A Bioinformatics Model of Human Diseases on the Basis of Differentially Expressed Genes (of Domestic Versus Wild Animals) That Are Orthologs of Human Genes Associated with Reproductive-Potential Changes
Abstract
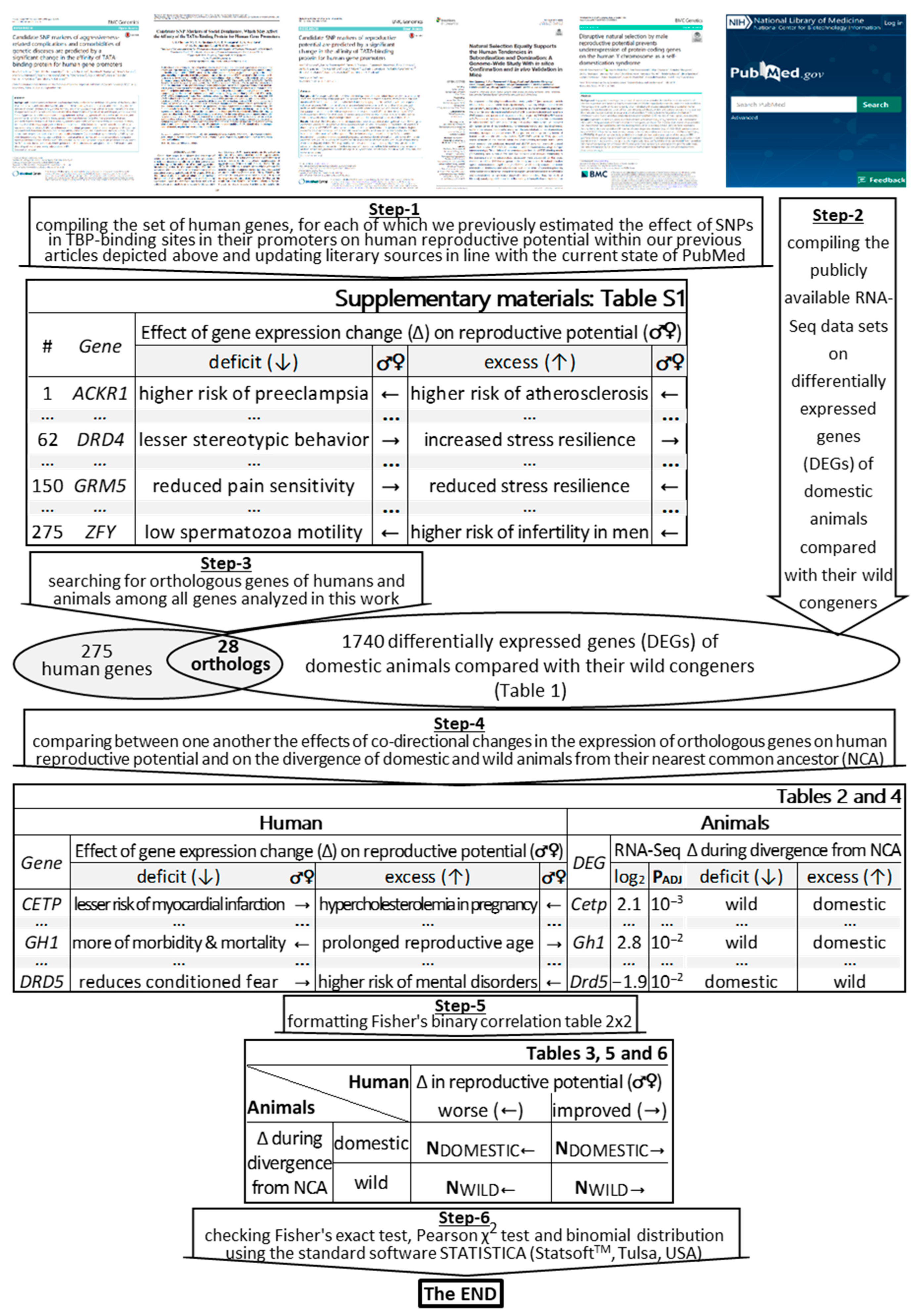
1. Introduction
2. Results and Discussion
2.1. The Bioinformatics Model Developed
2.2. DEGs of the Guinea Pig Versus Cavy and How Their Human Orthologous Genes Change Reproductive Potential
2.3. DEGs of Domestic Versus Wild Animals and How the Human Orthologous Genes Alter Reproductive Potential
2.4. DEGs in Domestic Animals Reliably Correspond to Their Human Orthologs Reducing Reproductive Potential
3. Materials and Methods
3.1. Human Genes under Study
3.2. DEGs of Domestic Animals Compared to Their Wild Congeners
3.3. Statistical Analysis
3.4. The Knowledge Base on Domestic Animals’ DEGs Whose Human Orthologous Genes Can Change Reproductive Potential
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DEG | differentially expressed gene |
| NCA | nearest common ancestor |
| SNP | single-nucleotide polymorphism |
| WHO | World Health Organization |
References
- Dobzhansky, T. Genetic entities in hominid evolution. In Classification and Human Evolution; Washburn, S.L., Ed.; Aldine Publishing Co.: Chicago, IL, USA, 1963; pp. 347–362. [Google Scholar]
- Darwin, C.R. A Naturalist’s Voyage Round the World: The Voyage of the Beagle; John Murray Publishing: London, UK, 1913. [Google Scholar]
- Henry, J.P. Genetics and origin of Homo sapiens. Med. Sci. 2019, 35, 39–45. [Google Scholar]
- Darwin, C.R. On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life; John Murray Publishing: London, UK, 1859. [Google Scholar]
- Hammond, T.T.; Ortiz-Jimenez, C.A.; Smith, J.E. Anthropogenic change alters ecological relationships via interactive changes in stress physiology and behavior within and among organisms. Integr. Comp. Biol. 2020, 60, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Charuta, A.; Mankowska-Pliszka, H.; Bartyzel, B.J.; Wysocki, J. Size of heart of the domestic Pekin duck (Anas platyrhynchos f. domestica) and wild duck (Anas platyrhynchos, Linnaeus, 1758). Acta Sci. Pol. Med. Vet. 2005, 4, 11–19. [Google Scholar]
- Barquera, S.; Pedroza-Tobias, A.; Medina, C.; Hernandez-Barrera, L.; Bibbins-Domingo, K.; Lozano, R.; Moran, A.E. Global overview of the epidemiology of atherosclerotic cardiovascular disease. Arch. Med. Res. 2015, 46, 328–338. [Google Scholar] [CrossRef]
- McGonigle, P.; Ruggeri, B. Animal models of human disease: Challenges in enabling translation. Biochem. Pharmacol. 2014, 87, 1621–1671. [Google Scholar] [CrossRef]
- Lutz, C.; Maher, L.; Lee, C.; Kang, W. COVID-19 preclinical models: Human angiotensin-converting enzyme 2 transgenic mice. Hum. Genom. 2020, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Mayr, E. Systematics and the Origin of Species, from the Viewpoint of a Zoologist; Harvard University Press: Cambridge, MA, USA, 1942. [Google Scholar]
- World Health Organization. Towards more objectivity in diagnosis and management of male infertility: Results of a World Health Organization multicentre study. Int. J. Androl. 1987, 7, 1–53. [Google Scholar]
- Hanson, B.M.; Hotaling, J.M. Limitations and opportunities in male fertility databases. Transl. Urol. 2018, 7, S292–S302. [Google Scholar] [CrossRef] [PubMed]
- Bellott, D.W.; Hughes, J.F.; Skaletsky, H.; Brown, L.G.; Pyntikova, T.; Cho, T.-J.; Koutseva, N.; Zaghlul, S.; Graves, T.; Rock, S.; et al. Mammalian Y chromosomes retain widely expressed dosage-sensitive regulators. Nature 2014, 508, 494–499. [Google Scholar] [CrossRef]
- Chapman, R.N. Animal Ecology with Special Reference to Insects; McGraw-Hill Book Co.: New York, NY, USA, 1931. [Google Scholar]
- Pianka, E.R. Natural selection of optimal reproductive tactics. Am. Zool. 1976, 16, 775–784. [Google Scholar] [CrossRef]
- du Fosse, N.A.; van der Hoorn, M.P.; van Lith, J.M.M.; le Cessie, S.; Lashley, E.E.L.O. Advanced paternal age is associated with an increased risk of spontaneous miscarriage: A systematic review and meta-analysis. Hum. Reprod. Update 2020, 26, 650–669. [Google Scholar] [CrossRef] [PubMed]
- Lam, D. How the world survived the population bomb: Lessons from 50 years of extraordinary demographic history. Demography 2011, 48, 1231–1262. [Google Scholar] [CrossRef]
- Lorenz, K. On Aggression; Psychology Press: Hove, UK, 2002. [Google Scholar]
- Michopoulos, V.; Higgins, M.; Toufexis, D.; Wilson, M.E. Social subordination produces distinct stress-related phenotypes in female rhesus monkeys. Psychoneuroendocrinology 2012, 37, 1071–1085. [Google Scholar] [CrossRef]
- Louise, S.; Warrington, N.M.; McCaskie, P.A.; Oddy, W.H.; Zubrick, S.R.; Hands, B.; Mori, T.A.; Briollais, L.; Silburn, S.; Palmer, L.J.; et al. Associations between aggressive behaviour scores and cardiovascular risk factors in childhood. Pediatr. Obes. 2012, 7, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.R.; Winkleby, M.A.; Boyce, W.T.; McLaughlin, R.; Broadwin, R.; Goldman, L. The association between hemoglobin and behavior problems in a sample of low-income Hispanic preschool children. J. Dev. Behav. Pediatr. 1992, 13, 209–214. [Google Scholar] [CrossRef]
- Ji, N.Y.; Findling, R.L. Pharmacotherapy for mental health problems in people with intellectual disability. Curr. Opin. Psychiatry 2016, 29, 103–125. [Google Scholar] [CrossRef]
- Christie, M.R.; Marine, M.L.; Fox, S.E.; French, R.A.; Blouin, M.S. A single generation of domestication heritably alters the expression of hundreds of genes. Nat. Commun. 2016, 7, 10676. [Google Scholar] [CrossRef] [PubMed]
- Natt, D.; Rubin, C.J.; Wright, D.; Johnsson, M.; Belteky, J.; Andersson, L.; Jensen, P. Heritable genome-wide variation of gene expression and promoter methylation between wild and domesticated chickens. BMC Genom. 2012, 13, 59. [Google Scholar] [CrossRef]
- Kukekova, A.V.; Johnson, J.L.; Xiang, X.; Feng, S.; Liu, S.; Rando, H.M.; Kharlamova, A.V.; Herbeck, Y.; Serdyukova, N.A.; Xiong, Z.; et al. Red fox genome assembly identifies genomic regions associated with tame and aggressive behaviors. Nat. Ecol. Evol. 2018, 2, 1479–1491. [Google Scholar] [CrossRef]
- Hekman, J.P.; Johnson, J.L.; Edwards, W.; Vladimirova, A.V.; Gulevich, R.G.; Ford, A.L.; Kharlamova, A.V.; Herbeck, Y.; Acland, G.M.; Raetzman, L.T.; et al. Anterior pituitary transcriptome suggests differences in ACTH release in tame and aggressive foxes. G3 2018, 8, 859–873. [Google Scholar] [CrossRef]
- Zapata, I.; Serpell, J.A.; Alvarez, C.E. Genetic mapping of canine fear and aggression. BMC Genom. 2016, 17, 572. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, H.; Shang, J.; Liu, G.; Xia, T.; Zhao, C.; Sun, G.; Dou, H. Comparative analysis of the blood transcriptomes between wolves and dogs. Anim. Genet. 2018, 49, 291–302. [Google Scholar] [CrossRef]
- Albert, F.W.; Somel, M.; Carneiro, M.; Aximu-Petri, A.; Halbwax, M.; Thalmann, O.; Blanco-Aguiar, J.A.; Plyusnina, I.Z.; Trut, L.; Villafuerte, R.; et al. A comparison of brain gene expression levels in domesticated and wild animals. PLoS Genet. 2012, 8, e1002962. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Li, A.M.; Adeola, A.C.; Liu, Y.H.; Irwin, D.M.; Xie, H.B.; Zhang, Y.P. Genome-wide identification of long intergenic noncoding RNA genes and their potential association with domestication in pigs. Genome Biol. Evol. 2014, 6, 138713–138792. [Google Scholar] [CrossRef]
- Alttoa, A.; Koiv, K.; Hinsley, T.A.; Brass, A.; Harro, J. Differential gene expression in a rat model of depression based on persistent differences in exploratory activity. Eur. Neuropsychopharmacol. 2010, 20, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtseva, N.N.; Smagin, D.A.; Kovalenko, I.L.; Galyamina, A.G.; Vishnivetskaya, G.B.; Babenko, V.N.; Orlov, Y.L. Serotonergic genes in the development of anxiety/depression-like state and pathology of aggressive behavior in male mice: RNA-seq data. Mol. Biol. 2017, 51, 288–300. [Google Scholar] [CrossRef]
- Telenti, A.; Pierce, L.; Biggs, W.; di Iulio, J.; Wong, E.; Fabani, M.; Kirkness, E.; Moustafa, A.; Shah, N.; Xie, C.; et al. Deep sequencing of 10,000 human genomes. Proc. Natl. Acad. Sci. USA 2016, 113, 11901–11906. [Google Scholar] [CrossRef]
- Sherry, S.; Ward, M.; Kholodov, M.; Baker, J.; Phan, L.; Smigielski, E.; Sirotkin, K. dbSNP: The NCBI database of genetic variation. Nucleic Acids Res. 2001, 29, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Pocai, B. The ICD-11 has been adopted by the World Health Assembly. World Psychiatry 2019, 18, 371–372. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, S.; Hemami, M.R.; Goheen, J.R. Human dimensions of wildlife conservation in Iran: Assessment of human-wildlife conflict in restoring a wide-ranging endangered species. PLoS ONE 2019, 14, e0220702. [Google Scholar] [CrossRef]
- Chadaeva, I.; Ponomarenko, M.; Rasskazov, D.; Sharypova, E.; Kashina, E.; Matveeva, M.; Arshinova, T.; Ponomarenko, P.; Arkova, O.; Bondar, N.; et al. Candidate SNP markers of aggressiveness-related complications and comorbidities of genetic diseases are predicted by a significant change in the affinity of TATA-binding protein for human gene promoters. BMC Genom. 2016, 17, 995. [Google Scholar] [CrossRef]
- Chadaeva, I.; Rasskazov, D.; Sharypova, E.; Savinkova, L.; Ponomarenko, P.; Ponomarenko, M. Candidate SNP markers of social dominance, which may affect the affinity of the TATA-binding protein for human gene promoters. Russ. J. Genet. Appl. Res. 2017, 7, 523–537. [Google Scholar] [CrossRef]
- Chadaeva, I.; Ponomarenko, P.; Rasskazov, D.; Sharypova, E.; Kashina, E.; Zhechev, D.; Drachkova, I.; Arkova, O.; Savinkova, L.; Ponomarenko, M.; et al. Candidate SNP markers of reproductive potential are predicted by a significant change in the affinity of TATA-binding protein for human gene promoters. BMC Genom. 2018, 19, 15–37. [Google Scholar] [CrossRef]
- Chadaeva, I.; Ponomarenko, P.; Rasskazov, D.; Sharypova, E.; Kashina, E.; Kleshchev, M.; Ponomarenko, M.; Naumenko, V.; Savinkova, L.; Kolchanov, N.; et al. Natural selection equally supports the human tendencies in subordination and domination: A genome-wide study with in silico confirmation and in vivo validation in mice. Front. Genet. 2019, 10, 73. [Google Scholar] [CrossRef]
- Ponomarenko, M.; Kleshchev, M.; Ponomarenko, P.; Chadaeva, I.; Sharypova, E.; Rasskazov, D.; Kolmykov, S.; Drachkova, I.; Vasiliev, G.; Gutorova, N.; et al. Disruptive natural selection by male reproductive potential prevents underexpression of protein-coding genes on the human Y chromosome as a self-domestication syndrome. BMC Genet. 2020, 21, 89. [Google Scholar] [CrossRef]
- Theofanopoulou, C.; Gastaldon, S.; O’Rourke, T.; Samuels, B.D.; Martins, P.T.; Delogu, F.; Alamri, S.; Boeckx, C. Self-domestication in Homo sapiens: Insights from comparative genomics. PLoS ONE 2017, 12, e0185306. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z. PubMed and beyond: A survey of web tools for searching biomedical literature. Database 2011, 2011, baq036. [Google Scholar] [CrossRef]
- Samet, H. A top-down quadtree traversal algorithm. IEEE Trans. Pattern Anal. Mach. Intell. 1985, 7, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.L.; Shen, W.; Wen, J.F. Triosephosphate isomerase genes in two trophic modes of euglenoids (euglenophyceae) and their phylogenetic analysis. J. Eukaryot. Microbiol. 2008, 55, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Morozova, O.V.; Alekseeva, A.E.; Sashina, T.A.; Brusnigina, N.F.; Epifanova, N.V.; Kashnikov, A.U.; Zverev, V.V.; Novikova, N.A. Phylodynamics of G4P[8] and G2P[4] strains of rotavirus A isolated in Russia in 2017 based on full-genome analyses. Virus Genes 2020, 56, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Plengpanich, W.; Le Goff, W.; Poolsuk, S.; Julia, Z.; Guerin, M.; Khovidhunkit, W. CETP deficiency due to a novel mutation in the CETP gene promoter and its effect on cholesterol efflux and selective uptake into hepatocytes. Atherosclerosis 2011, 216, 370–373. [Google Scholar] [CrossRef]
- Silliman, K.; Tall, A.R.; Kretchmer, N.; Forte, T.M. Unusual high-density lipoprotein subclass distribution during late pregnancy. Metabolism 1993, 42, 1592–1599. [Google Scholar] [CrossRef]
- Louder, M.I.M.; Hauber, M.E.; Balakrishnan, C.N. Early social experience alters transcriptomic responses to species-specific song stimuli in female songbirds. Behav. Brain Res. 2018, 347, 69–76. [Google Scholar] [CrossRef]
- Grondahl, M.L.; Borup, R.; Vikesa, J.; Ernst, E.; Andersen, C.Y.; Lykke-Hartmann, K. The dormant and the fully competent oocyte: Comparing the transcriptome of human oocytes from primordial follicles and in metaphase II. Mol. Hum. Reprod. 2013, 19, 600–617. [Google Scholar] [CrossRef]
- Mann, P.E. Gene expression profiling during pregnancy in rat brain tissue. Brain Sci. 2014, 4, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Tomas-Roig, J.; Piscitelli, F.; Gil, V.; Quintana, E.; Ramio-Torrenta, L.L.; Del Rio, J.A.; Moore, T.P.; Agbemenyah, H.; Salinas, G.; Pommerenke, C.; et al. Effects of repeated long-term psychosocial stress and acute cannabinoid exposure on mouse corticostriatal circuitries: Implications for neuropsychiatric disorders. CNS Neurosci. Ther. 2018, 24, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Izumi, T.; Maki, Y.; Muraki, I.; Koyama, T. Effect of the dopamine D(1/5) antagonist SCH 23390 on the acquisition of conditioned fear. Pharmacol. Biochem. Behav. 2000, 66, 573–578. [Google Scholar] [CrossRef]
- Schneider, T.; Ilott, N.; Brolese, G.; Bizarro, L.; Asherson, P.J.; Stolerman, I.P. Prenatal exposure to nicotine impairs performance of the 5-choice serial reaction time task in adult rats. Neuropsychopharmacology 2011, 36, 1114–1125. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Hong, S.H.; Shin, M.; Heo, H.R.; Jang, I.H. Blockade of FLT4 suppresses metastasis of melanoma cells by impaired lymphatic vessels. Biochem. Biophys. Res. Commun. 2016, 478, 733–738. [Google Scholar] [CrossRef]
- Leedom, A.J.; Sullivan, A.B.; Dong, B.; Lau, D.; Gronert, K. Endogenous LXA4 circuits are determinants of pathological angiogenesis in response to chronic injury. Am. J. Pathol. 2010, 176, 74–84. [Google Scholar] [CrossRef]
- Wang, S.; Albers, K.M. Behavioral and cellular level changes in the aging somatosensory system. Ann. N. Y. Acad. Sci. 2009, 1170, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, M.P.; Rau, K.K.; Soneji, D.J.; Anderson, C.E.; Koerber, H.R. Enhanced artemin/GFRα3 levels regulate mechanically insensitive, heat-sensitive C-fiber recruitment after axotomy and regeneration. J. Neurosci. 2010, 30, 16272–16283. [Google Scholar] [CrossRef]
- Lindfors, P.H.; Lindahl, M.; Rossi, J.; Saarma, M.; Airaksinen, M.S. Ablation of persephin receptor glial cell line-derived neurotrophic factor family receptor alpha4 impairs thyroid calcitonin production in young mice. Endocrinology 2006, 147, 2237–2244. [Google Scholar] [CrossRef]
- Yang, J.; Lindahl, M.; Lindholm, P.; Virtanen, H.; Coffey, E.; Runeberg-Roos, P.; Saarma, M. PSPN/GFRalpha4 has a significantly weaker capacity than GDNF/GFRalpha1 to recruit RET to rafts, but promotes neuronal survival and neurite outgrowth. FEBS Lett. 2004, 569, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Oh, C.M.; Park, J.; Park, H.; Cui, S.; Kim, H.S.; Namkung, J.; Park, S.K.; Pak, H.N.; Lee, M.H.; et al. Deletion of the serotonin receptor type 3a in mice leads to sudden cardiac death during pregnancy. Circ. J. 2015, 79, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; Nakamura, Y.; Ishida, Y.; Shimada, S. The 5-HT3 receptor is essential for exercise-induced hippocampal neurogenesis and antidepressant effects. Mol. Psychiatry 2015, 20, 1428–1437. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Otsuka, K.; Arimochi, H.; Tsukumo, S.I.; Yasutomo, K. Distinct roles of IL-1β and IL-18 in NLRC4-induced autoinflammation. Front. Immunol. 2020, 11, 591713. [Google Scholar] [CrossRef]
- Olkkonen, J.; Kouri, V.P.; Hynninen, J.; Konttinen, Y.T.; Mandelin, J. Differentially expressed in chondrocytes 2 (DEC2) increases the expression of IL-1β and is abundantly present in synovial membrane in rheumatoid arthritis. PLoS ONE 2015, 10, e0145279. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, M.R.; Gubbala, S.P.; Delphine Silvia, C.R.W.; Amanchy, R. Molecular diagnostics of disorders of sexual development: An Indian survey and systems biology perspective. Syst. Biol. Reprod. Med. 2019, 65, 105–120. [Google Scholar] [CrossRef]
- Jameson, J.L. Of mice and men: The tale of steroidogenic factor-1. J. Clin. Endocrinol. Metab. 2004, 89, 5927–5929. [Google Scholar] [CrossRef][Green Version]
- Li, L.; Chen, X.; Hao, L.; Chen, Q.; Liu, H.; Zhou, Q. Exploration of immune-related genes in high and low tumor mutation burden groups of chromophobe renal cell carcinoma. Biosci. Rep. 2020, 40, bsr20201491. [Google Scholar] [CrossRef] [PubMed]
- Coan, P.M.; Hummel, O.; Garcia Diaz, A.; Barrier, M.; Alfazema, N.; Norsworthy, P.J.; Pravenec, M.; Petretto, E.; Hubner, N.; Aitman, T.J. Genetic, physiological and comparative genomic studies of hypertension and insulin resistance in the spontaneously hypertensive rat. Dis. Model Mech. 2017, 10, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Nakao, K.; Iwata, K.; Takeshita, T.; Ozawa, H. Expression of hypothalamic kisspeptin, neurokinin B, and dynorphin A neurons attenuates in female Zucker fatty rats. Neurosci. Lett. 2018, 665, 135–139. [Google Scholar] [CrossRef]
- Szklarczyk, K.; Korostynski, M.; Golda, S.; Solecki, W.; Przewlocki, R. Genotype-dependent consequences of traumatic stress in four inbred mouse strains. Genes Brain Behav. 2012, 11, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Greig, C.J.; Zhang, L.; Cowles, R.A. Potentiated serotonin signaling in serotonin re-uptake transporter knockout mice increases enterocyte mass and small intestinal absorptive function. Physiol. Rep. 2019, 7, e14278. [Google Scholar] [CrossRef]
- Morgan, J.A.; Singhal, G.; Corrigan, F.; Jaehne, E.J.; Jawahar, M.C.; Breen, J.; Pederson, S.; Baune, B.T. Ceasing exercise induces depression-like, anxiety-like, and impaired cognitive-like behaviours and altered hippocampal gene expression. Brain Res. Bull. 2019, 148, 118–130. [Google Scholar] [CrossRef]
- Senol, B.K.; Zulfikar, B. Clinical problems and surgical interventions in inherited factor VII deficiency. Turk Pediatri Ars. 2020, 55, 184–190. [Google Scholar]
- Burad, J.; Bhakta, P.; Sharma, J. Timely ‘off-label’ use of recombinant activated factor VII (NovoSeven(®)) can help in avoiding hysterectomy in intractable obstetric bleeding complicated with disseminated intravascular coagulation: A case report and review of the literature. Indian J. Anaesth. 2012, 56, 69–71. [Google Scholar]
- Qian, C.; Wong, C.W.Y.; Wu, Z.; He, Q.; Xia, H.; Tam, P.K.H.; Wong, K.K.Y.; Lui, V.C.H. Stage specific requirement of platelet-derived growth factor receptor-α in embryonic development. PLoS ONE 2017, 12, e0184473. [Google Scholar] [CrossRef]
- Foster, D.S.; Marshall, C.D.; Gulati, G.S.; Chinta, M.S.; Nguyen, A.; Salhotra, A.; Jones, R.E.; Burcham, A.; Lerbs, T.; Cui, L.; et al. Elucidating the fundamental fibrotic processes driving abdominal adhesion formation. Nat. Commun. 2020, 11, 4061. [Google Scholar] [CrossRef]
- Brigger, D.; Torbett, B.E.; Chen, J.; Fey, M.F.; Tschan, M.P. Inhibition of GATE-16 attenuates ATRA-induced neutrophil differentiation of APL cells and interferes with autophagosome formation. Biochem. Biophys. Res. Commun. 2013, 438, 283–288. [Google Scholar] [CrossRef]
- Cho, H.S.; Park, S.Y.; Kim, S.M.; Kim, W.J.; Jung, J.Y. Autophagy-related protein MAP1LC3C plays a crucial role in odontogenic differentiation of human dental pulp cells. Tissue Eng. Regen. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, J.O.L.; Juul, A. Therapy of endocrine disease: Growth hormone replacement therapy in adults: 30 years of personal clinical experience. Eur. J. Endocrinol. 2018, 179, R47–R56. [Google Scholar] [CrossRef] [PubMed]
- Regan, S.L.P.; Knight, P.G.; Yovich, J.L.; Arfuso, F.; Dharmarajan, A. Growth hormone during in vitro fertilization in older women modulates the density of receptors in granulosa cells, with improved pregnancy outcomes. Fertil. Steril. 2018, 110, 1298–1310. [Google Scholar] [CrossRef]
- Takhviji, V.; Zibara, K.; Azarkeivan, A.; Mehrvar, N.; Mehrvar, N.; Mezginejad, F.; Khosravi, A. Fertility and pregnancy in Iranian thalassemia patients: An update on transfusion complications. Transfus. Med. 2020, 30, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yu, H.; Huang, S.; Zheng, L.; Zheng, P.; Zhang, S.; Li, S.; Chen, J. Jian-Pi-Yi-Shen regulates EPO and iron recycling protein expressions in anemic rats with chronic kidney disease: Accumulation of hypoxia inducible factor-2α via ERK signaling. Evid. Based Complement. Alternat. Med. 2020, 2020, 8894257. [Google Scholar] [CrossRef] [PubMed]
- Schulz, A.; Gorodetska, I.; Behrendt, R.; Fuessel, S.; Erdmann, K.; Foerster, S.; Datta, K.; Mayr, T.; Dubrovska, A.; Muders, M.H. Linking NRP2 with EMT and chemoradioresistance in bladder cancer. Front. Oncol. 2020, 9, 1461. [Google Scholar] [CrossRef]
- Pellet-Many, C.; Mehta, V.; Fields, L.; Mahmoud, M.; Lowe, V.; Evans, I.; Ruivo, J.; Zachary, I. Neuropilins 1 and 2 mediate neointimal hyperplasia and re-endothelialization following arterial injury. Cardiovasc. Res. 2015, 108, 288–298. [Google Scholar] [CrossRef]
- Yang, J.J.; Caligioni, C.S.; Chan, Y.M.; Seminara, S.B. Uncovering novel reproductive defects in neurokinin B receptor null mice: Closing the gap between mice and men. Endocrinology 2012, 153, 1498–1508. [Google Scholar] [CrossRef]
- Nilsson, L.L.; Hornstrup, M.B.; Perin, T.L.; Lindhard, A.; Funck, T.; Bjerrum, P.J.; Mule, H.T.; Scheike, T.; Nielsen, H.S.; Hviid, T.V.F. Soluble HLA-G and TGF-β in couples attending assisted reproduction—A possible role of TGF-β isoforms in semen? J. Reprod. Immunol. 2020, 137, 102857. [Google Scholar] [CrossRef]
- Ciebiera, M.; Wlodarczyk, M.; Wrzosek, M.; Męczekalski, B.; Nowicka, G.; Lukaszuk, K.; Ciebiera, M.; Słabuszewska-Jozwiak, A.; Jakiel, G. Role of transforming growth factor β in uterine fibroid biology. Int. J. Mol. Sci. 2017, 18, 2435. [Google Scholar] [CrossRef]
- Ivanski, F.; de Oliveira, V.M.; de Oliveira, I.M.; de Araujo Ramos, A.T.; de Oliveira Tonete, S.T.; de Oliveira Hykavei, G.; Bargi-Souza, P.; Schiessel, D.L.; Martino-Andrade, A.J.; Romano, M.A.; et al. Prepubertal acrylamide exposure causes dose-response decreases in spermatic production and functionality with modulation of genes involved in the spermatogenesis in rats. Toxicology 2020, 436, 152428. [Google Scholar] [CrossRef]
- Creed, M.; Kaufling, J.; Fois, G.R.; Jalabert, M.; Yuan, T.; Luscher, C.; Georges, F.; Bellone, C. Cocaine exposure enhances the activity of ventral tegmental area dopamine neurons via calcium-impermeable NMDARs. J. Neurosci. 2016, 36, 10759–10768. [Google Scholar] [CrossRef]
- Dela Pena, I.; Bang, M.; Lee, J.; de la Pena, J.B.; Kim, B.N.; Han, D.H.; Noh, M.; Shin, C.Y.; Cheong, J.H. Common prefrontal cortical gene expression profiles between adolescent SHR/NCrl and WKY/NCrl rats which showed inattention behavior. Behav. Brain Res. 2015, 291, 268–276. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, H.; Gao, J.; Wei, S.; Song, C.; Sun, P.; Qiao, M. Study of genes associated with the ‘anger-in’ and ‘anger-out’ emotions of humans using a rat model. Exp. Ther. Med. 2015, 9, 1448–1454. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Wang, L.; Duan, Q.; Gong, Z.; Yang, F.; Song, Y. Differential expression of 5-HT-related genes in symptomatic pulmonary embolism patients. Int. J. Clin. Exp. Med. 2015, 8, 512–518. [Google Scholar]
- Sackett, S.D.; Otto, T.; Mohs, A.; Sander, L.E.; Strauch, S.; Streetz, K.L.; Kroy, D.C.; Trautwein, C. Myeloid cells require gp130 signaling for protective anti-inflammatory functions during sepsis. FASEB J. 2019, 33, 6035–6044. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.R.; Robinson, M.; Nimmo, M.A. Response of plasma IL-6 and its soluble receptors during submaximal exercise to fatigue in sedentary middle-aged men. Cell Stress Chaperones 2008, 13, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, S.; Hu, R.; Zhou, Q.; Li, X. Decreased placental IL9 and IL9R in preeclampsia impair trophoblast cell proliferation, invasion, and angiogenesis. Hypertens. Pregnancy 2020, 39, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Osterfeld, H.; Ahrens, R.; Strait, R.; Finkelman, F.D.; Renauld, J.C.; Hogan, S.P. Differential roles for the IL-9/IL-9 receptor alpha-chain pathway in systemic and oral antigen-induced anaphylaxis. J. Allergy Clin. Immunol. 2010, 125, 469–476.e2. [Google Scholar] [CrossRef]
- Hahm, S.; Mizuno, T.M.; Wu, T.J.; Wisor, J.P.; Priest, C.A.; Kozak, C.A.; Boozer, C.N.; Peng, B.; McEvoy, R.C.; Good, P.; et al. Targeted deletion of the Vgf gene indicates that the encoded secretory peptide precursor plays a novel role in the regulation of energy balance. Neuron 1999, 23, 537–548. [Google Scholar] [CrossRef]
- Sainsbury, A.; Schwarzer, C.; Couzens, M.; Herzog, H. Y2 receptor deletion attenuates the type 2 diabetic syndrome of ob/ob mice. Diabetes 2002, 51, 3420–3427. [Google Scholar] [CrossRef] [PubMed]
- Sanford, L.P.; Ormsby, I.; Gittenberger-de Groot, A.C.; Sariola, H.; Friedman, R.; Boivin, G.P.; Cardell, E.L.; Doetschman, T. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development 1997, 124, 2659–2670. [Google Scholar] [PubMed]
- Kim, J.H.; Lee, Y.W.; Park, Y.M.; Park, K.A.; Park, S.H.; Lee, W.T.; Lee, J.E. Agmatine-reduced collagen scar area accompanied with surface righting reflex recovery after complete transection spinal cord injury. Spine 2011, 36, 2130–2138. [Google Scholar] [CrossRef]
- Osadchuk, L.V. Endocrine gonadal function in silver fox under domestication. Scientifur 1992, 16, 116–121. [Google Scholar]
- Osadchuk, L.V. Some peculiarities in reproduction in silver fox males under domestication. Scientifur 1992, 16, 285–288. [Google Scholar]
- Osadchuk, L.V. Black-silver fox Vulpes vulpes male reproductive potential after longitudinal selection on domestic behavior. Zh. Evol. Biokhim. Fiziol. 2006, 42, 146–152. [Google Scholar]
- Osadchuk, L.V.; Krass, P.M.; Trut, L.N.; Belyaev, D.K. Effects of selection for behavior on the endocrine function of the gonads in male silver-black foxes. Dokl. Akad. Nauk USSR 1978, 240, 1255–1258. [Google Scholar]
- Osadchuk, L.V.; Krass, P.M.; Trut, L.N.; Ivanova, L.N. Gonadal endocrine function in male silver foxes with different hereditary determined forms of defensive behavior. Proc. Sib. Branch Acad. Nauk USSR 1978, 79–86. [Google Scholar]
- Osadchuk, L.V. Biosynthesis of testosterone in the gonads in silver fox embryos after long-term selection for domesticated behavior. Genetika 1998, 34, 941–946. [Google Scholar] [PubMed]
- Prasolova, L.A.; Gerbek, Y.E.; Gulevich, R.G.; Shikhevich, S.G.; Konoshenko, M.Y.; Kozhemyakina, R.V.; Oskina, I.N.; Plyusnina, I.Z. The effects of prolonged selection for behavior on the stress response and activity of the reproductive system of male grey mice (Rattus norvegicus). Russ. J. Genet. 2014, 50, 846–852. [Google Scholar] [CrossRef]
- Liu, X.; Mawolo, J.B.; Du, X.; Zhou, Y.; Wang, H.; Liu, F.; He, Z.; Marela, H.A. Investigation of biochemical and physiological parameters of the newborn Saiga antelope (Saiga tatarica) in Gansu Province, China. PLoS ONE 2019, 14, e0224822. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Feng, L.; Mou, P.; Miquelle, D.G.; Hebblewhite, M.; Goldberg, J.F.; Robinson, H.S.; Zhao, X.; Zhou, B.; Wang, T.; et al. Estimating abundance and density of Amur tigers along the Sino-Russian border. Integr. Zool. 2016, 11, 322–332. [Google Scholar] [CrossRef]
- Almeida, F.F.; Leal, M.C.; Franca, L.R. Testis morphometry, duration of spermatogenesis, and spermatogenic efficiency in the wild boar (Sus scrofa scrofa). Biol. Reprod. 2006, 75, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Yang, J.; Wang, J.; Yang, Y.; Fu, W.; Zheng, C.; Cheng, J.; Zeng, Y.; Zhang, Y.; Xu, L.; et al. Changes in the population genetic structure of captive forest musk deer (Moschus berezovskii) with the increasing number of generation under closed breeding conditions. Animals 2020, 10, 255. [Google Scholar] [CrossRef]
| # | Domestic Animals | Wild Animals | Tissue | Number of DEGs | [Ref] |
|---|---|---|---|---|---|
| 1 | tame foxes (Vulpes vulpes) 6 males: | aggressive foxes (V. vulpes) 6 males: | pituitary | 327 | [26] |
| 2 | dogs (Canis familiaris): 1 female and 1 male | wolves (C. lupus): 2 females and 1 male | blood | 450 | [28] |
| 3 | dogs (C. familiaris): 2 females and 3 males | wolves (C. lupus): 2 females and 1 male | frontal cortex | 13 | [29] |
| 4 | pigs (Sus scrofa): 5 females | boars (S. scrofa): 5 females | frontal cortex | 30 | [29] |
| 5 | guinea pigs (Cavia porcellus): 3 females and 3 males | cavy (C. aperea): 3 females and 3 males | frontal cortex | 883 | [29] |
| 6 | domesticated rabbits (Oryctolagus cuniculus domesticus): 3 females and 3 males | wild rabbits (Oryctolagus cuniculus): 3 females and 3 males | frontal cortex | 17 | [29] |
| 7 | tame rats (Rattus norvegicus): 3 females and 3 males | aggressive rats (R. norvegicus): 3 females and 3 males | frontal cortex | 20 | [29] |
| Σ | 6 domesticated animal species: 17 females and 19 males | 6 wild animal species: 18 females and 17 males | 3 tissues | 1740 | 3 Refs |
| Humans | Animals | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gene | Effect of Gene Expression Change (Δ) on Reproductive Potential (♂♀) | DEG | RNA-Seq | Δ during Diver-Gence from NCA | |||||
| Deficit (↓) | ♂♀ | Excess (↑) | ♂♀ | log | PADJJ | Deficit (↓) | Excess (↑) | ||
| CETP | lesser risk of myo-cardial infarction [47] | → | hypercholesterolemia in pregnancy [48] | ← | Cetp | 2.1 | 10−3 | wild | domestic |
| CHRNA3 | improved finding oppo-site sex congeners [49] | → | greater nicotine effects on oocytes [50] | ← | Chrna3 | 0.9 | 0.05 | wild | domestic |
| CHRNA6 | improved maternal behavior [51] | → | higher risk of social defeats [52] | ← | Chrna6 | 0.9 | 0.05 | wild | domestic |
| DRD5 | reduces conditioned fear [53] | → | higher risk of mental disorders [54] | ← | Drd5 | –1.9 | 10−2 | domestic | wild |
| FLT4 | suppressed melanoma metastasis [55] | → | worse post-injury neo- vascularization [56] | ← | Flt4 | 0.8 | 0.05 | wild | domestic |
| GFRA3 | accelerated neuro-degeneration [57] | ← | improved neural regeneration [58] | → | Gfra3 | –1.0 | 0.05 | domestic | wild |
| GFRA4 | premature adolescent bone formation [59] | ← | improved neuronal survival [60] | → | Gfra4 | 1.5 | 0.05 | wild | domestic |
| HTR3A | higher risk of death during pregnancy [61] | ← | improved mood and behavior [62] | → | Htr3a | –2.9 | 10−14 | domestic | wild |
| IL1B | less bone deformation in infections [63] | → | circadian pain hypersensitivity [64] | ← | Il1b | 2.3 | 10−2 | wild | domestic |
| NR5A1 | higher risk of gonadal dysgenesis in men [65] | ← | improved gonadal development [66] | → | Nr5a1 | –2.2 | 0.05 | domestic | wild |
| PDGFRL | reduced tumor mutation burden [67] | → | myocardial hypertrophy [68] | ← | Pdgfrl | 1.3 | 10−8 | wild | domestic |
| PDYN | obesity-related subfertility [69] | ← | prevented conditioned fear behavior [70] | → | Pdyn | 0.9 | 10−2 | wild | domestic |
| SLC6A4 | improved small-intestinal function [71] | → | worse depression, anxiety, inertia [72] | ← | Slc6a4 | 2.9 | 10−2 | wild | domestic |
| Humans | Change in Reproductive Potential (♂♀) | Binomial Distribution | χ2-Test | Fisher’s Test | ||||
|---|---|---|---|---|---|---|---|---|
| Animals | Worse (←) | Improved (→) | χ2 | p | Value | p | ||
| change during divergence from NCA | domestic | 11 | 2 | 0.05 | 9.91 | 10−2 | 0.003 | 0.05 |
| wild | 3 | 10 | 0.06 | |||||
| Humans | Animals | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gene | Effect of Gene Expression Change (Δ) on Reproductive Potential (♂♀) | DEG | RNA-Seq | Δ during Diver-Gence from NCA | |||||
| Deficit (↓) | ♂♀ | Excess (↑) | ♂♀ | log | PADJJ | Deficit (↓) | Excess (↑) | ||
| domesticated versus wild rabbits [29] | |||||||||
| F7 | higher risk of bleeding [73] | ← | recombinant F7 treats obstetric bleeding [74] | → | F7 | –2.7 | 0.05 | domestic | wild |
| dog versus wolf (frontal cortex) [29] | |||||||||
| PDGFRA | skeletal defects in newborns [75] | ← | higher risk of infertility [76] | ← | Pdgfra | 1.5 | 10−3 | wild | domestic |
| dog versus wolf (blood) [28] | |||||||||
| GABARAPL2 | impaired wound healing [77] | ← | improved tooth injury healing [78] | → | Gabarapl2 | –2.0 | 10−2 | domestic | wild |
| GH1 | higher risk of mortality [79] | ← | prolonged reproductive age in women [80] | → | Gh1 | 2.8 | 10−2 | wild | domestic |
| HBB | worse reproductive health in women [81] | ← | relieved anemia in kidney diseases [82] | → | Hbbl | –5.9 | 10−8 | domestic | wild |
| NRP2 | better survival after radiochemotherapy [83] | → | vascular neointimal hyperplasia [84] | ← | Nrp2 | 1.8 | 0.05 | wild | domestic |
| TAC3 | higher risk of subfertility [85] | ← | lesser socially induced subfertility [85] | → | Tac3 | –4.7 | 10−8 | domestic | wild |
| TGFB3 | lowers semen quality and infertility [86] | ← | higher risk of female infertility [87] | ← | Tgfb3 | 3.3 | 0.05 | wild | domestic |
| tame versus aggressive foxes [26] | |||||||||
| ESR2 | impaired spermatogenesis [88] | ← | impaired spermatogenesis [88] | ← | Esr2 | –0.3 | 0.05 | domestic | wild |
| GRIN3A | prevents cocaine addiction [89] | → | higher risk of inatten-tive behavior [90] | ← | Grin3a | 0.5 | 10−2 | wild | domestic |
| HTR3B | reduced anger-reso-lutive behavior [91] | ← | lesser risk of pulmo-nary embolism [92] | → | Htr3b | –0.5 | 0.05 | domestic | wild |
| IL6ST | higher risk of mortality during sepsis [93] | ← | increased sensitivity to fatigue [94] | ← | Il6st | 0.3 | 0.05 | wild | domestic |
| IL9R | impaired trophoblast implantation [95] | ← | higher risk of anaphylaxis [96] | ← | Il9r | 0.4 | 0.05 | wild | domestic |
| NPY | higher risk of infertility [97] | ← | higher risk of obesity and subfertility [98] | ← | Npy | 0.4 | 10−2 | wild | domestic |
| TGFB2 | higher risk of perinatal mortality [99] | ← | impaired wound healing [100] | ← | Tgfb2 | 0.5 | 10−2 | wild | domestic |
| Humans | Change in Reproductive Potential (♂♀) | Binomial Distribution | χ2-Test | Fisher’s Test | ||||
|---|---|---|---|---|---|---|---|---|
| Animals | Worse (←) | Improved (→) | χ2 | p | Value | p | ||
| change during divergence from NCA | domestic | 14 | 1 | 10−3 | 6.14 | 0.05 | 0.04 | 0.05 |
| wild | 8 | 7 | 0.5 | |||||
| Humans | Change in Reproductive Potential (♂♀) | Binomial Distribution | χ2-Test | Fisher’s Test | ||||
|---|---|---|---|---|---|---|---|---|
| Animals | Worse (←) | Improved (→) | χ2 | p | Value | p | ||
| change during divergence from NCA | domestic | 25 | 3 | 10−4 | 15.2 | 10−3 | 10−4 | 0.05 |
| wild | 11 | 17 | 0.1 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasiliev, G.; Chadaeva, I.; Rasskazov, D.; Ponomarenko, P.; Sharypova, E.; Drachkova, I.; Bogomolov, A.; Savinkova, L.; Ponomarenko, M.; Kolchanov, N.; et al. A Bioinformatics Model of Human Diseases on the Basis of Differentially Expressed Genes (of Domestic Versus Wild Animals) That Are Orthologs of Human Genes Associated with Reproductive-Potential Changes. Int. J. Mol. Sci. 2021, 22, 2346. https://doi.org/10.3390/ijms22052346
Vasiliev G, Chadaeva I, Rasskazov D, Ponomarenko P, Sharypova E, Drachkova I, Bogomolov A, Savinkova L, Ponomarenko M, Kolchanov N, et al. A Bioinformatics Model of Human Diseases on the Basis of Differentially Expressed Genes (of Domestic Versus Wild Animals) That Are Orthologs of Human Genes Associated with Reproductive-Potential Changes. International Journal of Molecular Sciences. 2021; 22(5):2346. https://doi.org/10.3390/ijms22052346
Chicago/Turabian StyleVasiliev, Gennady, Irina Chadaeva, Dmitry Rasskazov, Petr Ponomarenko, Ekaterina Sharypova, Irina Drachkova, Anton Bogomolov, Ludmila Savinkova, Mikhail Ponomarenko, Nikolay Kolchanov, and et al. 2021. "A Bioinformatics Model of Human Diseases on the Basis of Differentially Expressed Genes (of Domestic Versus Wild Animals) That Are Orthologs of Human Genes Associated with Reproductive-Potential Changes" International Journal of Molecular Sciences 22, no. 5: 2346. https://doi.org/10.3390/ijms22052346
APA StyleVasiliev, G., Chadaeva, I., Rasskazov, D., Ponomarenko, P., Sharypova, E., Drachkova, I., Bogomolov, A., Savinkova, L., Ponomarenko, M., Kolchanov, N., Osadchuk, A., Oshchepkov, D., & Osadchuk, L. (2021). A Bioinformatics Model of Human Diseases on the Basis of Differentially Expressed Genes (of Domestic Versus Wild Animals) That Are Orthologs of Human Genes Associated with Reproductive-Potential Changes. International Journal of Molecular Sciences, 22(5), 2346. https://doi.org/10.3390/ijms22052346








