Abstract
The SNX-PXA-RGS-PXC subfamily of sorting nexins (SNXs) belongs to the superfamily of SNX proteins. SNXs are characterized by the presence of a common phox-homology (PX) domain, along with other functional domains that play versatile roles in cellular signaling and membrane trafficking. In addition to the PX domain, the SNX-PXA-RGS-PXC subfamily, except for SNX19, contains a unique RGS (regulators of G protein signaling) domain that serves as GTPase activating proteins (GAPs), which accelerates GTP hydrolysis on the G protein α subunit, resulting in termination of G protein-coupled receptor (GPCR) signaling. Moreover, the PX domain selectively interacts with phosphatidylinositol-3-phosphate and other phosphoinositides found in endosomal membranes, while also associating with various intracellular proteins. Although SNX19 lacks an RGS domain, all members of the SNX-PXA-RGS-PXC subfamily serve as dual regulators of receptor cargo signaling and endosomal trafficking. This review discusses the known and proposed functions of the SNX-PXA-RGS-PXC subfamily and how it participates in receptor signaling (both GPCR and non-GPCR) and endosomal-based membrane trafficking. Furthermore, we discuss the difference of this subfamily of SNXs from other subfamilies, such as SNX-BAR nexins (Bin-Amphiphysin-Rvs) that are associated with retromer or other retrieval complexes for the regulation of receptor signaling and membrane trafficking. Emerging evidence has shown that the dysregulation and malfunction of this subfamily of sorting nexins lead to various pathophysiological processes and disorders, including hypertension.
1. Introduction
Receptor-mediated signaling and membrane trafficking processes are intimately interconnected with the endosomes [1]. Internalized receptors, including G protein-coupled receptors (GPCRs) and non-GPCRs, are sorted at endosomes, from which receptors are either delivered to the lysosome for degradation, recycled back to the plasma membrane, or delivered to the trans-Golgi network (TGN) and other organelles by receptor-specific pathways [2,3]. Sorting nexins (SNXs) play critical roles in these processes [4].
The SNX family has a phox homology (PX) domain, capable of phosphoinositide binding, which enables SNX targeting to endosomal membranes by binding to phosphatidylinositols, most commonly phosphatidylinositol 3-phosphate (PI(3)P) [5,6]. SNXs are widely expressed from yeast to mammals, whose PX domain, first identified in two subunits of the NADPH oxidase, p40phox and p47phox, actively engages in protein–lipid and protein–protein interactions [5,6]. To date, 10 yeast and 33 mammalian SNXs have been identified [5,6,7,8]. Based on their domain architectures, the mammalian SNXs are divided into five subfamilies: SNX-PXA-RGS-PXC, SNX-FERM (protein 4.1/ezrin/radixin/moesin), SNX-BAR (Bin/Amphiphysin/Rvs), SNX-PX, and the unclassified SNX subfamilies [7,8] (Table 1).

Table 1.
Summary of mammalian sorting nexin (SNX) subfamilies [5,6,7,8].
2. SNX-PXA-RGS-PXC Subfamily Domain Structure and Biochemical Properties
The SNX-PXA-RGS-PXC subfamily is comprised of SNX13 (also known as RGS-PX1), SNX14, SNX19, and SNX25. This subfamily of SNXs contains two N-terminal helical transmembrane domains, followed by a PX-associated domain (PXA), a regulators of G protein signaling (RGS) domain, the PX domain, and a C-terminal PX-associated (PXC) domain [6,8].
Integrated transmembrane domains (IMDs), which are two, close short hydrophobic sequences, are involved in membrane tethering [4,6]. RGS domain, a unique domain compared with other subfamily SNXs, is a conserved, approximately 130 amino acid residue-domain with a specific molecular configuration (Figure 1A). The PXA and PXC domains are largely uncharacterized.
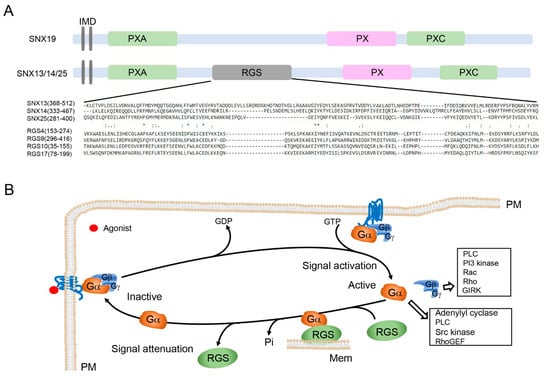
Figure 1.
Unique RGS domain in the structure of SNX-PXA-RGS-PXC subfamily. (A) Domain organization of SNX-PXA-RGS-PXC subfamily. All members of this subfamily, except SNX19, have unique RGS domains, which are aligned with RGS proteins, as shown. Asterisk denotes identical amino acid residues among all of the seven peptides, one dot indicates the weakly conserved amino acid residues, and double dots indicate the well-conserved amino acid residues among all of the peptides. IMD, integrated transmembrane domain. (B) RGS proteins in the G protein nucleotide cycle. Upon agonist binding, receptors activate heterotrimeric G proteins, which induce the exchange of GDP for GTP and dissociation of Gα from Gβγ, attenuation of Gα subunit’s activation (Gαs) or inhibition (Gαi) of its downstream effectors. The effect is terminated by GTPase or the intrinsic GTPase activity of Gα, where RGS is separated from Gαs. RGS proteins or SNX13 facilitates the hydrolysis of GTP by Gαs, as a GTPase-activating protein. Gα, G alpha subunit; Gβ, G beta subunit; GDP, guanosine diphosphate; Gγ, G gamma subunit; GIRK, G protein-coupled inwardly rectifying potassium; GTP, guanosine triphosphate; Mem, intracellular membranes; PI3 kinase, phosphoinositide 3-kinase; PLC, phospholipase C; PM, plasma membrane; Rac, Rac G protein; RGS, regulators of G protein signaling; Rho, Rho G protein; RhoGEF, guanine nucleotide exchange factor for Rho; Scr kinase, Src family kinases.
The PX domain of the SNX-PXA-RGS-PXC subfamily is similar to the PX domains of all other SNX subfamilies, with around 100-130 residues, comprised of three β-strands and three α-helices [8]. The conserved sequence ΨPxxPxK (Ψ refers to any large aliphatic amino acid V, I, L, or M) forms a shallow, positively charged proline-rich loop that is considered to be the binding site of the negatively charged phosphate groups of phosphoinositides [4]. Phosphatidylinositol 3-phosphate (PI(3)P), primarily found in early endosome membranes, is a common target of SNXs [9]. This was confirmed from the analysis of the crystal structure of the SNX PX domains [4]. Although PI(3)P is the most common phosphoinositide bound to SNX, many other phosphoinositide interactions have also been demonstrated (Table 2) [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27]. The PX domain acts not only as a lipid recognition module [7,11], but also plays a key role in protein–protein interactions, such as the interaction of SNX13 and SNX14 with Gαs [4,6] and SNX19 with IA2 [4,6] and D1R [20]. However, the molecular details of these interactions remain to be characterized further.

Table 2.
Summary of the characteristics of SNX-PXA-RGS-PXC subfamily members.
The RGS domain is present in SNX13, SNX14, and SNX25, but not SNX19 [6] (Table 3). This domain is found in a number of molecules, including 20 canonical mammalian RGS proteins and an additional 19 proteins that mediate the interaction with GPCRs or Gα subunits [28]. G proteins are activated by the binding of GTP to Gα and separation from the Gβγ dimer; the deactivation of G proteins occurs when GTP is hydrolyzed by the action of the GTPase-activating proteins (GAPs) (Figure 1B). RGS proteins bind to Gα to facilitate the GTP hydrolysis, accelerating the termination of G protein signaling [29,30]. The SNX-PXA-RGS-PXC subfamily belongs to 19 noncanonical proteins that were previously considered nonfunctional [31]. Recent findings demonstrated that the RGS domain in SNX proteins, like canonical RGS proteins, is involved in the attenuation of GPCR and related G protein signaling [13,32,33].

Table 3.
Examples of RGS domain in SNX-PXA-RGS-PXC subfamily members.
3. SNX-PXA-RGS-PXC Subfamily in Receptor Signaling
Similar to the canonical RGS proteins [34], the RGS domain in this subfamily functions as a GAP module, which potentially attenuates GPCR signaling (Table 3 [13,20,23,27,33,35,36]). SNX13 is the first identified SNX that contains the RGS domain, which regulates signaling triggered by GPCRs [33]. Zheng et al. reported that SNX13, through its RGS domain, interacts with the constitutively active form of Gαs, accelerating the hydrolysis of GTP by Gαs [33]. Exogenous expression of the RGS domain of SNX13 reduces the agonist-mediated cAMP increase in HEK293 cells and adenylate cyclase activity in rat cardiac membranes [32,33], while no effect is observed on forskolin-induced cAMP production and adenylate cyclase activity [33], which does not require Gαs. These studies confirm the role of SNX13, as a GAP, in attenuating Gαs-mediated signaling, indicating that SNX13 plays a critical role in the regulation of the duration of GPCR signaling [32]. SNX13 and D1R may interact because SNX13 105820C and DRD1 G-94 have been associated with an increase in albumin excretion in a twin pair study [37]. Therefore, SNX 13 may have a role in D1R signaling.
The RGS domain does not have to function as a GAP to regulate GPCR signaling in all cases. For example, the RGS domain of SNX14 does not have GAP activity, but specifically binds to and sequesters Gαs, inhibiting the downstream cAMP production caused by the activation of serotonin receptor 6 (5-HT6R) [13]. The binding affinity of SNX14 for Gαs is markedly attenuated by the phosphorylation of the RGS domain [13]. This suggests that SNX14 negatively regulates 5-HT6R signaling by sequestering Gαs.
As discussed above, the RGS domain facilitates the SNX-PXA-RGS-PXC subfamily in the regulation of GPCR signaling by sequestering Gαs with [33] or without [13] GAP function. To confirm further that RGS domain is not always required for SNX regulation of GPCR signaling, it is critical to study SNX19, a member of this family without RGS domain. SNX19 is essential for the lipid raft residence of D1R, cAMP production, and promotion of effective D1R signaling [20]. SNX19 also regulates the signaling of histamine receptor H4 (HRH4), a GPCR that is important in the initiation and maintenance of inflammation in mouse lung, following ammonia exposure [38].
In addition to GPCRs, the SNX-PXA-RGS-PXC subfamily also regulates the signaling of non-GPCRs. In mouse insulinoma cells exposed to high glucose concentration, SNX19 inhibits the conversion of PI(4,5)P2 to PI(3,4,5)P3 and suppresses the phosphorylation of Akt/protein kinase B (PKB), playing critical roles in insulin receptor signaling [22]. In NIH3T3 fibroblasts, SNX25 negatively interacts with transforming growth factor-β receptor 1 (TGF-β1) and downregulates its signaling by increasing the degradation of its receptor [23]. Of note, RGS domain is not necessarily responsible for the regulation of signaling [20,23]. Deletion of either PX or PXA domain abolishes the interaction of SNX25 with TGF-β1 and inhibits TGF-β1 signaling [23]. However, the RGS domain is not critical for the regulation of receptor signaling in this context [23]. SNX25 may also be involved in the circadian rhythmic regulation of vasopressin secretion in the mouse suprachiasmatic nucleus [24].
4. SNX-PXA-RGS-PXC Subfamily in Membrane Trafficking
Upon endocytosis, receptors (GPCR or non-GPCR) are trafficked to early endosomes, and then sorted to distinct destinations: lysosomal-mediated degradation or recycling to the plasma membrane or other organelle compartments for reuse [39]. As discussed previously, the SNX-PXA-RGS-PXC subfamily has a conserved PX domain, which enables the SNX to be targeted effectively to endosomal membranes, most frequently by binding to PI(3)P [6]. Therefore, the SNX-PXA-RGS-PXC subfamily represents a core regulator for mediating receptor-endocytic membrane trafficking.
4.1. SNX-PXA-RGS-PXC Subfamily in Lysosomal-Mediated Degradation
Endolysosomal trafficking is the major pathway by which transmembrane receptors are downregulated. Membrane contact sites (MCS) between lysosomes and endosomes, as well as mitochondria and endoplasmic reticulum (ER), are regions of phospholipid exchange, which regulate the sorting of receptors at late endosomes for degradation [40,41]. In yeast, Mdm1 (mitochondrial distribution and morphology 1), equivalent to the mammalian SNX-PXA-RGS-PXC subfamily, is a tethering protein that localizes to ER-vacuole/lysosome MCS [42]. Mdm1 PX domain is required and sufficient for its association with the vacuole/lysosome surface [42]. Overexpression of Mdm1 induces ER-vacuole/lysosome tethering and truncation of Mdm1, which removes the PXA domain, disrupts the ER-vacuole tethering, and suppresses lipid exchange and endolysosomal sorting [42].
SNX13 binds to a wide range of phosphoinositides (Table 2) and plays an important role in receptor-endosome-lysosomal degradation. In zebrafish cardiomyocytes, a reduction in SNX13 expression promotes the endolysosomal sorting of apoptosis repressor with caspase recruitment domain (ARC) for its lysosomal degradation [12]. SNX13 interacts with ARC and regulates the interaction between ARC and caspase-8. The increase in the lysosomal degradation of ARC results in the removal of ARC-mediated inhibition and the activation of caspase-8, leading to the activation of the extrinsic apoptotic pathway and subsequent apoptotic cardiomyocyte death [12]. In HEK293 cells, overexpression of SNX13 delays the ligand-dependent EGFR lysosomal targeting, trafficking, and degradation [33], similar to the knockdown of Gαs by RNA interference [35]. SNX13 colocalizes with Gαs and hepatocyte growth factor-regulated tyrosine kinase substrate (Hrs) [35], a critical component of the endosomal sorting machinery for sequestration into multivesicular bodies and subsequent degradation in lysosomes [43]. Henceforth, SNX13 effectively promotes EGFR lysosomal degradation.
Morphological evidence also demonstrated the critical role of SNX13 in lysosomal degradation. Two distinct endosome morphologies, vesicular and tubular, are involved in receptor degradation and recycling pathways, respectively [44]. An unusually abundant amount of tubular endosome structures was observed in the visceral yolk sac endoderm cells of systemic Snx13-null mice [45]. This indicates that the receptor is rerouted from endosomes to recycling or TGN pathways due to the defect in the sorting of the lysosomal pathway from early endosomes caused by the knockout of SNX13.
The SNX14 PX domain preferentially binds to PI(3,5)P2 [16], a key component of late endosomes/lysosomes [9,10], implicating its role in lysosomal degradation [16]. Similar to the yeast homologue of SNX14, Mdm1, which mediates the formation of ER-vacuole contact sites [42], SNX14 tethers for ER localization through its N-terminal transmembrane helices [14]. Knockdown of SNX14 causes accumulation of aberrant cytoplasmic vacuoles, suggesting defects in endolysosomal homeostasis [14]. SNX14 localizes at the interface between the ER and lipid droplets (LDs); SNX14, overexpressed in human bone osteosarcoma epithelial cells (U2OS), mediates LD budding and growth from the ER surface, after which the LDs are released following its maturation [15]. SNX14 also interacts with 5-HT6R, facilitating its endolysosomal degradation [13]. In yeast, Mdm1 not only tethers ER and LDs together, but also generates a high concentration of activated lipids proximal to the vacuole that may facilitate LDs’ autophagic lysosomal degradation [46].
Knockdown of Snx19 decreases the transmembrane protein, insulinoma-associated protein 2 (IA-2), and the number of dense core vesicles (DCV) in MIN6 cells, a mouse pancreatic β-cell line. The decrease in the IA-2 protein expression and the amount of DCV correlate with the increase in autophagic lysosomal activity [47], which is rescued with the re-introduction of SNX19, indicating a critical role of SNX19 in DCV autophagic lysosomal degradation in MIN6 cells [47].
SNX25 interacts with tropomyosin receptor kinase B (TrkB) in early endosomes, late endosomes, and lysosomes in hippocampal neurons and HEK293T cells [48]. SNX25 overexpression remarkedly reduces the expression of ligand dependent TrkB protein in HEK293T cells [48]. These findings suggest that SNX25 is important in the endolysosomal degradation of TrkB.
4.2. SNX-PXA-RGS-PXC Subfamily in Membrane Recycling
Besides mediating endolysosomal degradation, as described above [49], the SNX-PXA-RGS-PXC subfamily, like other SNXs, also regulates receptor membrane recycling. SNX19 plays an important role in D1R plasma membrane recycling [20]. In renal proximal tubule cells, SNX19 interacts and colocalizes with D1R at the plasma membrane, specifically in lipid rafts. This colocalization is increased by treatment with fenoldopam, a D1-like receptor agonist [20]. The increase in their colocalization starts within a few minutes and returns to the basal level after one hour [20]. Depletion of SNX19 by its specific siRNA decreases D1R lipid raft localization, plasma membrane expression, and signaling [20]. All of these results indicate the critical role of SNX19 in D1R recycling, probably via palmitoylation and lipid raft targeting.
SNX25 interacts with D1R and D2R in HEK293 cells, and overexpression of SNX25 perturbs the endocytosis of D1R and D2R and recycling of the D2R. Moreover, knockdown of SNX25 causes a subsequent decrease in D2R plasma membrane expression, suggesting that SNX25 plays a role in D2R membrane recycling [27].
5. Comparison of SNX-PXA-RGS-PXC Subfamily with SNX-BAR Subfamily in Receptor Signaling and Membrane Trafficking
SNX-BAR, another subfamily of SNXs, is known to regulate receptor signaling and orchestrate membrane trafficking through distinct mechanisms. Although there are few overlaps with the SNX-PXA-RGS-PXC subfamily, the SNX-BAR sorting nexin subfamily regulates different types of receptor cargoes. For example, SNX1 is important for D5R signaling [50], while the SNX5 regulates the signaling and trafficking of D1R [51], insulin receptors [52], and insulin-degrading enzyme [53] in renal proximal tubule cells. Likewise, SNX1, SNX2, and SNX6 have been found to regulate the membrane trafficking of cation-independent mannose phosphate receptor (CI-MPR) [54,55], cell surface receptor CED-1 [56], TGN38 [57], vacuolar sorting receptor [58], β-site amyloid precursor protein-cleaving enzyme 1 (BACE1) [59], PIN1 [60], and PIN2 [60]. SNX4 regulates the transferrin receptor [61], BACE1 [62], and E-cadherin recycling [63]. SNX18 regulates the transfer of LC3 from the recycling endosome to the autophagosome [64].
Distinct from the SNX-PXA-RGS-PXC subfamily, the SNX-BAR subfamily shares a close relationship with retromers and other retrieval machineries. SNX-BAR subfamily contains a dimeric Bin-Amphiphysin-Rvs (BAR) domain with a positively charged curved surface that binds to membranes [65]. The BAR domain confers targeting to the tubular domain of the endosome, and the endosome aids the transition from a spherical vacuole to a tubule membrane through the interaction of the BAR domains with endosomes, forming a tubular transport carrier [7]. In yeast, the SNX-BAR dimer forms a stable complex with the retromer, a heterotrimer of Vps26-Vps29-Vps35 [65]. In mammalian cells, the association of SNX-BAR dimer with the retromer is relatively weak, but SNX-BAR still relies on the retromer to orchestrate the recognition and capture of specific cargoes [2,3]. The weak association of SNX-BAR with the retromer in mammalian cells may reflect the large diversity of cargoes and the need for other proteins, such as Rab GTPases [66,67], ubiquitin [68], actin filaments [67], and WASH complex [69], to coordinate in the regulation of receptor signaling and trafficking [3,7]. The retromer is also critical for SNX-BAR regulation of receptor endocytic trafficking, retromer-independent receptor plasma membrane recycling, and endosome-to-TGN retrograde trafficking [61,70,71]. Because the SNX-PXA-RGS-PXC subfamily lacks the BAR domain, it does not depend on the retromer or other retrieval machineries to regulate receptor cargo signaling and trafficking.
Different from SNX-BAR, the SNX-PXA-RGS-PXC subfamily (except SNX19) plays some roles similar to RGS proteins. Canonical RGS proteins regulate the signaling of their GPCR cargo, by binding directly to Gαs, and function as a GAP [72]. SNX13, like canonical RGS proteins, can function as a GAP [34], but more studies are needed to determine if this function extends to all members of this subfamily of SNXs, i.e., SNX-PXA-RGS-PXC. As aforementioned, D1R signaling is regulated by SNX5, a member of SNX-BAR without the RGS domain. Both SNX5 and SNX19 regulate D1R internalization in early endosomes [20,51]. It is unknown whether the two SNXs regulate D1R subsequent trafficking and lysosomal degradation. SNX5 and SNX19 differently regulate D1R recycling (Figure 2).
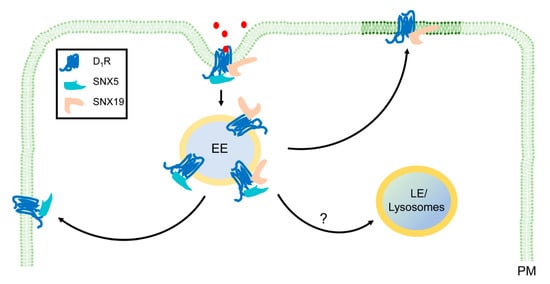
Figure 2.
Regulation of D1R signaling and membrane trafficking by SNX5 and SNX19 in human renal proximal tubule cells. D1R is stimulated by dopamine or D1R agonists, resulting in the activation of Gαs and increase in CAMP production (not shown). Both SNX5 and SNX19 interact to internalize D1R in early endosomes (EE). It is not clear if SNX5 and SNX19 participate in the subsequent trafficking of D1R in late endosomes (LE) and lysosomes. SNX5 and SNX19 differently regulate D1R recycling; SNX5 may regulate D1R recycling through phosphorylation (not shown), while SNX19 regulates D1R recycling through palmitoylation and targeting D1R to lipid rafts (dark green). Red dots, dopamine, fenoldopam, or other D1R agonists.
SNX5 regulates D1R signaling, probably through G protein-coupled receptor kinase (GRK) 4-mediated phosphorylation and desensitization of D1R, but not by targeting D1R to lipid rafts [51]. As previously stated, SNX19, a member of the SNX-PXA-RGS-PXC subfamily without the RGS domain, is required for the D1R-stimulated cAMP production [20]. Therefore, the RGS domain and its GAP function are not essential for the regulation of GPCR signaling by SNX19. SNX19 interacts with the Golgi-associated DHHC-type zinc finger enzyme for D1R palmitoylation and targeting into lipid rafts, where adenylate cyclase 6 is located [8], to regulate D1R signaling [20]. How SNX5 and SNX19, individually or synergistically regulate D1R signaling and internalization and if they regulate the degradation of D1R in lysosomes remains to be determined.
In contrast to the SNX-BAR subfamily, the SNX-PXA-RGS-PXC subfamily has a different preference for trafficking routes for its receptor cargoes. Based on recent limited studies, the SNX-PXA-RGS-PXC subfamily mainly transports receptor cargoes, via the endolysosomal pathway for degradation [12,16,35,42,43,45], while the SNX-BAR family mainly retrieves cargoes away from lysosomal degradation, via recycling pathways from the endosome to the plasma membrane, or retrograde pathways from the endosome-to-TGN [2,3,4,5,6,7,8,73,74,75]. The different trafficking pathways could be due to the distinct microdomain localization of retrieval machineries (e.g., retromers) for retrieval of receptor cargoes from ESCRT (endosomal sorting complex required for transport proteins) for degradation, as demonstrated in the Caenorhabditis elegans coelomocyte [76,77]. Whether a particular receptor cargo is sorted for recycling or endosomal degradation is governed largely by the SNX associated with retrieval complexes or the ESCRT machinery [2,3,5]. It is plausible for SNX-BAR family to regulate plasma membrane recycling or retrograde trafficking from endosomes to TGN through the retromer-dependent or retromer-independent (e.g.,: ESCPE-1, endosomal SNX-BAR sorting complex for promoting exit-1) protein machineries [69,78]. Ubiquitination [79,80] and palmitoylation [81,82] are important mechanisms for receptor cargo sorting into the ESCRT-mediated degradation. The D1R is regulated by ubiquitination [83] and palmitoylation [20], and as aforementioned, SNX5 [51] and SNX19 [20] regulate D1R signaling and trafficking. Therefore, it is possible that ubiquitin-tagged or palmitoylated D1R is sequestered by different ESCRT-subunits, using distinct mechanisms for its lysosomal degradation [84].
6. Comparison of SNX-PXA-RGS-PXC Subfamily with Other SNX Subfamilies
The SNX-PXA-RGS-PXC subfamily has differences from the other SNX subfamilies in its role in receptor signaling and trafficking. For example, SNX3, a member of the SNX-PX subfamily, interacts with the retromer complex to regulate cargoes, such as the divalent metal transporter 1-II (DMT1-II) recycling from the endosome to TGN [85]. SNX17, a member of the SNX-FERM subfamily, interacts via its FERM domain with cargoes, such as integrins, for endosomal recycling to the plasma membrane [86]. During this process, SNX17 is associated with the Commander complex, an assembly comprised of at least fifteen proteins, including the retriever, a retromer-like structure, consisting of three proteins VPS35L, VPS26C, and VPS29 [87]. SNX27, another member of the SNX-FERM subfamily, interacts simultaneously, via its unique PDZ domain, with retromer subunit and cargo receptors, such as the β2AR, to regulate their recycling [88].
7. SNX-PXA-RGS-PXC Subfamily in Physiology and Pathophysiology
As aforementioned, the SNX-PXA-RGS-PXC subfamily regulates the signaling and trafficking of internalized cargoes, including GPCRs and non-GPCRs, mainly leading them to endolysosomal degradation [10,13,42]. There is a dynamic coordinated interaction among the recycling, retrograde, and degradative pathways, which maintains normal cellular functions [2,3]. However, if the SNX-PXA-RGS-PXC subfamily, like all other SNX subfamilies, is dysfunctional and disabled to transport receptor cargoes to their appropriate cellular destinations, there will be the impairment of the above-mentioned pathways, which will negatively affect cellular functions, causing disorders, such as those listed in Table 4 [17,18,19,20,23,27,33,38,89,90,91,92,93,94,95,96,97,98].

Table 4.
SNX-PXA-RGS-PXC subfamily in cellular physiology and implications in diseases.
SNX13 forms a heterotrimeric complex with Gαs and Hrs in endosomes, critical in targeting ubiquitinated membrane cargoes, such as EGFR, for sequestration into multivesicular bodies and subsequent degradation in lysosomes [35,42]. Germline deletion of Snx13 in mice is embryonically lethal, indicating that SNX13-regulated endocytosis dynamics is essential in mouse development [45]. SNX13 plays a crucial role in preserving cardiomyocyte survival by targeting ARC endolysosomal degradation [12]. SNX13 is associated with skin pigmentation variation in humans [89,99], indicating that SNX13 plays a role in melanin cellular transport and trafficking.
SNX14 is important in normal neuronal excitability and synaptic transmission [90]. SNX14, localized in the lysosome [16], functions as a negative regulator of the signaling and trafficking of 5-HT6R [13] and probably other receptor cargoes, as well. SNX14 is also localized at the membrane contact site of ER-lipid droplets in yeast, drosophila, and mammals [14,42,91,100], indicating important roles of SNX14 in lipid drop biogenesis and trafficking of lipid transfer proteins [101].
SNX19 interacts with D1R and Golgi-associated DHHC-type zinc finger [20], a palmitoyltransferase in Golgi [102] and, as previously stated, facilitates D1R palmitoylation, trafficking from anterograde trafficking, and recycling [20]. This promotes the residence of D1R in the lipid rafts [20], where other D1R signaling complex components are localized, including GRK4, G proteins, adenylyl cyclases, and effector proteins, such as NADPH oxidase, Na+-K+-ATPase, and Na+-H+ exchanger (NHE) 3, for appropriate cellular responses and functions [103,104,105,106]. The PX domain of SNX19 is required for D1R targeting to lipid rafts because the deletion of the PX domain results in the D1R mistargeting to non-lipid rafts [20]. Moreover, SNX19 knockdown not only decreases the D1R-induced increase in cAMP production, but also abrogates the ability of the D1R to inhibit renal tubular sodium reabsorption [20]. Importantly, renal Snx19 knockdown increases the systolic blood pressure of C57BL/6J mice [20], indicating critical roles of SNX19 on the regulation of blood pressure. SNX19 also interacts with Islet antigen-2 [92], a major autoantigen in type 1 diabetes, and is located in dense-core secretory vesicles that regulate insulin secretion [23]. SNX19 may function as a protective factor against cartilage degradation [21]. A single nucleotide polymorphism of SNX19, rs2298566, increases the risk of coronary heart disease [18].
SNX25 is involved in the lysosomal degradation of the TGF-β receptor [23] and the development of temporal lobe epilepsy [25]. SNX25 interacts with and accelerates tropomyosin-related kinase B degradation [48]. SNX25 may also be involved in the regulation of genes associated with mesothelioma [93]. SNX25 is a potential candidate gene for distal hereditary motor neuropathies [94] and a genetic modifier of the age of onset of familial Alzheimer’s disease [26].
8. Conclusions and Perspectives
Emerging evidence has demonstrated that the SNX-PXA-RGS-PXC subfamily and their interacting partners are critical regulators for receptor signaling and membrane trafficking. The receptor cargoes can be GPCRs and non-GPCRs through which cells respond to both extracellular and intracellular stimulation. The complex interaction between cellular signaling and endosomal-based membrane trafficking plays an essential role in maintaining cellular homeostasis and versatile functions. SNX13, 14, and 25 have a unique RGS domain, which presumably serves as GAP, attenuating signals associated with GPCR. It is important to examine the molecular mechanisms of GAP both in vitro and in vivo for all three SNXs of the above subfamily. Current evidence suggests that SNX 19 lacks an RGS domain, indicating that it is unable to serve as a GAP. However, SNX19 has emerged to regulate GPCR in other ways, for example, facilitating D1R signaling through palmitoylation. Further studies are needed to determine the precise molecular mechanisms by which SNX19 regulates palmitoylation in the Golgi and the plasma membrane.
Different from retromer-dependent SNXs, which retrieve their cargoes through recycling to plasma membrane, TGN or other organelles in retromer-dependent and -independent mechanisms, the SNX-PXA-RGS-PXC subfamily mainly regulates their cargo receptors for endolysosomal degradation. The SNX-PXA-RGS-PXC subfamily regulates receptor recycling for certain cargoes as well, but the molecular switch that controls the different post-endocytic trafficking routes remains to be identified.
While cellular signaling directs the distinct receptor cargo trafficking routes, cargo trafficking actively shapes the cellular signaling response as well, by altering the location and time of specific signaling events. The incomplete understanding of the role that RGS-PXC SNX plays in cell polarity warrants further research. For example, it is important to understand the exact function of the SNX-PXA-RGS-PXC subfamily in the sorting of D1R and renal sodium transporters to different cell surface domains. We need to study how such processes can control polarized apical and basolateral locations and cellular function for sodium transport in the renal proximal tubule and other nephron segments. It is expected that the SNX-PXA-RGS-PXC subfamily, as with other SNXs, plays diverse roles on the regulation of the intricately linked signaling and trafficking for precise cellular functional outputs. Studies in appropriate conditional or non-conditional global knockout and transgenic or gene rescue animal models will advance our understanding of the physiological functions in vivo of the SNX-PXA-RGS-PXC subfamily and their associated pathophysiological disorders, which could lead to potential novel therapies targeting this SNX subfamily.
Author Contributions
B.A., H.L. and P.A.J. wrote the manuscript; B.A., H.L. and P.A.J. prepared the figures and tables. All authors (B.A., H.L., L.D.A., P.K., I.A., R.A.F., P.A.J.) edited and revised the manuscript and approved its final version. All authors have read and agreed to the published version of the manuscript.
Funding
The work was funded by grants from the US National Institutes of Health R01DK119652, R37HL023081, R01DK039308, R01HL092196, R01DK090918, P01HL068686, P01HL074940, and U01GM074492.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| Akt/PKB | protein kinase B |
| AR | adrenergic receptor |
| ARC | apoptosis receptor with caspase recruitment domain |
| BACE 1 | β-site amyloid precursor protein-cleaving enzyme 1 |
| BAR | Bin-Amphiphysin-Rvs |
| cAMP/PKA | cyclic adenosine monophosphate/protein kinase A |
| CED-1 | cell death abnormality protein-1 |
| CI-MPR | cation-independent mannose 6-phosphate receptor |
| CNS | central nervous system |
| DCV | dense core vesicles |
| dHMN | distal hereditary motor neuropathies |
| DMT1-II | divalent metal transporter 1-II |
| D1R | dopamine receptor 1 |
| D2R | dopamine receptor 2 |
| EGFR | epithelial growth factor receptor |
| EOAD | early-onset Alzheimer’s Disease |
| ER | endoplasmic reticulum |
| ESCPE-1 | endosomal SNX-BAR sorting complex for promoting exit-1 |
| ESCRT | endosomal sorting complex required for transport proteins |
| FERM | protein 4.1/ezrin/radixin/ moesin |
| GAP | GTPase-activating proteins |
| Gαs | G protein alpha stimulatory subunit |
| GPCR | G protein-coupled receptor |
| Hrs | hepatocyte growth factor-regulated tyrosine kinase substrate |
| 5HT6R | serotonin receptor 6 |
| IMD | integrated transmembrane domain |
| LC3 | microtubule-associated proteins 1A/1B light chain 3B |
| LD | lipid droplet |
| LOAD | late-onset Alzheimer’s Disease |
| MCS | membrane contact sites |
| Mdm1 | mitochondrial distribution and morphology 1 |
| NHE3 | sodium hydrogen exchanger 3 |
| PI | phosphoinositide |
| PI(3)P | phosphatidylinositol 3-phosphate |
| PLC | phospholipase C |
| PM | plasma membrane |
| PX | Phox-homology domain |
| PXA | PX-associated domain |
| PXC | C-terminal PX-associated domain |
| RGS | regulators of G protein signaling |
| GEF | guanine nucleotide exchange factor |
| SCAR20 | autosomal recessive spinocerebellar ataxia 20 |
| SXN | sorting nexin |
| TGF | transforming growth factor |
| TGN | trans-Golgi network |
| TrkB | tropomyosin receptor kinase B |
| U2OS | human bone osteosarcoma epithelial cells |
| WASH | Wiskott Aldrich Syndrome protein and scar homologue |
References
- Lobingier, B.T.; von Zastrow, M. When trafficking and signaling mix: How subcellular location shapes G protein-coupled receptor activation of heterotrimeric G proteins. Traffic 2019, 20, 130–136. [Google Scholar] [CrossRef]
- Wang, J.; Fedoseienko, A.; Chen, B.; Burstein, E.; Jia, D.; Billadeau, D.D. Endosomal receptor trafficking: Retromer and beyond. Traffic 2018, 19, 578–590. [Google Scholar] [CrossRef] [PubMed]
- Naslavsky, N.; Caplan, S. The enigmatic endosome-sorting the ins and outs of endocytic trafficking. J. Cell Sci. 2018, 131, jcs216499. [Google Scholar] [CrossRef] [PubMed]
- Chandra, M.; Collins, B.M. The phox homology (PX) domain. Adv. Exp. Med. Biol. 2019, 1111, 1–17. [Google Scholar] [PubMed]
- Cullen, P.J.; Korswagen, H.C. Sorting nexins provide diversity for retromer-dependent trafficking events. Nat. Cell Biol. 2012, 14, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Teasdale, R.D.; Collins, B.M. Insights into the PX (phox-homology) domain and SNX (sorting nexin) protein families: Structures, functions and roles in disease. Biochem. J. 2012, 441, 39–59. [Google Scholar] [CrossRef]
- Gallon, M.; Cullen, P.J. Retromer and sorting nexins in endosomal sorting. Biochem. Soc. Trans. 2015, 43, 33–47. [Google Scholar] [CrossRef]
- Yang, J.; Villar, V.A.M.; Rozyyev, S.; Jose, P.A.; Zeng, C. The emerging role of sorting nexins in cardiovascular diseases. Clin. Sci. 2019, 133, 723–737. [Google Scholar] [CrossRef]
- Falasca, M.; Maffucci, T. Rethinking phosphatidylinositol 3-monophosphate. Biochim. Biophys. Acta 2009, 1793, 1795–1803. [Google Scholar] [CrossRef]
- Mas, C.; Norwood, S.J.; Bugarcic, A.; Kinna, G.; Leneva, N.; Kovtun, O.; Ghai, R.; Ona Yanez, L.E.; Davis, J.L.; Teasdale, R.D.; et al. Structural basis for different phosphoinositide specificities of the PX domains of sorting nexins regulating G-protein signaling. J. Biol. Chem. 2014, 289, 28554–28568. [Google Scholar] [CrossRef] [PubMed]
- Chandra, M.; Chin, Y.K.; Mas, C.; Feathers, J.R.; Paul, B.; Datta, S.; Chen, K.E.; Jia, X.; Yang, Z.; Norwood, S.J.; et al. Classification of the human phox homology (PX) domains based on their phosphoinositide binding specificities. Nat. Commun. 2019, 10, 1528. [Google Scholar] [CrossRef]
- Li, J.; Li, C.; Zhang, D.; Shi, D.; Qi, M.; Feng, J.; Yuan, T.; Xu, X.; Liang, D.; Xu, L.; et al. SNX13 reduction mediates heart failure through degradative sorting of apoptosis repressor with caspase recruitment domain. Nat. Commun. 2014, 5, 5177. [Google Scholar] [CrossRef]
- Ha, C.M.; Park, D.; Kim, Y.; Na, M.; Panda, S.; Won, S.; Kim, H.; Ryu, H.; Park, Z.Y.; Rasenick, M.M.; et al. SNX14 is a bifunctional negative regulator for neuronal 5-HT6 receptor signaling. J. Cell Sci. 2015, 128, 1848–1861. [Google Scholar] [CrossRef]
- Bryant, D.; Liu, Y.; Datta, S.; Hariri, H.; Seda, M.; Anderson, G.; Peskett, E.; Demetriou, C.; Sousa, S.; Jenkins, D.; et al. SNX14 mutations affect endoplasmic reticulum-associated neutral lipid metabolism in autosomal recessive spinocerebellar ataxia 20. Hum. Mol. Genet. 2018, 27, 1927–1940. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Liu, Y.; Hariri, H.; Bowerman, J.; Henne, W.M. Cerebellar ataxia disease-associated Snx14 promotes lipid droplet growth at ER-droplet contacts. J. Cell Biol. 2019, 218, 1335–1351. [Google Scholar] [CrossRef] [PubMed]
- Akizu, N.; Cantagrel, V.; Zaki, M.S.; Al-Gazali, L.; Wang, X.; Rosti, R.O.; Dikoglu, E.; Gelot, A.B.; Rosti, B.; Vaux, K.K.; et al. Biallelic mutations in SNX14 cause a syndromic form of cerebellar atrophy and lysosome-autophagosome dysfunction. Nat. Genet. 2015, 47, 528–534. [Google Scholar] [CrossRef]
- Thomas, A.C.; Williams, H.; Setó-Salvia, N.; Bacchelli, C.; Jenkins, D.; O’Sullivan, M.; Mengrelis, K.; Ishida, M.; Ocaka, L.; Chanudet, E.; et al. Mutations in SNX14 cause a distinctive autosomal-recessive cerebellar ataxia and intellectual disability syndrome. Am. J. Hum. Genet. 2014, 95, 611–621. [Google Scholar] [CrossRef]
- Bare, L.A.; Morrison, A.C.; Rowland, C.M.; Shiffman, D.; Luke, M.M.; Iakoubova, O.A.; Kane, J.P.; Malloy, M.J.; Ellis, S.G.; Pankow, J.S.; et al. Five common gene variants identify elevated genetic risk for coronary heart disease. Genet. Med. 2007, 9, 682–689. [Google Scholar] [CrossRef]
- Fullard, J.F.; Giambartolomei, C.; Hauberg, M.E.; Xu, K.; Voloudakis, G.; Shao, Z.; Bare, C.; Dudley, J.T.; Mattheisen, M.; Robakis, N.K.; et al. Open chromatin profiling of human postmortem brain infers functional roles for non-coding schizophrenia loci. Hum. Mol. Genet. 2017, 26, 1942–1951. [Google Scholar] [CrossRef] [PubMed]
- Tiu, A.C.; Yang, J.; Asico, L.D.; Konkalmatt, P.; Zheng, X.; Cuevas, S.; Wang, X.; Lee, H.; Mazhar, M.; Felder, R.A.; et al. Lipid rafts are required for effective renal D1 dopamine receptor function. FASEB J. 2020, 34, 6999–7017. [Google Scholar] [CrossRef]
- Kan, A.; Ikeda, T.; Saito, T.; Yano, F.; Fukai, A.; Hojo, H.; Ogasawara, T.; Ogata, N.; Nakamura, K.; Chung, U.I.; et al. Screening of chondrogenic factors with a real-time fluorescence-monitoring cell line ATDC5-C2ER: Identification of sorting nexin 19 as a novel factor. Arthritis Rheum. 2009, 60, 3314–3323. [Google Scholar] [CrossRef] [PubMed]
- Harashima, S.I.; Harashima, C.; Nishimura, T.; Hu, Y.; Notkins, A.L. Overexpression of the autoantigen IA-2 puts beta cells into a pre-apoptotic state: Autoantigen-induced, but non-autoimmune-mediated, tissue destruction. Clin. Exp. Immunol. 2007, 150, 49–60. [Google Scholar] [CrossRef]
- Hao, X.; Wang, Y.; Ren, F.; Zhu, S.; Ren, Y.; Jia, B.; Li, Y.P.; Shi, Y.; Chang, Z. SNX25 regulates TGF-β signaling by enhancing the receptor degradation. Cell Signal. 2011, 23, 935–946. [Google Scholar] [CrossRef]
- Takemura, S.; Nagano, M.; Isonishi, A.; Tanaka, T.; Tatsumi, K.; Yamano, M.; Minami, Y.; Shigeyoshi, Y.; Wanaka, A. Circadian rhythms of sorting nexin 25 in the mouse suprachiasmatic nucleus. Neurosci. Lett. 2020, 727, 134897. [Google Scholar] [CrossRef]
- Du, Y.; Zou, Y.; Yu, W.; Shi, R.; Zhang, M.; Yang, W.; Duan, J.; Deng, Y.; Wang, X.; Lü, Y. Expression pattern of sorting Nexin 25 in temporal lobe epilepsy: A study on patients and pilocarpine-induced rats. Brain Res. 2013, 1509, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Cheng, R.; Vardarajan, B.; Lantigua, R.; Reyes-Dumeyer, D.; Ortmann, W.; Graham, R.R.; Bhangale, T.; Behrens, T.W.; Medrano, M.; et al. Genetic modifiers of age at onset in carriers of the G206A mutation in PSEN1 with Familial Alzheimer Disease among Caribbean Hispanics. JAMA Neurol. 2015, 72, 1043–1051. [Google Scholar] [CrossRef]
- Free, R.B.; Namkung, Y.; Hazelwood, L.A.; Sibley, D.R. Sorting nexin-25 interacts with D1 and D2 dopamine receptors to regulate receptor expression and signaling. FASEB J. 2010, 24, 771–778. [Google Scholar]
- Tesmer, J.J. Structure and function of regulator of G protein signaling homology domains. Prog. Mol. Biol. Transl. Sci. 2009, 86, 75–113. [Google Scholar]
- Squires, K.E.; Montañez-Miranda, C.; Pandya, R.R.; Torres, M.P.; Hepler, J.R. Genetic analysis of rare human variants of regulators of G protein signaling proteins and their role in human physiology and disease. Pharmacol. Rev. 2018, 70, 446–474. [Google Scholar] [CrossRef] [PubMed]
- Willars, G.B. Mammalian RGS proteins: Multifunctional regulators of cellular signalling. Semin. Cell Dev. Biol. 2006, 17, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.; Fisher, R.A. Introduction: G protein-coupled receptors and RGS proteins. Prog. Mol. Biol. Transl. Sci. 2015, 133, 1–11. [Google Scholar] [PubMed]
- Worby, C.A.; Dixon, J.E. Sorting out the cellular functions of sorting nexins. Nat. Rev. Mol. Cell Biol. 2002, 3, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Ma, Y.C.; Ostrom, R.S.; Lavoie, C.; Gill, G.N.; Insel, P.A.; Huang, X.Y.; Farquhar, M.G. RGS-PX1, a GAP for Gαs and sorting nexin in vesicular trafficking. Science 2001, 294, 1939–1942. [Google Scholar] [CrossRef]
- Ross, E.M.; Wilkie, T.M. GTPase-activating proteins for heterotrimeric G proteins: Regulators of G protein signaling (RGS) and RGS-like proteins. Annu. Rev. Biochem. 2000, 69, 795–827. [Google Scholar] [CrossRef]
- Zheng, B.; Lavoie, C.; Tang, T.D.; Ma, P.; Meerloo, T.; Beas, A.; Farquhar, M.G. Regulation of epidermal growth factor receptor degradation by heterotrimeric Gαs protein. Mol. Biol. Cell 2004, 15, 5538–5550. [Google Scholar] [CrossRef]
- Su, K.; Xu, T.; Yu, Z.; Zhu, J.; Zhang, Y.; Wu, M.; Xiong, Y.; Liu, J.; Xu, J. Structure of the PX domain of SNX25 reveals a novel phospholipid recognition model by dimerization in the PX domain. FEBS Lett. 2017, 591, 2011–2018. [Google Scholar] [CrossRef]
- Rao, F.; Wessel, J.; Wen, G.; Zhang, L.; Rana, B.K.; Kennedy, B.P.; Greenwood, T.A.; Salem, R.M.; Chen, Y.; Khandrika, S.; et al. Renal albumin excretion: Twin studies identify influences of heredity, environment, and adrenergic pathway polymorphism. Hypertension 2007, 49, 1015–1031. [Google Scholar] [CrossRef] [PubMed]
- Bein, K.; Ganguly, K.; Martin, T.M.; Concel, V.J.; Brant, K.A.; Di, Y.P.; Upadhyay, S.; Fabisiak, J.P.; Vuga, L.J.; Kaminski, N.; et al. Genetic determinants of ammonia-induced acute lung injury in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Irannejad, R.; Tsvetanova, N.G.; Lobingier, B.T.; von Zastrow, M. Effects of endocytosis on receptor-mediated signaling. Curr. Opin. Cell Biol. 2015, 35, 137–143. [Google Scholar] [CrossRef]
- Ballabio, A.; Bonifacino, J.S. Lysosomes as dynamic regulators of cell and organismal homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Ebner, M.; Koch, P.A.; Haucke, V. Phosphoinositides in the control of lysosome function and homeostasis. Biochem. Soc. Trans. 2019, 47, 1173–1185. [Google Scholar] [CrossRef] [PubMed]
- Henne, W.M.; Zhu, L.; Balogi, Z.; Stefan, C.; Pleiss, J.A.; Emr, S.D. Mdm1/Snx13 is a novel ER-endolysosomal interorganelle tethering protein. J. Cell Biol. 2015, 210, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Bache, K.G.; Brech, A.; Mehlum, A.; Stenmark, H. Hrs regulates multivesicular body formation via ESCRT recruitment to endosomes. J. Cell Biol. 2003, 162, 435–442. [Google Scholar] [CrossRef]
- Klumperman, J.; Raposo, G. The complex ultrastructure of the endolysosomal system. Cold Spring Harb. Perspect Biol. 2014, 6, a016857. [Google Scholar] [CrossRef]
- Zheng, B.; Tang, T.; Tang, N.; Kudlicka, K.; Ohtsubo, K.; Ma, P.; Marth, J.D.; Farquhar, M.G.; Lehtonen, E. Essential role of RGS-PX1/sorting nexin 13 in mouse development and regulation of endocytosis dynamics. Proc. Natl. Acad. Sci. USA 2006, 103, 16776–16781. [Google Scholar] [CrossRef]
- Hariri, H.; Speer, N.; Bowerman, J.; Rogers, S.; Fu, G.; Reetz, E.; Datta, S.; Feathers, J.R.; Ugrankar, R.; Nicastro, D.; et al. Mdm1 maintains endoplasmic reticulum homeostasis by spatially regulating lipid droplet biogenesis. J. Cell Biol. 2019, 218, 1319–1334. [Google Scholar] [CrossRef] [PubMed]
- Harashima, S.; Horiuchi, T.; Wang, Y.; Notkins, A.L.; Seino, Y.; Inagaki, N. Sorting nexin 19 regulates the number of dense core vesicles in pancreatic β-cells. J. Diabetes Investig. 2012, 3, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Takemura, S.; Isonishi, A.; Tanaka, T.; Okuda, H.; Tatsumi, K.; Yamano, M.; Wanaka, A. Neural expression of sorting nexin 25 and its regulation of tyrosine receptor kinase B trafficking. Brain Struct. Funct. 2020, 225, 2615–2642. [Google Scholar] [CrossRef]
- Weeratunga, S.; Paul, B.; Collins, B.M. Recognising the signals for endosomal trafficking. Curr. Opin. Cell Biol. 2020, 65, 17–27. [Google Scholar] [CrossRef]
- Villar, V.A.; Jones, J.E.; Armando, I.; Asico, L.D.; Escano, C.S., Jr.; Lee, H.; Wang, X.; Yang, Y.; Pascua-Crusan, A.M.; Palmes-Saloma, C.P.; et al. Sorting nexin 1 loss results in D5 dopamine receptor dysfunction in human renal proximal tubule cells and hypertension in mice. J. Biol. Chem. 2013, 288, 152–163. [Google Scholar] [CrossRef]
- Villar, V.A.; Armando, I.; Sanada, H.; Frazer, L.C.; Russo, C.M.; Notario, P.M.; Lee, H.; Comisky, L.; Russell, H.A.; Yang, Y.; et al. Novel role of sorting nexin 5 in renal D(1) dopamine receptor trafficking and function: Implications for hypertension. FASEB J. 2013, 27, 1808–1819. [Google Scholar] [CrossRef]
- Li, F.; Yang, J.; Jones, J.E.; Villar, V.A.; Yu, P.; Armando, I.; Felder, R.A.; Jose, P.A. Sorting nexin 5 and dopamine D1 receptor regulate the expression of the insulin receptor in human renal proximal tubule cells. Endocrinology 2015, 156, 2211–2221. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, F.; Yang, J.; Villar, V.A.M.; Asico, L.D.; Ma, X.; Armando, I.; Sanada, H.; Yoneda, M.; Felder, R.A.; Jose, P.A.; et al. Loss of renal SNX5 results in impaired IDE activity and insulin resistance in mice. Diabetologia 2018, 61, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Rojas, R.; Kametaka, S.; Haft, C.R.; Bonifacino, J.S. Interchangeable but essential functions of SNX1 and SNX2 in the association of retromer with endosomes and the trafficking of mannose 6-phosphate receptors. Mol. Cell Biol. 2007, 27, 1112–1124. [Google Scholar] [CrossRef] [PubMed]
- Mellado, M.; Cuartero, Y.; Brugada, R.; Verges, M. Subcellular localisation of retromer in post-endocytic pathways of polarised Madin-Darby canine kidney cells. Biol. Cell 2014, 106, 377–393. [Google Scholar] [CrossRef]
- Chen, D.; Xiao, H.; Zhang, K.; Wang, B.; Gao, Z.; Jian, Y.; Qi, X.; Sun, J.; Miao, L.; Yang, C. Retromer is required for apoptotic cell clearance by phagocytic receptor recycling. Science 2010, 327, 1261–1264. [Google Scholar] [CrossRef]
- Lieu, Z.Z.; Gleeson, P.A. Identification of different itineraries and retromer components for endosome-to-Golgi transport of TGN38 and Shiga toxin. Eur. J. Cell Biol. 2010, 89, 379–393. [Google Scholar] [CrossRef]
- Robinson, D.G.; Neuhaus, J.M. Receptor-mediated sorting of soluble vacuolar proteins: Myths, facts, and a new model. J. Exp. Bot. 2016, 67, 4435–4449. [Google Scholar] [CrossRef]
- Okada, H.; Zhang, W.; Peterhoff, C.; Hwang, J.C.; Nixon, R.A.; Ryu, S.H.; Kim, T.W. Proteomic identification of sorting nexin 6 as a negative regulator of BACE1-mediated APP processing. FASEB J. 2010, 24, 2783–2794. [Google Scholar] [CrossRef]
- Jaillais, Y.; Santambrogio, M.; Rozier, F.; Fobis-Loisy, I.; Miege, C.; Gaude, T. The retromer protein VPS29 links cell polarity and organ initiation in plants. Cell 2007, 130, 1057–1070. [Google Scholar] [CrossRef]
- Traer, C.J.; Rutherford, A.C.; Palmer, K.J.; Wassmer, T.; Oakley, J.; Attar, N.; Carlton, J.G.; Kremerskothen, J.; Stephens, D.J.; Cullen, P.J. SNX4 coordinates endosomal sorting of TfnR with dynein-mediated transport into the endocytic recycling compartment. Nat. Cell Biol. 2007, 9, 1370–1380. [Google Scholar] [CrossRef]
- Kim, N.Y.; Cho, M.H.; Won, S.H.; Kang, H.J.; Yoon, S.Y.; Kim, D.H. Sorting nexin-4 regulates β-amyloid production by modulating β-site-activating cleavage enzyme-1. Alzheimers Res. Ther. 2017, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Solis, G.P.; Hulsbusch, N.; Radon, Y.; Katanaev, V.L.; Plattner, H.; Stuermer, C.A. Reggies/flotillins interact with Rab11a and SNX4 at the tubulovesicular recycling compartment and function in transferrin receptor and E-cadherin trafficking. Mol. Biol. Cell 2013, 24, 2689–2702. [Google Scholar] [CrossRef] [PubMed]
- Knaevelsrud, H.; Carlsson, S.R.; Simonsen, A. SNX18 tubulates recycling endosomes for autophagosome biogenesis. Autophagy 2013, 9, 1639–1641. [Google Scholar] [CrossRef] [PubMed]
- van Weering, J.R.; Verkade, P.; Cullen, P.J. SNX-BAR proteins in phosphoinositide-mediated, tubular-based endosomal sorting. Semin. Cell Dev. Biol. 2010, 21, 371–380. [Google Scholar] [CrossRef]
- Li, H.; Li, H.F.; Felder, R.A.; Periasamy, A.; Jose, P.A. Rab4 and Rab11 coordinately regulate the recycling of angiotensin II type I receptor as demonstrated by fluorescence resonance energy transfer microscopy. J. Biomed. Opt. 2008, 13, 031206. [Google Scholar] [CrossRef]
- Li, H.; Yu, P.; Sun, Y.; Felder, R.A.; Periasamy, A.; Jose, P.A. Actin cytoskeleton-dependent Rab GTPase-regulated angiotensin type I receptor lysosomal degradation studied by fluorescence lifetime imaging microscopy. J. Biomed. Opt. 2010, 15, 056003. [Google Scholar] [CrossRef]
- Li, H.; Armando, I.; Yu, P.; Escano, C.; Mueller, S.C.; Asico, L.; Pascua, A.; Lu, Q.; Wang, X.; Villar, V.A.; et al. Dopamine 5 receptor mediates Ang II type 1 receptor degradation via a ubiquitin-proteasome pathway in mice and human cells. J. Clin. Invest. 2008, 118, 2180–2189. [Google Scholar] [CrossRef]
- McNally, K.E.; Cullen, P.J. Endosomal retrieval of cargo: Retromer is not alone. Trends Cell Biol. 2018, 28, 807–822. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, B.; Danson, C.M.; Heesom, K.J.; Cullen, P.J. Sequence-dependent cargo recognition by SNX-BARs mediates retromer-independent transport of CI-MPR. J. Cell Biol. 2017, 216, 3695–3712. [Google Scholar] [CrossRef]
- Yong, X.; Zhao, L.; Deng, W.; Sun, H.; Zhou, X.; Mao, L.; Hu, W.; Shen, X.; Sun, Q.; Billadeau, D.D.; et al. Mechanism of cargo recognition by retromer-linked SNX-BAR proteins. PLoS. Biol. 2020, 18, e3000631. [Google Scholar] [CrossRef] [PubMed]
- Masuho, I.; Balaji, S.; Muntean, B.S.; Skamangas, N.K.; Chavali, S.; Tesmer, J.J.G.; Babu, M.M.; Martemyanov, K.A. A global map of G protein signaling regulation by RGS proteins. Cell 2020, 183, 503–521. [Google Scholar] [CrossRef]
- Ma, M.; Burd, C.G. Retrograde trafficking and plasma membrane recycling pathways of the budding yeast Saccharomyces cerevisiae. Traffic 2020, 21, 45–59. [Google Scholar] [CrossRef]
- van Weering, J.R.; Sessions, R.B.; Traer, C.J.; Kloer, D.P.; Bhatia, V.K.; Stamou, D.; Carlsson, S.R.; Hurley, J.H.; Cullen, P.J. Molecular basis for SNX-BAR-mediated assembly of distinct endosomal sorting tubules. EMBO J. 2012, 31, 4466–4480. [Google Scholar] [CrossRef]
- Seaman, M.N.J. Retromer and the cation-independent mannose 6-phosphate receptor-time for a trial separation? Traffic 2018, 19, 150–152. [Google Scholar] [CrossRef] [PubMed]
- Norris, A.; Tammineni, P.; Wang, S.; Gerdes, J.; Murr, A.; Kwan, K.Y.; Cai, Q.; Grant, B.D. SNX-1 and RME-8 oppose the assembly of HGRS-1/ESCRT-0 degradative microdomains on endosomes. Proc. Natl. Acad. Sci. USA 2017, 114, E307–E316. [Google Scholar] [CrossRef]
- Norris, A.; Grant, B.D. Endosomal microdomains: Formation and function. Curr. Opin. Cell Biol. 2020, 65, 86–95. [Google Scholar] [CrossRef]
- Simonetti, B.; Paul, B.; Chaudhari, K.; Weeratunga, S.; Steinberg, F.; Gorla, M.; Heesom, K.J.; Bashaw, G.J.; Collins, B.M.; Cullen, P.J. Molecular identification of a BAR domain-containing coat complex for endosomal recycling of transmembrane proteins. Nat. Cell Biol. 2019, 21, 1219–1233. [Google Scholar] [CrossRef] [PubMed]
- Dores, M.R.; Trejo, J. Endo-lysosomal sorting of G-protein-coupled receptors by ubiquitin: Diverse pathways for G-protein-coupled receptor destruction and beyond. Traffic 2019, 20, 101–109. [Google Scholar] [CrossRef]
- Schwihla, M.; Korbei, B. The Beginning of the end: Initial steps in the degradation of plasma membrane proteins. Front Plant Sci. 2020, 11, 680. [Google Scholar] [CrossRef] [PubMed]
- Greaves, J.; Prescott, G.R.; Gorleku, O.A.; Chamberlain, L.H. The fat controller: Roles of palmitoylation in intracellular protein trafficking and targeting to membrane microdomains (Review). Mol. Membr. Biol. 2009, 26, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Tortosa, E.; Hoogenraad, C.C. Polarized trafficking: The palmitoylation cycle distributes cytoplasmic proteins to distinct neuronal compartments. Curr. Opin. Cell Biol. 2018, 50, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Jean-Charles, P.Y.; Snyder, J.C.; Shenoy, S.K. Chapter One—Ubiquitination and deubiquitination of G protein-coupled receptors. Prog. Mol. Biol. Transl. Sci. 2016, 141, 1–55. [Google Scholar]
- Williams, R.L.; Urbe, S. The emerging shape of the ESCRT machinery. Nat. Rev. Mol. Cell Biol. 2007, 8, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.; Gershlick, D.C.; Vidaurrazaga, A.; Rojas, A.L.; Bonifacino, J.S.; Hierro, A. Structural mechanism for cargo recognition by the retromer complex. Cell 2016, 167, 1623–1635.e1614. [Google Scholar] [CrossRef]
- Bottcher, R.T.; Stremmel, C.; Meves, A.; Meyer, H.; Widmaier, M.; Tseng, H.Y.; Fassler, R. Sorting nexin 17 prevents lysosomal degradation of beta1 integrins by binding to the β1- integrin tail. Nat. Cell Biol. 2012, 14, 584–592. [Google Scholar] [CrossRef]
- McNally, K.E.; Faulkner, R.; Steinberg, F.; Gallon, M.; Ghai, R.; Pim, D.; Langton, P.; Pearson, N.; Danson, C.M.; Nagele, H.; et al. Retriever is a multiprotein complex for retromer-independent endosomal cargo recycling. Nat. Cell Biol. 2017, 19, 1214–1225. [Google Scholar] [CrossRef]
- Clairfeuille, T.; Mas, C.; Chan, A.S.M.; Yang, Z.; Tello-Lafoz, M.; Chandra, M.; Widagdo, J.; Kerr, M.C.; Paul, B.; Mérida, I.; et al. A molecular code for endosomal recycling of phosphorylated cargos by the SNX27-retromer complex. Nat. Struct. Mol. Biol. 2016, 23, 921. [Google Scholar] [CrossRef] [PubMed]
- Zechi-Ceide, R.M.; Rodrigues, M.G.; Jehee, F.S.; Kokitsu-Nakata, N.M.; Passos-Bueno, M.R.; Guion-Almeida, M.L. Saethre-Chotzen phenotype with learning disability and hyper IgE phenotype in a patient due to complex chromosomal rearrangement involving chromosomes 3 and 7. Am. J. Med. Genet. A 2012, 158, 1680–1685. [Google Scholar] [CrossRef]
- Huang, H.S.; Yoon, B.J.; Brooks, S.; Bakal, R.; Berrios, J.; Larsen, R.S.; Wallace, M.L.; Han, J.E.; Chung, E.H.; Zylka, M.J.; et al. Snx14 regulates neuronal excitability, promotes synaptic transmission, and is imprinted in the brain of mice. PLoS ONE 2014, 9, e98383. [Google Scholar] [CrossRef]
- Bryant, D.; Seda, M.; Peskett, E.; Maurer, C.; Pomeranz, G.; Ghosh, M.; Hawkins, T.A.; Cleak, J.; Datta, S.; Hariri, H.; et al. Diverse species-specific phenotypic consequences of loss of function sorting nexin 14 mutations. Sci. Rep. 2020, 10, 13763. [Google Scholar] [CrossRef]
- Hu, Y.F.; Zhang, H.L.; Cai, T.; Harashima, S.; Notkins, A.L. The IA-2 interactome. Diabetologia 2005, 48, 2576–2581. [Google Scholar] [CrossRef] [PubMed]
- Bard, M.P.; Hegmans, J.P.; Hemmes, A.; Luider, T.M.; Willemsen, R.; Severijnen, L.A.; van Meerbeeck, J.P.; Burgers, S.A.; Hoogsteden, H.C.; Lambrecht, B.N. Proteomic analysis of exosomes isolated from human malignant pleural effusions. Am J Respir Cell Mol Biol. 2004, 31, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Muglia, M.; Magariello, A.; Citrigno, L.; Passamonti, L.; Sprovieri, T.; Conforti, F.L.; Mazzei, R.; Patitucci, A.; Gabriele, A.L.; Ungaro, C.; et al. A novel locus for dHMN with pyramidal features maps to chromosome 4q34.3–q35.2. Clin. Genet. 2008, 73, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, H.K.; Gudmundsdottir, V.; Brunak, S. Pancreatic islet protein complexes and their dysregulation in type 2 diabetes. Front Genet. 2017, 8, 43. [Google Scholar] [CrossRef]
- Mulindwa, J.; Noyes, H.; Ilboudo, H.; Pagani, L.; Nyangiri, O.; Kimuda, M.P.; Ahouty, B.; Asina, O.F.; Ofon, E.; Kamoto, K.; et al. High levels of genetic diversity within Nilo-Saharan populations: Implications for human adaptation. Am. J. Hum. Genet. 2020, 107, 473–486. [Google Scholar] [CrossRef]
- Ebrahimi-Fakhari, D. Congenital disorders of autophagy: What a pediatric neurologist should know. Neuropediatrics 2018, 49, 18–25. [Google Scholar] [CrossRef]
- Ma, L.; Semick, S.A.; Chen, Q.; Li, C.; Tao, R.; Price, A.J.; Shin, J.H.; Jia, Y. BrainSeq Consortium, Brandon, N.J.; Cross, A.J.; et al. Schizophrenia risk variants influence multiple classes of transcripts of sorting nexin 19 (SNX19). Mol. Psychiatry 2020, 25, 831–843. [Google Scholar] [CrossRef]
- Martin, A.R.; Lin, M.; Granka, J.M.; Myrick, J.W.; Liu, X.; Sockell, A.; Atkinson, E.G.; Werely, C.J.; Möller, M.; Sandhu, M.S.; et al. An unexpectedly complex architecture for skin pigmentation in Africans. Cell 2017, 171, 1340–1353.e14. [Google Scholar] [CrossRef]
- Ugrankar, R.; Bowerman, J.; Hariri, H.; Chandra, M.; Chen, K.; Bossanyi, M.F.; Datta, S.; Rogers, S.; Eckert, K.M.; Vale, G.; et al. Drosophila snazarus regulates a lipid droplet population at plasma membrane-droplet contacts in adipocytes. Dev. Cell 2019, 50, 557–572.e5. [Google Scholar] [CrossRef] [PubMed]
- Hugenroth, M.; Bohnert, M. Come a little bit closer! Lipid droplet-ER contact sites are getting crowded. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118603. [Google Scholar] [CrossRef] [PubMed]
- Hines, R.M.; Kang, R.; Goytain, A.; Quamme, G.A. Golgi-specific DHHC zinc finger protein GODZ mediates membrane Ca2+ transport. J. Biol. Chem. 2010, 285, 4621–4628. [Google Scholar] [CrossRef] [PubMed]
- Felder, R.A.; Sanada, H.; Xu, J.; Yu, P.Y.; Wang, Z.; Watanabe, H.; Asico, L.D.; Wang, W.; Zheng, S.; Yamaguchi, I.; et al. G protein-coupled receptor kinase 4 gene variants in human essential hypertension. Proc. Natl. Acad. Sci. USA 2002, 99, 3872–3877. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Han, W.; Villar, V.A.; Keever, L.B.; Lu, Q.; Hopfer, U.; Quinn, M.T.; Felder, R.A.; Jose, P.A.; Yu, P. D1-like receptors regulate NADPH oxidase activity and subunit expression in lipid raft microdomains of renal proximal tubule cells. Hypertension 2009, 53, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Li, H.; Villar, V.A.; Pascua, A.M.; Dajani, M.I.; Wang, X.; Natarajan, A.; Quinn, M.T.; Felder, R.A.; Jose, P.A.; et al. Lipid rafts keep NADPH oxidase in the inactive state in human renal proximal tubule cells. Hypertension 2008, 51, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, X.X.; Albrecht, F.E.; Hopfer, U.; Carey, R.M.; Jose, P.A. Dopamine(1) receptor, Gsα, and Na(+)-H(+) exchanger interactions in the kidney in hypertension. Hypertension 2000, 36, 395–399. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).