The Association of Human Herpesviruses with Malignant Brain Tumor Pathology and Therapy: Two Sides of a Coin
Abstract
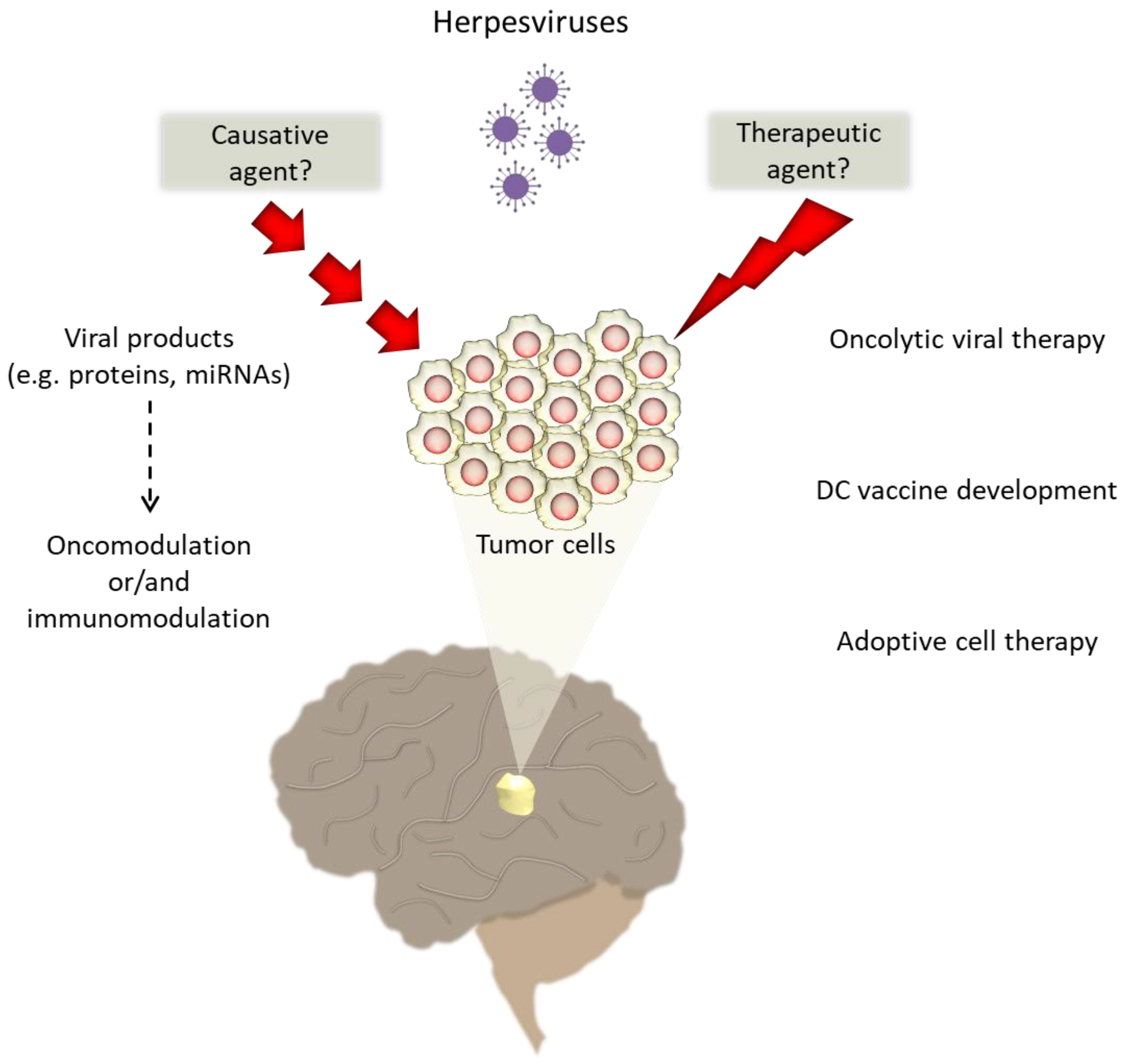
1. Introduction
2. Herpesviruses as Potent Causative Agents in Brain Tumors
2.1. CMV and Brain Tumors
2.2. EBV and Brain Tumors
2.3. HHV-6 and Brain Tumors
3. Therapeutic Approaches against Malignant Brain Tumors That Take Advantage of HHVs Neurotropism
3.1. Oncolytic Viral Therapy for Brain Tumors
3.2. Vaccine-Based Strategies against Brain Tumors
3.3. Adoptive Cell Therapy
4. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brain, G.B.D.; Other, C.N.S.C.C. Global, regional, and national burden of brain and other CNS cancer, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 376–393. [Google Scholar] [CrossRef]
- Duke, E.S.; Packer, R.J. Update on Pediatric Brain Tumors: The Molecular Era and Neuro-immunologic Beginnings. Curr. Neurol. Neurosci. Rep. 2020, 20, 30. [Google Scholar] [CrossRef] [PubMed]
- Lapointe, S.; Perry, A.; Butowski, N.A. Primary brain tumours in adults. Lancet 2018, 392, 432–446. [Google Scholar] [CrossRef]
- Weller, M.; Wick, W.; Aldape, K.; Brada, M.; Berger, M.; Pfister, S.M.; Nishikawa, R.; Rosenthal, M.; Wen, P.Y.; Stupp, R.; et al. Glioma. Nat. Rev. Dis. Primers 2015, 1, 15017. [Google Scholar] [CrossRef] [PubMed]
- Northcott, P.A.; Robinson, G.W.; Kratz, C.P.; Mabbott, D.J.; Pomeroy, S.L.; Clifford, S.C.; Rutkowski, S.; Ellison, D.W.; Malkin, D.; Taylor, M.D.; et al. Medulloblastoma. Nat. Rev. Dis. Primers 2019, 5, 11. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Adel Fahmideh, M.; Cote, D.J.; Muskens, I.S.; Schraw, J.M.; Scheurer, M.E.; Bondy, M.L. Risk factors for childhood and adult primary brain tumors. Neuro Oncol. 2019, 21, 1357–1375. [Google Scholar] [CrossRef]
- Vienne-Jumeau, A.; Tafani, C.; Ricard, D. Environmental risk factors of primary brain tumors: A review. Rev. Neurol. (Paris) 2019, 175, 664–678. [Google Scholar] [CrossRef]
- White, M.K.; Pagano, J.S.; Khalili, K. Viruses and human cancers: A long road of discovery of molecular paradigms. Clin. Microbiol. Rev. 2014, 27, 463–481. [Google Scholar] [CrossRef]
- Kofman, A.; Marcinkiewicz, L.; Dupart, E.; Lyshchev, A.; Martynov, B.; Ryndin, A.; Kotelevskaya, E.; Brown, J.; Schiff, D.; Abounader, R. The roles of viruses in brain tumor initiation and oncomodulation. J. Neurooncol. 2011, 105, 451–466. [Google Scholar] [CrossRef]
- Whitley, R.J. Herpesviruses. In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Lan, K.; Luo, M.H. Herpesviruses: Epidemiology, pathogenesis, and interventions. Virol. Sin. 2017, 32, 347–348. [Google Scholar] [CrossRef]
- Huang, E.S.; Roche, J.K. Cytomegalovirus D.N.A. and adenocarcinoma of the colon: Evidence for latent viral infection. Lancet 1978, 1, 957–960. [Google Scholar] [CrossRef]
- Salyakina, D.; Tsinoremas, N.F. Viral expression associated with gastrointestinal adenocarcinomas in TCGA high-throughput sequencing data. Hum. Genom. 2013, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Harkins, L.E.; Matlaf, L.A.; Soroceanu, L.; Klemm, K.; Britt, W.J.; Wang, W.; Bland, K.I.; Cobbs, C.S. Detection of human cytomegalovirus in normal and neoplastic breast epithelium. Herpesviridae 2010, 1, 8. [Google Scholar] [CrossRef] [PubMed]
- Taher, C.; de Boniface, J.; Mohammad, A.A.; Religa, P.; Hartman, J.; Yaiw, K.C.; Frisell, J.; Rahbar, A.; Soderberg-Naucler, C. High prevalence of human cytomegalovirus proteins and nucleic acids in primary breast cancer and metastatic sentinel lymph nodes. PLoS ONE 2013, 8, e56795. [Google Scholar] [CrossRef] [PubMed]
- Melnick, M.; Sedghizadeh, P.P.; Allen, C.M.; Jaskoll, T. Human cytomegalovirus and mucoepidermoid carcinoma of salivary glands: Cell-specific localization of active viral and oncogenic signaling proteins is confirmatory of a causal relationship. Exp. Mol. Pathol. 2012, 92, 118–125. [Google Scholar] [CrossRef]
- Cobbs, C.S.; Harkins, L.; Samanta, M.; Gillespie, G.Y.; Bharara, S.; King, P.H.; Nabors, L.B.; Cobbs, C.G.; Britt, W.J. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res. 2002, 62, 3347–3350. [Google Scholar]
- Dziurzynski, K.; Chang, S.M.; Heimberger, A.B.; Kalejta, R.F.; McGregor Dallas, S.R.; Smit, M.; Soroceanu, L.; Cobbs, C.S.; HCMV and Gliomas Symposium. Consensus on the role of human cytomegalovirus in glioblastoma. Neuro Oncol. 2012, 14, 246–255. [Google Scholar] [CrossRef]
- Bartek, J., Jr.; Fornara, O.; Merchut-Maya, J.M.; Maya-Mendoza, A.; Rahbar, A.; Stragliotto, G.; Broholm, H.; Svensson, M.; Sehested, A.; Soderberg Naucler, C.; et al. Replication stress, DNA damage signalling, and cytomegalovirus infection in human medulloblastomas. Mol. Oncol. 2017, 11, 945–964. [Google Scholar] [CrossRef]
- Bartek, J., Jr.; Merchut-Maya, J.M.; Maya-Mendoza, A.; Fornara, O.; Rahbar, A.; Brochner, C.B.; Sehested, A.; Soderberg-Naucler, C.; Bartek, J.; Bartkova, J. Cancer cell stemness, responses to experimental genotoxic treatments, cytomegalovirus protein expression and DNA replication stress in pediatric medulloblastomas. Cell Cycle 2020, 19, 727–741. [Google Scholar] [CrossRef]
- Baryawno, N.; Rahbar, A.; Wolmer-Solberg, N.; Taher, C.; Odeberg, J.; Darabi, A.; Khan, Z.; Sveinbjornsson, B.; FuskevAg, O.M.; Segerstrom, L.; et al. Detection of human cytomegalovirus in medulloblastomas reveals a potential therapeutic target. J. Clin. Investig. 2011, 121, 4043–4055. [Google Scholar] [CrossRef] [PubMed]
- Limam, S.; Missaoui, N.; Hmissa, S.; Yacoubi, M.T.; Krifa, H.; Mokni, M.; Selmi, B. Investigation of Human Cytomegalovirus and Human Papillomavirus in Glioma. Cancer Investig. 2020, 38, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Yang, S.; Li, X.; Chen, F.; Li, W. Viral infection and glioma: A meta-analysis of prognosis. BMC Cancer 2020, 20, 549. [Google Scholar] [CrossRef]
- Baumgarten, P.; Michaelis, M.; Rothweiler, F.; Starzetz, T.; Rabenau, H.F.; Berger, A.; Jennewein, L.; Braczynski, A.K.; Franz, K.; Seifert, V.; et al. Human cytomegalovirus infection in tumor cells of the nervous system is not detectable with standardized pathologico-virological diagnostics. Neuro Oncol. 2014, 16, 1469–1477. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.W.; Hellstrand, K.; Larsson, E. Absence of cytomegalovirus in high-coverage DNA sequencing of human glioblastoma multiforme. Int. J. Cancer 2015, 136, 977–981. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Ito, Y.; Isomura, H.; Takemura, N.; Okamoto, A.; Motomura, K.; Tsujiuchi, T.; Natsume, A.; Wakabayashi, T.; Toyokuni, S.; et al. Lack of presence of the human cytomegalovirus in human glioblastoma. Mod. Pathol. 2014, 27, 922–929. [Google Scholar] [CrossRef]
- Habibi, Z.; Hajizadeh, M.; Nozarian, Z.; Safavi, M.; Monajemzadeh, M.; Meybodi, K.T.; Nejat, F.; Vasei, M. Cytomegalovirus DNA in non-glioblastoma multiforme brain tumors of infants. Childs Nerv. Syst. 2021. [Google Scholar] [CrossRef]
- Yuan, Z.; Ye, X.; Zhu, L.; Zhang, N.; An, Z.; Zheng, W.J. Virome assembly and annotation in brain tissue based on next-generation sequencing. Cancer Med. 2020, 9, 6776–6790. [Google Scholar] [CrossRef]
- Joseph, G.P.; McDermott, R.; Baryshnikova, M.A.; Cobbs, C.S.; Ulasov, I.V. Cytomegalovirus as an oncomodulatory agent in the progression of glioma. Cancer Lett. 2017, 384, 79–85. [Google Scholar] [CrossRef]
- Heukers, R.; Fan, T.S.; de Wit, R.H.; van Senten, J.R.; De Groof, T.W.M.; Bebelman, M.P.; Lagerweij, T.; Vieira, J.; de Munnik, S.M.; Smits-de Vries, L.; et al. The constitutive activity of the virally encoded chemokine receptor US28 accelerates glioblastoma growth. Oncogene 2018, 37, 4110–4121. [Google Scholar] [CrossRef]
- Krenzlin, H.; Behera, P.; Lorenz, V.; Passaro, C.; Zdioruk, M.; Nowicki, M.O.; Grauwet, K.; Zhang, H.; Skubal, M.; Ito, H.; et al. Cytomegalovirus promotes murine glioblastoma growth via pericyte recruitment and angiogenesis. J. Clin. Investig. 2019, 129, 1671–1683. [Google Scholar] [CrossRef]
- Matlaf, L.A.; Harkins, L.E.; Bezrookove, V.; Cobbs, C.S.; Soroceanu, L. Cytomegalovirus pp71 protein is expressed in human glioblastoma and promotes pro-angiogenic signaling by activation of stem cell factor. PLoS ONE 2013, 8, e68176. [Google Scholar] [CrossRef]
- Soroceanu, L.; Matlaf, L.; Bezrookove, V.; Harkins, L.; Martinez, R.; Greene, M.; Soteropoulos, P.; Cobbs, C.S. Human cytomegalovirus US28 found in glioblastoma promotes an invasive and angiogenic phenotype. Cancer Res. 2011, 71, 6643–6653. [Google Scholar] [CrossRef]
- Soroceanu, L.; Matlaf, L.; Khan, S.; Akhavan, A.; Singer, E.; Bezrookove, V.; Decker, S.; Ghanny, S.; Hadaczek, P.; Bengtsson, H.; et al. Cytomegalovirus Immediate-Early Proteins Promote Stemness Properties in Glioblastoma. Cancer Res. 2015, 75, 3065–3076. [Google Scholar] [CrossRef]
- Ulasov, I.V.; Kaverina, N.V.; Ghosh, D.; Baryshnikova, M.A.; Kadagidze, Z.G.; Karseladze, A.I.; Baryshnikov, A.Y.; Cobbs, C.S. CMV70-3P miRNA contributes to the CMV mediated glioma stemness and represents a target for glioma experimental therapy. Oncotarget 2017, 8, 25989–25999. [Google Scholar] [CrossRef]
- Vischer, H.F.; Siderius, M.; Leurs, R.; Smit, M.J. Herpesvirus-encoded GPCRs: Neglected players in inflammatory and proliferative diseases? Nat. Rev. Drug Discov. 2014, 13, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Avdic, S.; McSharry, B.P.; Steain, M.; Poole, E.; Sinclair, J.; Abendroth, A.; Slobedman, B. Human Cytomegalovirus-Encoded Human Interleukin-10 (IL-10) Homolog Amplifies Its Immunomodulatory Potential by Upregulating Human IL-10 in Monocytes. J. Virol. 2016, 90, 3819–3827. [Google Scholar] [CrossRef] [PubMed]
- Dziurzynski, K.; Wei, J.; Qiao, W.; Hatiboglu, M.A.; Kong, L.Y.; Wu, A.; Wang, Y.; Cahill, D.; Levine, N.; Prabhu, S.; et al. Glioma-associated cytomegalovirus mediates subversion of the monocyte lineage to a tumor propagating phenotype. Clin. Cancer Res. 2011, 17, 4642–4649. [Google Scholar] [CrossRef]
- Abou-Ghazal, M.; Yang, D.S.; Qiao, W.; Reina-Ortiz, C.; Wei, J.; Kong, L.Y.; Fuller, G.N.; Hiraoka, N.; Priebe, W.; Sawaya, R.; et al. The incidence, correlation with tumor-infiltrating inflammation, and prognosis of phosphorylated STAT3 expression in human gliomas. Clin. Cancer Res. 2008, 14, 8228–8235. [Google Scholar] [CrossRef] [PubMed]
- Brantley, E.C.; Benveniste, E.N. Signal transducer and activator of transcription-3: A molecular hub for signaling pathways in gliomas. Mol. Cancer Res. 2008, 6, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.; Vranic, S.; Cyprian, F.S.; Al Moustafa, A.E. Epstein-Barr Virus in Gliomas: Cause, Association, or Artifact? Front. Oncol. 2018, 8, 123. [Google Scholar] [CrossRef] [PubMed]
- Freixo, C.; Hermouet, S.; Neves, A.M. Epstein-Barr Virus and Astrocytoma. Crit. Rev. Oncog. 2019, 24, 339–347. [Google Scholar] [CrossRef]
- Epstein, M.A.; Achong, B.G.; Barr, Y.M. Virus Particles in Cultured Lymphoblasts from Burkitt’s Lymphoma. Lancet 1964, 1, 702–703. [Google Scholar] [CrossRef]
- Munz, C. Latency and lytic replication in Epstein-Barr virus-associated oncogenesis. Nat. Rev. Microbiol. 2019, 17, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Bharucha, T.; Houlihan, C.F.; Breuer, J. Herpesvirus Infections of the Central Nervous System. Semin. Neurol. 2019, 39, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Bar-Or, A.; Piehl, F.; Preziosa, P.; Solari, A.; Vukusic, S.; Rocca, M.A. Multiple sclerosis. Nat. Rev. Dis. Primers 2018, 4, 43. [Google Scholar] [CrossRef]
- Sugita, Y.; Masuoka, J.; Kameda, K.; Takahashi, K.; Kimura, Y.; Higaki, K.; Furuta, T.; Ohshima, K. Primary central nervous system lymphomas associated with chronic inflammation: Diagnostic pitfalls of central nervous system lymphomas. Brain Tumor Pathol. 2020, 37, 127–135. [Google Scholar] [CrossRef]
- Sugita, Y.; Terasaki, M.; Niino, D.; Ohshima, K.; Fumiko, A.; Shigemori, M.; Sato, Y.; Asano, N. Epstein-Barr virus-associated primary central nervous system lymphomas in immunocompetent elderly patients: Analysis for latent membrane protein-1 oncogene deletion and EBNA-2 strain typing. J. Neurooncol. 2010, 100, 271–279. [Google Scholar] [CrossRef]
- Herman, A.; Gruden, K.; Blejec, A.; Podpecan, V.; Motaln, H.; Rozman, P.; Hren, M.; Zupancic, K.; Veber, M.; Verbovsek, U.; et al. Analysis of Glioblastoma Patients’ Plasma Revealed the Presence of MicroRNAs with a Prognostic Impact on Survival and Those of Viral Origin. PLoS ONE 2015, 10, e0125791. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.M.; Thompson, G.; Carvalheira, J.; Trindade, J.C.; Rueff, J.; Caetano, J.M.; Casey, J.W.; Hermouet, S. Detection and quantitative analysis of human herpesvirus in pilocytic astrocytoma. Brain Res. 2008, 1221, 108–114. [Google Scholar] [CrossRef]
- Cimino, P.J.; Zhao, G.; Wang, D.; Sehn, J.K.; Lewis, J.S., Jr.; Duncavage, E.J. Detection of viral pathogens in high grade gliomas from unmapped next-generation sequencing data. Exp. Mol. Pathol. 2014, 96, 310–315. [Google Scholar] [CrossRef]
- Lin, C.T.; Leibovitch, E.C.; Almira-Suarez, M.I.; Jacobson, S. Human herpesvirus multiplex ddPCR detection in brain tissue from low- and high-grade astrocytoma cases and controls. Infect. Agent Cancer 2016, 11, 32. [Google Scholar] [CrossRef]
- Fonseca, R.F.; Rosas, S.L.; Oliveira, J.A.; Teixeira, A.; Alves, G.; Carvalho Mda, G. Frequency of Epstein-Barr virus DNA sequences in human gliomas. Sao Paulo Med. J. 2015, 133, 51–54. [Google Scholar] [CrossRef]
- Strojnik, T.; Duh, D.; Lah, T.T. Prevalence of Neurotropic Viruses in Malignant Glioma and Their Onco-Modulatory Potential. In Vivo 2017, 31, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Zavala-Vega, S.; Castro-Escarpulli, G.; Hernandez-Santos, H.; Salinas-Lara, C.; Palma, I.; Mejia-Arangure, J.M.; Gelista-Herrera, N.; Rembao-Bojorquez, D.; Ochoa, S.A.; Cruz-Cordova, A.; et al. An overview of the infection of CMV, HSV 1/2 and EBV in Mexican patients with glioblastoma multiforme. Pathol. Res. Pract. 2017, 213, 271–276. [Google Scholar] [CrossRef]
- Strong, M.J.; Blanchard, E.t.; Lin, Z.; Morris, C.A.; Baddoo, M.; Taylor, C.M.; Ware, M.L.; Flemington, E.K. A comprehensive next generation sequencing-based virome assessment in brain tissue suggests no major virus-tumor association. Acta Neuropathol. Commun. 2016, 4, 71. [Google Scholar] [CrossRef] [PubMed]
- Cosset, E.; Petty, T.J.; Dutoit, V.; Cordey, S.; Padioleau, I.; Otten-Hernandez, P.; Farinelli, L.; Kaiser, L.; Bruyere-Cerdan, P.; Tirefort, D.; et al. Comprehensive metagenomic analysis of glioblastoma reveals absence of known virus despite antiviral-like type I interferon gene response. Int. J. Cancer 2014, 135, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Hashida, Y.; Taniguchi, A.; Yawata, T.; Hosokawa, S.; Murakami, M.; Hiroi, M.; Ueba, T.; Daibata, M. Prevalence of human cytomegalovirus, polyomaviruses, and oncogenic viruses in glioblastoma among Japanese subjects. Infect. Agent Cancer 2015, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Khoury, J.D.; Tannir, N.M.; Williams, M.D.; Chen, Y.; Yao, H.; Zhang, J.; Thompson, E.J.; Network, T.; Meric-Bernstam, F.; Medeiros, L.J.; et al. Landscape of DNA virus associations across human malignant cancers: Analysis of 3,775 cases using RNA-Seq. J. Virol. 2013, 87, 8916–8926. [Google Scholar] [CrossRef] [PubMed]
- Salahuddin, S.Z.; Ablashi, D.V.; Markham, P.D.; Josephs, S.F.; Sturzenegger, S.; Kaplan, M.; Halligan, G.; Biberfeld, P.; Wong-Staal, F.; Kramarsky, B.; et al. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science 1986, 234, 596–601. [Google Scholar] [CrossRef]
- Ablashi, D.; Agut, H.; Alvarez-Lafuente, R.; Clark, D.A.; Dewhurst, S.; DiLuca, D.; Flamand, L.; Frenkel, N.; Gallo, R.; Gompels, U.A.; et al. Classification of HHV-6A and HHV-6B as distinct viruses. Arch. Virol. 2014, 159, 863–870. [Google Scholar] [CrossRef]
- Collin, V.; Flamand, L. HHV-6A/B Integration and the Pathogenesis Associated with the Reactivation of Chromosomally Integrated HHV-6A/B. Viruses 2017, 9, 160. [Google Scholar] [CrossRef]
- Eliassen, E.; Lum, E.; Pritchett, J.; Ongradi, J.; Krueger, G.; Crawford, J.R.; Phan, T.L.; Ablashi, D.; Hudnall, S.D. Human Herpesvirus 6 and Malignancy: A Review. Front. Oncol. 2018, 8, 512. [Google Scholar] [CrossRef]
- Cuomo, L.; Trivedi, P.; Cardillo, M.R.; Gagliardi, F.M.; Vecchione, A.; Caruso, R.; Calogero, A.; Frati, L.; Faggioni, A.; Ragona, G. Human herpesvirus 6 infection in neoplastic and normal brain tissue. J. Med. Virol. 2001, 63, 45–51. [Google Scholar] [CrossRef]
- Zheng, X.; Li, S.; Zang, Z.; Hu, J.; An, J.; Pei, X.; Zhu, F.; Zhang, W.; Yang, H. Evidence for possible role of toll-like receptor 3 mediating virus-induced progression of pituitary adenomas. Mol. Cell. Endocrinol. 2016, 426, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Crawford, J.R.; Santi, M.R.; Thorarinsdottir, H.K.; Cornelison, R.; Rushing, E.J.; Zhang, H.; Yao, K.; Jacobson, S.; Macdonald, T.J. Detection of human herpesvirus-6 variants in pediatric brain tumors: Association of viral antigen in low grade gliomas. J. Clin. Virol. 2009, 46, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Crawford, J.R.; Santi, M.R.; Cornelison, R.; Sallinen, S.L.; Haapasalo, H.; MacDonald, T.J. Detection of human herpesvirus-6 in adult central nervous system tumors: Predominance of early and late viral antigens in glial tumors. J. Neurooncol. 2009, 95, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.; Gu, B.; Zhang, C.; Peng, G.; Zhou, F.; Chen, Y.; Zhang, G.; Guo, Y.; Guo, D.; Qin, J.; et al. Human herpesvirus 6 latent infection in patients with glioma. J. Infect. Dis. 2012, 206, 1394–1398. [Google Scholar] [CrossRef]
- Gu, B.; Li, M.; Zhang, Y.; Li, L.; Yao, K.; Wang, S. DR7 encoded by human herpesvirus 6 promotes glioma development and progression. Cancer Manag. Res. 2019, 11, 2109–2118. [Google Scholar] [CrossRef]
- Kashanchi, F.; Araujo, J.; Doniger, J.; Muralidhar, S.; Hoch, R.; Khleif, S.; Mendelson, E.; Thompson, J.; Azumi, N.; Brady, J.N.; et al. Human herpesvirus 6 (HHV-6) ORF-1 transactivating gene exhibits malignant transforming activity and its protein binds to p53. Oncogene 1997, 14, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, A.; Collot-Teixeira, S.; Mardivirin, L.; Jaccard, A.; Petit, B.; Piguet, C.; Sturtz, F.; Preux, P.M.; Bordessoule, D.; Ranger-Rogez, S. Involvement of human herpesvirus-6 variant B in classic Hodgkin’s lymphoma via DR7 oncoprotein. Clin. Cancer Res. 2010, 16, 4711–4721. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sang, Y.; Zhang, R.; Scott, W.R.; Creagh, A.L.; Haynes, C.A.; Straus, S.K. U24 from Roseolovirus interacts strongly with Nedd4 WW Domains. Sci. Rep. 2017, 7, 39776. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Deng, J.; Li, G.; Wang, B.; Cao, Y.; Tu, Y. Down-regulation of Nedd4L is associated with the aggressive progression and worse prognosis of malignant glioma. Jpn. J. Clin. Oncol. 2012, 42, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Nie, W.; Zhang, X.; Zhang, G.; Li, Z.; Wu, H.; Shi, Q.; Chen, Y.; Ding, Z.; Zhou, X.; et al. NEDD4-1 regulates migration and invasion of glioma cells through CNrasGEF ubiquitination in vitro. PLoS ONE 2013, 8, e82789. [Google Scholar] [CrossRef]
- Wells, M.J.; Jacobson, S.; Levine, P.H. An evaluation of HHV-6 as an etiologic agent in Hodgkin lymphoma and brain cancer using IARC criteria for oncogenicity. Infect. Agents Cancer 2019, 14, 31. [Google Scholar] [CrossRef] [PubMed]
- Krull, K.R.; Hardy, K.K.; Kahalley, L.S.; Schuitema, I.; Kesler, S.R. Neurocognitive Outcomes and Interventions in Long-Term Survivors of Childhood Cancer. J. Clin. Oncol. 2018, 36, 2181–2189. [Google Scholar] [CrossRef]
- Altinoz, M.A.; Elmaci, I.; Bolukbasi, F.H.; Ekmekci, C.G.; Yenmis, G.; Sari, R.; Sav, A. MGMT gene variants, temozolomide myelotoxicity and glioma risk. A concise literature survey including an illustrative case. J. Chemother. 2017, 29, 238–244. [Google Scholar] [CrossRef]
- Yovino, S.; Grossman, S.A. Severity, etiology and possible consequences of treatment-related lymphopenia in patients with newly diagnosed high-grade gliomas. CNS Oncol. 2012, 1, 149–154. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.H.; Gunn, M.D.; Fecci, P.E.; Ashley, D.M. Brain immunology and immunotherapy in brain tumours. Nat. Rev. Cancer 2020, 20, 12–25. [Google Scholar] [CrossRef]
- Kemp, V.; Lamfers, M.L.M.; van der Pluijm, G.; van den Hoogen, B.G.; Hoeben, R.C. Developing oncolytic viruses for clinical use: A consortium approach. Cytokine Growth Factor Rev. 2020. [Google Scholar] [CrossRef]
- Lemos de Matos, A.; Franco, L.S.; McFadden, G. Oncolytic Viruses and the Immune System: The Dynamic Duo. Mol. Ther. Methods Clin. Dev. 2020, 17, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Marelli, G.; Howells, A.; Lemoine, N.R.; Wang, Y. Oncolytic Viral Therapy and the Immune System: A Double-Edged Sword Against Cancer. Front. Immunol. 2018, 9, 866. [Google Scholar] [CrossRef]
- Su, K.Y.; Balasubramaniam, V. Zika Virus as Oncolytic Therapy for Brain Cancer: Myth or Reality? Front. Microbiol. 2019, 10, 2715. [Google Scholar] [CrossRef]
- Steiner, I.; Kennedy, P.G.; Pachner, A.R. The neurotropic herpes viruses: Herpes simplex and varicella-zoster. Lancet Neurol. 2007, 6, 1015–1028. [Google Scholar] [CrossRef]
- Martuza, R.L.; Malick, A.; Markert, J.M.; Ruffner, K.L.; Coen, D.M. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science 1991, 252, 854–856. [Google Scholar] [CrossRef] [PubMed]
- Grigg, C.; Blake, Z.; Gartrell, R.; Sacher, A.; Taback, B.; Saenger, Y. Talimogene laherparepvec (T-Vec) for the treatment of melanoma and other cancers. Semin. Oncol. 2016, 43, 638–646. [Google Scholar] [CrossRef]
- Totsch, S.K.; Schlappi, C.; Kang, K.D.; Ishizuka, A.S.; Lynn, G.M.; Fox, B.; Beierle, E.A.; Whitley, R.J.; Markert, J.M.; Gillespie, G.Y.; et al. Oncolytic herpes simplex virus immunotherapy for brain tumors: Current pitfalls and emerging strategies to overcome therapeutic resistance. Oncogene 2019, 38, 6159–6171. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, S.; Chen, X.; Shi, H.; Chen, C.; Sun, L.; Chen, Z.J. cGAS is essential for the antitumor effect of immune checkpoint blockade. Proc. Natl. Acad. Sci. USA 2017, 114, 1637–1642. [Google Scholar] [CrossRef]
- Froechlich, G.; Caiazza, C.; Gentile, C.; D’Alise, A.M.; De Lucia, M.; Langone, F.; Leoni, G.; Cotugno, G.; Scisciola, V.; Nicosia, A.; et al. Integrity of the Antiviral STING-mediated DNA Sensing in Tumor Cells Is Required to Sustain the Immunotherapeutic Efficacy of Herpes Simplex Oncolytic Virus. Cancers (Basel) 2020, 12, 3407. [Google Scholar] [CrossRef]
- Gujar, S.; Pol, J.G.; Kim, Y.; Lee, P.W.; Kroemer, G. Antitumor Benefits of Antiviral Immunity: An Underappreciated Aspect of Oncolytic Virotherapies. Trends Immunol. 2018, 39, 209–221. [Google Scholar] [CrossRef]
- Herbein, G.; Nehme, Z. Tumor Control by Cytomegalovirus: A Door Open for Oncolytic Virotherapy? Mol. Ther. Oncolytics 2020, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.C.; Parker, J.N.; Gillespie, G.Y.; Lakeman, F.D.; Meleth, S.; Markert, J.M.; Cassady, K.A. Enhanced antiglioma activity of chimeric HCMV/HSV-1 oncolytic viruses. Gene Ther. 2007, 14, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Ghonime, M.G.; Jackson, J.; Shah, A.; Roth, J.; Li, M.; Saunders, U.; Coleman, J.; Gillespie, G.Y.; Markert, J.M.; Cassady, K.A. Chimeric HCMV/HSV-1 and Deltagamma134.5 oncolytic herpes simplex virus elicit immune mediated antigliomal effect and antitumor memory. Transl. Oncol. 2018, 11, 86–93. [Google Scholar] [CrossRef]
- Friedman, G.K.; Nan, L.; Haas, M.C.; Kelly, V.M.; Moore, B.P.; Langford, C.P.; Xu, H.; Han, X.; Beierle, E.A.; Markert, J.M.; et al. gamma(1)34.5-deleted HSV-1-expressing human cytomegalovirus IRS1 gene kills human glioblastoma cells as efficiently as wild-type HSV-1 in normoxia or hypoxia. Gene Ther. 2015, 22, 348–355. [Google Scholar] [CrossRef]
- Menotti, L.; Avitabile, E. Herpes Simplex Virus Oncolytic Immunovirotherapy: The Blossoming Branch of Multimodal Therapy. Int. J. Mol. Sci. 2020, 21, 8310. [Google Scholar] [CrossRef] [PubMed]
- Sasso, E.; D’Alise, A.M.; Zambrano, N.; Scarselli, E.; Folgori, A.; Nicosia, A. New viral vectors for infectious diseases and cancer. Semin. Immunol. 2020, 50, 101430. [Google Scholar] [CrossRef]
- Sivanandam, V.; LaRocca, C.J.; Chen, N.G.; Fong, Y.; Warner, S.G. Oncolytic Viruses and Immune Checkpoint Inhibition: The Best of Both Worlds. Mol. Ther. Oncolytics 2019, 13, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Twumasi-Boateng, K.; Pettigrew, J.L.; Kwok, Y.Y.E.; Bell, J.C.; Nelson, B.H. Oncolytic viruses as engineering platforms for combination immunotherapy. Nat. Rev. Cancer 2018, 18, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Kishi, Y.; Inui, S.; Sakamoto, Y. Colposcopic findings of gland openings in cervical carcinoma: Their histological backgrounds. Int. J. Gynaecol. Obstet. 1987, 25, 223–233. [Google Scholar] [CrossRef]
- Samson, A.; Scott, K.J.; Taggart, D.; West, E.J.; Wilson, E.; Nuovo, G.J.; Thomson, S.; Corns, R.; Mathew, R.K.; Fuller, M.J.; et al. Intravenous delivery of oncolytic reovirus to brain tumor patients immunologically primes for subsequent checkpoint blockade. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef]
- Saha, D.; Martuza, R.L.; Rabkin, S.D. Macrophage Polarization Contributes to Glioblastoma Eradication by Combination Immunovirotherapy and Immune Checkpoint Blockade. Cancer Cell 2017, 32, 253–267.e255. [Google Scholar] [CrossRef]
- Smith, C.C.; Selitsky, S.R.; Chai, S.; Armistead, P.M.; Vincent, B.G.; Serody, J.S. Alternative tumour-specific antigens. Nat. Rev. Cancer 2019, 19, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Yarchoan, M.; Johnson, B.A., 3rd; Lutz, E.R.; Laheru, D.A.; Jaffee, E.M. Targeting neoantigens to augment antitumour immunity. Nat. Rev. Cancer 2017, 17, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.A.; Batich, K.A.; Gunn, M.D.; Huang, M.N.; Sanchez-Perez, L.; Nair, S.K.; Congdon, K.L.; Reap, E.A.; Archer, G.E.; Desjardins, A.; et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature 2015, 519, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.K.; De Leon, G.; Boczkowski, D.; Schmittling, R.; Xie, W.; Staats, J.; Liu, R.; Johnson, L.A.; Weinhold, K.; Archer, G.E.; et al. Recognition and killing of autologous, primary glioblastoma tumor cells by human cytomegalovirus pp65-specific cytotoxic T cells. Clin. Cancer Res. 2014, 20, 2684–2694. [Google Scholar] [CrossRef] [PubMed]
- Batich, K.A.; Reap, E.A.; Archer, G.E.; Sanchez-Perez, L.; Nair, S.K.; Schmittling, R.J.; Norberg, P.; Xie, W.; Herndon, J.E., 2nd; Healy, P.; et al. Long-term Survival in Glioblastoma with Cytomegalovirus pp65-Targeted Vaccination. Clin. Cancer Res. 2017, 23, 1898–1909. [Google Scholar] [CrossRef] [PubMed]
- Fruh, K.; Picker, L. CD8+ T cell programming by cytomegalovirus vectors: Applications in prophylactic and therapeutic vaccination. Curr. Opin. Immunol. 2017, 47, 52–56. [Google Scholar] [CrossRef]
- Luo, X.H.; Meng, Q.; Rao, M.; Liu, Z.; Paraschoudi, G.; Dodoo, E.; Maeurer, M. The impact of inflationary cytomegalovirus-specific memory T cells on anti-tumour immune responses in patients with cancer. Immunology 2018, 155, 294–308. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, M.O.; Ossmann, S.; Kaufmann, A.M.; Leitner, J.; Steinberger, P.; Willimsky, G.; Raftery, M.J.; Schonrich, G. Development of a Human Cytomegalovirus (HCMV)-Based Therapeutic Cancer Vaccine Uncovers a Previously Unsuspected Viral Block of MHC Class I Antigen Presentation. Front. Immunol. 2019, 10, 1776. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Mao, G.; Gokaslan, Z.S.; Sampath, P. Chimeric antigen receptors for treatment of glioblastoma: A practical review of challenges and ways to overcome them. Cancer Gene Ther. 2017, 24, 121–129. [Google Scholar] [CrossRef]
- Crough, T.; Beagley, L.; Smith, C.; Jones, L.; Walker, D.G.; Khanna, R. Ex vivo functional analysis, expansion and adoptive transfer of cytomegalovirus-specific T-cells in patients with glioblastoma multiforme. Immunol. Cell Biol. 2012, 90, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Schuessler, A.; Smith, C.; Beagley, L.; Boyle, G.M.; Rehan, S.; Matthews, K.; Jones, L.; Crough, T.; Dasari, V.; Klein, K.; et al. Autologous T-cell therapy for cytomegalovirus as a consolidative treatment for recurrent glioblastoma. Cancer Res. 2014, 74, 3466–3476. [Google Scholar] [CrossRef]
- Reap, E.A.; Suryadevara, C.M.; Batich, K.A.; Sanchez-Perez, L.; Archer, G.E.; Schmittling, R.J.; Norberg, P.K.; Herndon, J.E., 2nd; Healy, P.; Congdon, K.L.; et al. Dendritic Cells Enhance Polyfunctionality of Adoptively Transferred T Cells That Target Cytomegalovirus in Glioblastoma. Cancer Res. 2018, 78, 256–264. [Google Scholar] [CrossRef]
- Choi, B.D.; Curry, W.T.; Carter, B.S.; Maus, M.V. Chimeric antigen receptor T-cell immunotherapy for glioblastoma: Practical insights for neurosurgeons. Neurosurg. Focus 2018, 44, E13. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.D.; Maus, M.V.; June, C.H.; Sampson, J.H. Immunotherapy for Glioblastoma: Adoptive T-cell Strategies. Clin. Cancer Res. 2019, 25, 2042–2048. [Google Scholar] [CrossRef]
- Pule, M.A.; Savoldo, B.; Myers, G.D.; Rossig, C.; Russell, H.V.; Dotti, G.; Huls, M.H.; Liu, E.; Gee, A.P.; Mei, Z.; et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: Persistence and antitumor activity in individuals with neuroblastoma. Nat. Med. 2008, 14, 1264–1270. [Google Scholar] [CrossRef]
- Ahmed, N.; Brawley, V.; Hegde, M.; Bielamowicz, K.; Kalra, M.; Landi, D.; Robertson, C.; Gray, T.L.; Diouf, O.; Wakefield, A.; et al. HER2-Specific Chimeric Antigen Receptor-Modified Virus-Specific T Cells for Progressive Glioblastoma: A Phase 1 Dose-Escalation Trial. JAMA Oncol. 2017, 3, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Markert, J.M.; Medlock, M.D.; Rabkin, S.D.; Gillespie, G.Y.; Todo, T.; Hunter, W.D.; Palmer, C.A.; Feigenbaum, F.; Tornatore, C.; Tufaro, F.; et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: Results of a phase I trial. Gene Ther. 2000, 7, 867–874. [Google Scholar] [CrossRef]
- Markert, J.M.; Razdan, S.N.; Kuo, H.C.; Cantor, A.; Knoll, A.; Karrasch, M.; Nabors, L.B.; Markiewicz, M.; Agee, B.S.; Coleman, J.M.; et al. A phase 1 trial of oncolytic HSV-1, G207, given in combination with radiation for recurrent GBM demonstrates safety and radiographic responses. Mol. Ther. 2014, 22, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov Home Page. Available online: https://clinicaltrials.gov/ct2/show/NCT03911388 (accessed on 11 February 2021).
- ClinicalTrials.gov Home Page. Available online: https://clinicaltrials.gov/ct2/show/NCT02457845 (accessed on 11 February 2021).
- ClinicalTrials.gov Home Page. Available online: https://clinicaltrials.gov/ct2/show/NCT02062827 (accessed on 11 February 2021).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03657576 (accessed on 11 February 2021).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04482933 (accessed on 11 February 2021).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03299309 (accessed on 11 February 2021).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/results/NCT02529072 (accessed on 11 February 2021).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03927222 (accessed on 11 February 2021).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03688178 (accessed on 11 February 2021).
- Badhiwala, J.; Decker, W.K.; Berens, M.E.; Bhardwaj, R.D. Clinical trials in cellular immunotherapy for brain/CNS tumors. Expert Rev. Neurother. 2013, 13, 405–424. [Google Scholar] [CrossRef]
- Alibek, K.; Kakpenova, A.; Baiken, Y. Role of infectious agents in the carcinogenesis of brain and head and neck cancers. Infect. Agent Cancer 2013, 8, 7. [Google Scholar] [CrossRef] [PubMed]
| CMV Product | Oncomodulatory Role | Supporting Evidence |
|---|---|---|
| US28 | Activation of several proliferative, inflammatory and angiogenic signaling pathways through binding human chemokines [33,36] | Growth acceleration of glioblastoma cells in a murine orthotopic intracranial glioblastoma model [30]; VEGF suppression by RNA silencing of US28 in glioma cells [32] |
| pp71 | Activation of endothelium tube formation [32] | Enhanced cancer cell proliferation in CD133+ glioma-initiated cells [32] |
| Immediate early proteins, CMV70-3P miRNA | Stemness of glioma-initiated stem cells | Increased expression of the stemness markers sox2 and nestin in glioblastoma cells [34,35,41] |
| Therapy | Cancer Type | Phase | Status | Clinical Trial Identifier | Reference |
|---|---|---|---|---|---|
| Oncolytic Viruses | |||||
| G207 (genetically engineered HSV type I) | Glioma, astrocytoma, glioblastoma | I/II | Completed | NCT00028158 | [119] |
| G207 (genetically engineered HSV type I) | Glioblastoma | I | Completed | NCT00157703 | [120] |
| G207 (genetically engineered HSV type I) | Malignant cerebellar brain tumors | I | Recruiting | NCT03911388 | [121] |
| G207 (genetically engineered HSV type I) | Malignant supratentorial brain tumors | I | Active | NCT02457845 | [122] |
| M032 (second generation genetically engineered HSV type I) | Glioblastoma multiforme, anaplastic astrocytoma, gliosarcoma | I | Recruiting | NCT02062827 | [123] |
| C134 (genetically engineered HSV type I) | Glioblastoma multiforme, anaplastic astrocytoma, gliosarcoma | I | Active | NCT03657576 | [124] |
| G207 (genetically engineered HSV type I) | Malignant high-grade glioma | II | Not yet recruiting | NCT04482933 | [125] |
| Peptide and Dendritic Cells Vaccines | |||||
| PEP-CMV (synthetic long peptide of 26 amino acid residues from human pp65) | Medulloblastoma, malignant glioma | I | Recruiting | NCT03299309 | [126] |
| Nivolumab combined with CMV pp65 DC vaccination | Grade III or IV glioma or astrocytoma | I | Completed | NCT02529072 | [127] |
| CMV pp65 DC vaccination combined with GM-CSF | Glioblastoma multiforme | I | Active | NCT00639639 | [107] |
| CMV RNA-loaded DC vaccine | Glioblastoma | II | Recruiting | NCT03927222 | [128] |
| CMV RNA-loaded DC vaccine +/– varlilumab | Glioblastoma | II | Recruiting | NCT03688178 | [129] |
| Adoptive Cell Therapy | |||||
| Human epidermal growth factor receptor type2-Chimeric antigen receptor (HER2-CAR) virus specific T cells | HER2-positive glioblastoma | I | Completed | NCT01109095 | [130] |
| HHVs (Human Herpesviruses) | Role |
|---|---|
| CMV | Detection of nucleic acids and proteins in a high percentage of malignant gliomas and medulloblastomas |
| Absence in gliomas and tumor cells of the nervous system | |
| Oncomodulation by viral proteins and non-coding RNAs | |
| Immunomodulation by viral proteins and non-coding RNAs | |
| EBV (Epstein-Barr virus) | Detection of EBV DNA in high-grade gliomas |
| Presence in pilocytic astrocytomas | |
| EBV miRNAs in plasma of glioblastoma patients associated with impaired anti-tumor immune response | |
| Absence in gliomas | |
| HHV-6 | Detection of viral DNA and viral proteins in adult and pediatric gliomas compared to healthy controls |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Athanasiou, E.; Gargalionis, A.N.; Boufidou, F.; Tsakris, A. The Association of Human Herpesviruses with Malignant Brain Tumor Pathology and Therapy: Two Sides of a Coin. Int. J. Mol. Sci. 2021, 22, 2250. https://doi.org/10.3390/ijms22052250
Athanasiou E, Gargalionis AN, Boufidou F, Tsakris A. The Association of Human Herpesviruses with Malignant Brain Tumor Pathology and Therapy: Two Sides of a Coin. International Journal of Molecular Sciences. 2021; 22(5):2250. https://doi.org/10.3390/ijms22052250
Chicago/Turabian StyleAthanasiou, Evita, Antonios N. Gargalionis, Fotini Boufidou, and Athanassios Tsakris. 2021. "The Association of Human Herpesviruses with Malignant Brain Tumor Pathology and Therapy: Two Sides of a Coin" International Journal of Molecular Sciences 22, no. 5: 2250. https://doi.org/10.3390/ijms22052250
APA StyleAthanasiou, E., Gargalionis, A. N., Boufidou, F., & Tsakris, A. (2021). The Association of Human Herpesviruses with Malignant Brain Tumor Pathology and Therapy: Two Sides of a Coin. International Journal of Molecular Sciences, 22(5), 2250. https://doi.org/10.3390/ijms22052250








