Current Perspective Regarding the Immunopathogenesis of Drug-Induced Hypersensitivity Syndrome/Drug Reaction with Eosinophilia and Systemic Symptoms (DIHS/DRESS)
Abstract
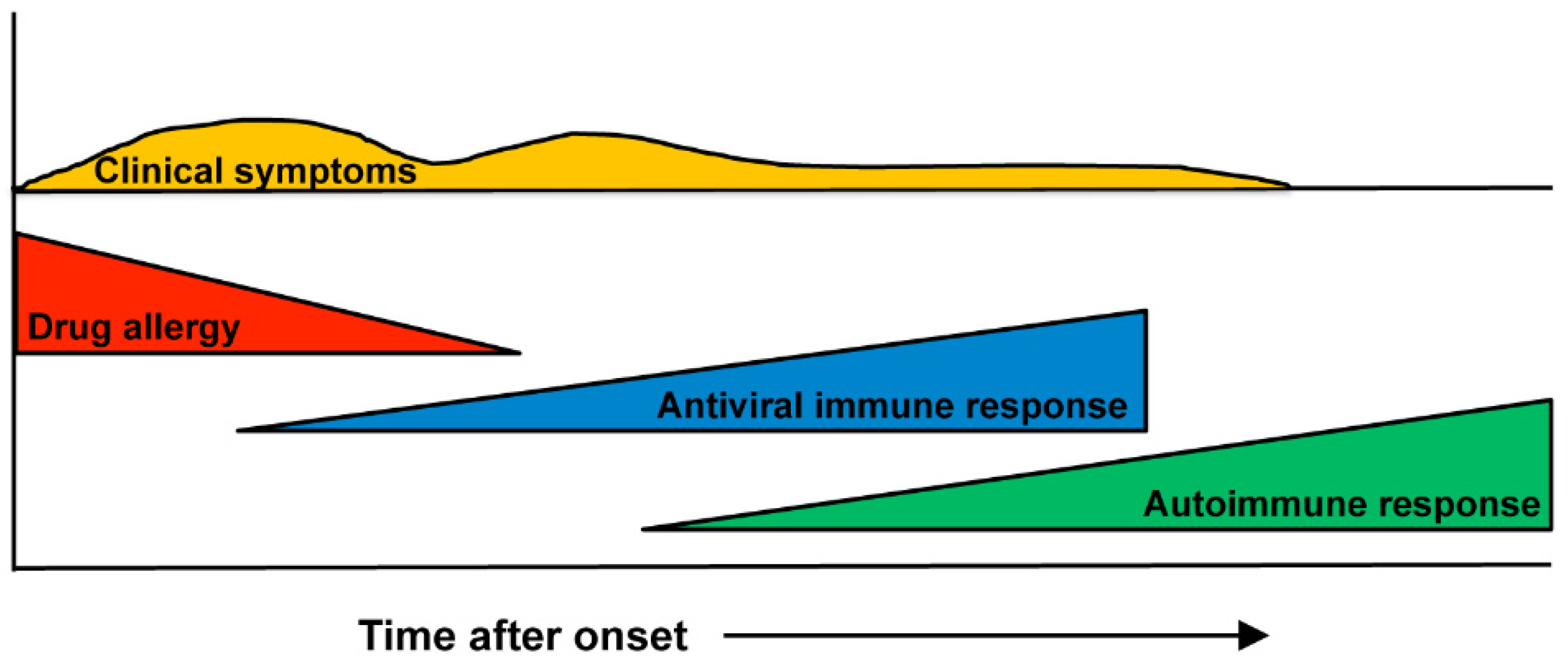
1. Introduction
2. Clinical Features
2.1. Clinical Symptoms
2.2. Complications
2.3. Sequelae (Autoimmune Disease)
3. Immunopathogenesis
3.1. Antigen Presentation
3.2. Human Leukocyte Antigens (HLAs)
3.3. Viruses
3.3.1. Roles of Herpesviruses in DIHS/DRESS
3.3.2. Immunological Mechanism of Virus Reactivation
3.4. Effector T cells
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shiohara, T.; Iijima, M.; Ikezawa, Z.; Hashimoto, K. The diagnosis of a DRESS syndrome has been sufficiently established on the basis of typical clinical features and viral reactivations. Br. J. Dermatol. 2007, 156, 1083–1084. [Google Scholar] [CrossRef]
- Kardaun, S.; Sidoroff, A.; Valeyrie-Allanore, L.; Halevy, S.; Davidovici, B.; Mockenhaupt, M.; Roujeau, J. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: Does a DRESS syndrome really exist? Br. J. Dermatol. 2007, 156, 609–611. [Google Scholar] [CrossRef]
- Bocquet, H.; Bagot, M.; Roujeau, J.C. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (drug rash with eosinophilia and systemic symptoms: DRESS). Semin. Cutan. Med. Surg. 1996, 15, 250–257. [Google Scholar] [CrossRef]
- Shiohara, T.; Mizukawa, Y. Drug-induced hypersensitivity syndrome (DiHS)/drug reaction with eosinophilia and systemic symptoms (DRESS): An update in 2019. Allergol. Int. 2019, 68, 301–308. [Google Scholar] [CrossRef]
- Chen, C.B.; Abe, R.; Pan, R.Y.; Wang, C.W.; Hung, S.I.; Tsai, Y.G.; Chung, W.H. An Updated Review of the Molecular Mechanisms in Drug Hypersensitivity. J. Immunol. Res. 2018, 2018, 6431694. [Google Scholar] [CrossRef]
- Ushigome, Y.; Kano, Y.; Hirahara, K.; Shiohara, T. Human herpesvirus 6 reactivation in drug-induced hypersensitivity syndrome and DRESS validation score. Am. J. Med. 2012, 125, e9–e10. [Google Scholar] [CrossRef] [PubMed]
- Shiohara, T.; Inaoka, M.; Kano, Y. Drug-induced Hypersensitivity Syndrome(DIHS): A Reaction Induced by a Complex Interplay among Herpesviruses and Antiviral and Antidrug Immune Responses. Allergol. Int. 2006, 55, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kardaun, S.H.; Sekula, P.; Valeyrie-Allanore, L.; Liss, Y.; Chu, C.Y.; Creamer, D.; Sidoroff, A.; Naldi, L.; Mockenhaupt, M.; Roujeau, J.C.; et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): An original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br. J. Dermatol. 2013, 169, 1071–1080. [Google Scholar] [CrossRef]
- Descamps, V.; Bouscarat, F.; Laglenne, S.; Aslangul, E.; Veber, B.; Saraux, J.-L.; Grange, M.; Grossin, M.; Navratil, E.; Crickx, B.; et al. Human herpesvirus 6 infection associated with anticonvulsant hypersensitivity syndrome and reactive haemophagocytic syndrome. Br. J. Dermatol. 1997, 137, 605–608. [Google Scholar] [CrossRef]
- Suzuki, Y.; Inagi, R.; Aono, T.; Yamanishi, K.; Shiohara, T. Human Herpesvirus 6 Infection as a Risk Factor for the Development of Severe Drug-Induced Hypersensitivity Syndrome. Arch. Dermatol. 1998, 134, 1108–1112. [Google Scholar] [CrossRef]
- Tohyama, M.; Yahata, Y.; Yasukawa, M.; Inagi, R.; Urano, Y.; Yamanishi, K.; Hashimoto, K. Severe hypersensitivity syndrome due to sulfasala-zine associated with reactivation of human herpesvirus 6. Arch. Dermatol. 1998, 134, 1113–1117. [Google Scholar] [CrossRef]
- Bourgeois, G.P.; Cafardi, J.A.; Groysman, V.; Hughey, L.C. A review of DRESS-associated myocarditis. J. Am. Acad. Dermatol. 2012, 66, e229–e236. [Google Scholar] [CrossRef] [PubMed]
- Asano, Y.; Kagawa, H.; Kano, Y.; Shiohara, T. Cytomegalovirus disease during severe drug eruptions: Report of 2 cases and ret-rospective study of 18 patients with drug-induced hypersensitivity syndrome. Arch. Dermatol. 2009, 145, 1030–1036. [Google Scholar] [CrossRef]
- Mizukawa, Y.; Hirahara, K.; Kano, Y.; Shiohara, T. Drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms severity score: A useful tool for assessing disease severity and predicting fatal cytomegalovirus disease. J. Am. Acad. Dermatol. 2019, 80, 670–678.e2. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chiang, H.H.; Cho, Y.T.; Chang, C.Y.; Chen, K.L.; Yang, C.W.; Lee, Y.H.; Chu, C.Y. Human herpes virus reactivations and dynamic cytokine profiles in patients with cutaneous adverse drug reactions—A prospective comparative study. Allergy 2015, 70, 568–575. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chang, C.Y.; Cho, Y.T.; Chiu, H.C.; Chu, C.Y. Long-term sequelae of drug reaction with eosinophilia and systemic symptoms: A retrospective cohort study from Taiwan. J. Am. Acad. Dermatol. 2013, 68, 459–465. [Google Scholar] [CrossRef]
- Kano, Y.; Tohyama, M.; Aihara, M.; Matsukura, S.; Watanabe, H.; Sueki, H.; Iijima, M.; Morita, E.; Niihara, H.; Asada, H.; et al. Sequelae in 145 patients with drug-induced hyper-sensitivity syndrome/drug reaction with eosinophilia and systemic symptoms: Survey conducted by the Asian Research Committee on Severe Cutaneous Adverse Reactions (ASCAR). J. Dermatol. 2015, 42, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Kano, Y.; Sakuma, K.; Shiohara, T. Sclerodermoid graft-versus-host disease-like lesions occurring after drug-induced hypersensi-tivity syndrome. Br. J. Dermatol. 2007, 156, 1061–1063. [Google Scholar] [CrossRef]
- Iinuma, S.; Kanno, K.; Honma, M.; Kinouchi, M.; Ishida-Yamamoto, A. Drug-induced hypersensitivity syndrome followed by chronic inflammatory demyelinating polyneuropathy. J. Dermatol. 2018, 45, e310–e311. [Google Scholar] [CrossRef] [PubMed]
- Morita, C.; Yanase, T.; Shiohara, T.; Aoyama, Y. Aggressive treatment in paediatric or young patients with drug-induced hyper-sensitivity syndrome (DiHS)/drug reaction with eosinophilia and systemic symptoms (DRESS) is associated with future de-velopment of type III polyglandular autoimmune syndrome. BMJ Case Rep. 2018, 2018, bcr2018225528. [Google Scholar] [CrossRef]
- Yang, C.W.; Cho, Y.T.; Hsieh, Y.C.; Hsu, S.H.; Chen, K.L.; Chu, C.Y. The interferon-gamma-induced protein 10/CXCR3 axis is associated with human herpesvirus-6 reactivation and the development of sequelae in drug reaction with eosinophilia and systemic symptoms. Br. J. Dermatol. 2020, 183, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Pichler, W.J.; Tilch, J. The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy 2004, 59, 809–820. [Google Scholar] [CrossRef]
- Pavlos, R.; Mallal, S.; Ostrov, D.; Buus, S.; Metushi, I.; Peters, B.; Phillips, E. T Cell–Mediated Hypersensitivity Reactions to Drugs. Annu. Rev. Med. 2015, 66, 439–454. [Google Scholar] [CrossRef]
- Padovan, E.; Bauer, T.; Tongio, M.M.; Kalbacher, H.; Weltzien, H.U. Penicilloyl peptides are recognized as T cell antigenic determi-nants in penicillin allergy. Eur. J. Immunol. 1997, 27, 1303–1307. [Google Scholar] [CrossRef]
- Pichler, W.J. Pharmacological interaction of drugs with antigen-specific immune receptors: The p-i concept. Curr. Opin. Allergy Clin. Immunol. 2002, 2, 301–305. [Google Scholar] [CrossRef]
- Schnyder, B.; Mauri-Hellweg, D.; Zanni, M.; Bettens, F.; Pichler, W.J. Direct, MHC-dependent presentation of the drug sulfameth-oxazole to human alphabeta T cell clones. J. Clin. Investig. 1997, 100, 136–141. [Google Scholar] [CrossRef]
- Wei, C.-Y.; Chung, W.-H.; Huang, H.-W.; Chen, Y.-T.; Hung, S.-I. Direct interaction between HLA-B and carbamazepine activates T cells in patients with Stevens-Johnson syndrome. J. Allergy Clin. Immunol. 2012, 129, 1562–1569.e5. [Google Scholar] [CrossRef]
- Yun, J.; Marcaida, M.J.; Eriksson, K.K.; Jamin, H.; Fontana, S.; Pichler, W.J.; Yerly, D. Oxypurinol Directly and Immediately Activates the Drug-Specific T Cells via the Preferential Use of HLA-B*58:01. J. Immunol. 2014, 192, 2984–2993. [Google Scholar] [CrossRef] [PubMed]
- Illing, P.T.; Vivian, J.P.; Dudek, N.L.; Kostenko, L.; Chen, Z.; Bharadwaj, M.; Miles, J.J.; Kjer-Nielsen, L.; Gras, S.; Williamson, N.A.; et al. Immune self-reactivity triggered by drug-modified HLA-peptide repertoire. Nature 2012, 486, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Ostrov, D.A.; Grant, B.J.; Pompeu, Y.A.; Sidney, J.; Harndahl, M.; Southwood, S.; Oseroff, C.; Lu, S.; Jakoncic, J.; De Oliveira, C.A.F.; et al. Drug hypersensitivity caused by alteration of the MHC-presented self-peptide repertoire. Proc. Natl. Acad. Sci. USA 2012, 109, 9959–9964. [Google Scholar] [CrossRef]
- Mallal, S.; Nolan, D.; Witt, C.; Masel, G.; Martin, A.M.; Moore, C.; Sayer, D.; Castley, A.; Mamotte, C.; Maxwell, D.; et al. Association between presence of HLA-B*5701, HLA-DR7, and HLA-DQ3 and hypersensitivity to HIV-1 reverse-transcriptase inhibitor abacavir. Lancet 2002, 359, 727–732. [Google Scholar] [CrossRef]
- Hetherington, S.; Hughes, A.R.; Mosteller, M.; Shortino, D.; Baker, K.L.; Spreen, W.; Lai, E.; Davies, K.; Handley, A.; Dow, D.J.; et al. Genetic variations in HLA-B region and hy-persensitivity reactions to abacavir. Lancet 2002, 359, 1121–1122. [Google Scholar] [CrossRef]
- Chung, W.H.; Hung, S.I.; Hong, H.S.; Hsih, M.S.; Yang, L.C.; Ho, H.C.; Wu, J.Y.; Chen, Y.T. Medical genetics: A marker for Stevens-Johnson syndrome. Nature 2004, 428, 486. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.I.; Chung, W.H.; Liou, L.B.; Chu, C.C.; Lin, M.; Huang, H.P.; Lin, Y.L.; Lan, J.L.; Yang, L.C.; Hong, H.S.; et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc. Natl. Acad. Sci. USA 2005, 102, 4134–4139. [Google Scholar] [CrossRef]
- Miyashita, K.; Miyagawa, F.; Nakamura, Y.; Ommori, R.; Azukizawa, H.; Asada, H. Up-regulation of Human Herpesvirus 6B-derived microRNAs in the Serum of Patients with Drug-induced Hypersensitivity Syndrome/Drug Reaction with Eosinophilia and Systemic Symptoms. Acta Derm. Venereol. 2018, 98, 612–613. [Google Scholar] [CrossRef]
- Tohyama, M.; Hashimoto, K.; Yasukawa, M.; Kimura, H.; Horikawa, T.; Nakajima, K.; Urano, Y.; Matsumoto, K.; Iijima, M.; Shear, N.H. Association of human herpesvirus 6 re-activation with the flaring and severity of drug-induced hypersensitivity syndrome. Br. J. Dermatol. 2007, 157, 934–940. [Google Scholar] [CrossRef]
- Saraya, T.; Mikoshiba, M.; Kamiyama, H.; Yoshizumi, M.; Tsuchida, S.; Tsukagoshi, H.; Ishioka, T.; Terada, M.; Tanabe, E.; Tomioka, C.; et al. Evidence for reactivation of human her-pesvirus 6 in generalized lymphadenopathy in a patient with drug-induced hypersensitivity syndrome. J. Clin. Microbiol. 2013, 51, 1979–1982. [Google Scholar] [CrossRef][Green Version]
- Miyashita, K.; Shobatake, C.; Miyagawa, F.; Kobayashi, N.; Onmori, R.; Yonekawa, S.; Tanabe, K.; Kawate, K.; Morita, K.; Asada, H. Involvement of Human Herpesvirus 6 Infection in Renal Dysfunction Associated with DIHS/DRESS. Acta Derm. Venereol. 2016, 96, 114–115. [Google Scholar] [CrossRef]
- Hagiya, H.; Iwamuro, M.; Tanaka, T.; Hasegawa, K.; Hanayama, Y.; Kimura, M.; Otsuka, F. Reactivation of Human Herpes Virus-6 in the Renal Tissue of a Patient with Drug-induced Hypersensitivity Syndrome/Drug Rash with Eosinophilia and Systemic Symp-toms (DIHS/DRESS). Int. Med. 2016, 55, 1769–1774. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roujeau, J.C.; Dupin, N. Virus Reactivation in Drug Reaction with Eosinophilia and Systemic Symptoms (Dress) Results from a Strong Drug-Specific Immune Response. J. Allergy Clin. Immunol. Pr. 2017, 5, 811–812. [Google Scholar] [CrossRef]
- Descamps, V.; Mahe, E.; Houhou, N.; Abramowitz, L.; Rozenberg, F.; Ranger-Rogez, S.; Crickx, B. Drug-induced hypersensitivity syn-drome associated with Epstein-Barr virus infection. Br. J. Dermatol. 2003, 148, 1032–1034. [Google Scholar] [CrossRef] [PubMed]
- Aihara, M.; Sugita, Y.; Takahashi, S.; Nagatani, T.; Arata, S.; Takeuchi, K.; Ikezawa, Z. Anticonvulsant hypersensitivity syndrome associated with reactivation of cytomegalovirus. Br. J. Dermatol. 2001, 144, 1231–1234. [Google Scholar] [CrossRef]
- Kano, Y.; Hiraharas, K.; Sakuma, K.; Shiohara, T. Several herpesviruses can reactivate in a severe drug-induced multiorgan reac-tion in the same sequential order as in graft-versus-host disease. Br. J. Dermatol. 2006, 155, 301–306. [Google Scholar] [CrossRef]
- Ljungman, P.; Boeckh, M.; Hirsch, H.H.; Josephson, F.; Lundgren, J.; Nichols, G.; Pikis, A.; Razonable, R.R.; Miller, V.; Griffiths, P.D. Definitions of Cytomegalovirus Infection and Disease in Transplant Patients for Use in Clinical Trials: Table 1. Clin. Infect. Dis. 2017, 64, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Lusso, P.; Ensoli, B.; Markham, P.D.; Ablashi, D.V.; Salahuddin, S.Z.; Tschachler, E.; Wong-Staal, F.; Gallo, R.C. Productive dual infection of human CD4+ T lymphocytes by HIV-1 and HHV-6. Nature 1989, 337, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Sonoda, S.; Higashi, K.; Kondo, T.; Takahashi, H.; Takahashi, M.; Yamanishi, K. Predominant CD4 T-lymphocyte tropism of human herpesvirus 6-related virus. J. Virol. 1989, 63, 3161–3163. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Kondo, T.; Okuno, T.; Takahashi, M.; Yamanishi, K. Latent human herpesvirus 6 infection of human mono-cytes/macrophages. J. Gen. Virol. 1991, 72 Pt 6, 1401–1408. [Google Scholar] [CrossRef]
- Miyagawa, F.; Nakamura, Y.; Miyashita, K.; Iioka, H.; Himuro, Y.; Ogawa, K.; Nishimura, C.; Nishikawa, M.; Mitsui, Y.; Ito, Y.; et al. Preferential expression of CD134, an HHV-6 cel-lular receptor, on CD4T cells in drug-induced hypersensitivity syndrome (DIHS)/drug reaction with eosinophilia and systemic symptoms (DRESS). J. Dermatol. Sci. 2016, 83, 151–154. [Google Scholar] [CrossRef]
- Croft, M.; So, T.; Duan, W.; Soroosh, P. The significance of OX40 and OX40L to T-cell biology and immune disease. Immunol. Rev. 2009, 229, 173–191. [Google Scholar] [CrossRef]
- Tang, H.; Serada, S.; Kawabata, A.; Ota, M.; Hayashi, E.; Naka, T.; Yamanishi, K.; Mori, Y. CD134 is a cellular receptor specific for human herpesvirus-6B entry. Proc. Natl. Acad. Sci. USA 2013, 110, 9096–9099. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, H.; Aoshima, M.; Ito, T.; Seo, N.; Takigawa, M.; Yagi, H. Emergence of circulating monomyeloid precursors predicts reactivation of human herpesvirus-6 in drug-induced hypersensitivity syndrome. Br. J. Dermatol. 2009, 161, 486–488. [Google Scholar] [CrossRef]
- Hashizume, H.; Fujiyama, T.; Kanebayashi, J.; Kito, Y.; Hata, M.; Yagi, H. Skin recruitment of monomyeloid precursors involves human herpesvirus-6 reactivation in drug allergy. Allergy 2013, 68, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.; Kano, Y.; Yamazaki, Y.; Kimishima, M.; Mizukawa, Y.; Shiohara, T. Defective Regulatory T Cells In Patients with Severe Drug Eruptions: Timing of the Dysfunction Is Associated with the Pathological Phenotype and Outcome. J. Immunol. 2009, 182, 8071–8079. [Google Scholar] [CrossRef] [PubMed]
- Ushigome, Y.; Mizukawa, Y.; Kimishima, M.; Yamazaki, Y.; Takahashi, R.; Kano, Y.; Shiohara, T. Monocytes are involved in the balance be-tween regulatory T cells and Th17 cells in severe drug eruptions. Clin. Exp. Allergy 2018, 48, 1453–1463. [Google Scholar] [CrossRef]
- Kano, Y.; Inaoka, M.; Shiohara, T. Association between anticonvulsant hypersensitivity syndrome and human herpesvirus 6 re-activation and hypogammaglobulinemia. Arch Dermatol. 2004, 140, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, F.; Nakamura, Y.; Ommori, R.; Miyashita, K.; Iioka, H.; Miyashita, N.; Nishikawa, M.; Himuro, Y.; Ogawa, K.; Asada, H. Predominant Contribution of CD4 T Cells to Human Herpesvirus 6 (HHV-6) Load in the Peripheral Blood of Patients with Drug-induced Hypersensitivity Syndrome and Persistent HHV-6 Infection. Acta Derm. Venereol. 2018, 98, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Kanatani, Y.; Miyagawa, F.; Ogawa, K.; Arima, A.; Asada, H. Parallel changes in serum thymus and activation-regulated chemokine levels in response to flare-ups in drug-induced hypersensitivity syndrome. J. Dermatol. 2020, 47. [Google Scholar] [CrossRef]
- Chessman, D.; Kostenko, L.; Lethborg, T.; Purcell, A.W.; Williamson, N.A.; Chen, Z.; Kjer-Nielsen, L.; Mifsud, N.A.; Tait, B.D.; Holdsworth, R.; et al. Human Leukocyte Antigen Class I-Restricted Activation of CD8+ T Cells Provides the Immunogenetic Basis of a Systemic Drug Hypersensitivity. Immunity 2008, 28, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Lerch, M.; Pichler, W.J. The immunological and clinical spectrum of delayed drug-induced exanthems. Curr. Opin. Allergy Clin. Immunol. 2004, 4, 411–419. [Google Scholar] [CrossRef]
- Mauri-Hellweg, D.; Zanni, M.; Frei, E.; Bettens, F.; Brander, C.; Mauri, D.; Padovan, E.; Weltzien, H.-U.; Pichler, W.J. Cross-reactivity of T cell lines and clones to be-ta-lactam antibiotics. J. Immunol. 1996, 157, 1071–1079. [Google Scholar] [PubMed]
- Ko, T.M.; Chung, W.H.; Wei, C.Y.; Shih, H.Y.; Chen, J.K.; Lin, C.H.; Chen, Y.T.; Hung, S.I. Shared and restricted T-cell receptor use is crucial for carbam-azepine-induced Stevens-Johnson syndrome. J. Allergy Clin. Immunol. 2011, 128, 1266–1276.e11. [Google Scholar] [CrossRef]
- Hashizume, H.; Takigawa, M. Drug-induced hypersensitivity syndrome associated with cytomegalovirus reactivation: Immu-nological characterization of pathogenic T cells. Acta Derm. Venereol. 2005, 85, 47–50. [Google Scholar] [CrossRef]
- Naisbitt, D.J.; Farrell, J.; Wong, G.; Depta, J.P.; Dodd, C.C.; Hopkins, J.E.; Gibney, C.A.; Chadwick, D.W.; Pichler, W.J.; Pirmohamed, M.; et al. Characterization of drug-specific T cells in lamotrigine hypersensitivity. J. Allergy Clin. Immunol. 2003, 111, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Naisbitt, D.J.; Britschgi, M.; Wong, G.; Farrell, J.; Depta, J.P.; Chadwick, D.W.; Pichler, W.J.; Pirmohamed, M.; Park, B.K. Hypersensitivity reactions to carbamazepine: Characterization of the specificity, phenotype, and cytokine profile of drug-specific T cell clones. Mol. Pharmacol. 2003, 63, 732–741. [Google Scholar] [CrossRef]
- Hashizume, H.; Fujiyama, T.; Tokura, Y. Reciprocal contribution of Th17 and regulatory T cells in severe drug allergy. J. Dermatol. Sci. 2016, 81, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.M.; Hur, G.Y.; Kim, S.H.; Ban, G.Y.; Jee, Y.K.; Naisbitt, D.J.; Park, H.S.; Kim, S.H. Drug-specific CD4(+) T-cell immune responses are responsible for antituberculosis drug-induced maculopapular exanthema and drug reaction with eosinophilia and systemic symptoms syndrome. Br. J. Dermatol. 2017, 176, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Picard, D.; Janela, B.; Descamps, V.; D’Incan, M.; Courville, P.; Jacquot, S.; Rogez, S.; Mardivirin, L.; Moins-Teisserenc, H.; Toubert, A.; et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): A multiorgan antiviral T cell response. Sci. Transl. Med. 2010, 2, 46ra62. [Google Scholar] [CrossRef]
- Choquet-Kastylevsky, G.; Intrator, L.; Chenal, C.; Bocquet, H.; Revuz, J.; Roujeau, J.C. Increased levels of interleukin 5 are associated with the generation of eosinophilia in drug-induced hypersensitivity syndrome. Br. J. Dermatol. 1998, 139, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Morito, H.; Hasegawa, A.; Daikoku, N.; Miyagawa, F.; Okazaki, A.; Fukumoto, T.; Kobayashi, N.; Kasai, T.; Watanabe, H.; et al. Identification of thymus and activation-regulated chemokine (TARC/CCL17) as a potential marker for early indication of disease and prediction of disease activity in drug-induced hypersensitivity syndrome (DIHS)/drug rash with eosinophilia and systemic symptoms (DRESS). J. Dermatol. Sci. 2013, 69, 38–43. [Google Scholar]
- Ogawa, K.; Morito, H.; Hasegawa, A.; Miyagawa, F.; Kobayashi, N.; Watanabe, H.; Sueki, H.; Tohyama, M.; Hashimoto, K.; Kano, Y.; et al. Elevated serum thymus and activa-tion-regulated chemokine (TARC/CCL17) relates to reactivation of human herpesvirus 6 in drug reaction with eosinophilia and systemic symptoms (DRESS)/drug-induced hypersensitivity syndrome (DIHS). Br. J. Dermatol. 2014, 171, 425–457. [Google Scholar] [CrossRef]
- Miyagawa, F.; Hasegawa, A.; Imoto, K.; Ogawa, K.; Kobayashi, N.; Ito, K.; Fujita, H.; Aihara, M.; Watanabe, H.; Sueki, H.; et al. Differential expression profile of Th1/Th2-associated chemokines characterizes Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) and drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms (DIHS/DRESS) as distinct entities. Eur. J. Dermatol. 2015, 25, 87–89. [Google Scholar] [PubMed]
- Nakamura-Nishimura, Y.; Miyagawa, F.; Miyashita, K.; Ommori, R.; Azukizawa, H.; Asada, H. Serum thymus and activation-regulated chemokine is associated with the severity of drug reaction with eosinophilia and systemic symp-toms/drug-induced hypersensitivity syndrome. Br. J. Dermatol. 2018, 178, 1430–1432. [Google Scholar] [CrossRef] [PubMed]
- Teraki, Y.; Fukuda, T. Skin-Homing IL-13-Producing T Cells Expand in the Circulation of Patients with Drug Rash with Eosino-philia and Systemic Symptoms. Dermatology 2017, 233, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, F.; Nakamura-Nishimura, Y.; Kanatani, Y.; Asada, H. Correlation between Expression of CD134, a Human Herpesvirus 6 Cellular Receptor, on CD4+ T cells and Th2-type Immune Responses in Drug-induced Hypersensitivity Syn-drome/Drug Reaction with Eosinophilia and Systemic Symptoms. Acta Derm. Venereol. 2020, 100, adv00102. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, Y.; Yang, L.P.; Uchiyama, T.; Tanaka, Y.; Baum, P.; Sergerie, M.; Hermann, P.; Delespesse, G. OX40 costimulation enhances interleukin-4 (IL-4) expression at priming and promotes the differentiation of naive human CD4(+) T cells into high IL-4-producing effectors. Blood 1998, 92, 338–345. [Google Scholar] [CrossRef]

| 1 | Maculopapular rash developing > 3 weeks after starting with a limited number of drugs |
| 2 | Prolonged clinical symptoms 2 weeks after discontinuation of the causative drug |
| 3 | Fever (>38 °C) |
| 4 | Liver abnormalities (alanine aminotransferase > 100 U·L−1) a |
| 5 | Leukocyte abnormalities (at least one present) |
| a | Leukocytosis (>11 × 109 L−1) |
| b | Atypical lymphocytosis (>5%) |
| c | Eosinophilia (>1.5 × 109 L−1) |
| 6 | Lymphadenopathy |
| 7 | Human herpesvirus 6 reactivation |
| Hospitalization |
| Reaction suspected to be drug related |
| Acute skin rash a |
| Fever above 38 °C a |
| Enlarged lymph nodes at at least two sites a |
| Involvement of at least one internal organ a |
| Blood count abnormalities |
| Lymphocytes above or below the laboratory limits a |
| Eosinophils above the laboratory limits (in percentage or absolute count) a |
| Platelets below the laboratory limits a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyagawa, F.; Asada, H. Current Perspective Regarding the Immunopathogenesis of Drug-Induced Hypersensitivity Syndrome/Drug Reaction with Eosinophilia and Systemic Symptoms (DIHS/DRESS). Int. J. Mol. Sci. 2021, 22, 2147. https://doi.org/10.3390/ijms22042147
Miyagawa F, Asada H. Current Perspective Regarding the Immunopathogenesis of Drug-Induced Hypersensitivity Syndrome/Drug Reaction with Eosinophilia and Systemic Symptoms (DIHS/DRESS). International Journal of Molecular Sciences. 2021; 22(4):2147. https://doi.org/10.3390/ijms22042147
Chicago/Turabian StyleMiyagawa, Fumi, and Hideo Asada. 2021. "Current Perspective Regarding the Immunopathogenesis of Drug-Induced Hypersensitivity Syndrome/Drug Reaction with Eosinophilia and Systemic Symptoms (DIHS/DRESS)" International Journal of Molecular Sciences 22, no. 4: 2147. https://doi.org/10.3390/ijms22042147
APA StyleMiyagawa, F., & Asada, H. (2021). Current Perspective Regarding the Immunopathogenesis of Drug-Induced Hypersensitivity Syndrome/Drug Reaction with Eosinophilia and Systemic Symptoms (DIHS/DRESS). International Journal of Molecular Sciences, 22(4), 2147. https://doi.org/10.3390/ijms22042147






