Natural Products Targeting Amyloid Beta in Alzheimer’s Disease
Abstract
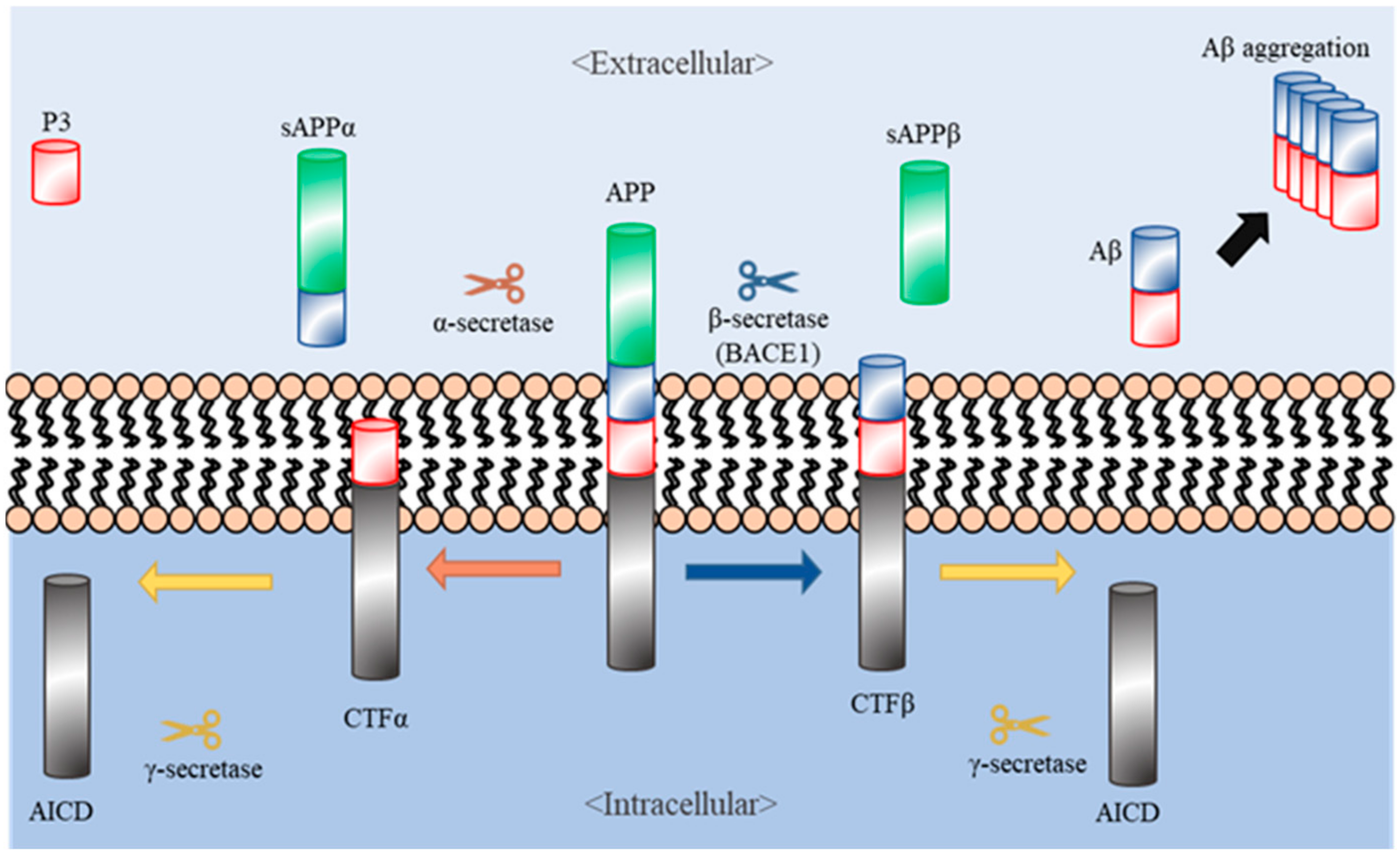
1. Introduction
2. Natural Products Targeting Secretases (Secretase-Dependent Approaches)
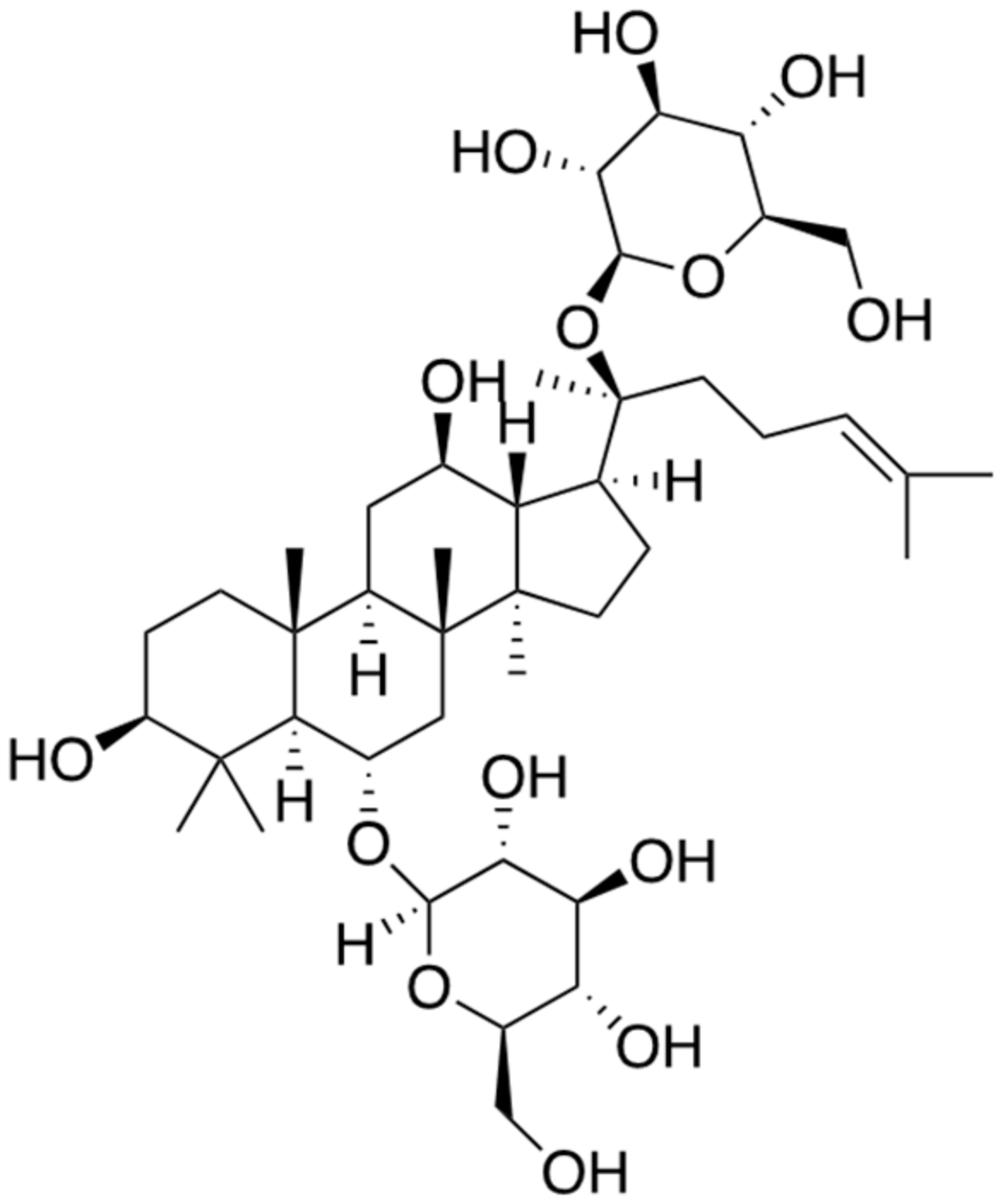
2.1. Ginsenoside Rg1
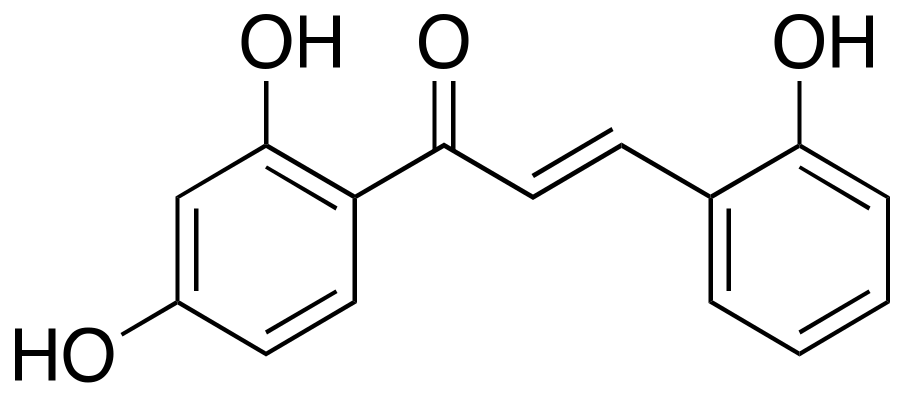
2.2. 2,2′,4′-Trihydroxychalcone (TDC)
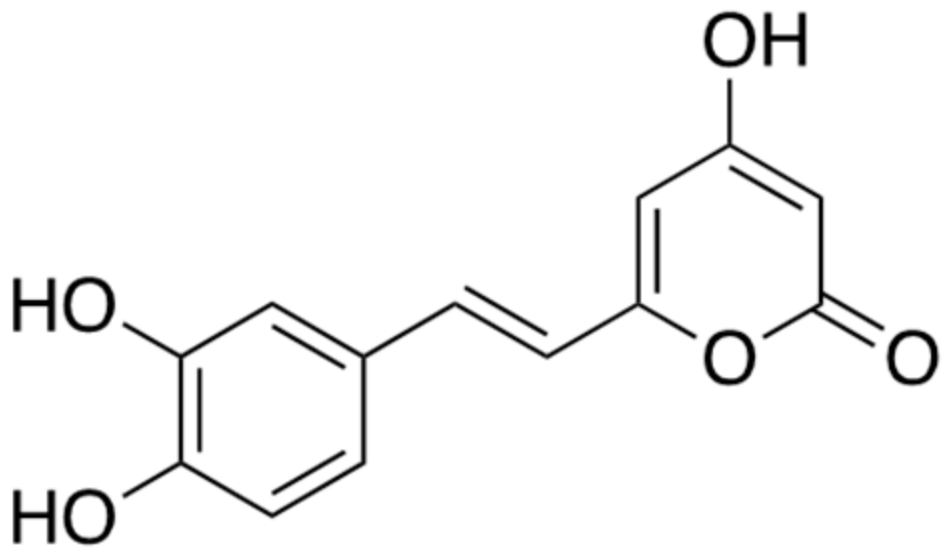
2.3. Hispidin
2.4. Berberine
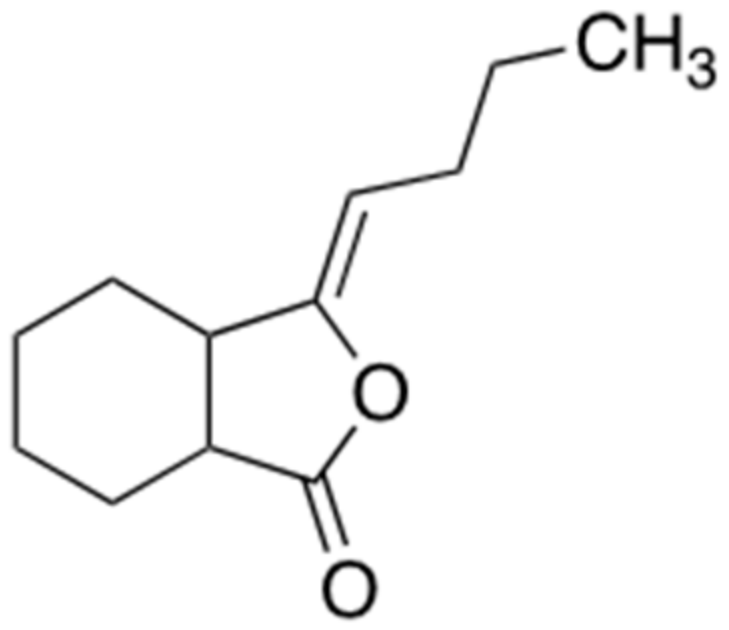
2.5. Ligustilide
2.6. Epigallochetchin-3-Galate (EGCG)
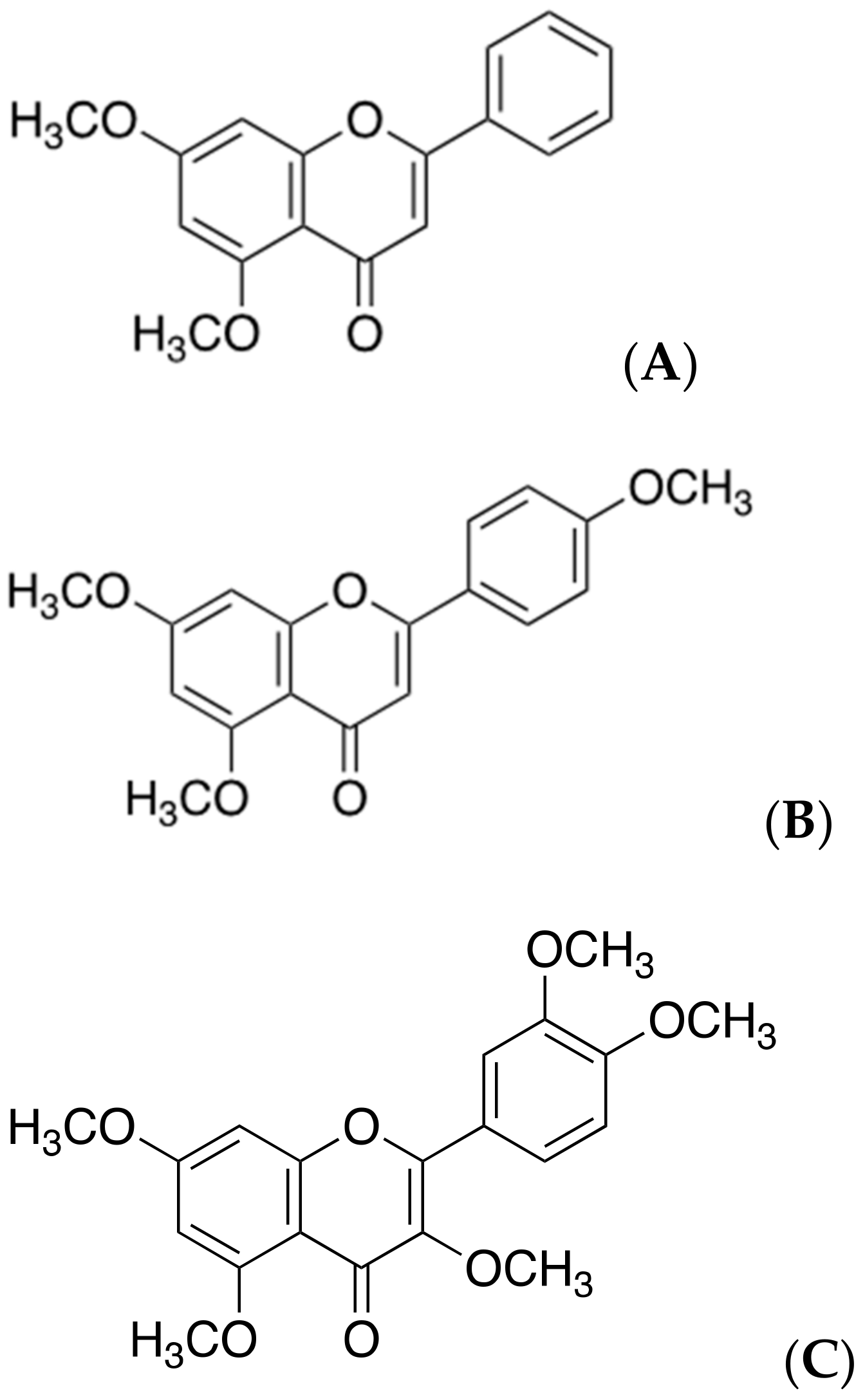
2.7. Polymethoxyflavones
3. Natural Products Targeting Abnormal Aβ Aggregates (Structure-Dependent Approaches)
3.1. Resveratrol
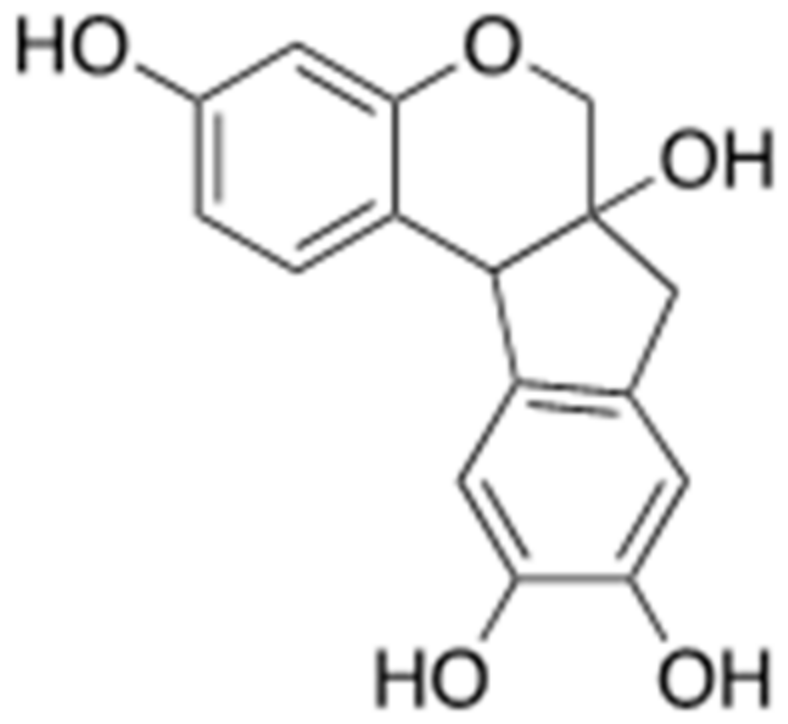
3.2. Brazilin
3.3. Curcumin
3.4. Tannic Acid
3.5. Theaflavin
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, S.H.; Lee, D.K.; Shin, J.; Lee, S.; Baek, S.; Kim, J.; Jung, H.; Hah, Y.; Kim, J.M. Nec-1 alleviates cognitive impairment with reduction of Abeta and tau abnormalities in APP/PS1 mice. EMBO Mol. Med. 2017, 9, 61–77. [Google Scholar] [CrossRef]
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef]
- Acx, H.; Serneels, L.; Radaelli, E.; Muyldermans, S.; Vincke, C.; Pepermans, E.; Muller, U.; Chavez-Gutierrez, L.; De Strooper, B. Inactivation of gamma-secretases leads to accumulation of substrates and non-Alzheimer neurodegeneration. EMBO Mol. Med. 2017, 9, 1088–1099. [Google Scholar] [CrossRef]
- Zolochevska, O.; Taglialatela, G. Non-Demented Individuals with Alzheimer’s Disease Neuropathology: Resistance to Cognitive Decline May Reveal New Treatment Strategies. Curr. Pharm. Des. 2016, 22, 4063–4068. [Google Scholar] [CrossRef]
- Briley, D.; Ghirardi, V.; Woltjer, R.; Renck, A.; Zolochevska, O.; Taglialatela, G.; Micci, M.A. Preserved neurogenesis in non-demented individuals with AD neuropathology. Sci. Rep. 2016, 6, 27812. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.; Vassar, L.R. The Alzheimer’s disease beta-secretase enzyme, BACE1. Mol. Neurodegener. 2007, 2, 22. [Google Scholar] [CrossRef] [PubMed]
- Satir, T.M.; Agholme, L.; Karlsson, A.; Karlsson, M.; Karila, P.; Illes, S.; Bergstrom, H.; Zetterberg, P. Partial reduction of amyloid beta production by beta-secretase inhibitors does not decrease synaptic transmission. Alzheimers Res. Ther. 2020, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Yang, H.; Shen, N.; Pham, P.; Brown, B.; Lin, X.; Hong, Y.; Sinu, P.; Cai, J.; Li, X.; et al. An Immunomodulatory Therapeutic Vaccine Targeting Oligomeric Amyloid-beta. J. Alzheimers Dis. 2020, 77, 1639–1653. [Google Scholar] [CrossRef] [PubMed]
- Egan, M.F.; Kost, J.; Tariot, P.N.; Aisen, P.S.; Cummings, J.L.; Vellas, B.; Sur, C.; Mukai, Y.; Voss, T.; Furtek, C.; et al. Randomized Trial of Verubecestat for Mild-to-Moderate Alzheimer’s Disease. N. Engl. J. Med. 2018, 378, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Morris-Natschke, S.L.; Lee, K.H. Strategies for the Optimization of Natural Leads to Anticancer Drugs or Drug Candidates. Med. Res. Rev. 2016, 36, 32–91. [Google Scholar] [CrossRef]
- Yuan, X.Z.; Sun, S.; Tan, C.C.; Yu, L.; Tan, J.T. The Role of ADAM10 in Alzheimer’s Disease. J. Alzheimers Dis. 2017, 58, 303–322. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.R.; Holler, C.J.; Webb, R.L.; Li, F.; Beckett, T.L.; Murphy, M.P. BACE1 and BACE2 enzymatic activities in Alzheimer’s disease. J. Neurochem. 2010, 112, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Das, B.; Hou, H.; He, W.; Yan, R. BACE1 deletion in the adult mouse reverses preformed amyloid deposition and improves cognitive functions. J. Exp. Med. 2018, 215, 927–940. [Google Scholar] [CrossRef]
- Tyler, S.J.; Dawbarn, D.; Wilcock, G.K.; Allen, S.J. alpha- and beta-secretase: Profound changes in Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2002, 299, 373–376. [Google Scholar] [CrossRef]
- Ameen-Ali, K.E.; Wharton, S.B.; Simpson, J.E.; Heath, P.R.; Sharp, P.; Berwick, J. Review: Neuropathology and behavioural features of transgenic murine models of Alzheimer’s disease. Neuropathol. Appl. Neurobiol. 2017, 43, 553–570. [Google Scholar] [CrossRef]
- Bai, L.T.; Gao, J.L.; Wei, F.; Zhao, J.; Wang, D.W.; Wei, J.P. Therapeutic Potential of Ginsenosides as an Adjuvant Treatment for Diabetes. Front. Pharmacol. 2018, 9, 423. [Google Scholar] [CrossRef]
- Choi, R.J.; Roy, A.; Jung, H.J.; Ali, M.Y.; Min, B.S.; Park, C.H.; Yokozawa, T.; Fan, T.P.; Choi, J.S.; Jung, H.A.; et al. BACE1 molecular docking and anti-Alzheimer’s disease activities of ginsenosides. J. Ethnopharmacol. 2016, 190, 219–230. [Google Scholar] [CrossRef]
- Li, F.L.; Wu, X.Q.; Li, J.; Niu, Q.L. Ginsenoside Rg1 ameliorates hippocampal long-term potentiation and memory in an Alzheimer’s disease model. Mol. Med. Rep. 2016, 13, 4904–4910. [Google Scholar] [CrossRef]
- Fang, F.; Chen, X.C.; Huang, T.W.; Lue, L.F.; Luddy, S.J.; Yan, S.S. Multi-faced neuroprotective effects of Ginsenoside Rg1 in an Alzheimer mouse model. BBA-Mol. Basis Dis. 2012, 1822, 286–292. [Google Scholar] [CrossRef]
- Guo, J.; Liu, A.; Cao, H.; Luo, Y.; Pezzuto, J.M.; van Breemen, R.B. Biotransformation of the chemopreventive agent 2’,4’,4-trihydroxychalcone (isoliquiritigenin) by UDP-glucuronosyltransferases. Drug Metab. Dispos. 2008, 36, 2104–2112. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.Y.; Li, C.J.; Wang, X.; Yang, Z.Y.; Chen, J.; Hu, L.H.; Jiang, X.; Shen, H.L. 2,2′,4′-Trihydroxychalcone from Glycyrrhiza glabra as a new specific BACE1 inhibitor efficiently ameliorates memory impairment in mice. J. Neurochem. 2010, 114, 374–385. [Google Scholar] [CrossRef]
- Park, I.H.; Chung, S.K.; Lee, K.B.; Yoo, Y.C.; Kim, S.K.; Kim, G.S.; Song, K.S. An antioxidant hispidin from the mycelial cultures of Phellinus linteus. Arch. Pharm. Res. 2004, 27, 615–618. [Google Scholar] [CrossRef]
- Park, I.H.; Jeon, S.Y.; Lee, H.J.; Kim, S.I.; Song, K.S. A beta 5-secretase (BACE1) inhibitor hispidin from the mycelial cultures of Phellinus linteus. Planta Med. 2004, 70, 143–146. [Google Scholar]
- Durairajan, S.S.; Liu, L.F.; Lu, J.H.; Chen, L.L.; Yuan, Q.; Chung, S.K.; Huang, L.; Li, X.S.; Huang, J.D.; Li, M.; et al. Berberine ameliorates beta-amyloid pathology, gliosis, and cognitive impairment in an Alzheimer’s disease transgenic mouse model. Neurobiol. Aging 2012, 33, 2903–2919. [Google Scholar] [CrossRef] [PubMed]
- Asai, M.; Iwata, N.; Yoshikawa, A.; Aizaki, Y.; Ishiura, S.; Saido, K.; Maruyama, T.C. Berberine alters the processing of Alzheimer’s amyloid precursor protein to decrease Abeta secretion. Biochem Biophys Res. Commun. 2007, 352, 498–502. [Google Scholar] [CrossRef]
- Panahi, N.; Mahmoudian, M.; Mortazavi, P.; Hashjin, G.S. Effects of berberine on beta-secretase activity in a rabbit model of Alzheimer’s disease. Arch. Med. Sci. 2013, 9, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Wang, C.; He, W.; Chen, Y. Berberine Alleviates Amyloid-Beta Pathology in the Brain of APP/PS1 Transgenic Mice via Inhibiting beta/gamma-Secretases Activity and Enhancing alpha-Secretases. Curr. Alzheimer Res. 2018, 15, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ning, Z.Q.; Shan, S.; Zhang, K.; Deng, T.; Lu, X.; Cheng, P.; Phthalide, Y.Y. Lactones from Ligusticum chuanxiong inhibit lipopolysaccharide-induced TNF-alpha production and TNF-alpha-mediated NF-kappaB Activation. Planta Med. 2005, 71, 808–813. [Google Scholar] [CrossRef]
- Kuang, X.; Du, J.R.; Liu, Y.X.; Zhang, G.Y.; Peng, H.Y. Postischemic administration of Z-Ligustilide ameliorates cognitive dysfunction and brain damage induced by permanent forebrain ischemia in rats. Pharmacol. Biochem. Behav. 2008, 88, 213–221. [Google Scholar] [CrossRef]
- Peng, H.Y.; Du, J.R.; Zhang, G.Y.; Kuang, X.; Liu, Y.X.; Qian, Z.M.; Wang, C.Y. Neuroprotective effect of Z-ligustilide against permanent focal ischemic damage in rats. Biol. Pharm. Bull. 2007, 30, 309–312. [Google Scholar] [CrossRef]
- Xu, Y.J.; Mei, Y.; Qu, Z.L.; Zhang, S.J.; Zhao, W.; Fang, J.S.; Wu, J.; Yang, C.; Liu, S.J.; Fang, Y.Q.; et al. Ligustilide Ameliorates Memory Deficiency in APP/PS1 Transgenic Mice via Restoring Mitochondrial Dysfunction. Biomed. Res. Int. 2018, 2018, 4606752. [Google Scholar] [CrossRef]
- Kuang, X.; Zhou, H.J.; Thorne, A.H.; Chen, X.N.; Li, L.J.; Du, J.R. Neuroprotective Effect of Ligustilide through Induction of alpha-Secretase Processing of Both APP and Klotho in a Mouse Model of Alzheimer’s Disease. Front. Aging Neurosci. 2017, 9, 353. [Google Scholar] [CrossRef]
- Mereles, D.; Hunstein, W. Epigallocatechin-3-gallate (EGCG) for clinical trials: More pitfalls than promises? Int. J. Mol. Sci. 2011, 12, 5592–5603. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Giunta, B.; Bickford, P.C.; Fountain, M.; Tan, J.; Shytle, R.D. Nanolipidic particles improve the bioavailability and alpha-secretase inducing ability of epigallocatechin-3-gallate (EGCG) for the treatment of Alzheimer’s disease. Int. J. Pharmaceut. 2010, 389, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.Y.; Bae, K.H.; Seong, Y.H.; Song, K.S. Green tea catechins as a BACE1 (beta-secretase) inhibitor. Bioorg. Med. Chem. Lett. 2003, 13, 3905–3908. [Google Scholar] [CrossRef]
- Bao, J.; Liu, W.; Zhou, H.Y.; Gui, Y.R.; Yang, Y.H.; Wu, M.J.; Xiao, Y.F.; Shang, J.T.; Long, G.F.; Shu, X.J.; et al. Epigallocatechin-3-gallate Alleviates Cognitive Deficits in APP/PS1 Mice. Curr. Med. Sci. 2020, 40, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.T.; Li, S.M. Polymethoxyflavones from citrus: Chemistry, metabolism and selected bioactivity. Abstr. Pap. Am. Chem Soc. 2019, 258. [Google Scholar]
- Youn, K.; Lee, J.; Ho, M.; Jun, C.T. Discovery of polymethoxyflavones from black ginger (Kaempferia parviflora) as potential beta-secretase (BACE1) inhibitors. J. Funct. Foods. 2016, 20, 567–574. [Google Scholar] [CrossRef]
- Stroud, J.C.; Liu, C.; Teng, D.; Eisenberg, P.K. Toxic fibrillar oligomers of amyloid-beta have cross-beta structure. Proc. Natl. Acad. Sci. USA 2012, 109, 7717–7722. [Google Scholar] [CrossRef]
- Ahmed, M.; Davis, J.; Aucoin, D.; Sato, T.; Ahuja, S.; Aimoto, S.; Elliott, J.I.; Van Nostrand, W.E.; Smith, S.O. Structural conversion of neurotoxic amyloid-beta(1-42) oligomers to fibrils. Nat. Struct. Mol. Biol. 2010, 17, 561–567. [Google Scholar] [CrossRef]
- Paravastu, A.K.; Leapman, R.D.; Yau, R.; Tycko, W.M. Molecular structural basis for polymorphism in Alzheimer’s beta-amyloid fibrils. Proc. Natl. Acad. Sci. USA 2008, 105, 18349–18354. [Google Scholar] [CrossRef]
- Hoyer, W.; Gronwall, C.; Jonsson, A.; Stahl, S.; Hard, T. Stabilization of a beta-hairpin in monomeric Alzheimer’s amyloid-beta peptide inhibits amyloid formation. Proc. Natl. Acad. Sci. USA 2008, 105, 5099–5104. [Google Scholar] [CrossRef]
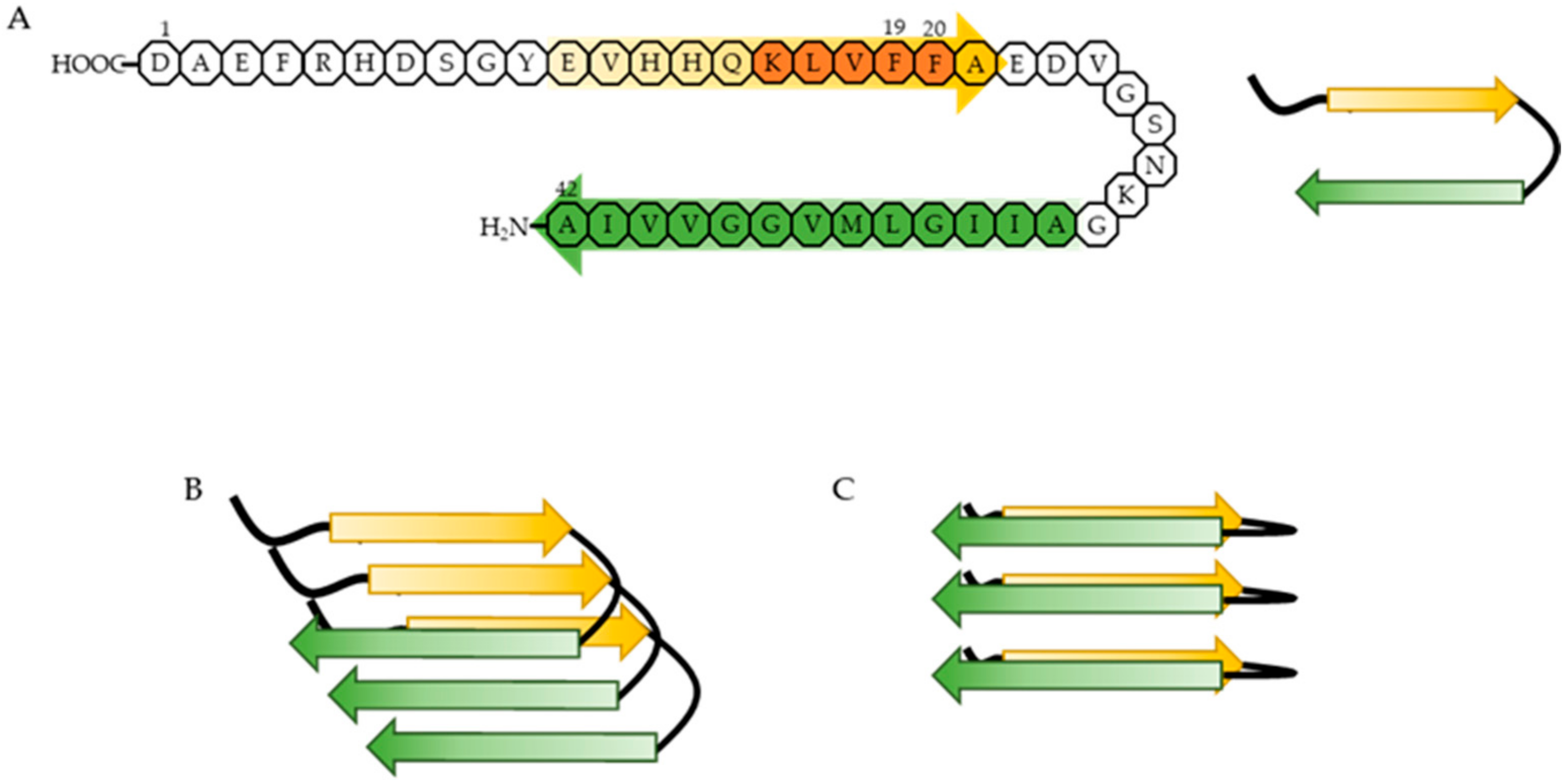
- Enache, T.A.; Chiorcea-Paquim, A.M.; Oliveira-Brett, A.M. Amyloid Beta Peptide VHHQ, KLVFF, and IIGLMVGGVV Domains Involved in Fibrilization: AFM and Electrochemical Characterization. Anal. Chem. 2018, 90, 2285–2292. [Google Scholar] [CrossRef]
- Gao, Y.; Guo, C.; Watzlawik, J.O.; Randolph, P.S.; Lee, E.J.; Huang, D.; Stagg, S.M.; Zhou, H.X.; Rosenberry, T.L.; Paravastu, A.K.; et al. Out-of-Register Parallel beta-Sheets and Antiparallel beta-Sheets Coexist in 150-kDa Oligomers Formed by Amyloid-beta(1-42). J. Mol. Biol. 2020, 432, 4388–4407. [Google Scholar] [CrossRef]
- Tamamis, P.; Adler-Abramovich, L.; Reches, M.; Marshall, K.; Sikorski, P.; Serpell, L.; Gazit, E.; Archontis, G. Self-assembly of phenylalanine oligopeptides: Insights from experiments and simulations. Biophys. J. 2009, 96, 5020–5029. [Google Scholar] [CrossRef] [PubMed]
- Marshall, K.E.; Morris, K.L.; Charlton, D.; O’Reilly, N.; Lewis, L.; Walden, H.; Serpell, L.C. Hydrophobic, aromatic, and electrostatic interactions play a central role in amyloid fibril formation and stability. Biochemistry 2011, 50, 2061–2071. [Google Scholar] [CrossRef]
- Korn, A.; McLennan, S.; Adler, J.; Krueger, M.; Surendran, D.; Maiti, D.; Huster, S. Amyloid beta (1–40) Toxicity Depends on the Molecular Contact between Phenylalanine 19 and Leucine 34. ACS Chem. Neurosci. 2018, 9, 790–799. [Google Scholar] [CrossRef]
- Vitrac, X.; Bornet, A.; Vanderlinde, R.; Valls, J.; Richard, T.; Delaunay, J.C.; Merillon, J.M.; Teissedre, P.L. Determination of stilbenes (delta-viniferin, trans-astringin, trans-piceid, cis- and trans-resveratrol, epsilon-viniferin) in Brazilian wines. J. Agric. Food Chem. 2005, 53, 5664–5669. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Ciccone, G.; Castiglione, A.; Gambino, R.; De Michieli, F.; Villois, P.; Durazzo, M.; Cavallo-Perin, P.; Cassader, M. Anti-inflammatory and antioxidant effects of resveratrol in healthy smokers a randomized, double-blind, placebo-controlled, cross-over trial. Curr. Med. Chem. 2013, 20, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Hung, V.W.S.; Cheng, X.R.; Li, N.; Veloso, K.; Kerman, A.J. Electrochemical Detection of Amyloid-Beta Aggregation in the Presence of Resveratrol. J. Electrochem. Soc. 2013, 160, G3097–G3101. [Google Scholar] [CrossRef]
- Phan, H.T.T.; Samarat, K.; Takamura, Y.; Azo-Oussou, A.F.; Nakazono, Y.; Vestergaard, M.C. Polyphenols Modulate Alzheimer’s Amyloid Beta Aggregation in a Structure-Dependent Manner. Nutrients 2019, 11, 756. [Google Scholar] [CrossRef]
- Al-Edresi, S.; Alsalahat, I.; Freeman, S.; Aojula, H.; Penny, J. Resveratrol-mediated cleavage of amyloid beta(1–42) peptide: Potential relevance to Alzheimer’s disease. Neurobiol. Aging 2020, 94, 24–33. [Google Scholar] [CrossRef]
- Gu, H.; Li, L.; Cui, C.; Zhao, Z.; Song, G. Overexpression of let-7a increases neurotoxicity in a PC12 cell model of Alzheimer’s disease via regulating autophagy. Exp. Ther. Med. 2017, 14, 3688–3698. [Google Scholar] [CrossRef]
- Andrade, S.; Ramalho, M.J.; Pereira, M.D.C.; Loureiro, J.A. Resveratrol Brain Delivery for Neurological Disorders Prevention and Treatment. Front. Pharmacol. 2018, 9, 1261. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.W.; Grossman, H.; Neugroschl, J.; Parker, S.; Burden, A.; Luo, X.; Sano, M. A randomized, double-blind, placebo-controlled trial of resveratrol with glucose and malate (RGM) to slow the progression of Alzheimer’s disease: A pilot study. Alzheimer’s Dement. 2018, 4, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.J.; Sun, W.Q.; Liu, F.F. Brazilin inhibits the Zn2+-mediated aggregation of amyloid beta-protein and alleviates cytotoxicity. J. Inorg. Biochem. 2017, 177, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Du, W.J.; Guo, J.J.; Gao, M.T.; Hu, S.Q.; Dong, X.Y.; Han, Y.F.; Liu, F.F.; Jiang, Y.; Sun, S.Y. Brazilin inhibits amyloid beta-protein fibrillogenesis, remodels amyloid fibrils and reduces amyloid cytotoxicity functional. Sci. Rep. 2015, 5, 1–10. [Google Scholar]
- Hatcher, H.; Planalp, R.; Cho, J.; Torti, F.M.; Torti, S.V. Curcumin: From ancient medicine to current clinical trials. Cell Mol. Life Sci. 2008, 65, 1631–1652. [Google Scholar] [CrossRef]
- Reinke, A.A.; Gestwicki, J.E. Structure-activity relationships of amyloid beta-aggregation inhibitors based on curcumin: Influence of linker length and flexibility. Chem. Biol. Drug Des. 2007, 70, 206–215. [Google Scholar] [CrossRef]
- Kumaraswamy, P.; Sethuraman, S.; Krishnan, U.M. Mechanistic insights of curcumin interactions with the core-recognition motif of beta-amyloid peptide. J. Agric. Food Chem. 2013, 61, 3278–3285. [Google Scholar] [CrossRef]
- Thapa, A.; Jett, S.D.; Chi, E.Y. Curcumin Attenuates Amyloid-beta Aggregate Toxicity and Modulates Amyloid-beta Aggregation Pathway. ACS Chem. Neurosci. 2016, 7, 56–68. [Google Scholar] [CrossRef]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A.; et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar] [CrossRef]
- Lim, G.P.; Chu, T.; Yang, F.; Beech, W.; Frautschy, S.A.; Cole, G.M. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J. Neurosci. 2001, 21, 8370–8377. [Google Scholar] [CrossRef]
- Estrada, L.; Soto, D.C. Disrupting beta-amyloid aggregation for Alzheimer disease treatment. Curr. Top. Med. Chem. 2007, 7, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Hasegawa, K.; Naiki, H.; Yamada, M. Anti-amyloidogenic activity of tannic acid and its activity to destabilize Alzheimer’s beta-amyloid fibrils in vitro. Biochim. Biophys. Acta 2004, 1690, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Ladiwala, A.R.; Dordick, J.S.; Tessier, P.M. Aromatic small molecules remodel toxic soluble oligomers of amyloid beta through three independent pathways. J. Biol. Chem. 2011, 286, 3209–3218. [Google Scholar] [CrossRef]
- Turkan, F.; Taslimi, P.; Saltan, F.Z. Tannic acid as a natural antioxidant compound: Discovery of a potent metabolic enzyme inhibitor for a new therapeutic approach in diabetes and Alzheimer’s disease. J. Biochem. Mol. Toxicol. 2019, 33, e22340. [Google Scholar] [CrossRef]
- Sharma, N.; Phan, H.T.; Chikae, M.; Takamura, Y.; Azo-Oussou, A.F.; Vestergaard, M.C. Black tea polyphenol theaflavin as promising antioxidant and potential copper chelator. J. Sci. Food Agric. 2020, 100, 3126–3135. [Google Scholar] [CrossRef] [PubMed]
- Anandhan, A.; Tamilselvam, K.; Radhiga, T.; Rao, S.; Essa, T.; Manivasagam, M.M. Theaflavin, a black tea polyphenol, protects nigral dopaminergic neurons against chronic MPTP/probenecid induced Parkinson’s disease. Brain Res. 2012, 1433, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Grelle, G.; Otto, A.; Lorenz, M.; Frank, R.F.; Wanker, J.; Bieschke, E.E. Black tea theaflavins inhibit formation of toxic amyloid-beta and alpha-synuclein fibrils. Biochemistry 2011, 50, 10624–10636. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z. The modification of natural products for medical use. Acta Pharm. Sin. B 2017, 7, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.D. Unlocking the potential of natural products in drug discovery. Microb. Biotechnol. 2019, 12, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Barbier, P.; Zejneli, O.; Martinho, M.; Lasorsa, A.; Belle, V.; Smet-Nocca, C.; Tsvetkov, P.O.; Devred, F.; Landrieu, I. Role of Tau as a Microtubule-Associated Protein: Structural and Functional Aspects. Front. Aging Neurosci. 2019, 11, 204. [Google Scholar] [CrossRef] [PubMed]
- Arendt, T.; Stieler, M.; Holzer, J.T. Tau and tauopathies. Brain Res. Bull. 2016, 126, 238–292. [Google Scholar] [CrossRef]
- Yang, S.H. Cellular and Molecular Mediators of Neuroinflammation in Alzheimer Disease. Int. Neurourol. J. 2019, 23, S54–S62. [Google Scholar] [CrossRef]
- Zheng, C.; Zhou, X.W.; Wang, J.Z. The dual roles of cytokines in Alzheimer’s disease: Update on interleukins, TNF-alpha, TGF-beta and IFN-gamma. Transl. Neurodegener. 2016, 5, 7. [Google Scholar] [CrossRef]



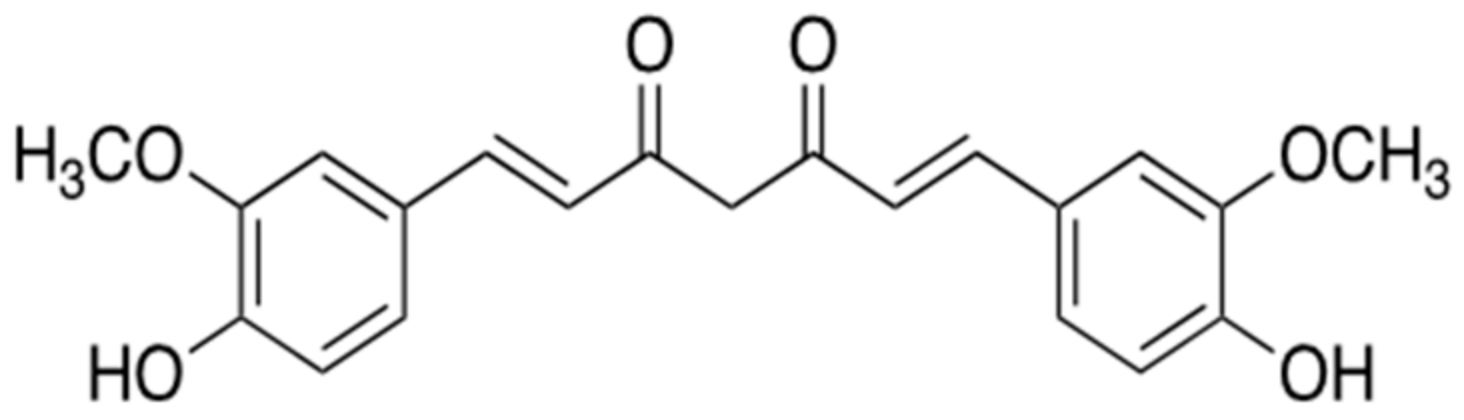

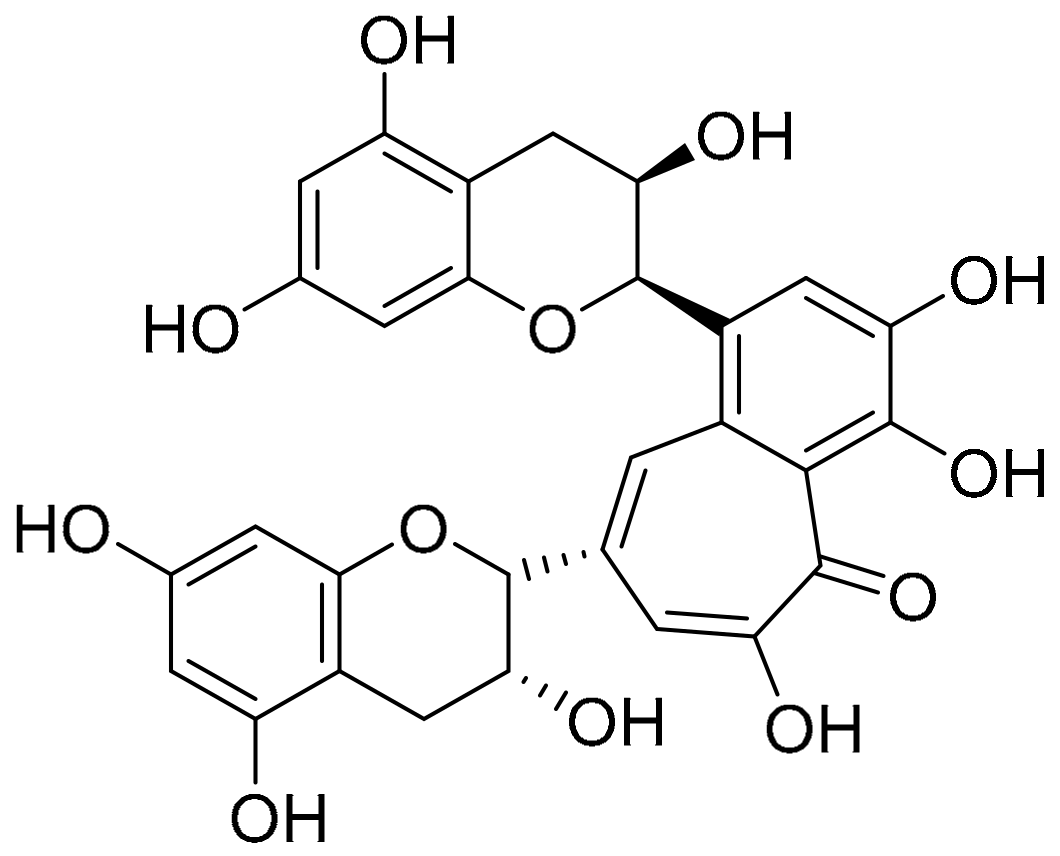
| Mechanism | Name (Origin) | ID | Concentration | Group | Time Span | Administration | Clinical Trial |
|---|---|---|---|---|---|---|---|
| Targeting to BACE1 or α-secretase (secretase-dependent ways) 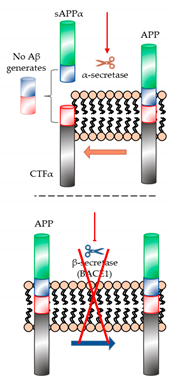 | Ginsenoside Rg1 (Panax ginseng) | - | - | - | - | - | Improvement of level Aβ in the transgenic AD mice, but clinical trials with patients was not reported yet. |
| 2,2′,4′-Trihydroxychalcone (TDC) (Glycyrrhiza glabra) | - | - | - | - | - | Have no known clinical trials. | |
| Hispidin (Phellinus linteus) | - | - | - | - | - | Ongoing trial is not reported yet. | |
| Berberine (Coptidis rhizoma) | NCT03221894 | Donepezil: 5~10 mg Berberine: 10 g/d | Donepezil group and Donepezil + grape granule group | 12 months | Each bag of granules with 150 mL warm water melt was taken orally twice a day. | Currently little planned or ongoing clinical trials. | |
| Ligustilide (Ligusticum chuanxiong Hort.) | - | - | - | - | Clinical trials have been rarely reported yet. | ||
| Epigallochetchin-3-galate (EGCG) (Camellia sinensis leaf) | NCT00951834 | EGCG: 200~800 mg/day (dependent on intake period) | Single group assignment (21 people) | 18 months | Co-medication with donepezil | in phase 3 | |
| Polymethoxyflavones (Kaempferia parviflora) | - | - | - | - | - | It is reported that there are not enough clinical trials using this. |
| Mechanism | Name (Origin) | ID | Concentration | Group | Time Span | Administration | Clinical Trial |
|---|---|---|---|---|---|---|---|
| Targeting to changes of structure. (structure-dependent way) 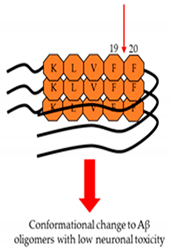 | Resveratrol (Arachis) | NCT00678431 | Resveratrol: 5 mg/day Dextrose and malate: 5 g/day | Placebo group and dextrose, malate, and resveratrol groups (32 people) | 3, 6, and 12 months of each group | Administered twice a day in liquid form and taken in a 15ml volume dissolved in unsweetened grape juice | Its effects on AD are less deterioration. |
| Brazilin (Caesalpinia sappan) | - | - | - | - | - | Was not reported yet. | |
| Curcumin (Zingiberaceae) | NCT00099710 | Each 2 g/day or 4 g/day of curcumin | Placebo group and 2 g/day and 4 g/day groups (36 people) | 24-weeks and 48-weeks of each group | Oral administration by capsules | Effects on Aβ aggregates was not observed in AD patients. | |
| Tannic acid (Fagaceae) | - | - | - | - | - | Clinical trials are not widely conducted yet. | |
| Theaflavin (the fermentation of tea into black tea) | - | - | - | - | - | The clinical trials for use of theaflavin as a drug candidate in AD treatment was little reported yet. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; Ahn, N.-H.; Choi, S.-B.; Kwon, Y.; Yang, S.-H. Natural Products Targeting Amyloid Beta in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 2341. https://doi.org/10.3390/ijms22052341
Lee J-H, Ahn N-H, Choi S-B, Kwon Y, Yang S-H. Natural Products Targeting Amyloid Beta in Alzheimer’s Disease. International Journal of Molecular Sciences. 2021; 22(5):2341. https://doi.org/10.3390/ijms22052341
Chicago/Turabian StyleLee, Joo-Hee, Na-Hyun Ahn, Su-Bin Choi, Youngeun Kwon, and Seung-Hoon Yang. 2021. "Natural Products Targeting Amyloid Beta in Alzheimer’s Disease" International Journal of Molecular Sciences 22, no. 5: 2341. https://doi.org/10.3390/ijms22052341
APA StyleLee, J.-H., Ahn, N.-H., Choi, S.-B., Kwon, Y., & Yang, S.-H. (2021). Natural Products Targeting Amyloid Beta in Alzheimer’s Disease. International Journal of Molecular Sciences, 22(5), 2341. https://doi.org/10.3390/ijms22052341






