Effect of Varying Amount of Polyethylene Glycol (PEG-600) and 3-Aminopropyltriethoxysilane on the Properties of Chitosan based Reverse Osmosis Membranes
Abstract
:1. Introduction
2. Results and Discussion
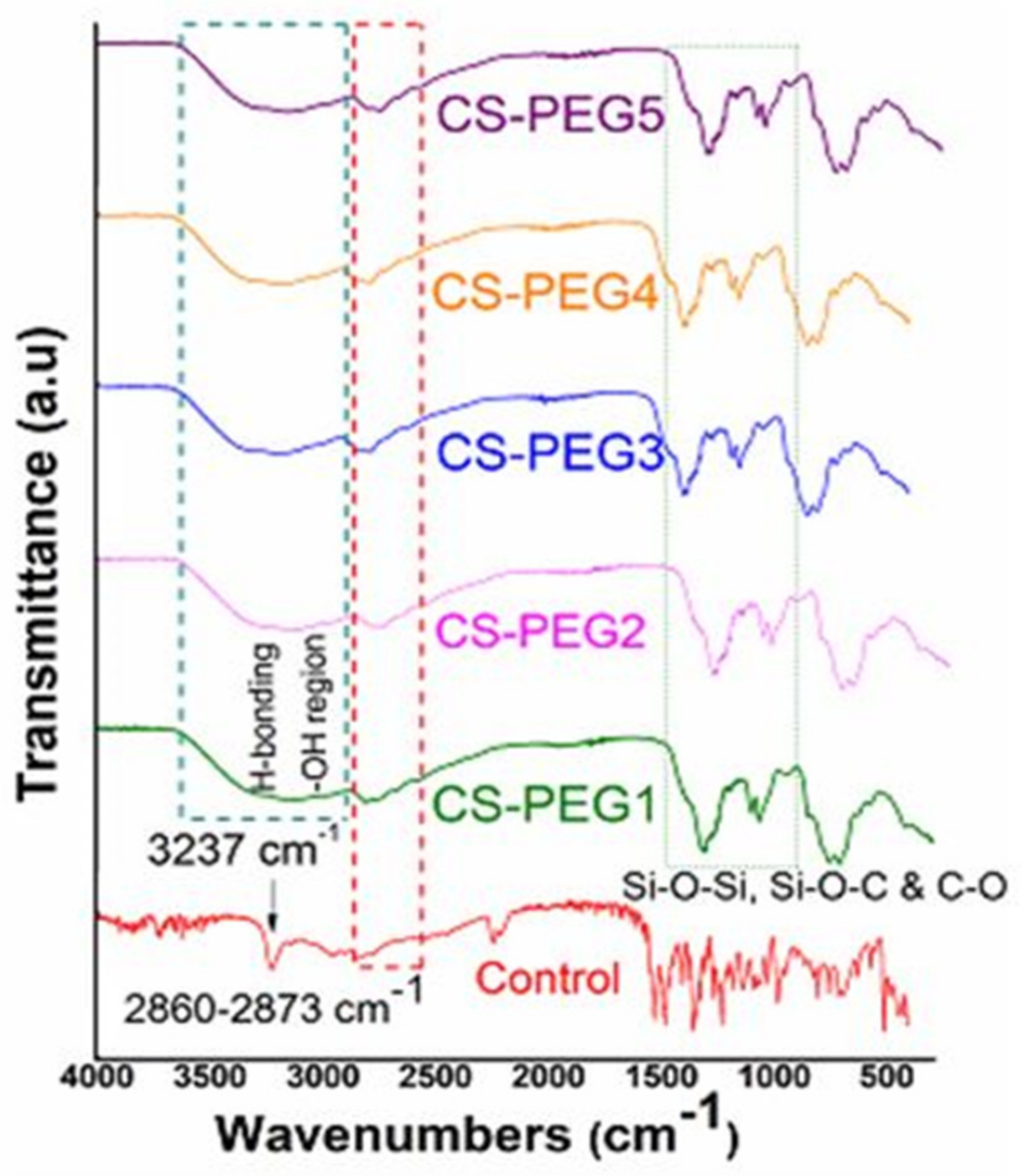
2.1. Fourier Transform Infrared Spectroscopy
2.2. Water Content Measurement
2.3. Reverse Osmosis Performance
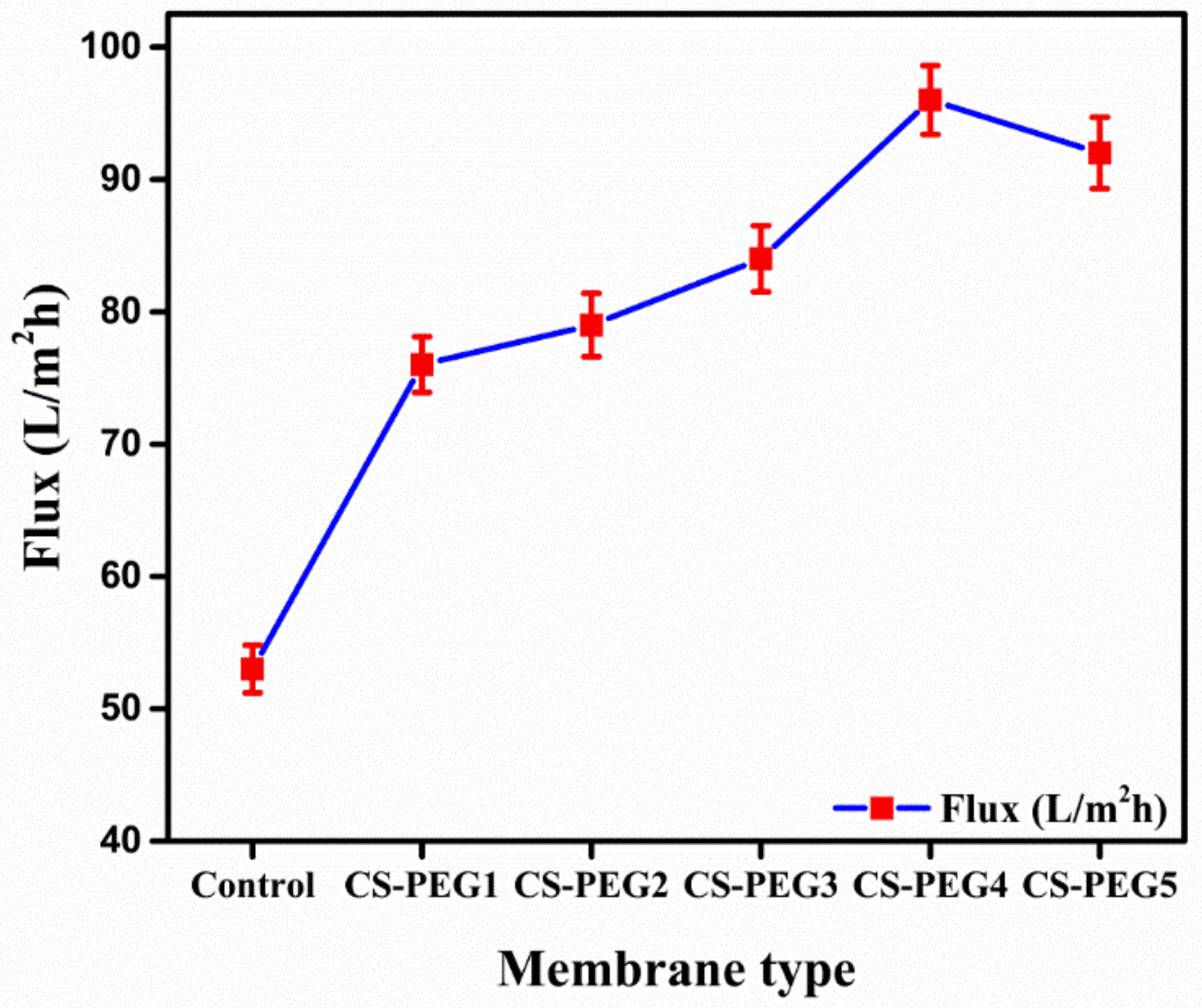
2.3.1. Flux
2.3.2. Salt Rejection
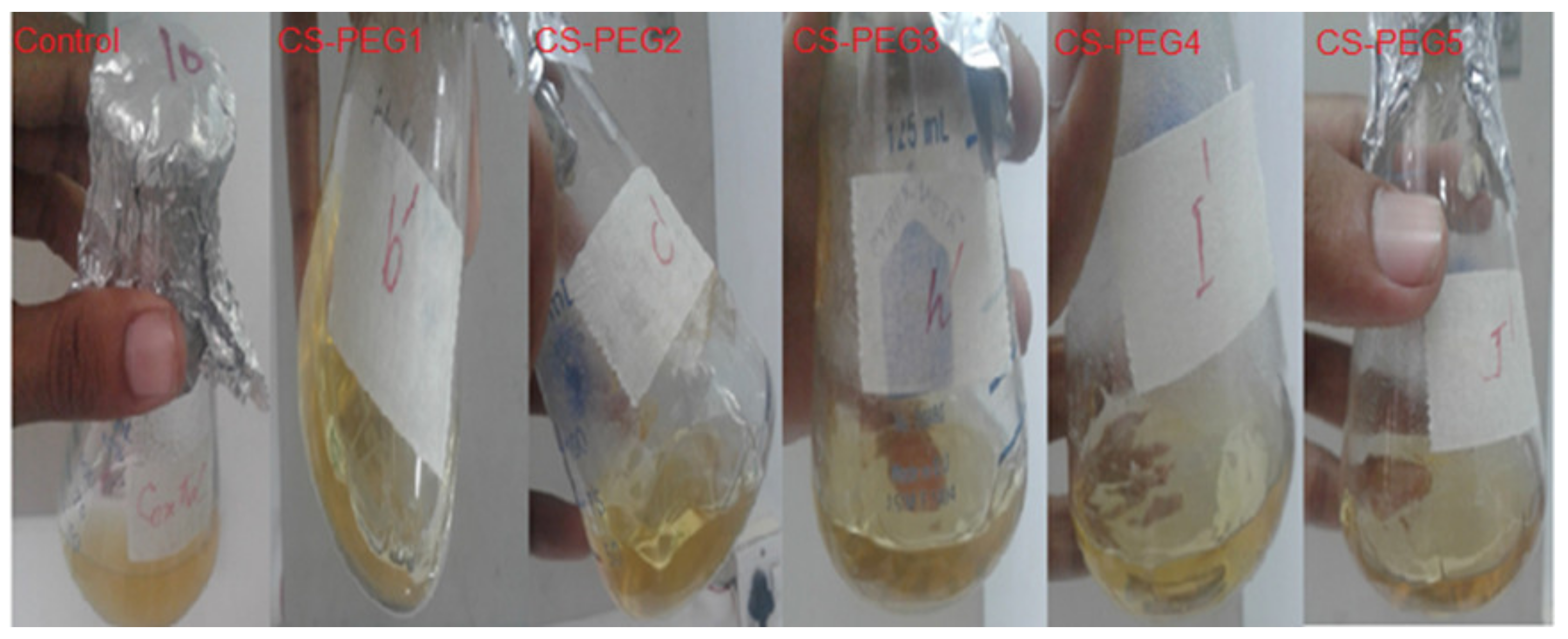
2.4. Antibacterial Activity
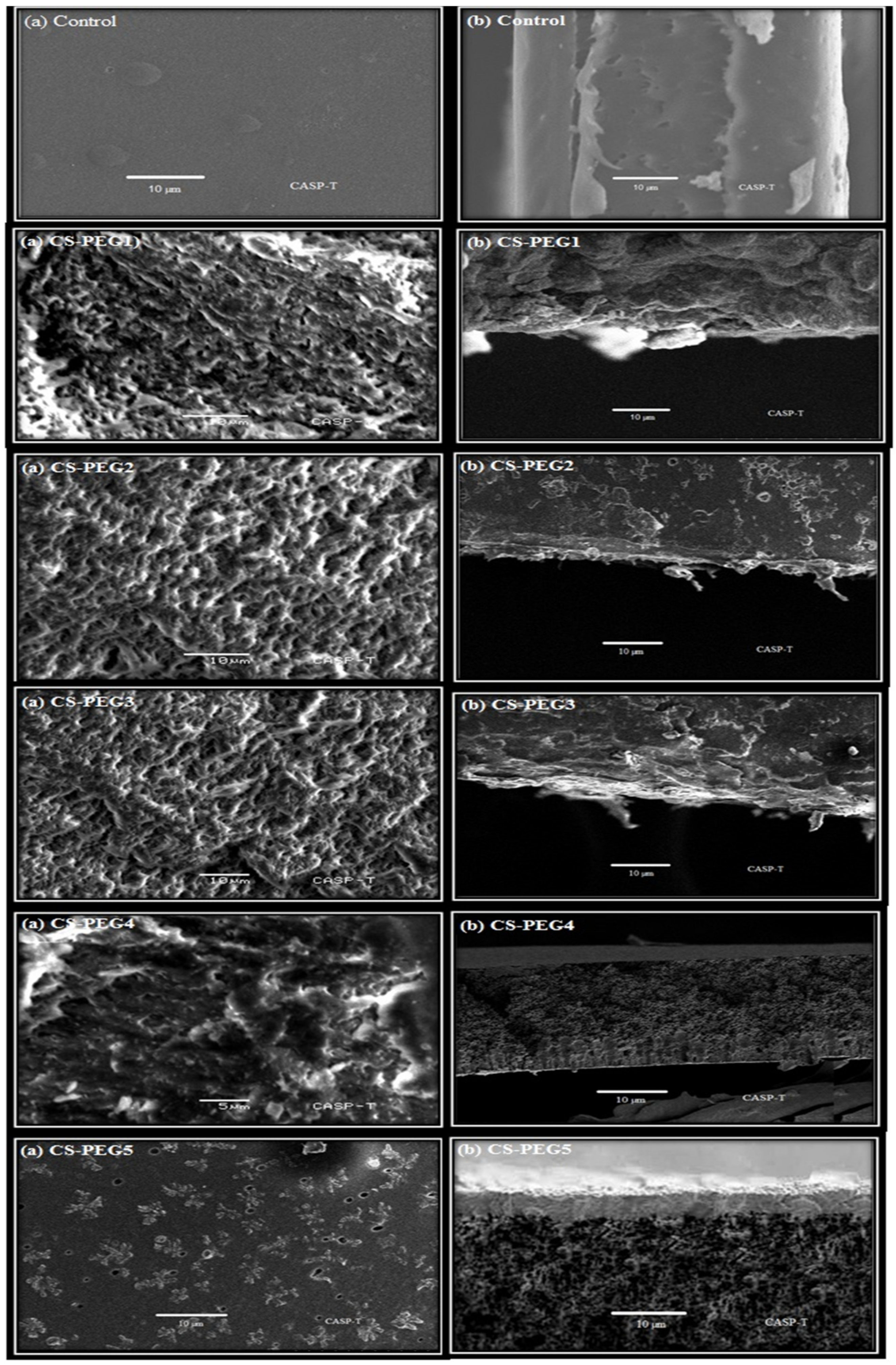
2.5. SEM Analysis
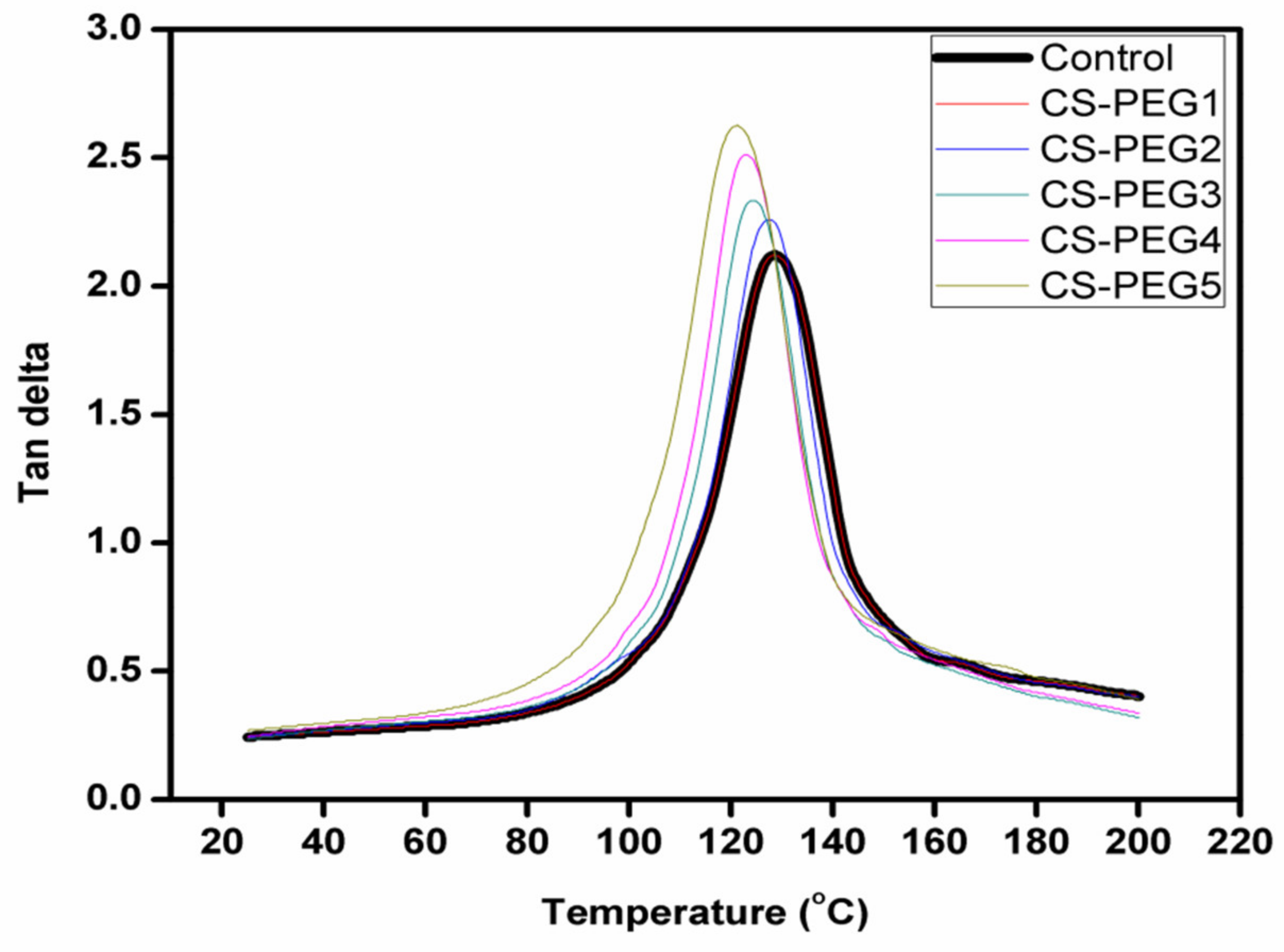
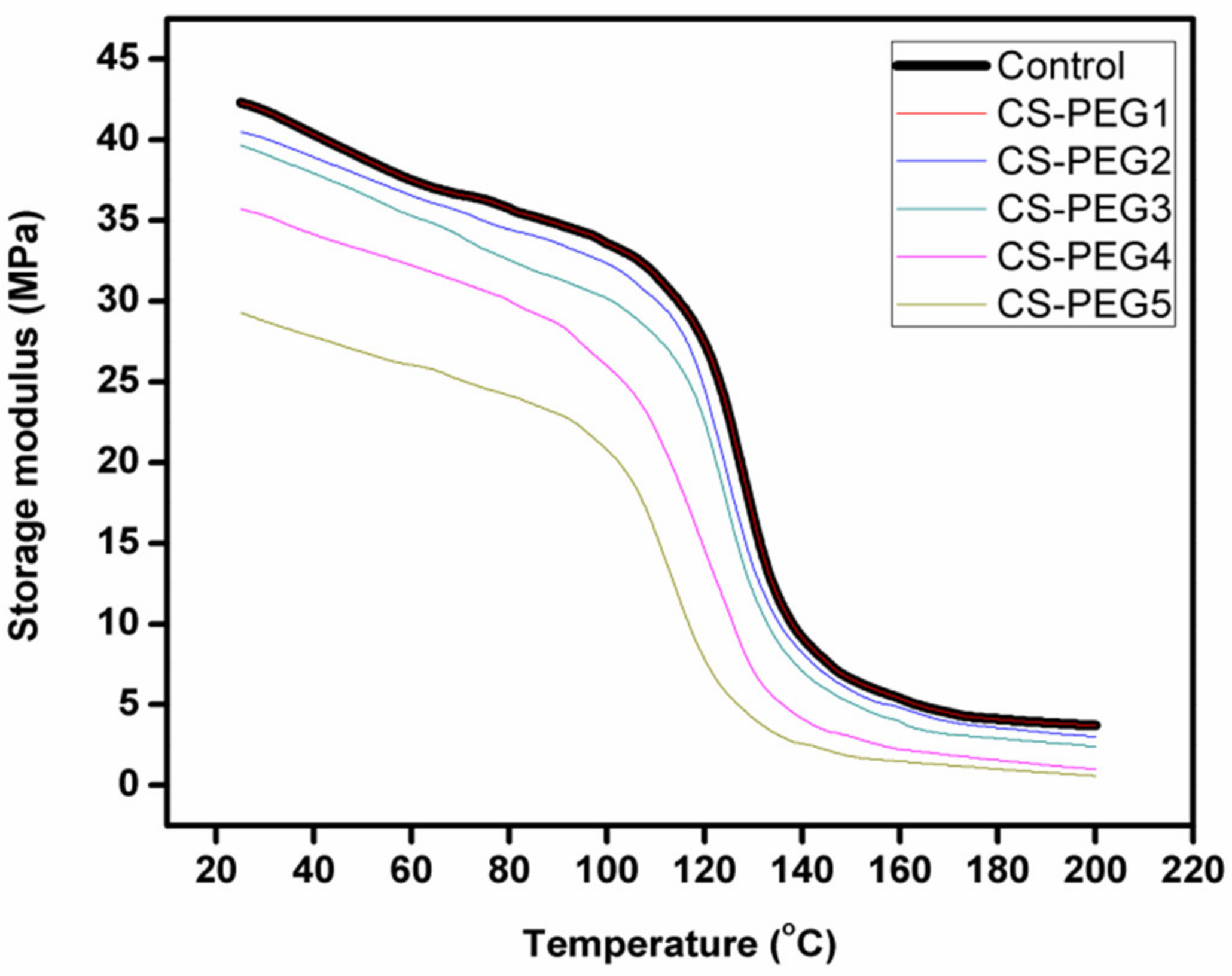
2.6. Dynamic Mechanical Analysis
3. Experimental
3.1. Materials
3.2. Membrane Preparation
3.2.1. Preparation of Control Membrane
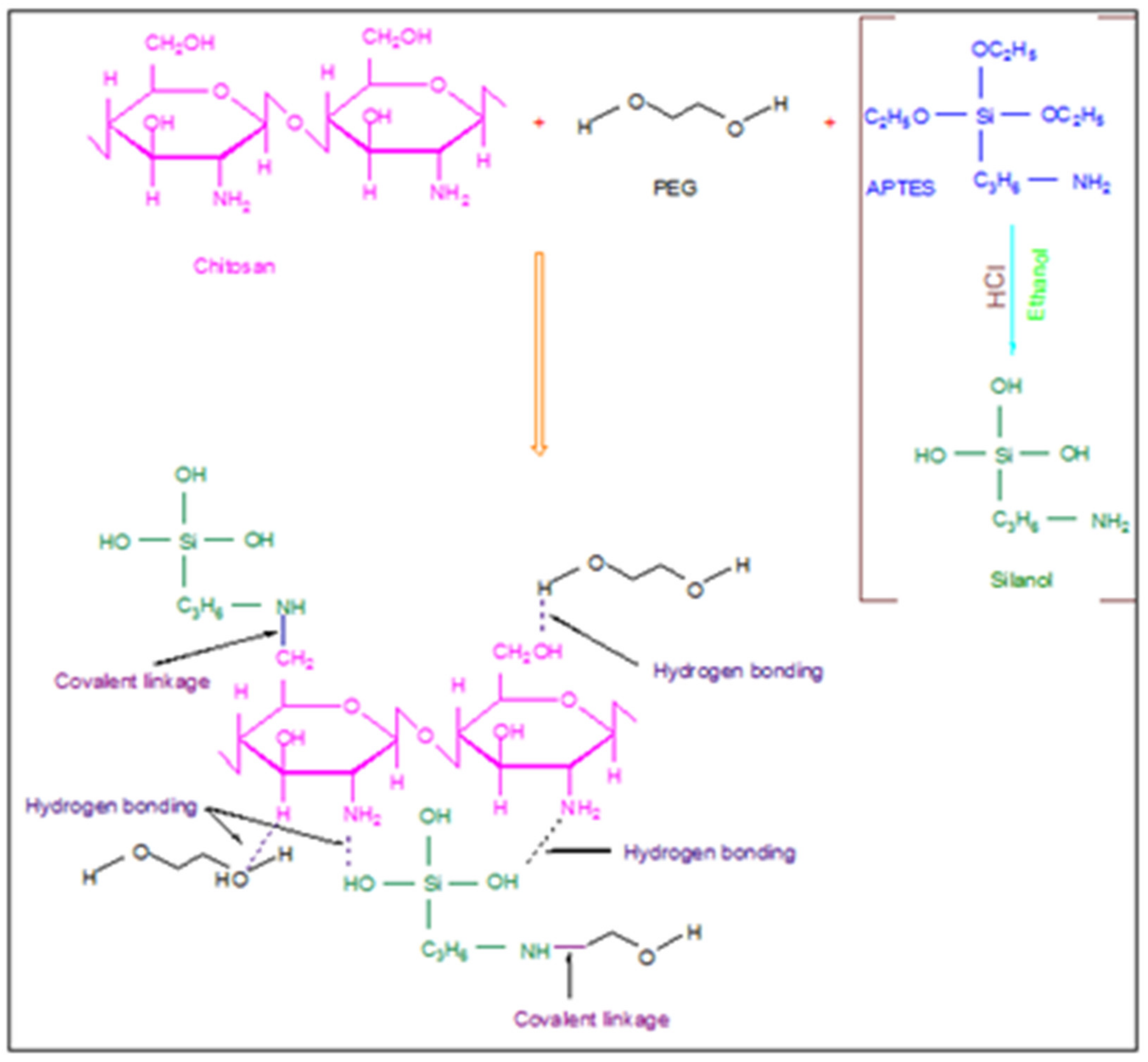
3.2.2. Preparation of Modified Membranes
4. Characterization
4.1. Fourier Transform Infrared Spectroscopy
4.2. Percentage Water Content
4.3. Reverse Osmosis Performance
4.3.1. Percentage of Salt Rejection
4.3.2. Permeate Flux
4.4. Antibacterial Analysis
4.5. Scanning Electron Microscopy
4.6. Dynamic Mechanical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability
Acknowledgments
Conflicts of Interest
References
- Adams, W.M. Green Development: Environment and sustainability in the Third World. Routledge: Abingdon-on-Thames, UK, 2003. [Google Scholar]
- Geise, G.M.; Lee, H.S.; Miller, D.J.; Freeman, B.D.; McGrath, J.E.; Paul, D.R. Water purification by membranes: The role of polymer science. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 1685–1718. [Google Scholar] [CrossRef]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nanoscience and technology: A collection of reviews from nature Journals; World Scientific: Sydney, Australia, 2010. [Google Scholar]
- Misdan, N.; Lau, W.; Ismail, A. Seawater Reverse Osmosis (SWRO) desalination by thin-film composite membrane—Current development, challenges and future prospects. Desalination 2012, 287, 228–237. [Google Scholar] [CrossRef] [Green Version]
- Wallace, R.M. Concentration and separation of ions by Donnan membrane equilibrium. Ind. Eng. Chem. Process Des. Dev. 1967, 6, 423–431. [Google Scholar] [CrossRef]
- Sabir, A.; Islam, A.; Shafiq, M.; Shafeeq, A.; Butt, M.T.Z.; Ahmad, N.M.; Sanaullah, K.; Jamil, T. Novel polymer matrix composite membrane doped with fumed silica particles for reverse osmosis desalination. Desalination 2015, 368, 159–170. [Google Scholar] [CrossRef]
- Xu, J.; Chang, C.-Y.; Gao, C. Performance of a ceramic ultrafiltration membrane system in pretreatment to seawater desalination. Sep. Purif. Technol. 2010, 75, 165–173. [Google Scholar] [CrossRef]
- Santoro, S.; Drioli, E.; Figoli, A. Development of novel ECTFE coated PP composite hollow-fiber membranes. Coatings 2016, 6, 40. [Google Scholar] [CrossRef] [Green Version]
- Greenlee, L.F.; Lawler, D.F.; Freeman, B.D.; Marrot, B.; Moulin, P. Reverse osmosis desalination: Water sources, technology, and today’s challenges. Water Res. 2009, 43, 2317–2348. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.W. Membrane technology and applications; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Van der Bruggen, B.; Vandecasteele, C. Distillation vs. membrane filtration: Overview of process evolutions in seawater desalination. Desalination 2002, 143, 207–218. [Google Scholar] [CrossRef]
- Sabir, A.; Shafiq, M.; Islam, A.; Sarwar, A.; Dilshad, M.R.; Shafeeq, A.; Butt, M.T.Z.; Jamil, T. Fabrication of tethered carbon nanotubes in cellulose acetate/polyethylene glycol-400 composite membranes for reverse osmosis. Carbohydr. Polym. 2015, 132, 589–597. [Google Scholar] [CrossRef]
- Sassi, J.-F.; Chanzy, H. Ultrastructural aspects of the acetylation of cellulose. Cellulose 1995, 2, 111–127. [Google Scholar] [CrossRef]
- Asim, S.; Wasim, M.; Sabir, A.; Shafiq, M.; Andlib, H.; Khuram, S.; Ahmad, A.; Jamil, T. The effect of Nanocrystalline cellulose/Gum Arabic conjugates in crosslinked membrane for antibacterial, chlorine resistance and boron removal performance. J. Hazard. Mater. 2018, 343, 68–77. [Google Scholar] [CrossRef]
- Sidra, W.; Adnan, A.; Khan, S.; Tahir, J.; Atif, I.; Tousif, H. Synthesis, characterization, permeation and antibacterial properties of cellulose acetate/polyethylene glycol membranes modified with chitosan. Desalination 2014, 351, 59–69. [Google Scholar]
- Kim, I.-Y.; Seo, S.-J.; Moon, H.-S.; Yoo, M.-K.; Park, I.-Y.; Kim, B.-C.; Cho, C.-S. Chitosan and its derivatives for tissue engineering applications. Biotechnol. Adv. 2008, 26, 1–21. [Google Scholar] [CrossRef]
- Sun, X.; Peng, B.; Ji, Y.; Chen, J.; Li, D. Chitosan (chitin)/cellulose composite biosorbents prepared using ionic liquid for heavy metal ions adsorption. Aiche J. 2009, 55, 2062–2069. [Google Scholar] [CrossRef]
- Hu, M.Z.C.; Reeves, M. Ligand-grafted biomaterials for adsorptive separations of uranium in solution. Aiche J. 1999, 45, 2333–2345. [Google Scholar] [CrossRef]
- Kumar, R.; Isloor, A.M.; Ismail, A.; Rashid, S.A.; Al Ahmed, A. Permeation, antifouling and desalination performance of TiO2 nanotube incorporated PSf/CS blend membranes. Desalination 2013, 316, 76–84. [Google Scholar] [CrossRef] [Green Version]
- Padaki, M.; Isloor, A.M.; Wanichapichart, P.; Ismail, A.F. Preparation and characterization of sulfonated polysulfone and N-phthloyl chitosan blend composite cation-exchange membrane for desalination. Desalination 2012, 298, 42–48. [Google Scholar] [CrossRef]
- Schmuhl, R.; Krieg, H.; Keizer, K. Adsorption of Cu (II) and Cr (VI) ions by chitosan: Kinetics and equilibrium studies. Water Sa 2001, 27, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Vårum, K.; Ottøy, M.; Smidsrød, O. Acid hydrolysis of chitosans. Carbohydr. Polym. 2001, 46, 89–98. [Google Scholar] [CrossRef]
- Gupta, B.; Saxena, S.; Arora, A.; Alam, M.S. Chitosan-polyethylene glycol coated cotton membranes for wound dressings; NISCAIR-CSIR: New Delhi, India, 2011; Volume 36, pp. 272–280. [Google Scholar]
- Ahumada, E.A.; Delgado, D.R.; Martínez, F. Solution thermodynamics of acetaminophen in some PEG 400+ water mixtures. Fluid Phase Equilibria 2012, 332, 120–127. [Google Scholar] [CrossRef]
- Zhou, J.; Fu, H.; Peng, G.; Cao, H.; Zhang, Y.; Liu, M.; Wu, W.; Qing, X.; Zhou, J. Solubility and solution thermodynamics of flofenicol in binary PEG 400+ water systems. Fluid Phase Equilibria 2014, 376, 159–164. [Google Scholar] [CrossRef]
- Pakdel, P.M.; Peighambardoust, S.J. Review on recent progress in chitosan-based hydrogels for wastewater treatment application. Carbohydr. Polym. 2018, 201, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Kariduraganavar, M.Y.; Kulkarni, S.S.; Kittur, A.A. Pervaporation separation of water-acetic acid mixtures through poly (vinyl alcohol)-silicone based hybrid membranes. J. Membr. Sci. 2005, 246, 83–93. [Google Scholar] [CrossRef]
- Park, S.-B.; You, J.-O.; Park, H.-Y.; Haam, S.J.; Kim, W.-S. A novel pH-sensitive membrane from chitosan—TEOS IPN; preparation and its drug permeation characteristics. Biomaterials 2001, 22, 323–330. [Google Scholar] [CrossRef]
- Lechevallier, S.V.; Hammer, P.; Caiut, J.M.; Mazeres, S.; Mauricot, R.; Verelst, M.; Dexpert, H.; Ribeiro, S.J.; Dexpert-Ghys, J. APTES-modified RE2O3: Eu3+ luminescent beads: Structure and properties. Langmuir 2012, 28, 3962–3971. [Google Scholar] [CrossRef]
- Huang, R.; Chen, G.; Sun, M.; Hu, Y.; Gao, C. Studies on nanofiltration membrane formed by diisocyanate cross-linking of quaternized chitosan on poly (acrylonitrile)(PAN) support. J. Membr. Sci. 2006, 286, 237–244. [Google Scholar] [CrossRef]
- Tuntikulwattana, S.; Sinchaipanid, N.; Ketjinda, W.; Williams, D.B.; Mitrevej, A. Fabrication of chitosan-polyacrylic acid complexes as polymeric osmogents for swellable micro/nanoporous osmotic pumps. Drug Dev. Ind. Pharm. 2011, 37, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Lin, H.; Wang, W.; Zhang, L.-C. Amphoteric composite membranes for nanofiltration prepared from sulfated chitosan crosslinked with hexamethylene diisocyanate. Chem. Eng. J. 2013, 234, 132–139. [Google Scholar] [CrossRef]
- Mucha, M.; Piekielna, J.; Wieczorek, A. Proceedings of the Macromolecular Symposia, Bali, Indonesia, 16–19 October 1999; p. 391.
- Zeng, M.; Fang, Z.; Xu, C. Novel method of preparing microporous membrane by selective dissolution of chitosan/polyethylene glycol blend membrane. J. Appl. Polym. Sci. 2004, 91, 2840–2847. [Google Scholar] [CrossRef]
- Zeng, X.; Ruckenstein, E. Control of pore sizes in macroporous chitosan and chitin membranes. Ind. Eng. Chem. Res. 1996, 35, 4169–4175. [Google Scholar] [CrossRef]
- Shafiq, M.; Sabir, A.; Islam, A.; Khan, S.; Hussain, S.; Butt, M.; Jamil, T. Development and performance characteristics of silane crosslinked poly (vinyl alcohol)/chitosan membranes for reverse osmosis. J. Ind. Eng. Chem. 2017, 48, 99–107. [Google Scholar] [CrossRef]
- Qi, G.-Q.; Liang, C.-L.; Bao, R.-Y.; Liu, Z.-Y.; Yang, W.; Xie, B.-H.; Yang, M.-B. Polyethylene glycol based shape-stabilized phase change material for thermal energy storage with ultra-low content of graphene oxide. Sol. Energy Mater. Sol. Cells 2014, 123, 171–177. [Google Scholar] [CrossRef]
- Mahdavinia, G.; Pourjavadi, A.; Hosseinzadeh, H.; Zohuriaan, M. Modified chitosan 4. Superabsorbent hydrogels from poly (acrylic acid-co-acrylamide) grafted chitosan with salt-and pH-responsiveness properties. Eur. Polym. J. 2004, 40, 1399–1407. [Google Scholar] [CrossRef]
- Sivakumar, M.; Mohan, D.R.; Rangarajan, R. Studies on cellulose acetate-polysulfone ultrafiltration membranes: II. Effect of additive concentration. J. Membr. Sci. 2006, 268, 208–219. [Google Scholar] [CrossRef]
- Lv, C.; Su, Y.; Wang, Y.; Ma, X.; Sun, Q.; Jiang, Z. Enhanced permeation performance of cellulose acetate ultrafiltration membrane by incorporation of Pluronic F127. J. Membr. Sci. 2007, 294, 68–74. [Google Scholar] [CrossRef]
- Sabir, A.; Shafiq, M.; Islam, A.; Jabeen, F.; Shafeeq, A.; Ahmad, A.; Butt, M.T.Z.; Jacob, K.I.; Jamil, T. Conjugation of silica nanoparticles with cellulose acetate/polyethylene glycol 300 membrane for reverse osmosis using MgSO4 solution. Carbohydr. Polym. 2016, 136, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Malaisamy, R.; Mahendran, R.; Mohan, D.; Rajendran, M.; Mohan, V. Cellulose acetate and sulfonated polysulfone blend ultrafiltration membranes. I. Preparation and characterization. J. Appl. Polym. Sci. 2002, 86, 1749–1761. [Google Scholar] [CrossRef]
- Mazid, M. Mechanisms of transport through reverse osmosis membranes. Sep. Sci. Technol. 1984, 19, 357–373. [Google Scholar] [CrossRef]
- Burghoff, H.G.; Lee, K.; Pusch, W. Characterization of transport across cellulose acetate membranes in the presence of strong solute-membrane interactions. J. Appl. Polym. Sci. 1980, 25, 323–347. [Google Scholar] [CrossRef]
- Arthanareeswaran, G.; Mohan, D.; Raajenthiren, M. Preparation, characterization and performance studies of ultrafiltration membranes with polymeric additive. J. Membr. Sci. 2010, 350, 130–138. [Google Scholar] [CrossRef]
- Arthanareeswaran, G.; Devi, T.S.; Raajenthiren, M. Effect of silica particles on cellulose acetate blend ultrafiltration membranes: Part I. Sep. Purif. Technol. 2008, 64, 38–47. [Google Scholar] [CrossRef]
- Eric, M.; Seungkwan, H.; Menachem, E. Influence of membrane surface properties on initial rate of colloidal fouling of reverse osmosis and nanofiltration membranes. J. Membr. Sci. 2001, 188, 115. [Google Scholar]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.-H.; Adibkia, K. Box-Behnken experimental design for preparation and optimization of ciprofloxacin hydrochloride-loaded CaCO3 nanoparticles. J. Drug Deliv. Sci. Technol. 2015, 29, 125–131. [Google Scholar] [CrossRef]
- Lonsdale, H.; Merten, U.; Riley, R. Transport properties of cellulose acetate osmotic membranes. J. Appl. Polym. Sci. 1965, 9, 1341–1362. [Google Scholar] [CrossRef]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; de Aberasturi, D.J.; de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Tripathi, B.P.; Shahi, V.K. Crosslinked chitosan/polyvinyl alcohol blend beads for removal and recovery of Cd (II) from wastewater. J. Hazard. Mater. 2009, 172, 1041–1048. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Fahmy, M.M. Synthesis and antimicrobial activity of some novel cross-linked chitosan hydrogels. Int. J. Mol. Sci. 2012, 13, 11194–11209. [Google Scholar] [CrossRef] [PubMed]
- Atta, S.; Khaliq, S.; Islam, A.; Javeria, I.; Jamil, T.; Athar, M.M.; Shafiq, M.I.; Ghaffar, A. Injectable biopolymer based hydrogels for drug delivery applications. Int. J. Biol. Macromol. 2015, 80, 240–245. [Google Scholar] [CrossRef]
- Padil, V.V.T.; Nguyen, N.H.; Rożek, Z.; Ševců, A.; Černík, M. Synthesis, fabrication and antibacterial properties of a plasma modified electrospun membrane consisting of gum Kondagogu, dodecenyl succinic anhydride and poly (vinyl alcohol). Surf. Coat. Technol. 2015, 271, 32–38. [Google Scholar] [CrossRef]
- He, L.-h.; Xue, R.; Yang, D.-b.; Liu, Y.; Song, R. Effects of blending chitosan with PEG on surface morphology, crystallization and thermal properties. Chin. J. Polym. Sci. 2009, 27, 501–510. [Google Scholar] [CrossRef]
- Zeng, M.; Fang, Z.; Xu, C. Effect of compatibility on the structure of the microporous membrane prepared by selective dissolution of chitosan/synthetic polymer blend membrane. J. Membr. Sci. 2004, 230, 175–181. [Google Scholar] [CrossRef]
- Bigger, S.; Scheirs, J.; Delatycki, O.; Billingham, N. Effects of frequency, molecular weight and thermal oxidation on the dynamic mechanical response of poly (ethylene oxide). Polym. Int. 1991, 26, 181–186. [Google Scholar] [CrossRef]
- Foreman, J.; Sauerbrunn, S.; Marcozzi, C. Exploring the sensitivity of thermal analysis techniques to the glass transition; TA Instruments. In Inc: New Castle, DE, USA, 2013. [Google Scholar]
- Lee, C.H.; VanHouten, D.; Lane, O.; McGrath, J.E.; Hou, J.; Madsen, L.A.; Spano, J.; Wi, S.; Cook, J.; Xie, W. Disulfonated poly (arylene ether sulfone) random copolymer blends tuned for rapid water permeation via cation complexation with poly (ethylene glycol) oligomers. Chem. Mater. 2011, 23, 1039–1049. [Google Scholar] [CrossRef]
- Hamciuc, E.; Hamciuc, C.; Olariu, M. Thermal and electrical behavior of polyimide/silica hybrid thin films. Polym. Eng. Sci. 2010, 50, 520–529. [Google Scholar] [CrossRef]
- Lin, B.P.; Pan, Y.; Qian, Y.; Yuan, C.W. Comparative study of silicon-containing polyimides from different oxydianilines. J. Appl. Polym. Sci. 2004, 94, 2363–2367. [Google Scholar] [CrossRef]
- Ahmad, A.; Jamshaid, F.; Adrees, M.; Iqbal, S.S.; Sabir, A.; Riaz, T.; Zaheer, H.; Islam, A.; Jamil, T. Novel Polyurethane/Polyvinyl chloride-co-vinyl acetate crosslinked membrane for reverse osmosis (RO). Desalination 2017, 420, 136–144. [Google Scholar] [CrossRef]
- Falath, W.; Sabir, A.; Jacob, K.I. Novel reverse osmosis membranes composed of modified PVA/Gum Arabic conjugates: Biofouling mitigation and chlorine resistance enhancement. Carbohydr. Polym. 2017, 155, 28–39. [Google Scholar] [CrossRef] [PubMed]
- French, S.S.; Neuman-Lee, L.A. Improved ex vivo method for microbiocidal activity across vertebrate species. Biol. Open 2012, 1, 482–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Sr. # | Membrane Type | Water Content (%) | Standard Deviation | Optical Density (OD) at 600 nm | Standard Deviation | Bacterial Killing Ability (%) | Standard deviation |
|---|---|---|---|---|---|---|---|
| 1 | Control | 52 | 16.10 | 0.532 | 0.04 | - | - |
| 2 | CS-PEG1 | 90.14 | 0.94 | 0.512 | 0.03 | 91.7 | 9.57 |
| 3 | CS-PEG2 | 93.56 | 2.47 | 0.488 | 0.02 | 96.2 | 7.32 |
| 4 | CS-PEG3 | 96.02 | 3.57 | 0.461 | 0.01 | 101.3 | 4.77 |
| 5 | CS-PEG4 | 97.71 | 4.33 | 0.322 | 0.04 | 127.4 | 8.28 |
| 6 | CS-PEG5 | 98.73 | 4.78 | 0.268 | 0.07 | 137.6 | 13.38 |
| Sr. # | Membrane Type | Tg |
|---|---|---|
| 1 | Control | 128.5 |
| 2 | CS-PEG1 | 128.5 |
| 3 | CS-PEG2 | 127.6 |
| 4 | CS-PEG3 | 124.5 |
| 5 | CS-PEG4 | 122.9 |
| 6 | CS-PEG5 | 120 |
| Sr. # | Compositions | Amount of Chitosan (g) | Volume of APTES (mL) | Volume of PEG (mL) |
|---|---|---|---|---|
| 1 | Control | 1.5 | 0 | 0 |
| 2 | CS-PEG1 | 1.5 | 0.9 | 0.1 |
| 3 | CS-PEG2 | 1.5 | 0.7 | 0.3 |
| 4 | CS-PEG3 | 1.5 | 0.5 | 0.5 |
| 5 | CS-PEG4 | 1.5 | 0.3 | 0.7 |
| 6 | CS-PEG5 | 1.5 | 0.1 | 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kayani, A.; Raza, M.A.; Raza, A.; Hussain, T.; Akram, M.S.; Sabir, A.; Islam, A.; Haider, B.; Khan, R.U.; Park, S.H. Effect of Varying Amount of Polyethylene Glycol (PEG-600) and 3-Aminopropyltriethoxysilane on the Properties of Chitosan based Reverse Osmosis Membranes. Int. J. Mol. Sci. 2021, 22, 2290. https://doi.org/10.3390/ijms22052290
Kayani A, Raza MA, Raza A, Hussain T, Akram MS, Sabir A, Islam A, Haider B, Khan RU, Park SH. Effect of Varying Amount of Polyethylene Glycol (PEG-600) and 3-Aminopropyltriethoxysilane on the Properties of Chitosan based Reverse Osmosis Membranes. International Journal of Molecular Sciences. 2021; 22(5):2290. https://doi.org/10.3390/ijms22052290
Chicago/Turabian StyleKayani, Anum, Muhammad Asim Raza, Arsalan Raza, Tajamal Hussain, Muhammad Sarfraz Akram, Aneela Sabir, Atif Islam, Bilal Haider, Rafi Ullah Khan, and Sang Hyun Park. 2021. "Effect of Varying Amount of Polyethylene Glycol (PEG-600) and 3-Aminopropyltriethoxysilane on the Properties of Chitosan based Reverse Osmosis Membranes" International Journal of Molecular Sciences 22, no. 5: 2290. https://doi.org/10.3390/ijms22052290
APA StyleKayani, A., Raza, M. A., Raza, A., Hussain, T., Akram, M. S., Sabir, A., Islam, A., Haider, B., Khan, R. U., & Park, S. H. (2021). Effect of Varying Amount of Polyethylene Glycol (PEG-600) and 3-Aminopropyltriethoxysilane on the Properties of Chitosan based Reverse Osmosis Membranes. International Journal of Molecular Sciences, 22(5), 2290. https://doi.org/10.3390/ijms22052290








