Regenerative Stem Cell Therapy for Neurodegenerative Diseases: An Overview
Abstract
1. Introduction
2. Stem Cell Classifications
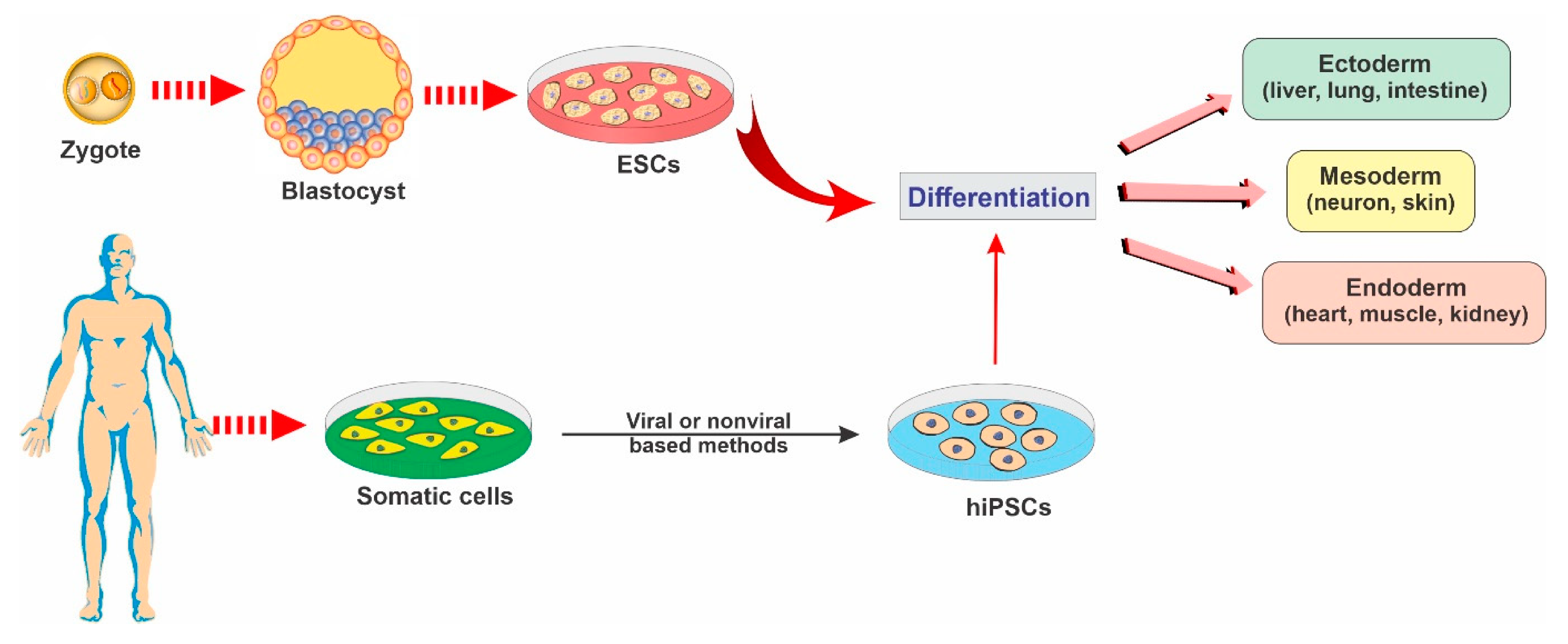
2.1. Embryonic Stem Cells
2.2. Induced Pluripotent Stem Cells
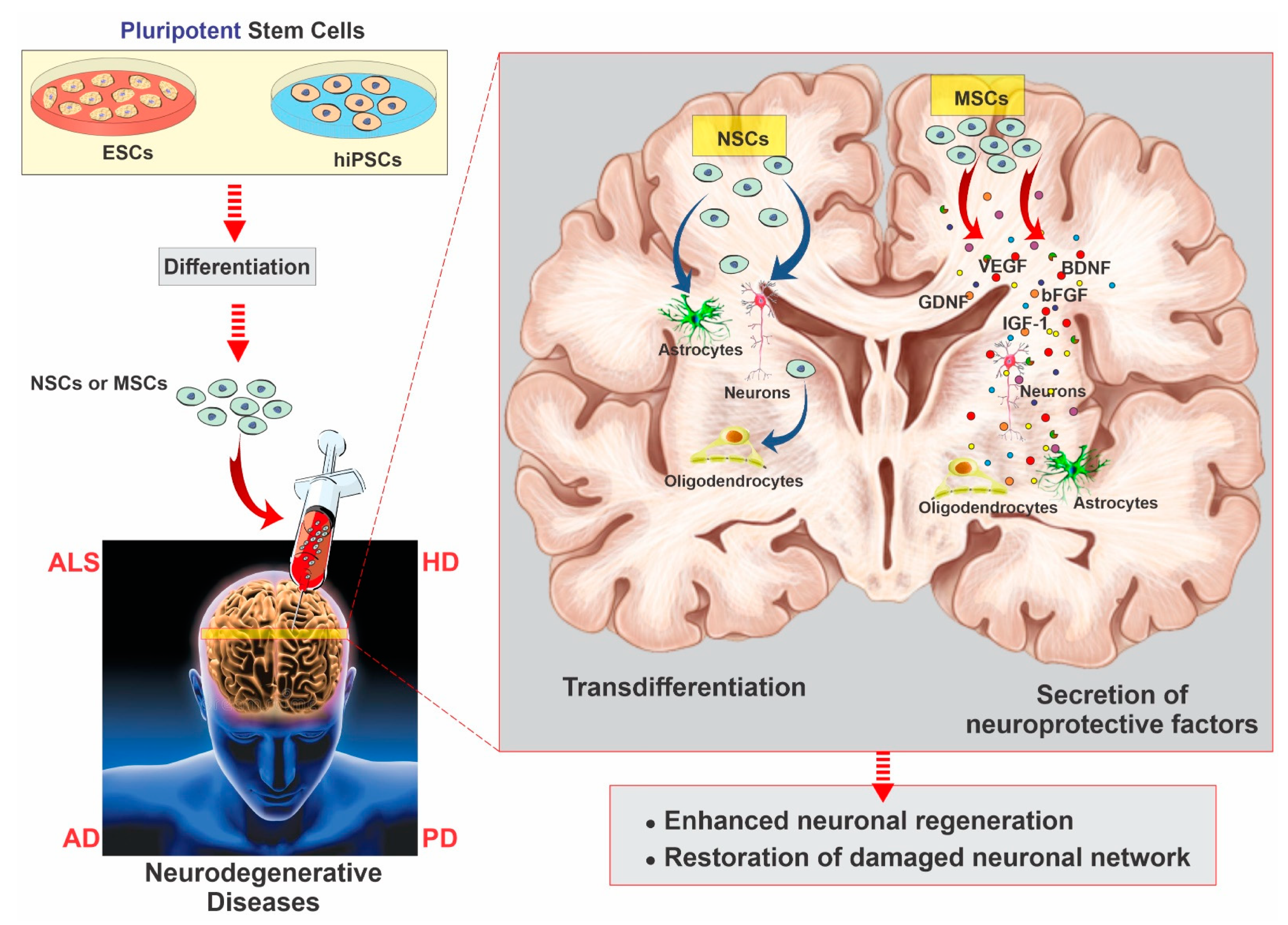
2.3. Mesenchymal Stem Cells
2.4. Neural Stem Cells
3. Neurodegenerative Diseases and Stem Cell Therapy Strategies for Regeneration
3.1. Stem Cell Therapy in Parkinson’s Disease
3.2. Stem Cell Therapy in Alzheimer’s Disease
3.3. Stem Cell Therapy in Huntington’s Disease
3.4. Stem Cell Therapy in Amyotrophic Lateral Sclerosis
3.5. Stem Cell Therapy in Frontotemporal Dementia
4. Challenges and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| ALS | amyotrophic lateral sclerosis |
| BDNF | brain-derived neurotrophic factor |
| CNS | central nervous system |
| DA | dopamine |
| ESCs | embryonic stem cells |
| FTD | frontotemporal dementia |
| GDNF | glial-cell-line-derived neurotrophic factor |
| HD | Huntington’s disease |
| IPSCs | induced pluripotent stem cells |
| MSCs | mesenchymal stem cells |
| NFTs | neurofibrillary tangles |
| NGF | nerve growth factor |
| NPCs | neural progenitor cells |
| NSCs | neural stem cells |
| PD | Parkinson’s disease |
| ROS | reactive oxygen species |
| SGZ | subgranular zone |
| SVZ | subventricular zone |
References
- De Gioia, R.; Biella, F.; Citterio, G.; Rizzo, F.; Abati, E.; Nizzardo, M.; Bresolin, N.; Comi, G.P.; Corti, S. Neural Stem Cell Transplantation for Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 3103. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-Y.; Ting, H.-C.; Liu, C.-A.; Su, H.-L.; Chiou, T.-W.; Lin, S.-Z.; Harn, H.-J.; Ho, T.-J. Induced pluripotent stem cell (iPSC)-based neurodegenerative disease models for phenotype recapitulation and drug screening. Molecules 2020, 25, 2000. [Google Scholar] [CrossRef] [PubMed]
- Helms, H.C.; Abbott, N.J.; Burek, M.; Cecchelli, R.; Couraud, P.-O.; Deli, M.A.; Förster, C.; Galla, H.J.; Romero, I.A.; Shusta, E.V. In vitro models of the blood–brain barrier: An overview of commonly used brain endothelial cell culture models and guidelines for their use. J. Cereb. Blood Flow Metab. 2016, 36, 862–890. [Google Scholar] [CrossRef]
- Sivandzade, F.; Cucullo, L. In-vitro blood–brain barrier modeling: A review of modern and fast-advancing technologies. J. Cereb. Blood Flow Metab. 2018, 38, 1667–1681. [Google Scholar] [CrossRef] [PubMed]
- Tam, V.H.; Sosa, C.; Liu, R.; Yao, N.; Priestley, R.D. Nanomedicine as a noninvasive strategy for drug delivery across the blood brain barrier. Int. J. Pharm. 2016, 515, 331–342. [Google Scholar] [CrossRef]
- Wen, M.M.; El-Salamouni, N.S.; El-Refaie, W.M.; Hazzah, H.A.; Ali, M.M.; Tosi, G.; Farid, R.M.; Blanco-Prieto, M.J.; Billa, N.; Hanafy, A.S. Nanotechnology-based drug delivery systems for Alzheimer’s disease management: Technical, industrial, and clinical challenges. J. Control. Release 2017, 245, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yan, W.; Zhu, Y.; Yang, W.; Le, W.; Chen, B.; Zhu, R.; Cheng, L. Nanomaterials in neural-stem-cell-mediated regenerative medicine: Imaging and treatment of neurological diseases. Adv. Mater. 2018, 30, 1705694. [Google Scholar] [CrossRef]
- Lunn, J.S.; Sakowski, S.A.; Hur, J.; Feldman, E.L. Stem cell technology for neurodegenerative diseases. Ann. Neurol. 2011, 70, 353–361. [Google Scholar] [CrossRef]
- Sakthiswary, R.; Raymond, A.A. Stem cell therapy in neurodegenerative diseases: From principles to practice. Neural Regen. Res. 2012, 7, 1822. [Google Scholar]
- Shariati, A.; Nemati, R.; Sadeghipour, Y.; Yaghoubi, Y.; Baghbani, R.; Javidi, K.; Zamani, M.; Hassanzadeh, A. Mesenchymal stromal cells (MSCs) for neurodegenerative disease; a promising frontier. Eur. J. Cell Biol. 2020, 151097. [Google Scholar] [CrossRef]
- Frenette, P.S.; Pinho, S.; Lucas, D.; Scheiermann, C. Mesenchymal stem cell: Keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu. Rev. Immunol. 2013, 31, 285–316. [Google Scholar] [CrossRef]
- Drouin-Ouellet, J.; Barker, R.A. Stem cell therapies for Parkinson’s disease: Are trials just around the corner? Regen. Med. 2014, 9, 553–555. [Google Scholar] [CrossRef]
- Dekmak, A.; Mantash, S.; Shaito, A.; Toutonji, A.; Ramadan, N.; Ghazale, H.; Kassem, N.; Darwish, H.; Zibara, K. Stem cells and combination therapy for the treatment of traumatic brain injury. Behav. Brain Res. 2018, 340, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Kazmerova, Z.; Zilka, N.; Cente, M.; Neradil, P.; Zilkova, M.; Novak, M. Can we teach old dogs new tricks? Neuroprotective cell therapy in Alzheimer’s and Parkinson’s disease. J. Alzheimer’s Dis. 2013, 37, 251–272. [Google Scholar] [CrossRef]
- de Lázaro, I.; Yilmazer, A.; Kostarelos, K. Induced pluripotent stem (iPS) cells: A new source for cell-based therapeutics? J. Control. Release 2014, 185, 37–44. [Google Scholar] [CrossRef]
- Sproul, A.A. Being human: The role of pluripotent stem cells in regenerative medicine and humanizing Alzheimer’s disease models. Mol. Asp. Med. 2015, 43, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Fujimori, K.; Andoh-Noda, T.; Ando, T.; Kuzumaki, N.; Toyoshima, M.; Tada, H.; Imaizumi, K.; Ishikawa, M.; Yamaguchi, R. Functional neurons generated from T cell-derived induced pluripotent stem cells for neurological disease modeling. Stem Cell Rep. 2016, 6, 422–435. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, X.; Leak, R.K.; Chen, F.; Cao, G. Stem cell therapies in age-related neurodegenerative diseases and stroke. Ageing Res. Rev. 2017, 34, 39–50. [Google Scholar] [CrossRef]
- Verma, A.; Verma, N. Induced pluripotent stem cells and promises of neuroregenerative medicine. Neurol. India 2011, 59, 555. [Google Scholar] [CrossRef]
- Barkho, B.Z.; Zhao, X. Adult neural stem cells: Response to stroke injury and potential for therapeutic applications. Curr. Stem Cell Res. Ther. 2011, 6, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, K.-C.; Song, B.; Lee, N.; Jung, J.H.; Cha, Y.; Leblanc, P.; Neff, C.; Kong, S.W.; Carter, B.S.; Schweitzer, J. Pluripotent stem cell-based therapy for Parkinson’s disease: Current status and future prospects. Prog. Neurobiol. 2018, 168, 1–20. [Google Scholar] [CrossRef]
- Glat, M.J.; Offen, D. Cell and gene therapy in Alzheimer’s disease. Stem Cells Dev. 2013, 22, 1490–1496. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, S.; Cao, W. Mesenchymal stem cell-mediated immunomodulation in cell therapy of neurodegenerative diseases. Cell. Immunol. 2018, 326, 8–14. [Google Scholar] [CrossRef]
- Vissers, C.; Ming, G.-l.; Song, H. Nanoparticle technology and stem cell therapy team up against neurodegenerative disorders. Adv. Drug Deliv. Rev. 2019, 148, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Aleynik, A.; Gernavage, K.M.; Mourad, Y.S.; Sherman, L.S.; Liu, K.; Gubenko, Y.A.; Rameshwar, P. Stem cell delivery of therapies for brain disorders. Clin. Transl. Med. 2014, 3, 24. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fan, D.; Xiong, D. Mesenchymal stem cells as delivery vectors for anti-tumor therapy. Stem Cell Investig. 2015, 2. [Google Scholar] [CrossRef]
- Hasan, A.; Deeb, G.; Rahal, R.; Atwi, K.; Mondello, S.; Marei, H.E.; Gali, A.; Sleiman, E. Mesenchymal stem cells in the treatment of traumatic brain injury. Front. Neurol. 2017, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, T.; Kibayashi, T.; Katayama, T.; Yamashita, Y.; Suzuki, S.; Kawamata, J.; Honmou, O.; Minami, M.; Shimohama, S. Mesenchymal stem cells transmigrate across brain microvascular endothelial cell monolayers through transiently formed inter-endothelial gaps. Neurosci. Lett. 2011, 502, 41–45. [Google Scholar] [CrossRef]
- Kar, M.; Shih, Y.-R.V.; Velez, D.O.; Cabrales, P.; Varghese, S. Poly (ethylene glycol) hydrogels with cell cleavable groups for autonomous cell delivery. Biomaterials 2016, 77, 186–197. [Google Scholar] [CrossRef]
- Murrell, W.; Palmero, E.; Bianco, J.; Stangeland, B.; Joel, M.; Paulson, L.; Thiede, B.; Grieg, Z.; Ramsnes, I.; Skjellegrind, H.K. Expansion of multipotent stem cells from the adult human brain. PLoS ONE 2013, 8, e71334. [Google Scholar] [CrossRef]
- Belenguer, G.; Domingo-Muelas, A.; Ferrón, S.R.; Morante-Redolat, J.M.; Fariñas, I. Isolation, culture and analysis of adult subependymal neural stem cells. Differentiation 2016, 91, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.; Zalucki, O.; Piper, M.; Heng, J.I.-T. Insights into the biology and therapeutic applications of neural stem cells. Stem Cells Int. 2016, 2016, 9745315. [Google Scholar] [CrossRef] [PubMed]
- Carradori, D.; Eyer, J.; Saulnier, P.; Préat, V.; Des Rieux, A. The therapeutic contribution of nanomedicine to treat neurodegenerative diseases via neural stem cell differentiation. Biomaterials 2017, 123, 77–91. [Google Scholar] [CrossRef]
- Li, X.; Peng, Z.; Long, L.; Tuo, Y.; Wang, L.; Zhao, X.; Le, W.; Wan, Y. Wnt4-modified NSC transplantation promotes functional recovery after spinal cord injury. FASEB J. 2020, 34, 82–94. [Google Scholar] [CrossRef]
- Araque, A.; Carmignoto, G.; Haydon, P.G.; Oliet, S.H.; Robitaille, R.; Volterra, A. Gliotransmitters travel in time and space. Neuron 2014, 81, 728–739. [Google Scholar] [CrossRef]
- Bond, A.M.; Ming, G.-l.; Song, H. Adult mammalian neural stem cells and neurogenesis: Five decades later. Cell Stem Cell 2015, 17, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Ming, G.-L.; Song, H. Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron 2011, 70, 687–702. [Google Scholar] [CrossRef]
- Nam, H.; Lee, K.-H.; Nam, D.-H.; Joo, K.M. Adult human neural stem cell therapeutics: Current developmental status and prospect. World J. Stem Cells 2015, 7, 126. [Google Scholar] [CrossRef]
- López-León, M.; Outeiro, T.F.; Goya, R.G. Cell reprogramming: Therapeutic potential and the promise of rejuvenation for the aging brain. Ageing Res. Rev. 2017, 40, 168–181. [Google Scholar] [CrossRef]
- Karussis, D.; Petrou, P.; Kassis, I. Clinical experience with stem cells and other cell therapies in neurological diseases. J. Neurol. Sci. 2013, 324, 1–9. [Google Scholar] [CrossRef]
- Yoo, J.; Kim, H.S.; Hwang, D.Y. Stem cells as promising therapeutic options for neurological disorders. J. Cell. Biochem. 2013, 114, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Sousa, B.R.; Parreira, R.C.; Fonseca, E.A.; Amaya, M.J.; Tonelli, F.M.; Lacerda, S.M.; Lalwani, P.; Santos, A.K.; Gomes, K.N.; Ulrich, H. Human adult stem cells from diverse origins: An overview from multiparametric immunophenotyping to clinical applications. Cytom. Part A 2014, 85, 43–77. [Google Scholar] [CrossRef]
- Stoll, E.A. Advances toward regenerative medicine in the central nervous system: Challenges in making stem cell therapy a viable clinical strategy. Mol. Cell. Ther. 2014, 2, 1–9. [Google Scholar] [CrossRef]
- Nicaise, C.; Mitrecic, D.; Falnikar, A.; Lepore, A.C. Transplantation of stem cell-derived astrocytes for the treatment of amyotrophic lateral sclerosis and spinal cord injury. World J. Stem Cells 2015, 7, 380. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.K.; Staff, N.P.; Knight, A.M.; Nesbitt, J.J.; Butler, G.W.; Padley, D.J.; Parisi, J.E.; Dietz, A.B.; Windebank, A.J. A safety study on intrathecal delivery of autologous mesenchymal stromal cells in rabbits directly supporting P hase I human trials. Transfusion 2015, 55, 1013–1020. [Google Scholar] [CrossRef]
- Fu, M.-H.; Li, C.-L.; Lin, H.-L.; Chen, P.-C.; Calkins, M.J.; Chang, Y.-F.; Cheng, P.-H.; Yang, S.-H. Stem cell transplantation therapy in Parkinson’s disease. SpringerPlus 2015, 4, 597. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.-W.; Moon, C.; Kim, H.Y.; Oh, S.-I.; Park, J.; Lee, J.H.; Chang, I.Y.; Kim, K.S.; Kim, S.H. Phase I trial of repeated intrathecal autologous bone marrow-derived mesenchymal stromal cells in amyotrophic lateral sclerosis. Stem Cells Transl. Med. 2015, 4, 590–597. [Google Scholar] [CrossRef]
- Ritfeld, G.J.; Roos, R.A.; Oudega, M. Stem cells for central nervous system repair and rehabilitation. PM&R 2011, 3, S117–S122. [Google Scholar]
- Sorrells, S.F.; Paredes, M.F.; Cebrian-Silla, A.; Sandoval, K.; Qi, D.; Kelley, K.W.; James, D.; Mayer, S.; Chang, J.; Auguste, K.I. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 2018, 555, 377–381. [Google Scholar] [CrossRef]
- Snyder, J.S. Questioning Human Neurogenesis; Nature: New York, NY, USA, 2018; Volume 555, pp. 315–316. [Google Scholar]
- Boldrini, M.; Fulmore, C.A.; Tartt, A.N.; Simeon, L.R.; Pavlova, I.; Poposka, V.; Rosoklija, G.B.; Stankov, A.; Arango, V.; Dwork, A.J. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell 2018, 22, 589–599.e5. [Google Scholar] [CrossRef]
- Gan, L.; Johnson, J.A. Oxidative damage and the Nrf2-ARE pathway in neurodegenerative diseases. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2014, 1842, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- Buddhala, C.; Loftin, S.K.; Kuley, B.M.; Cairns, N.J.; Campbell, M.C.; Perlmutter, J.S.; Kotzbauer, P.T. Dopaminergic, serotonergic, and noradrenergic deficits in Parkinson disease. Ann. Clin. Transl. Neurol. 2015, 2, 949–959. [Google Scholar] [CrossRef]
- Ford, E.; Pearlman, J.; Ruan, T.; Manion, J.; Waller, M.; Neely, G.G.; Caron, L. Human Pluripotent Stem Cells-Based Therapies for Neurodegenerative Diseases: Current Status and Challenges. Cells 2020, 9, 2517. [Google Scholar] [CrossRef]
- Reddy, A.P.; Ravichandran, J.; Carkaci-Salli, N. Neural regeneration therapies for Alzheimer’s and Parkinson’s disease-related disorders. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2020, 1866, 165506. [Google Scholar] [CrossRef]
- Sivandzade, F.; Prasad, S.; Bhalerao, A.; Cucullo, L. NRF2 and NF-κB interplay in cerebrovascular and neurodegenerative disorders: Molecular mechanisms and possible therapeutic approaches. Redox Biol. 2019, 21, 101059. [Google Scholar] [CrossRef]
- Raza, C.; Anjum, R. Parkinson’s disease: Mechanisms, translational models and management strategies. Life Sci. 2019, 226, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Trombetta-Lima, M.; Sabogal-Guáqueta, A.M.; Dolga, A.M. Mitochondrial dysfunction in neurodegenerative diseases: A focus on iPSC-derived neuronal models. Cell Calcium 2021, 94, 102362. [Google Scholar] [CrossRef]
- Kim, H.-J. Stem cell potential in Parkinson’s disease and molecular factors for the generation of dopamine neurons. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2011, 1812, 1–11. [Google Scholar] [CrossRef]
- Schwarz, J.; Storch, A. Transplantation in Parkinson’s disease: Will mesenchymal stem cells help to reenter the clinical arena? Transl. Res. 2009, 155, 55–56. [Google Scholar] [CrossRef] [PubMed]
- Takagi, Y.; Takahashi, J.; Saiki, H.; Morizane, A.; Hayashi, T.; Kishi, Y.; Fukuda, H.; Okamoto, Y.; Koyanagi, M.; Ideguchi, M. Dopaminergic neurons generated from monkey embryonic stem cells function in a Parkinson primate model. J. Clin. Investig. 2005, 115, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Garitaonandia, I.; Gonzalez, R.; Christiansen-Weber, T.; Abramihina, T.; Poustovoitov, M.; Noskov, A.; Sherman, G.; Semechkin, A.; Snyder, E.; Kern, R. Neural stem cell tumorigenicity and biodistribution assessment for phase I clinical trial in Parkinson’s disease. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Wang, Y.-K.; Zhu, W.-W.; Wu, M.-H.; Wu, Y.-H.; Liu, Z.-X.; Liang, L.-M.; Sheng, C.; Hao, J.; Wang, L.; Li, W. Human clinical-grade parthenogenetic ESC-derived dopaminergic neurons recover locomotive defects of nonhuman primate models of Parkinson’s disease. Stem Cell Rep. 2018, 11, 171–182. [Google Scholar] [CrossRef]
- Tom, C.M.; Younesi, S.; Meer, E.; Bresee, C.; Godoy, M.; Mattis, V.B. Survival of iPSC-derived grafts within the striatum of immunodeficient mice: Importance of developmental stage of both transplant and host recipient. Exp. Neurol. 2017, 297, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Deleidi, M.; Cooper, O.; Hargus, G.; Levy, A.; Isacson, O. Oct4-induced reprogramming is required for adult brain neural stem cell differentiation into midbrain dopaminergic neurons. PLoS ONE 2011, 6, e19926. [Google Scholar] [CrossRef] [PubMed]
- Yasuhara, T.; Matsukawa, N.; Hara, K.; Yu, G.; Xu, L.; Maki, M.; Kim, S.U.; Borlongan, C.V. Transplantation of human neural stem cells exerts neuroprotection in a rat model of Parkinson’s disease. J. Neurosci. 2006, 26, 12497–12511. [Google Scholar] [CrossRef]
- Soler, R.; Füllhase, C.; Hanson, A.; Campeau, L.; Santos, C.; Andersson, K.-E. Stem cell therapy ameliorates bladder dysfunction in an animal model of Parkinson disease. J. Urol. 2012, 187, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Venkataramana, N.K.; Kumar, S.K.; Balaraju, S.; Radhakrishnan, R.C.; Bansal, A.; Dixit, A.; Rao, D.K.; Das, M.; Jan, M.; Gupta, P.K. Open-labeled study of unilateral autologous bone-marrow-derived mesenchymal stem cell transplantation in Parkinson’s disease. Transl. Res. 2010, 155, 62–70. [Google Scholar] [CrossRef]
- Cave, J.; Wang, M.; Baker, H. Adult subventricular zone neural stem cells as a potential source of dopaminergic replacement neurons. Front. Neurosci. 2014, 8, 16. [Google Scholar] [CrossRef]
- Barker, R.A.; Drouin-Ouellet, J.; Parmar, M. Cell-based therapies for Parkinson disease—Past insights and future potential. Nat. Rev. Neurol. 2015, 11, 492. [Google Scholar] [CrossRef]
- Han, F.; Baremberg, D.; Gao, J.; Duan, J.; Lu, X.; Zhang, N.; Chen, Q. Development of stem cell-based therapy for Parkinson’s disease. Transl. Neurodegener. 2015, 4, 1–13. [Google Scholar] [CrossRef]
- Doi, D.; Magotani, H.; Kikuchi, T.; Ikeda, M.; Hiramatsu, S.; Yoshida, K.; Amano, N.; Nomura, M.; Umekage, M.; Morizane, A. Preclinical study of induced pluripotent stem cell-derived dopaminergic progenitor cells for Parkinson’s disease. Nat. Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Takahashi, J. Strategies for bringing stem cell-derived dopamine neurons to the clinic: The Kyoto trial. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2017; Volume 230, pp. 213–226. [Google Scholar]
- Okita, K.; Yamakawa, T.; Matsumura, Y.; Sato, Y.; Amano, N.; Watanabe, A.; Goshima, N.; Yamanaka, S. An efficient nonviral method to generate integration-free human-induced pluripotent stem cells from cord blood and peripheral blood cells. Stem Cells 2013, 31, 458–466. [Google Scholar] [CrossRef]
- Kim, H.J.; Seo, S.W.; Chang, J.W.; Lee, J.I.; Kim, C.H.; Chin, J.; Choi, S.J.; Kwon, H.; Yun, H.J.; Lee, J.M. Stereotactic brain injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer’s disease dementia: A phase 1 clinical trial. Alzheimer’s Dement. 2015, 1, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Politis, M.; Oertel, W.H.; Wu, K.; Quinn, N.P.; Pogarell, O.; Brooks, D.J.; Bjorklund, A.; Lindvall, O.; Piccini, P. Graft-induced dyskinesias in Parkinson’s disease: High striatal serotonin/dopamine transporter ratio. Mov. Disord. 2011, 26, 1997–2003. [Google Scholar] [CrossRef] [PubMed]
- Bangde, P.; Atale, S.; Dey, A.; Pandit, A.; Dandekar, P.; Jain, R. Potential Gene Therapy Towards Treating Neurodegenerative Disea ses Employing Polymeric Nanosystems. Curr. Gene Ther. 2017, 17, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Mucke, L. Alzheimer mechanisms and therapeutic strategies. Cell 2012, 148, 1204–1222. [Google Scholar] [CrossRef]
- Guo, J.-W.; Guan, P.-P.; Ding, W.-Y.; Wang, S.-L.; Huang, X.-S.; Wang, Z.-Y.; Wang, P. Erythrocyte membrane-encapsulated celecoxib improves the cognitive decline of Alzheimer’s disease by concurrently inducing neurogenesis and reducing apoptosis in APP/PS1 transgenic mice. Biomaterials 2017, 145, 106–127. [Google Scholar] [CrossRef] [PubMed]
- Association, A.S. 2016 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2016, 12, 459–509. [Google Scholar] [CrossRef]
- Murphy, S.L.; Xu, J.; Kochanek, K.D. Deaths: Final Data for 2010; National Vital Statistics Reports: Atlanta, GA, USA, 2013; Volume 61, pp. 1–117. [Google Scholar]
- Hebert, L.E.; Weuve, J.; Scherr, P.A.; Evans, D.A. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 2013, 80, 1778–1783. [Google Scholar] [CrossRef] [PubMed]
- Banigan, M.G.; Kao, P.F.; Kozubek, J.A.; Winslow, A.R.; Medina, J.; Costa, J.; Schmitt, A.; Schneider, A.; Cabral, H.; Cagsal-Getkin, O. Differential expression of exosomal microRNAs in prefrontal cortices of schizophrenia and bipolar disorder patients. PLoS ONE 2013, 8, e48814. [Google Scholar] [CrossRef]
- Yeo, R.W.Y.; Lai, R.C.; Zhang, B.; Tan, S.S.; Yin, Y.; Teh, B.J.; Lim, S.K. Mesenchymal stem cell: An efficient mass producer of exosomes for drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Duncan, T.; Valenzuela, M. Alzheimer’s disease, dementia, and stem cell therapy. Stem Cell Res. Ther. 2017, 8, 111. [Google Scholar] [CrossRef]
- Ager, R.R.; Davis, J.L.; Agazaryan, A.; Benavente, F.; Poon, W.W.; LaFerla, F.M.; Blurton-Jones, M. Human neural stem cells improve cognition and promote synaptic growth in two complementary transgenic models of Alzheimer’s disease and neuronal loss. Hippocampus 2015, 25, 813–826. [Google Scholar] [CrossRef]
- Kern, D.; Maclean, K.; Jiang, H.; Synder, E.; Sladek Jr, J.; Bjugstad, K. Neural stem cells reduce hippocampal tau and reelin accumulation in aged Ts65Dn Down syndrome mice. Cell Transplant. 2011, 20, 371–379. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, L.; Wu, Z.; Chen, Y.; Wang, F.; Chen, G. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer’s disease model. Cell Stem Cell 2014, 14, 188–202. [Google Scholar] [CrossRef]
- Cundiff, P.E.; Anderson, S.A. Impact of induced pluripotent stem cells on the study of central nervous system disease. Curr. Opin. Genet. Dev. 2011, 21, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Penney, J.; Ralvenius, W.T.; Tsai, L.-H. Modeling Alzheimer’s disease with iPSC-derived brain cells. Mol. Psychiatry 2020, 25, 148–167. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, K.; Ikeda, T.; Haruta, M.; Matsumura, K.; Ogi, Y.; Nakagata, N.; Uchino, M.; Ando, Y.; Nishimura, Y.; Senju, S. Degradation of amyloid beta by human induced pluripotent stem cell-derived macrophages expressing Neprilysin-2. Stem Cell Res. 2014, 13, 442–453. [Google Scholar] [CrossRef]
- Vertelov, G.; Kharazi, L.; Muralidhar, M.; Sanati, G.; Tankovich, T.; Kharazi, A. High targeted migration of human mesenchymal stem cells grown in hypoxia is associated with enhanced activation of RhoA. Stem Cell Res. Ther. 2013, 4, 1–9. [Google Scholar] [CrossRef]
- Garcia, K.d.O.; Ornellas, F.L.; Matsumoto, P.; Patti, C.d.L.; Mello, L.E.; Frussa-Filho, R.; Han, S.W.; Longo, B.M. Therapeutic effects of the transplantation of VEGF overexpressing bone marrow mesenchymal stem cells in the hippocampus of murine model of Alzheimer’s disease. Front. Aging Neurosci. 2014, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.M.; Ahmed, H.H.; Atta, H.M.; Ghazy, M.A.; Aglan, H.A. Potential of bone marrow mesenchymal stem cells in management of Alzheimer’s disease in female rats. Cell Biol. Int. 2014, 38, 1367–1383. [Google Scholar] [CrossRef]
- Urdzíková, L.M.; Růžička, J.; LaBagnara, M.; Kárová, K.; Kubinová, Š.; Jiráková, K.; Murali, R.; Syková, E.; Jhanwar-Uniyal, M.; Jendelová, P. Human mesenchymal stem cells modulate inflammatory cytokines after spinal cord injury in rat. Int. J. Mol. Sci. 2014, 15, 11275–11293. [Google Scholar] [CrossRef]
- Oh, S.H.; Kim, H.N.; Park, H.-J.; Shin, J.Y.; Lee, P.H. Mesenchymal stem cells increase hippocampal neurogenesis and neuronal differentiation by enhancing the Wnt signaling pathway in an Alzheimer’s disease model. Cell Transplant. 2015, 24, 1097–1109. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.P.; Flor-García, M.; Terreros-Roncal, J.; Rábano, A.; Cafini, F.; Pallas-Bazarra, N.; Ávila, J.; Llorens-Martín, M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 2019, 25, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Gong, K.; Ao, Q.; Yan, Y.; Song, B.; Huang, H.; Zhang, X.; Gong, Y. Intracerebral transplantation of adipose-derived mesenchymal stem cells alternatively activates microglia and ameliorates neuropathological deficits in Alzheimer’s disease mice. Cell Transplant. 2013, 22, 113–126. [Google Scholar] [CrossRef]
- Kan, I.; Barhum, Y.; Melamed, E.; Offen, D. Mesenchymal stem cells stimulate endogenous neurogenesis in the subventricular zone of adult mice. Stem Cell Rev. Rep. 2011, 7, 404–412. [Google Scholar] [CrossRef]
- Martínez-Morales, P.; Revilla, A.; Ocana, I.; Gonzalez, C.; Sainz, P.; McGuire, D.; Liste, I. Progress in stem cell therapy for major human neurological disorders. Stem Cell Rev. Rep. 2013, 9, 685–699. [Google Scholar] [CrossRef] [PubMed]
- Hunsberger, J.G.; Rao, M.; Kurtzberg, J.; Bulte, J.W.; Atala, A.; LaFerla, F.M.; Greely, H.T.; Sawa, A.; Gandy, S.; Schneider, L.S. Accelerating stem cell trials for Alzheimer’s disease. Lancet Neurol. 2016, 15, 219–230. [Google Scholar] [CrossRef]
- Bianchi, F.; Maioli, M.; Leonardi, E.; Olivi, E.; Pasquinelli, G.; Valente, S.; Mendez, A.J.; Ricordi, C.; Raffaini, M.; Tremolada, C. A new nonenzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte-like elements by mild mechanical forces from human lipoaspirates. Cell Transplant. 2013, 22, 2063–2077. [Google Scholar] [CrossRef] [PubMed]
- Connor, B. Concise review: The use of stem cells for understanding and treating Huntington’s disease. Stem Cells 2018, 36, 146–160. [Google Scholar] [CrossRef]
- Ebert, A.D.; Barber, A.E.; Heins, B.M.; Svendsen, C.N. Ex vivo delivery of GDNF maintains motor function and prevents neuronal loss in a transgenic mouse model of Huntington’s disease. Exp. Neurol. 2010, 224, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Dey, N.D.; Bombard, M.C.; Roland, B.P.; Davidson, S.; Lu, M.; Rossignol, J.; Sandstrom, M.I.; Skeel, R.L.; Lescaudron, L.; Dunbar, G.L. Genetically engineered mesenchymal stem cells reduce behavioral deficits in the YAC 128 mouse model of Huntington’s disease. Behav. Brain Res. 2010, 214, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Snyder, B.R.; Chiu, A.M.; Prockop, D.J.; Chan, A.W. Human multipotent stromal cells (MSCs) increase neurogenesis and decrease atrophy of the striatum in a transgenic mouse model for Huntington’s disease. PLoS ONE 2010, 5, e9347. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-T.; Chern, Y.; Shen, C.-K.J.; Wen, H.-L.; Chang, Y.-C.; Li, H.; Cheng, T.-H.; Hsieh-Li, H.M. Human mesenchymal stem cells prolong survival and ameliorate motor deficit through trophic support in Huntington’s disease mouse models. PLoS ONE 2011, 6, e22924. [Google Scholar] [CrossRef]
- Snyder, B.R.; Cheng, P.-H.; Yang, J.; Yang, S.-H.; Huang, A.H.; Chan, A.W. Characterization of dental pulp stem/stromal cells of Huntington monkey tooth germs. BMC Cell Biol. 2011, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jeon, I.; Lee, N.; Li, J.Y.; Park, I.H.; Park, K.S.; Moon, J.; Shim, S.H.; Choi, C.; Chang, D.J.; Kwon, J. Neuronal properties, in vivo effects, and pathology of a Huntington’s disease patient-derived induced pluripotent stem cells. Stem Cells 2012, 30, 2054–2062. [Google Scholar] [CrossRef]
- An, M.C.; Zhang, N.; Scott, G.; Montoro, D.; Wittkop, T.; Mooney, S.; Melov, S.; Ellerby, L.M. Genetic correction of Huntington’s disease phenotypes in induced pluripotent stem cells. Cell Stem Cell 2012, 11, 253–263. [Google Scholar] [CrossRef]
- Lin, C.Y.; Wu, C.L.; Lee, K.Z.; Chen, Y.J.; Zhang, P.H.; Chang, C.Y.; Harn, H.J.; Lin, S.Z.; Tsai, H.J. Extracellular Pgk1 enhances neurite outgrowth of motoneurons through Nogo66/NgR-independent targeting of NogoA. Elife 2019, 8, e49175. [Google Scholar] [CrossRef]
- Mattis, V.B.; Svendsen, C.N. Induced pluripotent stem cells: A new revolution for clinical neurology? Lancet Neurol. 2011, 10, 383–394. [Google Scholar] [CrossRef]
- Raore, B.; Federici, T.; Taub, J.; Wu, M.C.; Riley, J.; Franz, C.K.; Kliem, M.A.; Snyder, B.; Feldman, E.L.; Johe, K. Cervical multilevel intraspinal stem cell therapy: Assessment of surgical risks in Gottingen minipigs. Spine 2011, 36, E164–E171. [Google Scholar] [CrossRef]
- Martínez, H.R.; Molina-Lopez, J.F.; González-Garza, M.T.; Moreno-Cuevas, J.E.; Caro-Osorio, E.; Gil-Valadez, A.; Gutierrez-Jimenez, E.; Zazueta-Fierro, O.E.; Meza, J.A.; Couret-Alcaraz, P. Stem cell transplantation in amyotrophic lateral sclerosis patients: Methodological approach, safety, and feasibility. Cell Transplant. 2012, 21, 1899–1907. [Google Scholar] [CrossRef] [PubMed]
- Chiò, A.; Mora, G.; Bella, V.L.; Caponnetto, C.; Mancardi, G.; Sabatelli, M.; Siciliano, G.; Silani, V.; Corbo, M.; Moglia, C. Repeated courses of granulocyte colony-stimulating factor in amyotrophic lateral sclerosis: Clinical and biological results from a prospective multicenter study. Muscle Nerve 2011, 43, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Mazzini, L.; Mareschi, K.; Ferrero, I.; Miglioretti, M.; Stecco, A.; Servo, S.; Carriero, A.; Monaco, F.; Fagioli, F. Mesenchymal stromal cell transplantation in amyotrophic lateral sclerosis: A long-term safety study. Cytotherapy 2012, 14, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Minguell, J.J.; Allers, C.; Lasala, G.P. Mesenchymal stem cells and the treatment of conditions and diseases: The less glittering side of a conspicuous stem cell for basic research. Stem Cells Dev. 2013, 22, 193–203. [Google Scholar] [CrossRef]
- Blanquer, M.; Moraleda, J.M.; Iniesta, F.; Gómez-Espuch, J.; Meca-Lallana, J.; Villaverde, R.; Pérez-Espejo, M.Á.; Ruíz-López, F.J.; Garcia Santos, J.M.; Bleda, P. Neurotrophic bone marrow cellular nests prevent spinal motoneuron degeneration in amyotrophic lateral sclerosis patients: A pilot safety study. Stem Cells 2012, 30, 1277–1285. [Google Scholar] [CrossRef]
- Mazzini, L.; Gelati, M.; Profico, D.C.; Sgaravizzi, G.; Pensi, M.P.; Muzi, G.; Ricciolini, C.; Nodari, L.R.; Carletti, S.; Giorgi, C. Human neural stem cell transplantation in ALS: Initial results from a phase I trial. J. Transl. Med. 2015, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Petrou, P.; Gothelf, Y.; Argov, Z.; Gotkine, M.; Levy, Y.S.; Kassis, I.; Vaknin-Dembinsky, A.; Ben-Hur, T.; Offen, D.; Abramsky, O. Safety and clinical effects of mesenchymal stem cells secreting neurotrophic factor transplantation in patients with amyotrophic lateral sclerosis: Results of phase 1/2 and 2a clinical trials. JAMA Neurol. 2016, 73, 337–344. [Google Scholar] [CrossRef]
- Lunn, J.S.; Sakowski, S.A.; Feldman, E.L. Concise review: Stem cell therapies for amyotrophic lateral sclerosis: Recent advances and prospects for the future. Stem Cells 2014, 32, 1099–1109. [Google Scholar] [CrossRef]
- Karageorgiou, E.; Miller, B.L. Frontotemporal Lobar Degeneration: A Clinical approach. Semin Neurol. 2014, 34, 189–201. [Google Scholar] [CrossRef]
- Guo, W.; Fumagalli, L.; Prior, R.; Van Den Bosch, L. Current advances and limitations in modeling ALS/FTD in a dish using induced pluripotent stem cells. Front. Neurosci. 2017, 11, 671. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.; Spina, S.; Miller, B.L. Frontotemporal dementia. Lancet 2015, 386, 1672–1682. [Google Scholar] [CrossRef]
- Capozzo, R.; Sassi, C.; Hammer, M.B.; Arcuti, S.; Zecca, C.; Barulli, M.R.; Tortelli, R.; Gibbs, J.R.; Crews, C.; Seripa, D. Clinical and genetic analyses of familial and sporadic frontotemporal dementia patients in Southern Italy. Alzheimer’s Dement. 2017, 13, 858–869. [Google Scholar] [CrossRef]
- Greaves, C.V.; Rohrer, J.D. An update on genetic frontotemporal dementia. J. Neurol. 2019, 266, 2075–2086. [Google Scholar] [CrossRef]
- Lines, G.; Casey, J.M.; Preza, E.; Wray, S. Modelling Frontotemporal Dementia using patient-derived induced pluripotent stem cells. Mol. Cell. Neurosci. 2020, 109, 103553. [Google Scholar] [CrossRef]
- Ferrari, R.; Manzoni, C.; Hardy, J. Genetics and molecular mechanisms of frontotemporal lobar degeneration: An update and future avenues. Neurobiol. Aging 2019, 78, 98–110. [Google Scholar] [CrossRef]
- Tang, M.; Gu, X.; Wei, J.; Jiao, B.; Zhou, L.; Zhou, Y.; Weng, L.; Yan, X.; Tang, B.; Xu, J. Analyses MAPT, GRN, and C9orf72 mutations in Chinese patients with frontotemporal dementia. Neurobiol. Aging 2016, 46, 235.e211–235.e215. [Google Scholar] [CrossRef] [PubMed]
- Che, X.-Q.; Zhao, Q.-H.; Huang, Y.; Li, X.; Ren, R.-J.; Chen, S.-D.; Wang, G.; Guo, Q.-H. Genetic features of MAPT, GRN, C9orf72 and CHCHD10 gene mutations in Chinese patients with frontotemporal dementia. Curr. Alzheimer Res. 2017, 14, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-J.; Kim, Y.-E.; Jang, J.-H.; Cho, E.-H.; Na, D.L.; Seo, S.W.; Jung, N.-Y.; Jeong, J.H.; Kwon, J.C.; Park, K.H. Analysis of frontotemporal dementia, amyotrophic lateral sclerosis, and other dementia-related genes in 107 Korean patients with frontotemporal dementia. Neurobiol. Aging 2018, 72, 186.e181–186.e187. [Google Scholar] [CrossRef]
- Hedges, E.C.; Mehler, V.J.; Nishimura, A.L. The use of stem cells to model amyotrophic lateral sclerosis and frontotemporal dementia: From basic research to regenerative medicine. Stem Cells Int. 2016, 2016, 9279516. [Google Scholar] [CrossRef] [PubMed]
- Karch, C.M.; Kao, A.W.; Karydas, A.; Onanuga, K.; Martinez, R.; Argouarch, A.; Wang, C.; Huang, C.; Sohn, P.D.; Bowles, K.R. A comprehensive resource for induced pluripotent stem cells from patients with primary tauopathies. Stem Cell Rep. 2019, 13, 939–955. [Google Scholar] [CrossRef] [PubMed]
- Freibaum, B.D.; Lu, Y.; Lopez-Gonzalez, R.; Kim, N.C.; Almeida, S.; Lee, K.-H.; Badders, N.; Valentine, M.; Miller, B.L.; Wong, P.C. GGGGCC repeat expansion in C9orf72 compromises nucleocytoplasmic transport. Nature 2015, 525, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-K.; Morin, P.; Wells, J.; Hanlon, E.B.; Xia, W. Induced pluripotent stem cells (iPSCs) derived from frontotemporal dementia patient’s peripheral blood mononuclear cells. Stem Cell Res. 2015, 15, 325–327. [Google Scholar] [CrossRef]
- Rasmussen, M.A.; Hjermind, L.E.; Hasholt, L.F.; Waldemar, G.; Nielsen, J.E.; Clausen, C.; Hyttel, P.; Holst, B. Induced pluripotent stem cells (iPSCs) derived from a patient with frontotemporal dementia caused by a P301L mutation in microtubule-associated protein tau (MAPT). Stem Cell Res. 2016, 16, 70–74. [Google Scholar] [CrossRef]
- Rasmussen, M.A.; Hjermind, L.E.; Hasholt, L.F.; Waldemar, G.; Nielsen, J.E.; Clausen, C.; Hyttel, P.; Holst, B. Induced pluripotent stem cells (iPSCs) derived from a patient with frontotemporal dementia caused by a R406W mutation in microtubule-associated protein tau (MAPT). Stem Cell Res. 2016, 16, 75–78. [Google Scholar] [CrossRef]
- Nimsanor, N.; Jørring, I.; Rasmussen, M.A.; Clausen, C.; Mau-Holzmann, U.A.; Kitiyanant, N.; Nielsen, J.E.; Nielsen, T.T.; Hyttel, P.; Holst, B. Induced pluripotent stem cells (iPSCs) derived from a symptomatic carrier of a S305I mutation in the microtubule-associated protein tau (MAPT)-gene causing frontotemporal dementia. Stem Cell Res. 2016, 17, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Almeida, S.; Zhang, Z.; Coppola, G.; Mao, W.; Futai, K.; Karydas, A.; Geschwind, M.D.; Tartaglia, M.C.; Gao, F.; Gianni, D. Induced pluripotent stem cell models of progranulin-deficient frontotemporal dementia uncover specific reversible neuronal defects. Cell Rep. 2012, 2, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, M.; Hallmann, A.-L.; Reinhardt, P.; Araúzo-Bravo, M.J.; Korr, S.; Röpke, A.; Psathaki, O.E.; Ehling, P.; Meuth, S.G.; Oblak, A.L. Distinct neurodegenerative changes in an induced pluripotent stem cell model of frontotemporal dementia linked to mutant TAU protein. Stem Cell Res. 2015, 5, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, H.J.; Koh, W.; Li, L.; Heo, H.; Cho, H.; Lyoo, C.H.; Seo, S.W.; Kim, E.-J.; Nakanishi, M. Modeling of Frontotemporal Dementia Using iPSC Technology. Int. J. Mol. Sci. 2020, 21, 5319. [Google Scholar] [CrossRef]
- Raitano, S.; Ordovàs, L.; De Muynck, L.; Guo, W.; Espuny-Camacho, I.; Geraerts, M.; Khurana, S.; Vanuytsel, K.; Tóth, B.I.; Voets, T. Restoration of progranulin expression rescues cortical neuron generation in an induced pluripotent stem cell model of frontotemporal dementia. Stem Cell Rep. 2015, 4, 16–24. [Google Scholar] [CrossRef]
- Mirahmadi, M.; Rezanejadbardaji, H.; Irfan-Maqsood, M.; Mokhtari, M.J.; Naderi-Meshkin, H. Stem Cell Therapy for Neurodegenerative Diseases: Strategies for Regeneration against Degeneration. Cell Ther. Regen. Med. J. 2016, 1, 3. [Google Scholar] [CrossRef]
- Ding, D.-C.; Lin, C.-H.; Shyu, W.-C.; Lin, S.-Z. Neural stem cells and stroke. Cell Transplant. 2013, 22, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Carballo-Molina, O.A.; Sánchez-Navarro, A.; López-Ornelas, A.; Lara-Rodarte, R.; Salazar, P.; Campos-Romo, A.; Ramos-Mejía, V.; Velasco, I. Semaphorin 3C released from a biocompatible hydrogel guides and promotes axonal growth of rodent and human dopaminergic neurons. Tissue Eng. Part A 2016, 22, 850–861. [Google Scholar] [CrossRef]
- Garcia-Bennett, A.E.; König, N.; Abrahamsson, N.; Kozhevnikova, M.; Zhou, C.; Trolle, C.; Pankratova, S.; Berezin, V.; Kozlova, E.N. In vitro generation of motor neuron precursors from mouse embryonic stem cells using mesoporous nanoparticles. Nanomedicine 2014, 9, 2457–2466. [Google Scholar] [CrossRef]
- Kuo, Y.-C.; Liu, Y.-C.; Rajesh, R. Pancreatic differentiation of induced pluripotent stem cells in activin A-grafted gelatin-poly (lactide-co-glycolide) nanoparticle scaffolds with induction of LY294002 and retinoic acid. Mater. Sci. Eng. C 2017, 77, 384–393. [Google Scholar] [CrossRef]
- Kuo, Y.-C.; Rajesh, R. Nerve growth factor-loaded heparinized cationic solid lipid nanoparticles for regulating membrane charge of induced pluripotent stem cells during differentiation. Mater. Sci. Eng. C 2017, 77, 680–689. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Cai, L. Effects of treatment of cervical spinal cord injury without fracture and dislocation in a medium-to long-term follow-up study. World Neurosurg. 2018, 113, e515–e520. [Google Scholar] [CrossRef] [PubMed]
- Perale, G.; Rossi, F.; Santoro, M.; Peviani, M.; Papa, S.; Llupi, D.; Torriani, P.; Micotti, E.; Previdi, S.; Cervo, L. Multiple drug delivery hydrogel system for spinal cord injury repair strategies. J. Control. Release 2012, 159, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; George, J.K.; Sorger, V.J.; Zhang, L.G. 3D printing scaffold coupled with low level light therapy for neural tissue regeneration. Biofabrication 2017, 9, 025002. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z.; Xing, H.; Wang, Y.; Guo, Y. Exosomes derived from miR-188-3p-modified adipose-derived mesenchymal stem cells protect Parkinson’s disease. Mol. Ther. Nucleic Acids 2021. [Google Scholar] [CrossRef]
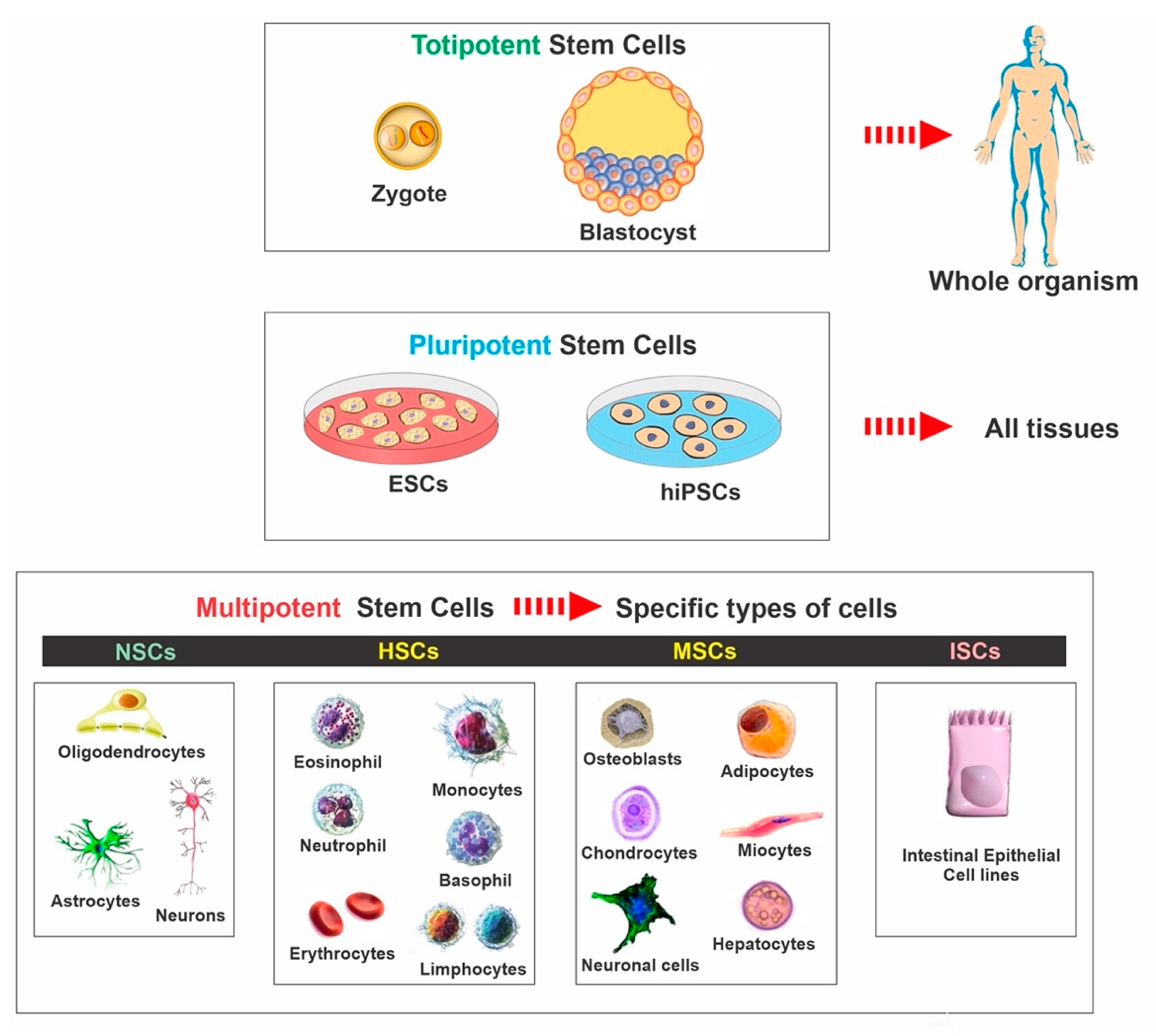
| Stem Cell Type | Origin | Advantages | Disadvantages |
|---|---|---|---|
| ESCs (pluripotent) | Embryo (blastocyst) |
|
|
| IPSCs (pluripotent) | Reprogrammed adult cells: fibroblasts, hepatocytes, circulating T cells, and keratinocytes |
|
|
| MSCs (multipotent) | Adult tissues (bone marrow, skin, blood, umbilical cord, etc.) |
|
|
| NSCs (Multipotent) | Embryo, human fetal brain and brain tissue of adults (SVZ and SGZ of hippocampus) |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivandzade, F.; Cucullo, L. Regenerative Stem Cell Therapy for Neurodegenerative Diseases: An Overview. Int. J. Mol. Sci. 2021, 22, 2153. https://doi.org/10.3390/ijms22042153
Sivandzade F, Cucullo L. Regenerative Stem Cell Therapy for Neurodegenerative Diseases: An Overview. International Journal of Molecular Sciences. 2021; 22(4):2153. https://doi.org/10.3390/ijms22042153
Chicago/Turabian StyleSivandzade, Farzane, and Luca Cucullo. 2021. "Regenerative Stem Cell Therapy for Neurodegenerative Diseases: An Overview" International Journal of Molecular Sciences 22, no. 4: 2153. https://doi.org/10.3390/ijms22042153
APA StyleSivandzade, F., & Cucullo, L. (2021). Regenerative Stem Cell Therapy for Neurodegenerative Diseases: An Overview. International Journal of Molecular Sciences, 22(4), 2153. https://doi.org/10.3390/ijms22042153







