The Role of Tenascin C in Cardiac Reverse Remodeling Following Banding–Debanding of the Ascending Aorta
Abstract
1. Introduction
2. Results
2.1. Experimental Groups
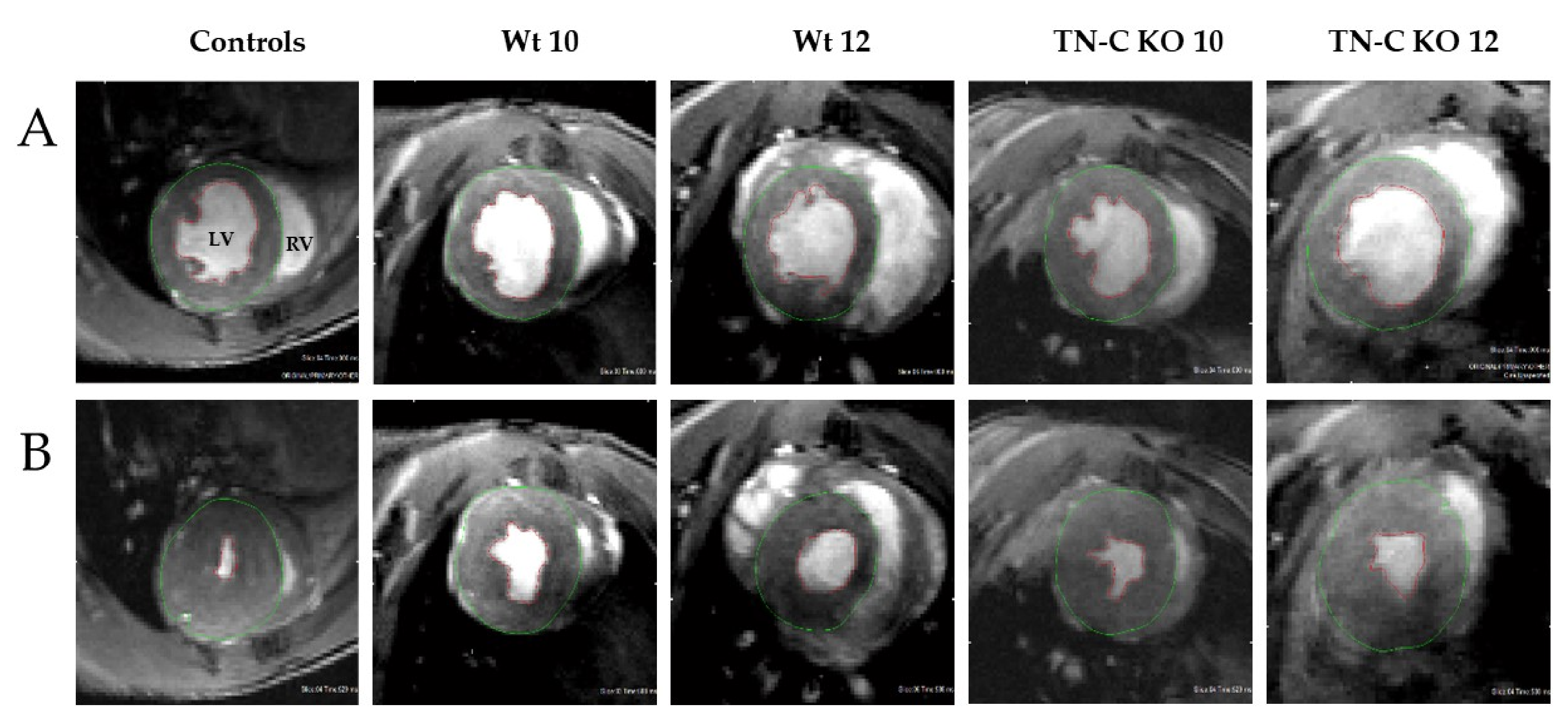
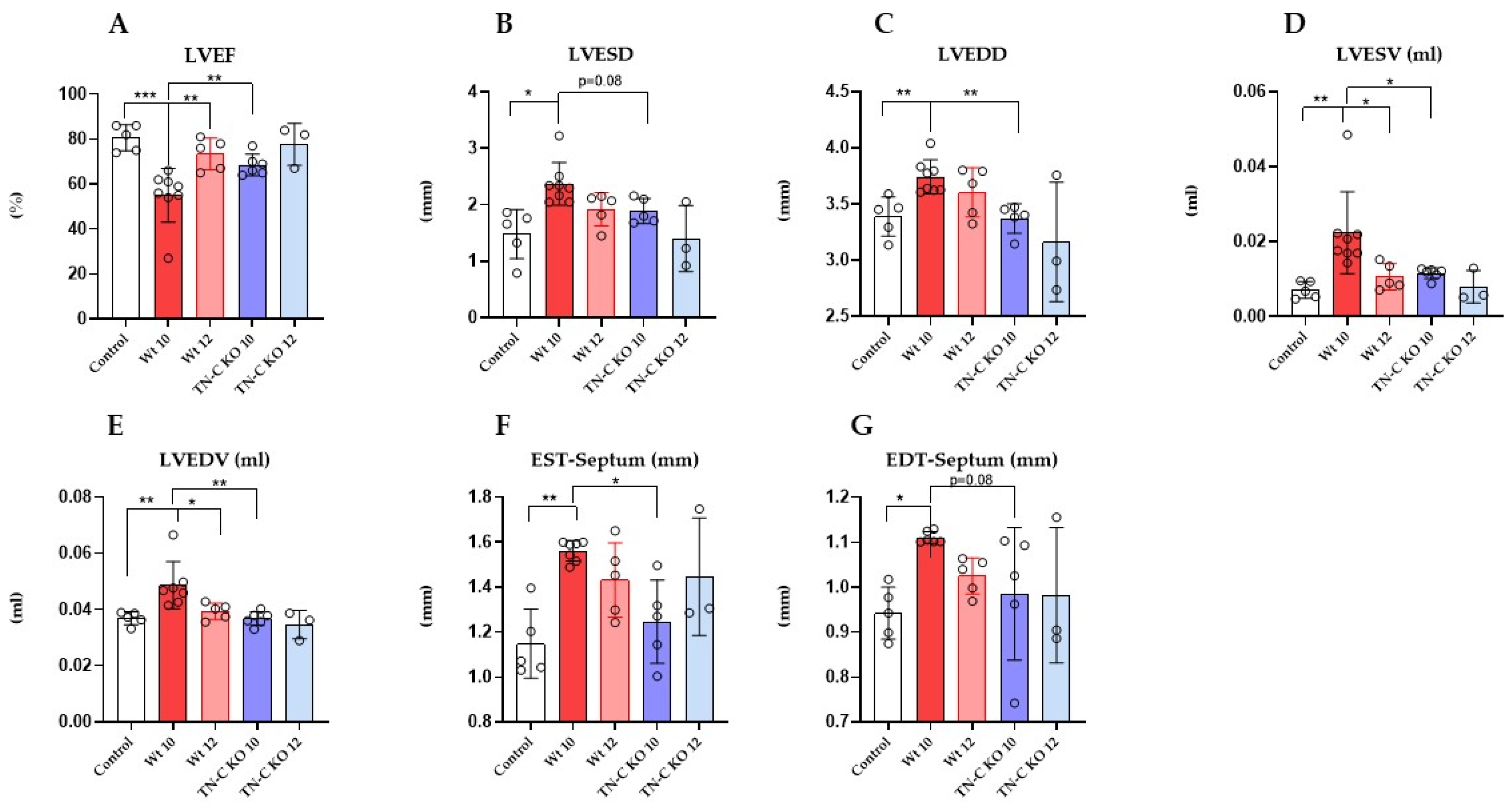
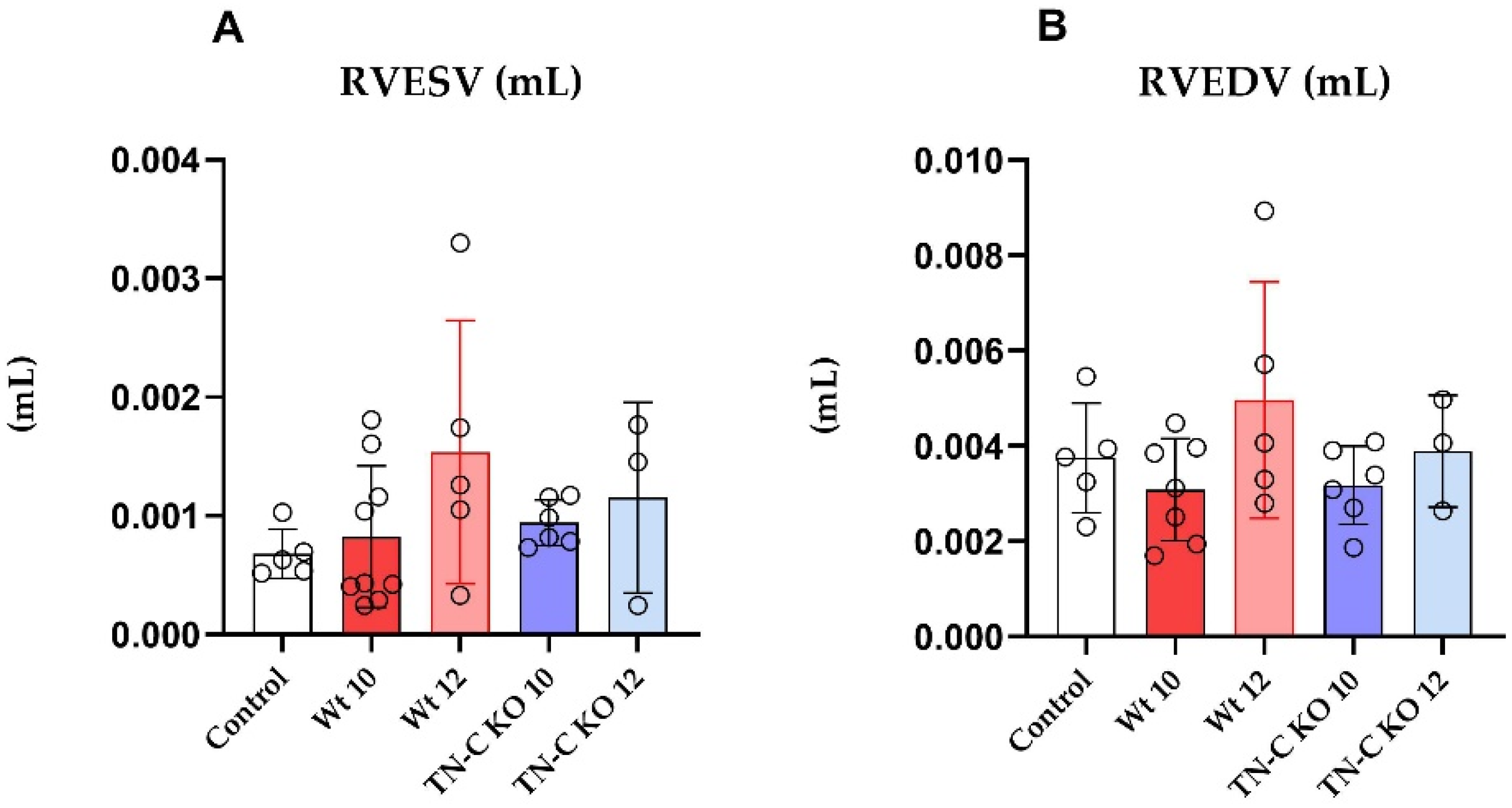
2.2. Functional and Morphological Changes of Left and Right Ventricular Function Following Aortic Constriction and Debanding in Mice
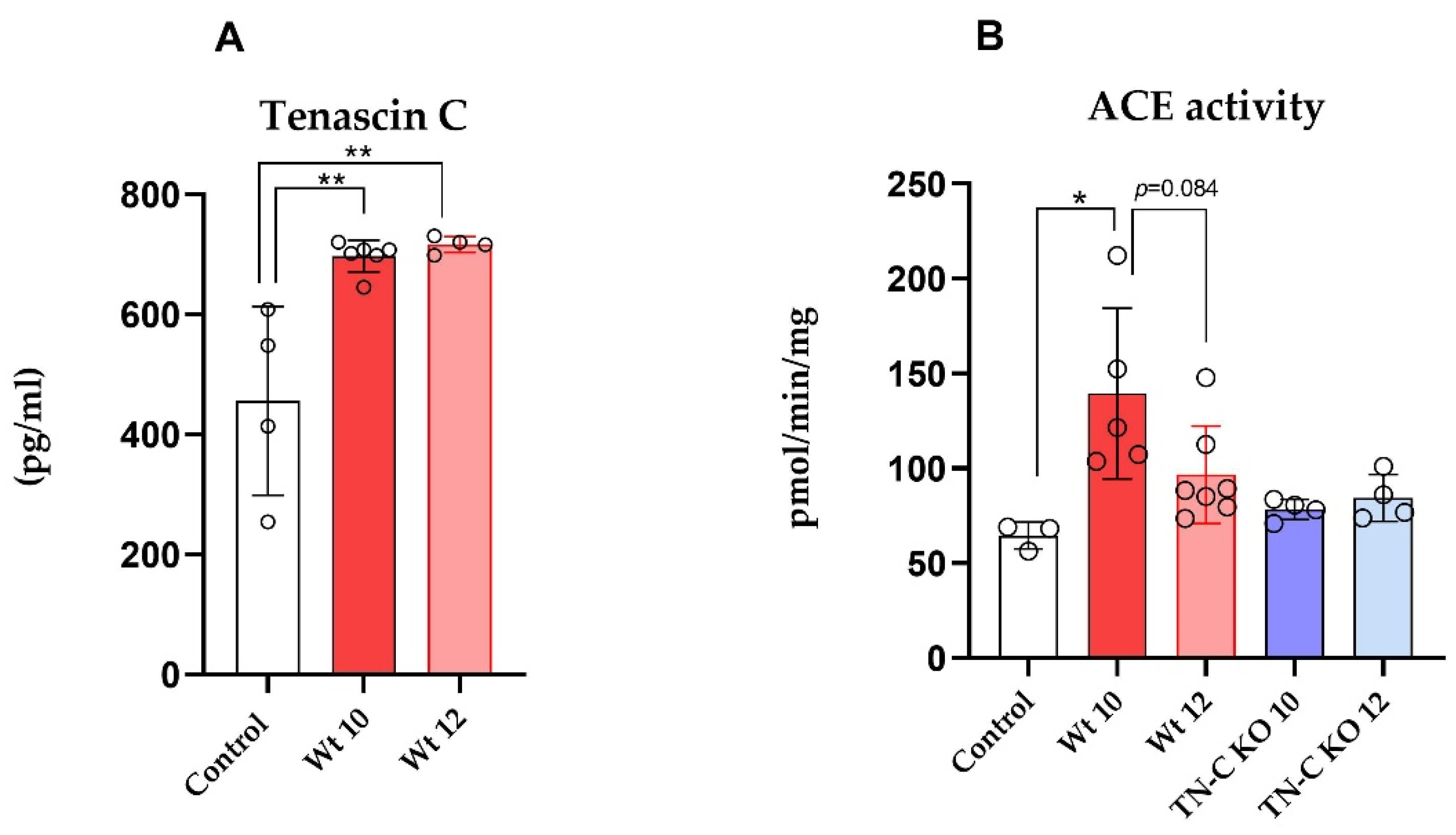
2.3. TN-C Levels and ACE Activity in Cardiac Tissue Samples
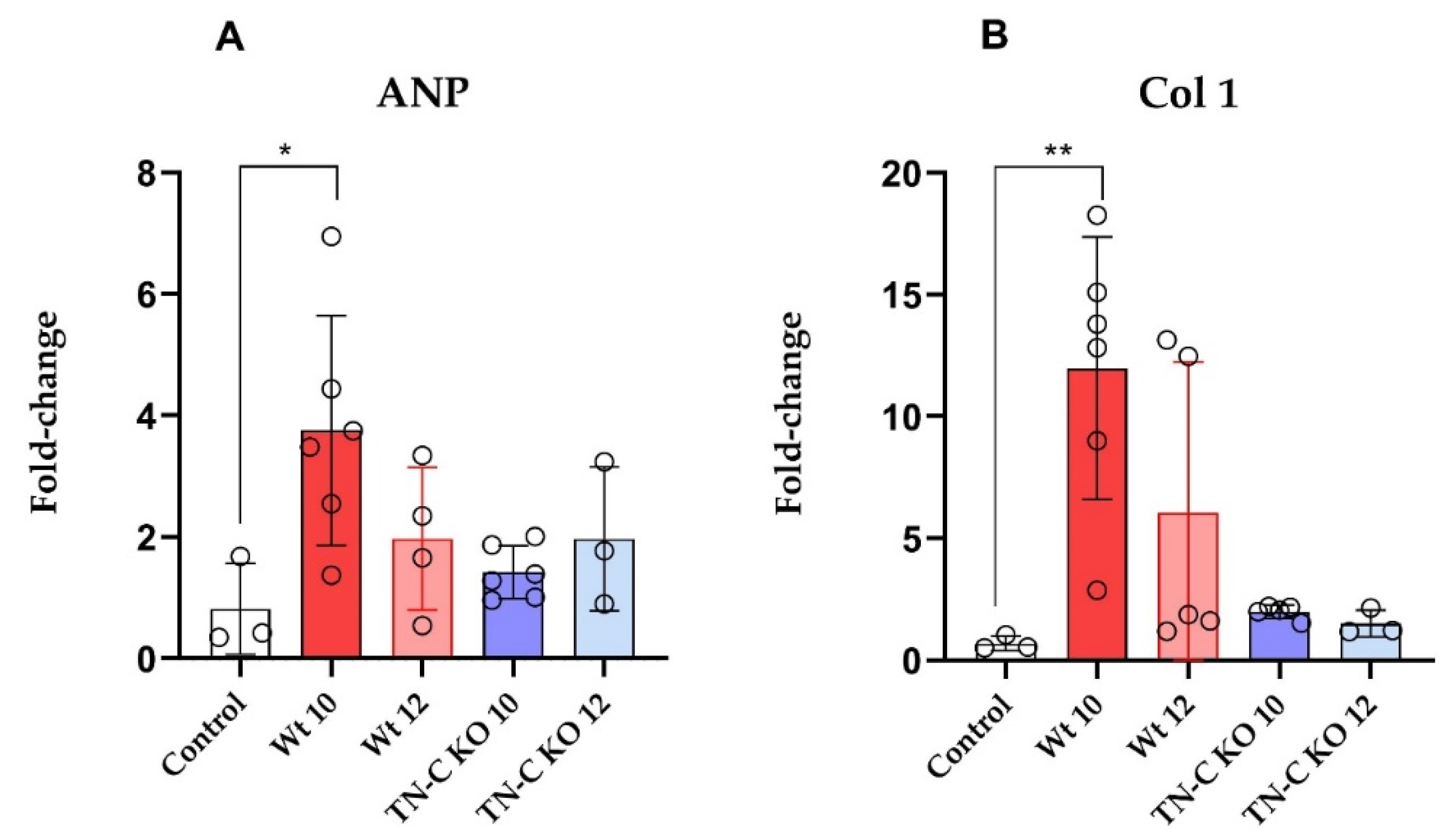
2.4. mRNA Expression of ANP and Collagen 1 (Col 1) in Left Ventricular Tissue Samples
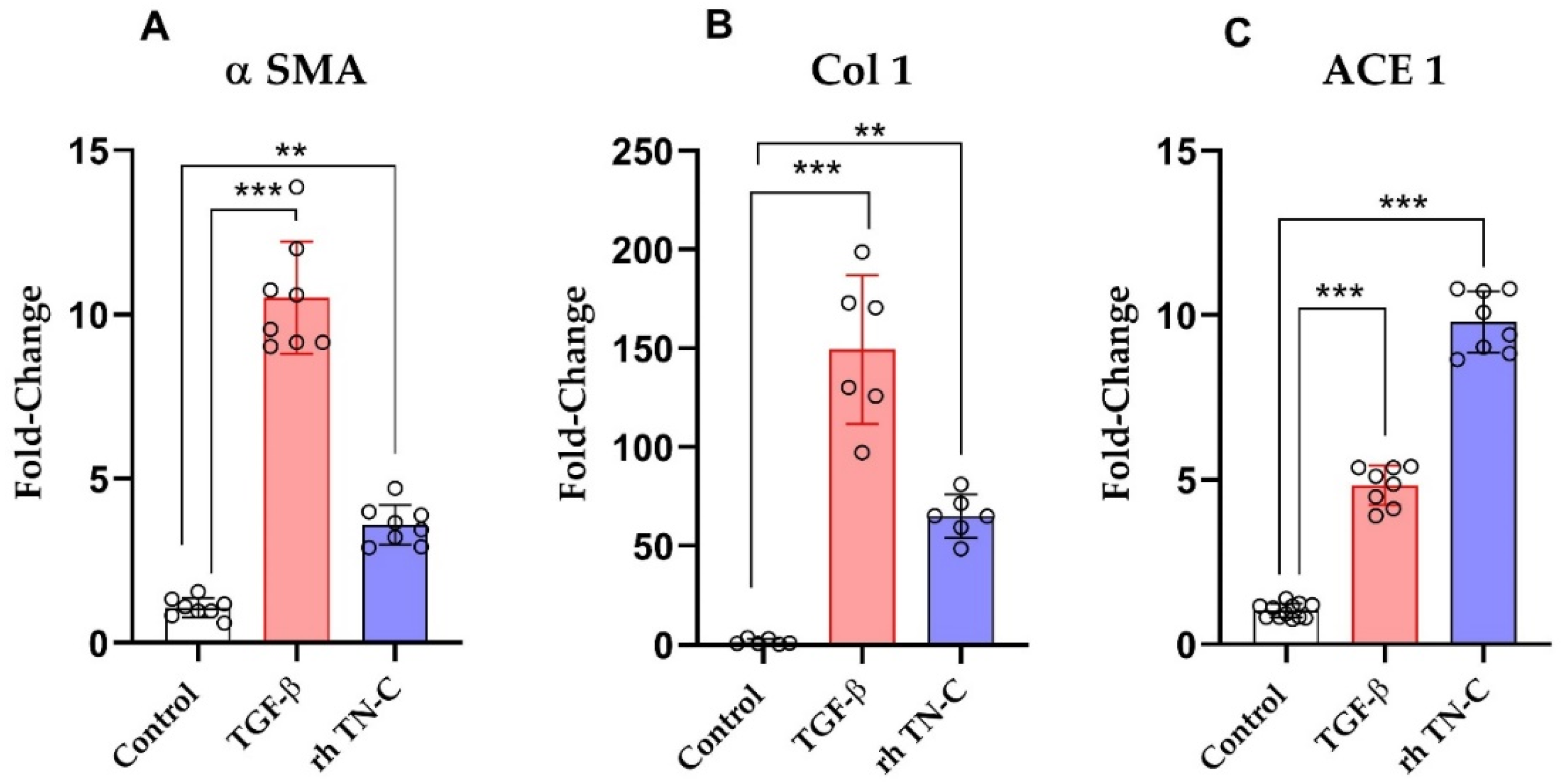
2.5. The Effect of TN-C on ACE Expression in Human Ventricular Cardiac Fibroblasts
3. Materials and Methods
3.1. Animals
3.2. Experimental Modeling of Cardiac Pressure Overload and Unloading
3.3. Cardiac Magnetic Resonance Imaging
3.4. Reverse Transcription and Quantitative Polymerase Chain Reaction (PCR)
3.5. Assessment of TN-C Levles in Cardiac Tissue Samples
3.6. Angiotensin-Converting Enzyme Activity Measurement
3.7. Human Ventricular Cardiac Fibroblast Experiments
3.8. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| α-SMA | Alpha smooth muscle actin |
| ACE | Angiotensin-converting enzyme |
| Ang II | Angiotensin II |
| BDNF | Brain-Derived Neurotrophic Factor |
| BNP | Brain natriuretic peptide |
| BP | Blood pressure |
| BW | Body weight |
| Col 1 | Collagen I |
| ECM | Extracellular matrix |
| EDV | End diastolic volume |
| EF | Ejection fraction |
| ESV | End systolic volume |
| FOV | Field of view |
| HCF | Human cardiac fibroblast |
| HW | Heart weight |
| LV | Left ventricular |
| LVEDD | Left ventricular end-diastolic diameter |
| LVEDV | Left ventricular end-diastolic volume |
| LVEF | Left ventricular ejection fraction |
| LVESD | Left ventricular end-systolic diameter |
| LVESV | Left Ventricular end-systolic volume |
| LVH | Left ventricular hypertrophy |
| LVM | Left ventricular mass |
| LW | Lung wet weight |
| MRI | Magnetic resonance imaging |
| RV | Right ventricular |
| RVEDV | Right ventricular end-diastolic volume |
| RVESV | Right ventricular end-systolic volume |
| RVM | Right ventricular mass |
| TAC | Transverse aortic constriction |
| TE | Echo time |
| TGF-β | Transforming growth factor beta |
| TN-C | Tenascin C |
References
- Grossman, W.; Jones, D.; McLaurin, L.P. Wall stress and patterns of hypertrophy in the human left ventricle. J. Clin. Investig. 1975, 56, 56–64. [Google Scholar] [CrossRef]
- Krayenbuehl, H.P.; Hess, O.M.; Monrad, E.S.; Schneider, J.; Mall, G.; Turina, M. Left-Ventricular Myocardial Structure in Aortic-Valve Disease before, Intermediate, and Late after Aortic-Valve Replacement. Circulation 1989, 79, 744–755. [Google Scholar] [CrossRef]
- Kapur, N.K.; Paruchuri, V.; Urbano-Morales, J.A.; Mackey, E.E.; Daly, G.H.; Qiao, X.; Pandian, N.; Perides, G.; Karas, R.H. Mechanically unloading the left ventricle before coronary reperfusion reduces left ventricular wall stress and myocardial infarct size. Circulation 2013, 128, 328–336. [Google Scholar] [CrossRef]
- Levin, H.R.; Oz, M.C.; Chen, J.M.; Packer, M.; Rose, E.A.; Burkhoff, D. Reversal of chronic ventricular dilation in patients with end-stage cardiomyopathy by prolonged mechanical unloading. Circulation 1995, 91, 2717–2720. [Google Scholar] [CrossRef]
- Kim, G.H.; Uriel, N.; Burkhoff, D. Reverse remodelling and myocardial recovery in heart failure. Nat. Rev. Cardiol. 2018, 15, 83–96. [Google Scholar] [CrossRef]
- Kassner, A.; Oezpeker, C.; Gummert, J.; Zittermann, A.; Gartner, A.; Tiesmeier, J.; Fox, H.; Morshuis, M.; Milting, H. Mechanical circulatory support does not reduce advanced myocardial fibrosis in patients with end-stage heart failure. Eur. J. Heart Fail. 2020. [Google Scholar] [CrossRef] [PubMed]
- Bjornstad, J.L.; Skrbic, B.; Sjaastad, I.; Bjornstad, S.; Christensen, G.; Tonnessen, T. A mouse model of reverse cardiac remodelling following banding-debanding of the ascending aorta. Acta Physiol. 2012, 205, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Imanaka-Yoshida, K. Tenascin-C in cardiovascular tissue remodeling: From development to inflammation and repair. Circ. J. 2012, 76, 2513–2520. [Google Scholar] [CrossRef] [PubMed]
- Erickson, H.P. Tenascin-C, tenascin-R and tenascin-X: A family of talented proteins in search of functions. Curr. Opin. Cell Biol. 1993, 5, 869–876. [Google Scholar] [CrossRef]
- Podesser, B.K.; Kreibich, M.; Dzilic, E.; Santer, D.; Forster, L.; Trojanek, S.; Abraham, D.; Krssak, M.; Klein, K.U.; Tretter, E.V.; et al. Tenascin-C promotes chronic pressure overload-induced cardiac dysfunction, hypertrophy and myocardial fibrosis. J. Hypertens 2018, 36, 847–856. [Google Scholar] [CrossRef]
- Santer, D.; Nagel, F.; Goncalves, I.F.; Kaun, C.; Wojta, J.; Fagyas, M.; Krssak, M.; Balogh, A.; Papp, Z.; Toth, A.; et al. Tenascin-C aggravates ventricular dilatation and angiotensin-converting enzyme activity after myocardial infarction in mice. ESC Heart Fail. 2020, 7, 2113–2122. [Google Scholar] [CrossRef]
- Wang, J.; Xiang, B.; Deng, J.; Lin, H.Y.; Freed, D.H.; Arora, R.C.; Tian, G. Preservation of Myocardial Perfusion and Function by Keeping Hypertrophied Heart Empty and Beating for Valve Surgery: An In Vivo MR Study of Pig Hearts. Biomed. Res. Int. 2017, 2017, 4107587. [Google Scholar] [CrossRef]
- Nakao, N.; Hiraiwa, N.; Yoshiki, A.; Ike, F.; Kusakabe, M. Tenascin-C promotes healing of Habu-snake venom-induced glomerulonephritis: Studies in knockout congenic mice and in culture. Am. J. Pathol. 1998, 152, 1237–1245. [Google Scholar]
- Matsuda, A.; Yoshiki, A.; Tagawa, Y.; Matsuda, H.; Kusakabe, M. Corneal wound healing in tenascin knockout mouse. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1071–1080. [Google Scholar]
- Koyama, Y.; Kusubata, M.; Yoshiki, A.; Hiraiwa, N.; Ohashi, T.; Irie, S.; Kusakabe, M. Effect of tenascin-C deficiency on chemically induced dermatitis in the mouse. J. Investig. Dermatol. 1998, 111, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Heiberg, E.; Wigstrom, L.; Carlsson, M.; Bolger, A.F.; Karlsson, M. Time Resolved Three-Dimensional Automated Segmentation of the Left Ventricle; Computers in Cardiology; IEEE: Lyon, France, 2005; pp. 599–602. [Google Scholar]
- Carmona, A.K.; Schwager, S.L.; Juliano, M.A.; Juliano, L.; Sturrock, E.D. A continuous fluorescence resonance energy transfer angiotensin I-converting enzyme assay. Nat. Protoc. 2006, 1, 1971–1976. [Google Scholar] [CrossRef]
- Fagyas, M.; Uri, K.; Siket, I.M.; Fulop, G.A.; Csato, V.; Darago, A.; Boczan, J.; Banyai, E.; Szentkiralyi, I.E.; Maros, T.M.; et al. New perspectives in the renin-angiotensin-aldosterone system (RAAS) II: Albumin suppresses angiotensin converting enzyme (ACE) activity in human. PLoS ONE 2014, 9, e87844. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, I.F.; Acar, E.; Costantino, S.; Szabo, P.L.; Hamza, O.; Tretter, E.V.; Klein, K.U.; Trojanek, S.; Abraham, D.; Paneni, F.; et al. Epigenetic modulation of tenascin C in the heart: Implications on myocardial ischemia, hypertrophy and metabolism. J. Hypertens. 2019, 37, 1861–1870. [Google Scholar] [CrossRef] [PubMed]
- Young, C.; Laufer, G.; Kocher, A.; Solinas, M.; Alamanni, F.; Polvani, G.; Podesser, B.K.; Aramendi, J.I.; Arribas, J.; Bouchot, O.; et al. One-year outcomes after rapid-deployment aortic valve replacement. J. Thorac. Cardiovasc. Surg. 2018, 155, 575–585. [Google Scholar] [CrossRef]
- Laufer, G.; Haverich, A.; Andreas, M.; Mohr, F.W.; Walther, T.; Shrestha, M.; Rahmanian, P.; Holzhey, D.; Roth, M.; Schmitz, C.; et al. Long-term outcomes of a rapid deployment aortic valve: Data up to 5 years. Eur. J. Cardiothorac. Surg. 2017, 52, 281–287. [Google Scholar] [CrossRef]
- Magne, J.; Guinot, B.; Le Guyader, A.; Begot, E.; Marsaud, J.P.; Mohty, D.; Aboyans, V. Relation Between Renin-Angiotensin System Blockers and Survival Following Isolated Aortic Valve Replacement for Aortic Stenosis. Am. J. Cardiol. 2018, 121, 455–460. [Google Scholar] [CrossRef]
- Falkenham, A.; de Antueno, R.; Rosin, N.; Betsch, D.; Lee, T.D.; Duncan, R.; Legare, J.F. Nonclassical resident macrophages are important determinants in the development of myocardial fibrosis. Am. J. Pathol. 2015, 185, 927–942. [Google Scholar] [CrossRef]
- Ma, F.; Li, Y.; Jia, L.; Han, Y.; Cheng, J.; Li, H.; Qi, Y.; Du, J. Macrophage-stimulated cardiac fibroblast production of IL-6 is essential for TGF beta/Smad activation and cardiac fibrosis induced by angiotensin II. PLoS ONE 2012, 7, e35144. [Google Scholar]
- Rubina Perestrelo, A.; Silva, A.C.; Oliver-De La Cruz, J.; Martino, F.; Horvath, V.; Caluori, G.; Polansky, O.; Vinarsky, V.; Azzato, G.; de Marco, G.; et al. Multiscale Analysis of Extracellular Matrix Remodeling in the Failing Heart. Circ. Res. 2020, 128, 24–38. [Google Scholar] [CrossRef]
- Yokokawa, T.; Sugano, Y.; Nakayama, T.; Nagai, T.; Matsuyama, T.A.; Ohta-Ogo, K.; Ikeda, Y.; Ishibashi-Ueda, H.; Nakatani, T.; Yasuda, S.; et al. Significance of myocardial tenascin-C expression in left ventricular remodelling and long-term outcome in patients with dilated cardiomyopathy. Eur. J. Heart Fail. 2016, 18, 375–385. [Google Scholar] [CrossRef]
- Yao, H.C.; Han, Q.F.; Zhao, A.P.; Yao, D.K.; Wang, L.X. Prognostic values of serum tenascin-C in patients with ischaemic heart disease and heart failure. Heart Lung Circ. 2013, 22, 184–187. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gao, W.; Li, J.; Ni, H.; Shi, H.; Qi, Z.; Zhu, S.; Hao, C.; Xie, Q.; Luo, X.; Xie, K. Tenascin C: A Potential Biomarker for Predicting the Severity of Coronary Atherosclerosis. J. Atheroscler. Thromb. 2019, 26, 31–38. [Google Scholar] [CrossRef]
- Sato, A.; Aonuma, K.; Imanaka-Yoshida, K.; Yoshida, T.; Isobe, M.; Kawase, D.; Kinoshita, N.; Yazaki, Y.; Hiroe, M. Serum tenascin-C might be a novel predictor of left ventricular remodeling and prognosis after acute myocardial infarction. J. Am. Coll. Cardiol. 2006, 47, 2319–2325. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Hiroe, M.; Akiyama, D.; Hikita, H.; Nozato, T.; Hoshi, T.; Kimura, T.; Wang, Z.; Sakai, S.; Imanaka-Yoshida, K.; et al. Prognostic value of serum tenascin-C levels on long-term outcome after acute myocardial infarction. J. Card Fail. 2012, 18, 480–486. [Google Scholar] [CrossRef] [PubMed]
| BW (g) | HW (g) | HW/BW (×1000) | LVM (g) | LVM/BW (×1000) | LW/BW (×1000) | |
|---|---|---|---|---|---|---|
| Controls | 24.82 ± 2.68 | 0.11 ± 0.02 | 4.6 ± 0.04 | 0.069 ± 0.006 | 2.72 ± 0.15 | 51 ± 3 |
| Wt 10 | 28.33 ± 1.90 | 0.19 ± 0.03 *** | 6.5 ± 0.01 ** | 0.097 ± 0.011 ** | 3.84 ± 1.10 ** | 90 ± 21 ** |
| Wt 12 | 26.74 ± 0.96 | 0.14 ± 0.02 ## | 5.2 ± 0.05 ## | 0.074 ± 0.006 ## | 2.82 ± 0.20 ## | 61 ± 28 |
| TN-C KO 10 | 26.68 ± 1.75 | 0.14 ± 0.03 | 5.0 ± 0.07 | 0.074 ± 0.011 | 2.85 ± 0.67 | 50 ± 9 |
| TN-C KO 12 | 26.54 ± 0.92 | 0.12 ± 0.01 | 5.1 ± 0.05 | 0.065 ± 0.011 | 2.54 ± 0.25 | 51 ± 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perera-Gonzalez, M.; Kiss, A.; Kaiser, P.; Holzweber, M.; Nagel, F.; Watzinger, S.; Acar, E.; Szabo, P.L.; Gonçalves, I.F.; Weber, L.; et al. The Role of Tenascin C in Cardiac Reverse Remodeling Following Banding–Debanding of the Ascending Aorta. Int. J. Mol. Sci. 2021, 22, 2023. https://doi.org/10.3390/ijms22042023
Perera-Gonzalez M, Kiss A, Kaiser P, Holzweber M, Nagel F, Watzinger S, Acar E, Szabo PL, Gonçalves IF, Weber L, et al. The Role of Tenascin C in Cardiac Reverse Remodeling Following Banding–Debanding of the Ascending Aorta. International Journal of Molecular Sciences. 2021; 22(4):2023. https://doi.org/10.3390/ijms22042023
Chicago/Turabian StylePerera-Gonzalez, Mireia, Attila Kiss, Philipp Kaiser, Michael Holzweber, Felix Nagel, Simon Watzinger, Eylem Acar, Petra Lujza Szabo, Inês Fonseca Gonçalves, Lukas Weber, and et al. 2021. "The Role of Tenascin C in Cardiac Reverse Remodeling Following Banding–Debanding of the Ascending Aorta" International Journal of Molecular Sciences 22, no. 4: 2023. https://doi.org/10.3390/ijms22042023
APA StylePerera-Gonzalez, M., Kiss, A., Kaiser, P., Holzweber, M., Nagel, F., Watzinger, S., Acar, E., Szabo, P. L., Gonçalves, I. F., Weber, L., Pilz, P. M., Budinsky, L., Helbich, T., & Podesser, B. K. (2021). The Role of Tenascin C in Cardiac Reverse Remodeling Following Banding–Debanding of the Ascending Aorta. International Journal of Molecular Sciences, 22(4), 2023. https://doi.org/10.3390/ijms22042023








