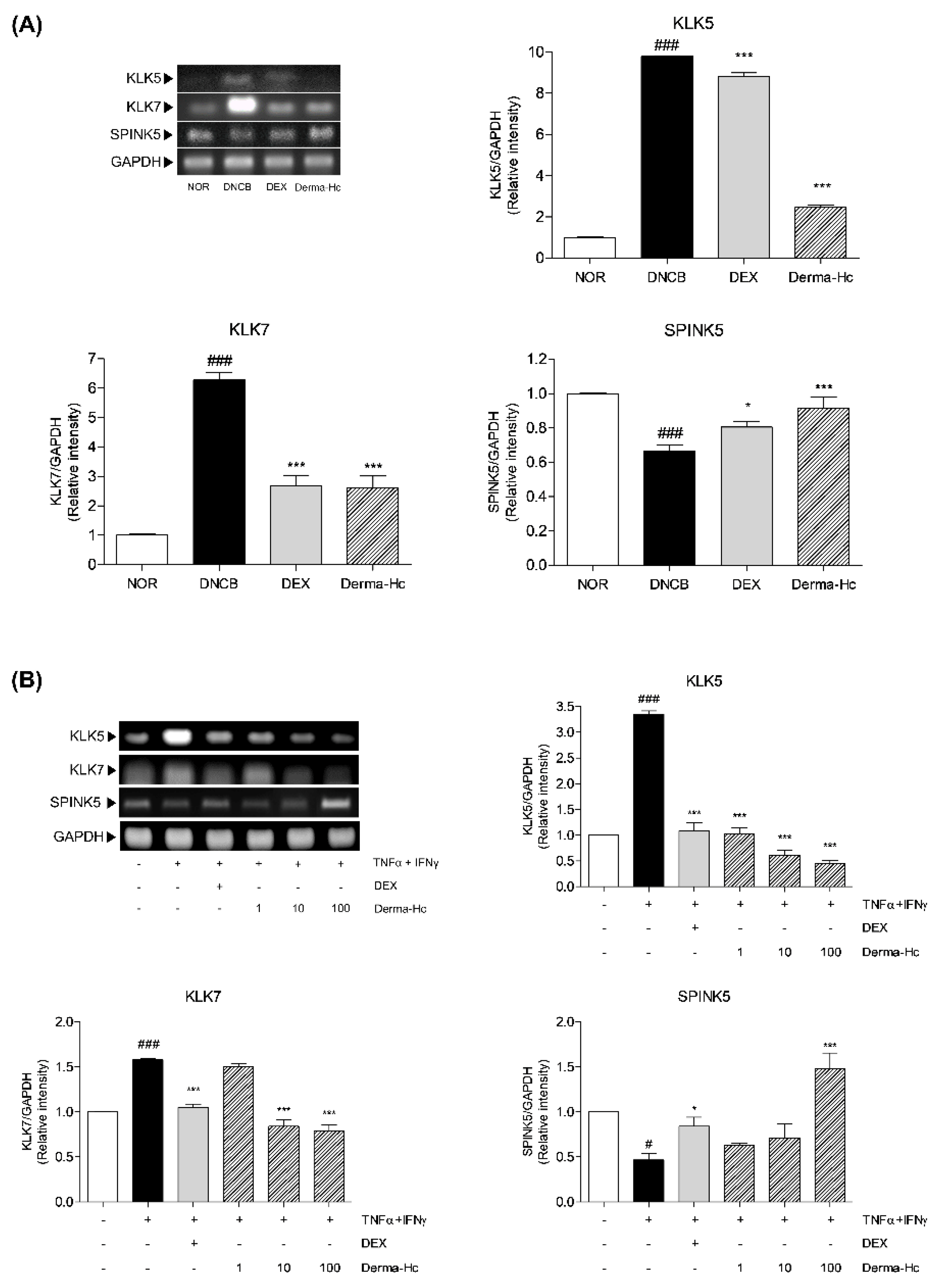
2.7. Effect of Derma-Hc on Expression Levels of Keratinocyte Differentiation-Related Factors
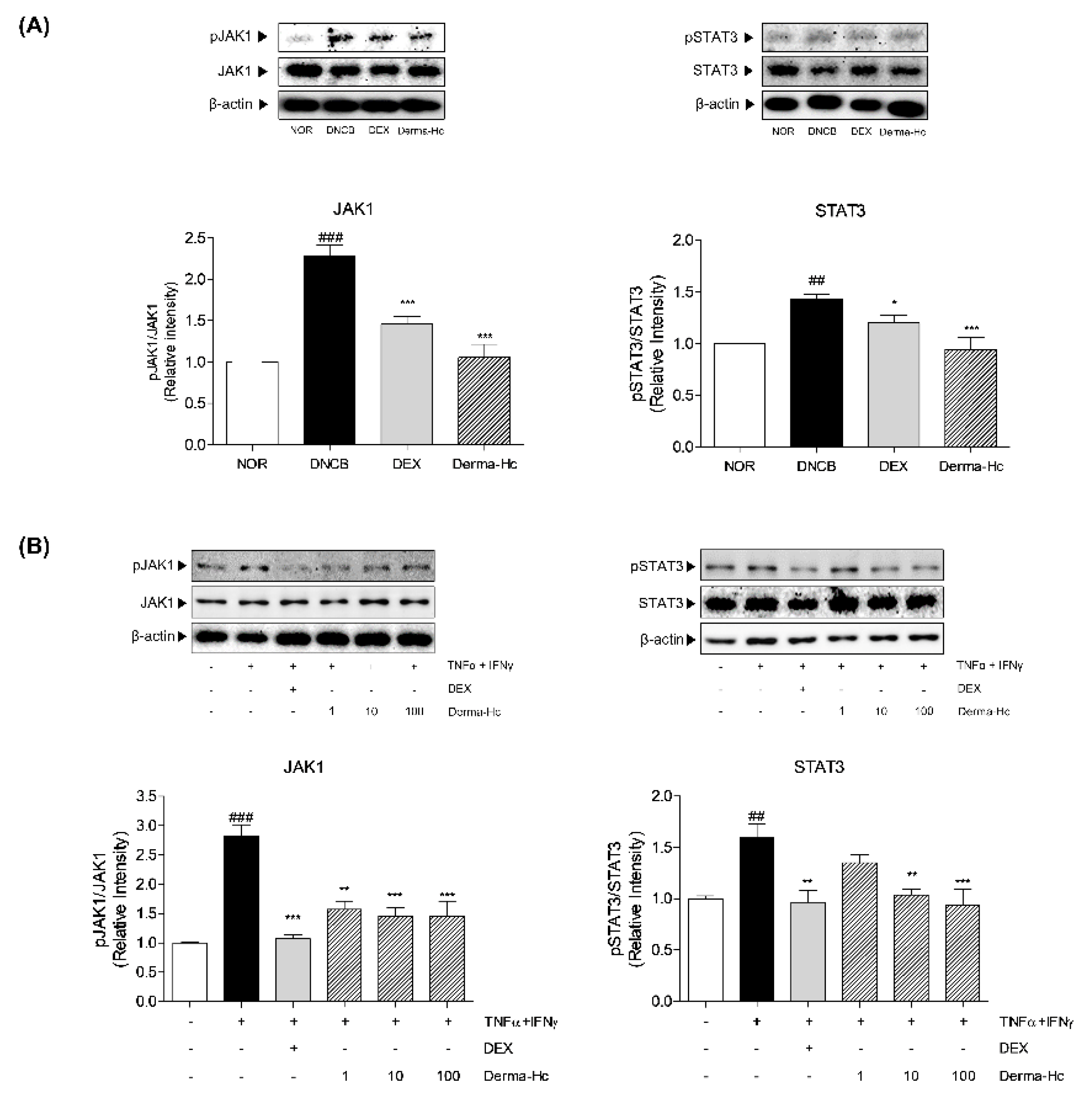
2.8. Effect of Derma-Hc on Expression Levels of JAK1-STAT3 Signal Pathway
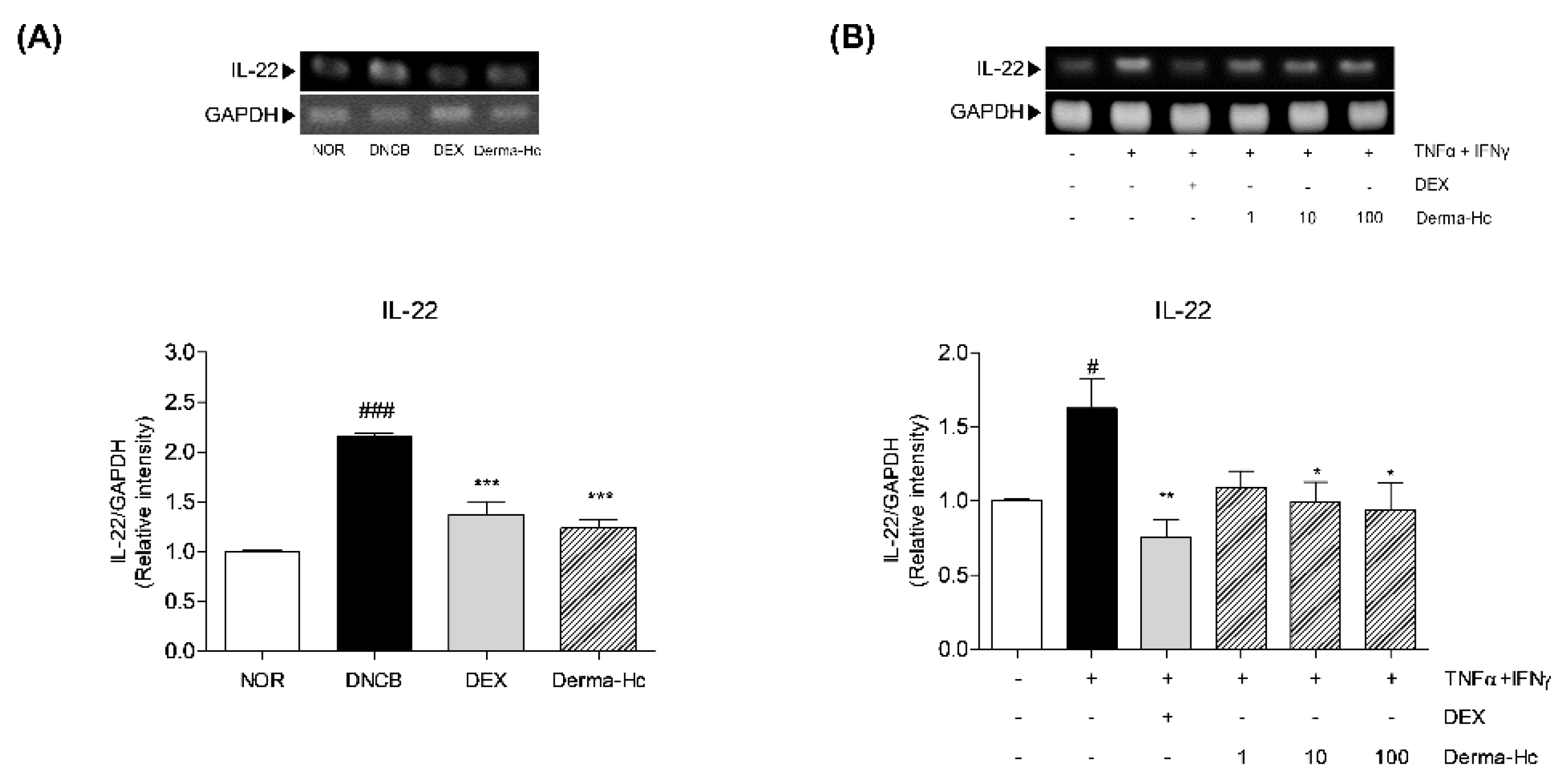
2.9. Effect of Derma-Hc on Expression Level of IL-22
3. Discussion
4. Materials and Methods
4.1. Derma-Hc Preparation
4.2. UPLC-ESI-QTOF MS/MS Analysis for Identification of Derma-Hc
4.3. Animal Treatment
4.4. Evaluation of Dermatitis Severity
4.5. Measurement of Scratching Behavior Frequency
4.6. Histopathological Examination
4.7. Immunofluorescence Analysis
4.8. Cell Culture
4.9. Western Blot Analysis
4.10. Reverse Transcription-Polymerase Chain Reaction (RT-PCR) Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Atopic dermatitis |
| AL | Arctium lappa |
| AM | Astragalus membranaceus |
| AS | Angelica sinensis |
| CP | Cryptotympana pustulata |
| DEX | Dexamethasone |
| DNCB | 2,4-Dinitrochlorobenzene |
| FLG | Filaggrin |
| IFN-γ | Interferon-gamma |
| Ig | Immunoglobulin |
| IL | Interleukin |
| JAK1 | Janus kinase 1 |
| KLK5 | Kallikrein-related peptidase 5 |
| KLK7 | Kallikrein-related peptidase 7 |
| QTOF | Quadrupole time-of-flight |
| RT | Retention time |
| SPINK5 | Serine protease inhibitor Kazal-type 5 |
| ST | Schizonepeta tenuifolia |
| STAT3 | Signal transducer and activator of transcription 3 |
| Th | T helper |
| TNF-α | Tumor necrosis factor-alpha |
References
- Jeon, J.H.; Kwon, S.C.; Park, D.; Shin, S.; Jeong, J.H.; Park, S.Y.; Hwang, S.Y.; Kim, Y.B.; Joo, S.S. Anti-allergic effects of white rose petal extract and anti-atopic properties of its hexane fraction. Arch. Pharm. Res. 2009, 32, 823–830. [Google Scholar] [CrossRef]
- Nettis, E.; Ortoncelli, M.; Pellacani, G.; Foti, C.; Di Leo, E.; Patruno, C.; Rongioletti, F.; Argenziano, G.; Ferrucci, S.M.; Macchia, L.; et al. A Multicenter Study on the Prevalence of Clinical Patterns and Clinical Phenotypes in Adult Atopic Dermatitis. J. Investig. Allergol. Clin. Immunol. 2020, 30, 448–450. [Google Scholar] [CrossRef]
- Lee, J.H.; Ki, H.H.; Kim, D.K.; Lee, Y.M. Triticum aestivum sprout extract attenuates 2,4dinitrochlorobenzeneinduced atopic dermatitislike skin lesions in mice and the expression of chemokines in human keratinocytes. Mol. Med. Rep. 2018, 18, 3461–3468. [Google Scholar] [CrossRef]
- De Benedetto, A.; Agnihothri, R.; McGirt, L.Y.; Bankova, L.G.; Beck, L.A. Atopic dermatitis: A disease caused by innate immune defects? J. Investig. Dermatol. 2009, 129, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Bylund, S.; von Kobyletzki, L.B.; Svalstedt, M.; Svensson, A. Prevalence and Incidence of Atopic Dermatitis: A Systematic Review. Acta Derm. Venereol. 2020, 100, adv00160. [Google Scholar] [CrossRef]
- Yarbrough, K.B.; Neuhaus, K.J.; Simpson, E.L. The effects of treatment on itch in atopic dermatitis. Dermatol. Ther. 2013, 26, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Carter, K.F.; Dufour, L.T.; Ballard, C.N. Identifying secondary skin lesions. Nursing 2004, 34, 68. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Lu, J.; Choi, E.B.; Oh, M.H.; Jeong, M.; Barmettler, S.; Zhu, Z.; Zheng, T. Expression of IL-22 in the Skin Causes Th2-Biased Immunity, Epidermal Barrier Dysfunction, and Pruritus via Stimulating Epithelial Th2 Cytokines and the GRP Pathway. J. Immunol. 2017, 198, 2543–2555. [Google Scholar] [CrossRef] [PubMed]
- Eyerich, K.; Eyerich, S. Th22 cells in allergic disease. Allergo J. Int. 2015, 24, 1–7. [Google Scholar] [CrossRef]
- Kawakami, T.; Ando, T.; Kimura, M.; Wilson, B.S.; Kawakami, Y. Mast cells in atopic dermatitis. Curr. Opin. Immunol. 2009, 21, 666–678. [Google Scholar] [CrossRef]
- Amin, K. The role of mast cells in allergic inflammation. Respir. Med. 2012, 106, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.X.; Xu Landen, N. New insights into T cells and their signature cytokines in atopic dermatitis. IUBMB Life 2015, 67, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Fujita, H. The role of IL-22 and Th22 cells in human skin diseases. J. Dermatol. Sci. 2013, 72, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Rerknimitr, P.; Otsuka, A.; Nakashima, C.; Kabashima, K. The etiopathogenesis of atopic dermatitis: Barrier disruption, immunological derangement, and pruritus. Inflamm. Regen. 2017, 37, 14. [Google Scholar] [CrossRef]
- Caubet, C.; Jonca, N.; Brattsand, M.; Guerrin, M.; Bernard, D.; Schmidt, R.; Egelrud, T.; Simon, M.; Serre, G. Degradation of corneodesmosome proteins by two serine proteases of the kallikrein family, SCTE/KLK5/hK5 and SCCE/KLK7/hK7. J. Invest. Dermatol. 2004, 122, 1235–1244. [Google Scholar] [CrossRef]
- Simpson, E.L. Atopic dermatitis: A review of topical treatment options. Curr. Med. Res. Opin. 2010, 26, 633–640. [Google Scholar] [CrossRef]
- Tidwell, W.J.; Fowler, J.F., Jr. T-cell inhibitors for atopic dermatitis. J. Am. Acad. Dermatol. 2018, 78, S67–S70. [Google Scholar] [CrossRef]
- Thomsen, S.F. Atopic dermatitis: Natural history, diagnosis, and treatment. ISRN Allergy 2014, 2014, 354250. [Google Scholar] [CrossRef] [PubMed]
- Dattola, A.; Bennardo, L.; Silvestri, M.; Nistico, S.P. What’s new in the treatment of atopic dermatitis? Dermatol. Ther. 2019, 32, e12787. [Google Scholar] [CrossRef]
- Mostow, E. A systematic review of the safety and efficacy of systemic corticosteroids in atopic dermatitis. J. Am. Acad. Dermatol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Thaci, D.; Salgo, R. Malignancy concerns of topical calcineurin inhibitors for atopic dermatitis: Facts and controversies. Clin. Dermatol. 2010, 28, 52–56. [Google Scholar] [CrossRef]
- Jeon, Y.C. Treatment for an Adult Patient with Psoriasis with Traditional Korean Medicine, Especially Sa-Am Acupuncture and Herbal Medicine. J. Acupunct. Meridian Stud. 2016, 9, 88–92. [Google Scholar] [CrossRef]
- Jo, S.Y.; Kim, M.H.; Lee, H.; Lee, S.H.; Yang, W.M. Ameliorative and Synergic Effects of Derma-H, a New Herbal Formula, on Allergic Contact Dermatitis. Front. Pharmacol. 2020, 11, 1019. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, M.H.; Yang, G.; Huh, Y.; Kim, S.H.; Yang, W.M. Effects of topical application of Astragalus membranaceus on allergic dermatitis. Immunopharmacol. Immunotoxicol. 2013, 35, 151–156. [Google Scholar] [CrossRef]
- Choi, Y.Y.; Kim, M.H.; Kim, J.H.; Jung, H.S.; Sohn, Y.; Choi, Y.J.; Hwang, M.K.; Kim, S.H.; Kim, J.; Yang, W.M. Schizonepeta tenuifolia inhibits the development of atopic dermatitis in mice. Phytother. Res. 2013, 27, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Lin, Y.H.; Huang, J.W.; Chen, Y.C. Chinese herbal medicine network and core treatments for allergic skin diseases: Implications from a nationwide database. J. Ethnopharmacol. 2015, 168, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Choi, Y.Y.; Kim, M.H.; Han, J.M.; Lee, J.E.; Kim, E.H.; Hong, J.; Kim, J.; Yang, W.M. Topical Application of Angelica sinensis Improves Pruritus and Skin Inflammation in Mice with Atopic Dermatitis-Like Symptoms. J. Med. Food 2016, 19, 98–105. [Google Scholar] [CrossRef]
- Sohn, E.H.; Jang, S.A.; Joo, H.; Park, S.; Kang, S.C.; Lee, C.H.; Kim, S.Y. Anti-allergic and anti-inflammatory effects of butanol extract from Arctium lappa L. Clin. Mol. Allergy 2011, 9, 4. [Google Scholar] [CrossRef]
- Petersen, T.K. In vivo pharmacological disease models for psoriasis and atopic dermatitis in drug discovery. Basic Clin. Pharmacol. Toxicol. 2006, 99, 104–115. [Google Scholar] [CrossRef]
- Kim, J.; Kim, B.E.; Leung, D.Y.M. Pathophysiology of atopic dermatitis: Clinical implications. Allergy Asthma Proc. 2019, 40, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L. Diagnostic clinical features of atopic dermatitis. Indian J. Dermatol. Venereol. Leprol. 2001, 67, 25–27. [Google Scholar] [PubMed]
- Vender, R.B. Alternative treatments for atopic dermatitis: A selected review. Skin Ther. Lett. 2002, 7, 1–5. [Google Scholar]
- Zhu, Y.; Yu, X.; Ge, Q.; Li, J.; Wang, D.; Wei, Y.; Ouyang, Z. Antioxidant and anti-aging activities of polysaccharides from Cordyceps cicadae. Int. J. Biol. Macromol. 2020, 157, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.S.; Cheng, L.N.; Wu, J.H.; Chan, E.; Kwan, Y.W.; Lee, S.M.; Leung, G.P.; Yu, P.H.; Chan, S.W. A review of the pharmacological effects of Arctium lappa (burdock). Inflammopharmacology 2011, 19, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Hanifin, J.M.; Thurston, M.; Omoto, M.; Cherill, R.; Tofte, S.J.; Graeber, M. The eczema area and severity index (EASI): Assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp. Dermatol. 2001, 10, 11–18. [Google Scholar] [CrossRef]
- Ikoma, A.; Steinhoff, M.; Stander, S.; Yosipovitch, G.; Schmelz, M. The neurobiology of itch. Nat. Rev. Neurosci. 2006, 7, 535–547. [Google Scholar] [CrossRef]
- Rinaldi, G. The Itch-Scratch Cycle: A Review of the Mechanisms. Dermatol. Pract. Concept. 2019, 9, 90–97. [Google Scholar] [CrossRef]
- Arima, K.; Ohta, S.; Takagi, A.; Shiraishi, H.; Masuoka, M.; Ontsuka, K.; Suto, H.; Suzuki, S.; Yamamoto, K.; Ogawa, M.; et al. Periostin contributes to epidermal hyperplasia in psoriasis common to atopic dermatitis. Allergol. Int. 2015, 64, 41–48. [Google Scholar] [CrossRef]
- Matsunaga, Y.; Ogura, Y.; Ehama, R.; Amano, S.; Nishiyama, T.; Tagami, H. Establishment of a mouse skin model of the lichenification in human chronic eczematous dermatitis. Br. J. Dermatol. 2007, 156, 884–891. [Google Scholar] [CrossRef]
- Nakahigashi, K.; Kabashima, K.; Ikoma, A.; Verkman, A.S.; Miyachi, Y.; Hara-Chikuma, M. Upregulation of aquaporin-3 is involved in keratinocyte proliferation and epidermal hyperplasia. J. Investig. Dermatol. 2011, 131, 865–873. [Google Scholar] [CrossRef]
- Gu, H.; Kim, W.H.; An, H.J.; Kim, J.Y.; Gwon, M.G.; Han, S.M.; Leem, J.; Park, K.K. Therapeutic effects of bee venom on experimental atopic dermatitis. Mol. Med. Rep. 2018, 18, 3711–3718. [Google Scholar] [CrossRef]
- Irani, A.M.; Sampson, H.A.; Schwartz, L.B. Mast cells in atopic dermatitis. Allergy 1989, 44 (Suppl. 9), 31–34. [Google Scholar] [CrossRef] [PubMed]
- Sehra, S.; Serezani, A.P.M.; Ocana, J.A.; Travers, J.B.; Kaplan, M.H. Mast Cells Regulate Epidermal Barrier Function and the Development of Allergic Skin Inflammation. J. Investig. Dermatol. 2016, 136, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Donetti, E.; Bedoni, M.; Boschini, E.; Dellavia, C.; Barajon, I.; Gagliano, N. Desmocollin 1 and desmoglein 1 expression in human epidermis and keratinizing oral mucosa: A comparative immunohistochemical and molecular study. Arch. Dermatol. Res. 2005, 297, 31–38. [Google Scholar] [CrossRef]
- Totsuka, A.; Omori-Miyake, M.; Kawashima, M.; Yagi, J.; Tsunemi, Y. Expression of keratin 1, keratin 10, desmoglein 1 and desmocollin 1 in the epidermis: Possible downregulation by interleukin-4 and interleukin-13 in atopic dermatitis. Eur. J. Dermatol. 2017, 27, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Amagai, M. Dissecting the formation, structure and barrier function of the stratum corneum. Int. Immunol. 2015, 27, 269–280. [Google Scholar] [CrossRef]
- Sandilands, A.; Sutherland, C.; Irvine, A.D.; McLean, W.H. Filaggrin in the frontline: Role in skin barrier function and disease. J. Cell Sci. 2009, 122, 1285–1294. [Google Scholar] [CrossRef]
- O’Regan, G.M.; Sandilands, A.; McLean, W.H.; Irvine, A.D. Filaggrin in atopic dermatitis. J. Allergy Clin. Immunol. 2009, 124, R2–R6. [Google Scholar] [CrossRef]
- Brown, S.J.; McLean, W.H. One remarkable molecule: Filaggrin. J. Investig. Dermatol. 2012, 132, 751–762. [Google Scholar] [CrossRef]
- Scharschmidt, T.C.; Man, M.Q.; Hatano, Y.; Crumrine, D.; Gunathilake, R.; Sundberg, J.P.; Silva, K.A.; Mauro, T.M.; Hupe, M.; Cho, S.; et al. Filaggrin deficiency confers a paracellular barrier abnormality that reduces inflammatory thresholds to irritants and haptens. J. Allergy Clin. Immunol. 2009, 124, 496–506.e6. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, S.H. Epidermal permeability barrier defects and barrier repair therapy in atopic dermatitis. Allergy Asthma Immunol. Res. 2014, 6, 276–287. [Google Scholar] [CrossRef]
- Ekholm, I.E.; Brattsand, M.; Egelrud, T. Stratum corneum tryptic enzyme in normal epidermis: A missing link in the desquamation process? J. Investig. Dermatol. 2000, 114, 56–63. [Google Scholar] [CrossRef]
- Morizane, S. The Role of Kallikrein-Related Peptidases in Atopic Dermatitis. Acta Med. Okayama 2019, 73, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Egawa, G.; Kabashima, K. Multifactorial skin barrier deficiency and atopic dermatitis: Essential topics to prevent the atopic march. J. Allergy Clin. Immunol. 2016, 138, 350–358.e1. [Google Scholar] [CrossRef] [PubMed]
- Fridman, J.S.; Scherle, P.A.; Collins, R.; Burn, T.; Neilan, C.L.; Hertel, D.; Contel, N.; Haley, P.; Thomas, B.; Shi, J.; et al. Preclinical evaluation of local JAK1 and JAK2 inhibition in cutaneous inflammation. J. Investig. Dermatol. 2011, 131, 1838–1844. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Guttman-Yassky, E. JAK Inhibitors for Atopic Dermatitis: An Update. Am. J. Clin. Dermatol. 2019, 20, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Fukada, T.; Nishida, K.; Nakayama, M.; Matsuda, M.; Miura, I.; Dainichi, T.; Fukuda, S.; Kabashima, K.; Nakaoka, S.; et al. Hyperactivation of JAK1 tyrosine kinase induces stepwise, progressive pruritic dermatitis. J. Clin. Investig. 2016, 126, 2064–2076. [Google Scholar] [CrossRef]
- Brunner, P.M.; Pavel, A.B.; Khattri, S.; Leonard, A.; Malik, K.; Rose, S.; Jim On, S.; Vekaria, A.S.; Traidl-Hoffmann, C.; Singer, G.K.; et al. Baseline IL-22 expression in patients with atopic dermatitis stratifies tissue responses to fezakinumab. J. Allergy Clin. Immunol. 2019, 143, 142–154. [Google Scholar] [CrossRef]
- Welsch, K.; Holstein, J.; Laurence, A.; Ghoreschi, K. Targeting JAK/STAT signalling in inflammatory skin diseases with small molecule inhibitors. Eur. J. Immunol. 2017, 47, 1096–1107. [Google Scholar] [CrossRef]
- Lejeune, D.; Dumoutier, L.; Constantinescu, S.; Kruijer, W.; Schuringa, J.J.; Renauld, J.C. Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. J. Biol. Chem. 2002, 277, 33676–33682. [Google Scholar] [CrossRef]
- Bernard, F.X.; Morel, F.; Camus, M.; Pedretti, N.; Barrault, C.; Garnier, J.; Lecron, J.C. Keratinocytes under Fire of Proinflammatory Cytokines: Bona Fide Innate Immune Cells Involved in the Physiopathology of Chronic Atopic Dermatitis and Psoriasis. J. Allergy 2012, 2012, 718725. [Google Scholar] [CrossRef] [PubMed]
- Hanel, K.H.; Cornelissen, C.; Luscher, B.; Baron, J.M. Cytokines and the skin barrier. Int. J. Mol. Sci. 2013, 14, 6720–6745. [Google Scholar] [CrossRef] [PubMed]



| Gene | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|
| IL-22 | TGAGTGAGCGCTGCTATCTG | TGTGCTTAGCCTGTTGCTGA |
| FLG | TGAAGCCTATGACACCACTGA | TCCCCTACGCTTTCTTGTCCT |
| KLK5 | GCCACACTGCAGGAAGAAA | GGATTTGACCCCCTGGAA |
| KLK7 | GCATCCCCGACTCCAAGAA | CAGGGTACCTCTGCACACCAA |
| SPINK5 | GATCCTATTGAGGGTCTAGAT | ATTACCATGTGTCTTGCCATC |
| GAPDH | GGCATGGACTGTGGTCATGA | TTCACCACCATGGAGAAGGC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, Y.K.; Kim, M.H.; Ha, I.J.; Yang, W.M. Derma-Hc, a New Developed Herbal Formula, Ameliorates Cutaneous Lichenification in Atopic Dermatitis. Int. J. Mol. Sci. 2021, 22, 2359. https://doi.org/10.3390/ijms22052359
Nam YK, Kim MH, Ha IJ, Yang WM. Derma-Hc, a New Developed Herbal Formula, Ameliorates Cutaneous Lichenification in Atopic Dermatitis. International Journal of Molecular Sciences. 2021; 22(5):2359. https://doi.org/10.3390/ijms22052359
Chicago/Turabian StyleNam, Yeon Kyung, Mi Hye Kim, In Jin Ha, and Woong Mo Yang. 2021. "Derma-Hc, a New Developed Herbal Formula, Ameliorates Cutaneous Lichenification in Atopic Dermatitis" International Journal of Molecular Sciences 22, no. 5: 2359. https://doi.org/10.3390/ijms22052359
APA StyleNam, Y. K., Kim, M. H., Ha, I. J., & Yang, W. M. (2021). Derma-Hc, a New Developed Herbal Formula, Ameliorates Cutaneous Lichenification in Atopic Dermatitis. International Journal of Molecular Sciences, 22(5), 2359. https://doi.org/10.3390/ijms22052359









