Female Germ Cell Development, Functioning and Associated Adversities under Unfavorable Circumstances
Abstract
1. Introduction
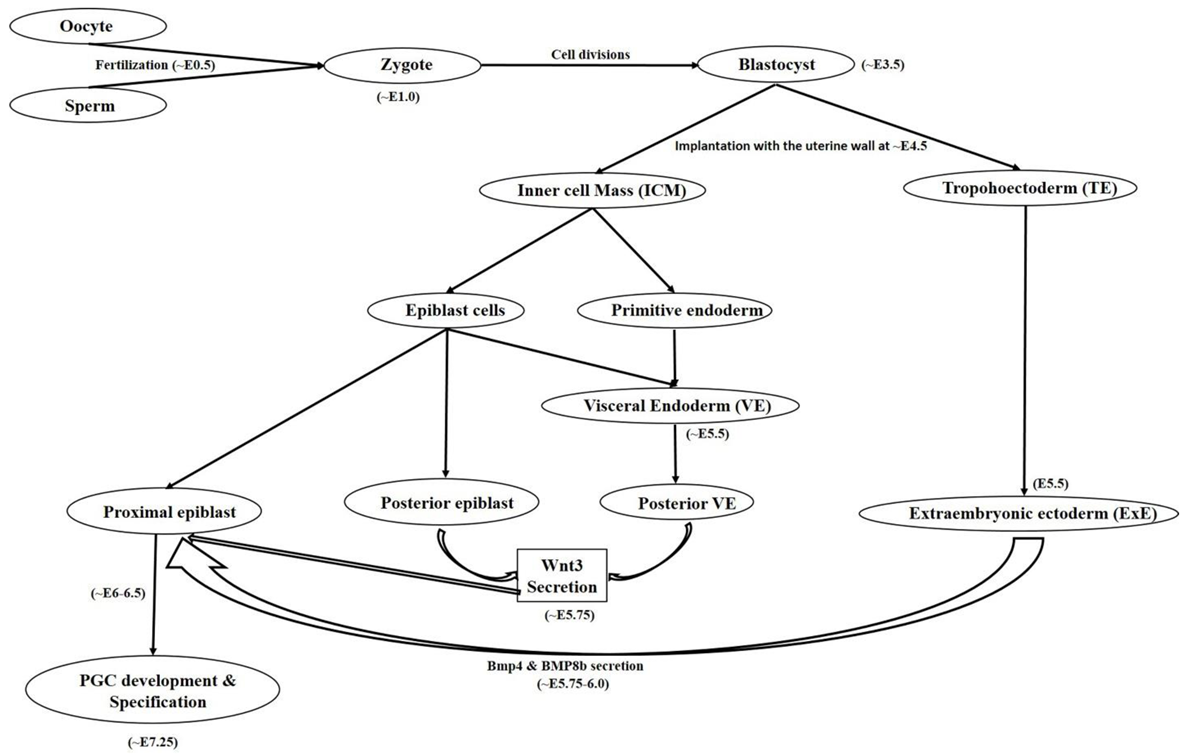
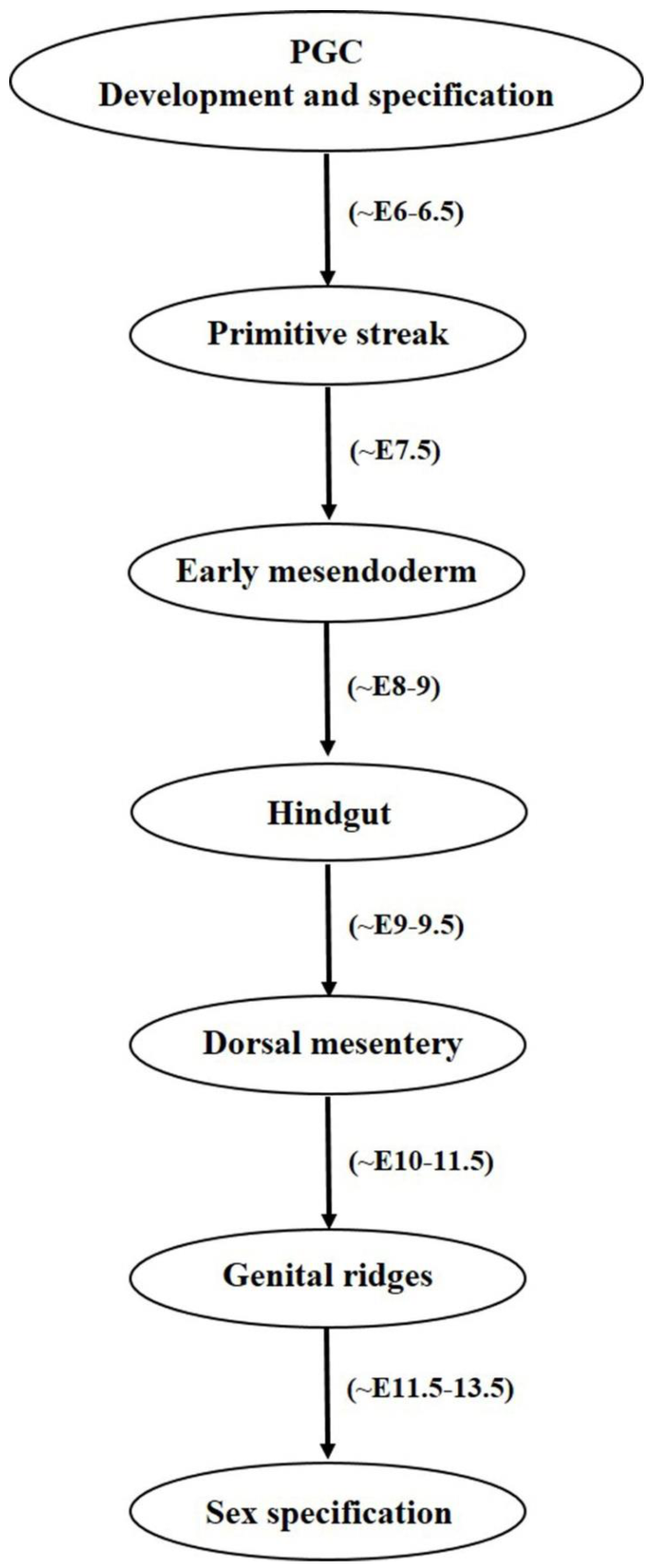
2. Female Germ Cell: In Vivo Developmental Stages (Primordial Germ Cells to Oocyte)
2.1. Primordial Germ Cell Development
2.2. Primordial Germ Cell Migration
3. Functional Efficacy of the Female Germ Cells
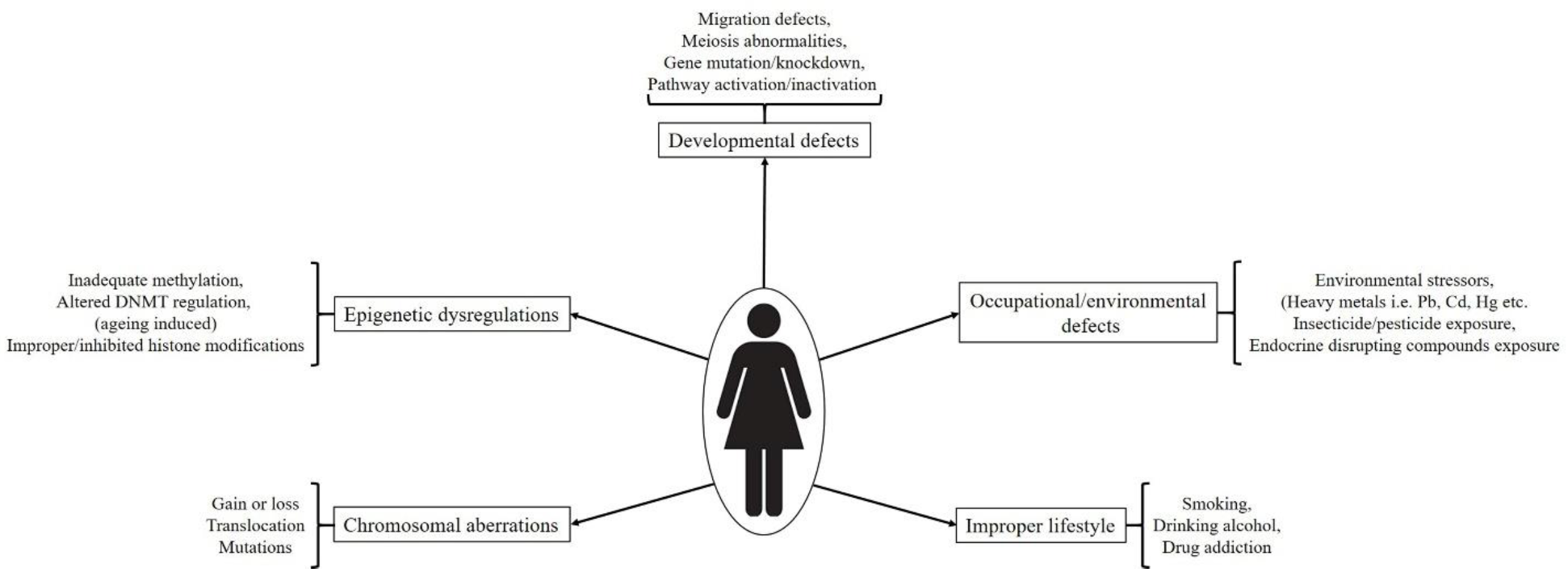
4. Developmental Impairments and Associated Abnormalities
4.1. Chromosomal Aberrations
4.2. Spontaneous Genetic and Epigenetic Regulations
4.3. External/Environmental Factors and Incorrect Lifestyle Associated Defects/Exposure to Toxicants In Utero
4.3.1. Chemical Intoxicants
4.3.2. Smoking, Alcoholism and Drug Abuse
5. Germ Cell Tumor Development and Their Molecular Regulation
5.1. Occurrence and Molecular Regulation
5.2. Types, Symptoms and Diagnosis
5.3. Treatment and Associated Complications
6. Present Experimental Needs and Future Expectations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hayashi, M.; Kawaguchi, T.; Durcova-Hills, G.; Imai, H. Generation of germ cells from pluripotent stem cells in mammals. Reprod. Med. Biol. 2018, 17, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Felici, M.D. Germ stem cells in the mammalian adult ovary: Considerations by a fan of the primordial germ cells. Mol. Hum. Reprod. 2010, 16, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, K.; Hayashi, Y.; Sekinaka, T.; Otsuka, K.; Matsuoka, Y.; Kobayashi, H.; Oki, S.; Takehara, A.; Kono, T.; Osumi, N.; et al. Repression of Somatic Genes by Selective Recruitment of HDAC3 by BLIMP1 Is Essential for Mouse Primordial Germ Cell Fate Determination. Cell. Rep. 2018, 24, 2682–2693.e6. [Google Scholar] [CrossRef]
- Bharti, D.; Jang, S.J.; Lee, S.Y.; Lee, S.L.; Rho, G.J. In Vitro Generation of Oocyte Like Cells and Their In Vivo Efficacy: How Far We have been Succeeded. Cells 2020, 9, 557. [Google Scholar] [CrossRef]
- Aramaki, S.; Hayashi, K.; Kurimoto, K.; Ohta, H.; Yabuta, Y.; Iwanari, H.; Mochizuki, Y.; Hamakubo, T.; Kato, Y.; Shirahige, K.; et al. A mesodermal factor, T, specifies mouse germ cell fate by directly activating germline determinants. Dev. Cell 2013, 27, 516–529. [Google Scholar] [CrossRef]
- Ying, Y.; Qi, X.; Zhao, G.Q. Induction of primordial germ cells from murine epiblasts by synergistic action of BMP4 and BMP8B signaling pathways. Proc. Natl. Acad. Sci. USA 2001, 98, 7858–7862. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.; Zhao, G.Q. Cooperation of Endoderm-Derived BMP2 and Extraembryonic Ectoderm-Derived BMP4 in Primordial Germ Cell Generation in the Mouse. Dev. Biol. 2001, 232, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Lawson, K.A.; Dunn, N.R.; Roelen, B.A.; Zeinstra, L.M.; Davis, A.M.; Wright, C.V.; Korving, J.P.; Hogan, B.L.M. Bmp4 is required for the generation of primordial germ cells in the mouse embryos. Genes. Dev. 1999, 13, 424–436. [Google Scholar] [CrossRef]
- Tremblay, K.D.; Dunn, N.R.; Robertson, E.J. Mouse embryos lacking Smad1 signals display defects in extra-embryonic tissues and germ cell formation. Development 2001, 128, 3609–3621. [Google Scholar] [PubMed]
- Chu, G.C.; Dunn, N.R.; Anderson, D.C.; Oxburgh, L.; Robertson, E.J. Differential requirements for Smad4 in TGFβ-dependent patterning of the early mouse embryo. Development 2004, 131, 3501–3512. [Google Scholar] [CrossRef]
- Chang, H.; Matzuk, M.M. Smad5 is required for mouse primordial germ cell development. Mech. Dev. 2001, 104, 61–67. [Google Scholar] [CrossRef]
- Cantú, A.V.; Laird, D.J. Primordial germ cell migration and the Wnt signaling pathway. Anim. Reprod. 2017, 14, 89–101. [Google Scholar] [CrossRef]
- Saitou, M.; Payer, B.; Lange, U.C.; Erhardt, S.; Barton, S.C.; Surani, M.A. Specification of germ cell fate in mice. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003, 358, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Lange, U.C.; Saitou, M.; Western, P.S.; Barton, S.C.; Surani, M.A. The Fragilis interferon-inducible gene family of transmembrane proteins is associated with germ cell specification in mice. BMC. Dev. Biol. 2003, 3, 1–11. [Google Scholar] [CrossRef]
- Tres, L.L.; Rosselot, C.; Kierszenbaum, A.L. Primordial Germ Cells What Does It Take to be alive? Mol. Reprod. Dev. 2004, 68, 1–4. [Google Scholar] [CrossRef]
- Saitou, M.; Kagiwada, S.; Kurimoto, K. Epigenetic reprogramming in mouse pre-implantation development and primordial germ cells. Development 2012, 139, 15–31. [Google Scholar] [CrossRef]
- Lee, H.J.; Hore, T.A.; Reik, W. Reprogramming the Methylome: Erasing Memory and Creating Diversity. Cell Stem Cell 2014, 14, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Gkountela, S.; Zhang, K.X.; Shafiq, T.A.; Liao, W.W.; Calvopiña, J.H.; Chen, P.Y.; Clark, A.T. DNA Demethylation Dynamics in the Human Prenatal Germline. Cell 2015, 161, 1425–1436. [Google Scholar] [CrossRef]
- Guo, F.; Yan, L.; Guo, H.; Li, L.; Hu, B.; Zhao, Y.; Yong, J.; Hu, Y.; Wang, X.; Wei, Y.; et al. The Transcriptome and DNA Methylome Landscapes of Human Primordial Germ Cells. Cell 2015, 161, 1437–1452. [Google Scholar] [CrossRef] [PubMed]
- Canovas, S.; Campos, R.; Aguilar, E.; Cibelli, J.B. Progress towards human primordial germ cell specification in vitro. Mol. Hum. Reprod. 2017, 23, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Sun, N.; Hou, L.; Kim, R.; Faith, J.; Aslanyan, M.; Tao, Y.; Zheng, Y.; Fu, J.; Liu, W.; et al. Human Primordial Germ Cells Are Specified from Lineage-Primed Progenitors. Cell Rep. 2019, 29, 4568–4582.e5. [Google Scholar] [CrossRef]
- Chen, D.; Liu, W.; Lukianchikov, A.; Hancock, G.V.; Zimmerman, J.; Lowe, M.G.; Kim, R.; Galic, Z.; Irie, N.; Surani, M.A.; et al. Germline competency of human embryonic stem cells depends on eomesodermin. Biol. Reprod. 2017, 97, 850–861. [Google Scholar] [CrossRef]
- Irie, N.; Weinberger, L.; Tang, W.W.C.; Kobayashi, T.; Viukov, S.; Manor, Y.S.; Dietmann, S.; Hanna, J.H.; Surani, M.A. SOX17 is a critical specifier of human primordial germ cell fate. Cell 2015, 160, 253–268. [Google Scholar] [CrossRef]
- Sasaki, K.; Yokobayashi, S.; Nakamura, T.; Okamoto, I.; Yabuta, Y.; Kurimoto, K.; Ohta, H.; Moritoki, Y.; Iwatani, C.; Tsuchiya, H.; et al. Robust In Vitro Induction of Human Germ Cell Fate from Pluripotent Stem Cells. Cell Stem Cell 2015, 17, 178–194. [Google Scholar] [CrossRef] [PubMed]
- Raz, E. Primordial germ-cell development: The zebrafish perspective. Nat. Rev. Genet. 2003, 4, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Richardson, B.E.; Lehmann, R. Mechanisms guiding primordial germ cell migration: Strategies from different organisms. Nat. Rev. Mol. Cell Biol. 2010, 11, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Saitou, M.; Payer, B.; O’Carroll, D.; Ohinata, Y.; Surani, M.A. Blimp1 and the emergence of the germ line during development in the mouse. Cell Cycle 2005, 4, 1736–1740. [Google Scholar] [CrossRef] [PubMed]
- Vincent, S.D.; Dunn, N.R.; Sciammas, R.; Sharpiro-Shalef, M.; Davis, M.M.; Calame, K.; Bikoff, E.K.; Robertson, E.J. The zinc finger transcriptional repressor Blimp1/Prdm1 is dispensable for early axis formation but is required for specification of primordial germ cells in the mouse. Development 2005, 132, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Kurimoto, K.; Yamaji, M.; Seki, Y.; Saitou, M. Specification of the germ cell lineage in mice: A process orchestrated by the PR-domain proteins, Blimp1 and Prdm14. Cell Cycle 2008, 7, 3514–3518. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, M.; Seki, Y.; Kurimoto, K.; Yabuta, Y.; Yuasa, M.; Shigeta, M.; Yamanaka, K.; Ohinata, Y.; Saitou, M. Critical function of Prdm14 for the establishment of the germ cell lineage in mice. Nat. Genet. 2008, 40, 1016–1022. [Google Scholar] [CrossRef]
- Tanaka, S.S.; Yamaguchi, Y.L.; Tsoi, B.; Lickert, H.; Tam, P.P. IFITM/Mil/fragilis family proteins IFITM1 and IFITM3 play distinct roles in mouse primordial germ cell homing and repulsion. Dev. Cell 2005, 9, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Lange, U.C.; Adams, D.J.; Lee, C.; Barton, S.; Schneider, R.; Bradley, A.; Surani, M.A. Normal germ line establishment in mice carrying a deletion of the Ifitm/Fragilis gene family cluster. Mol. Cell Biol. 2008, 28, 4688–4696. [Google Scholar] [CrossRef]
- Weber, S.; Eckert, D.; Nettersheim, D.; Gillis, A.J.; Schafer, S.; Kuckenberg, P.; Ehlermann, J.; Werling, U.; Biermann, K.; Looijenga, L.H.; et al. Critical function of AP-2g/TCFAP2C in mouse embryonic germ cell maintenance. Biol. Reprod. 2010, 82, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Julaton, V.T.A.; Pera, R.A.R. NANOS3 function in human germ cell development. Hum. Mol. Genet. 2011, 20, 2238–2250. [Google Scholar] [CrossRef]
- Shen, W.; Zhang, D.; Qing, T.; Cheng, J.; Bai, Z.; Shi, Y.; Ding, M.; Deng, H. Live Offspring Produced by Mouse Oocytes Derived from Premeiotic Fetal Germ Cells. Biol. Reprod. 2006, 75, 615–623. [Google Scholar] [CrossRef]
- Zou, K.; Yuan, Z.; Yang, Z.; Luo, H.; Sun, K.; Zhou, L.; Xiang, J.; Shi, L.; Yu, Q.; Zhang, Y.; et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat. Cell Biol. 2009, 11, 631–636. [Google Scholar] [CrossRef]
- Hayashi, K.; Ogushi, S.; Kurimoto, K.; Shimamoto, S.; Ohta, H.; Saitou, M. Offspring from Oocytes Derived from in Vitro Primordial Germ Cell–like Cells in Mice. Science 2012, 338, 971–975. [Google Scholar] [CrossRef]
- Morohaku, K.; Tanimoto, R.; Sasaki, K.; Kawahara-Miki, R.; Kono, T.; Hayashi, K.; Hiraoe, Y.; Obata, Y. Complete in vitro generation of fertile oocytes from mouse primordial germ cells. Proc. Natl. Acad. Sci. USA 2016, 113, 9021–9026. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Liu, L.; Ye, X.; Fu, H.; Sheng, X.; Wang, L.; Wang, H.; Heng, D.; Liu, L. Functional Oocytes Derived from Granulosa Cells. Cell Rep. 2019, 29, 4256–4267. [Google Scholar] [CrossRef] [PubMed]
- Matyakhina, L.; Lenherr, S.M.; Stratakis, C.A. Protein Kinase A and Chromosomal Stability. Ann. N. Y. Acad. Sci. 2002, 968, 148–157. [Google Scholar] [CrossRef]
- Kraggerud, S.M.; Szymanska, J.; Abeler, V.M.; Kaern, J.; Eknaes, M.; Heim, S.; Teixeira, M.R.; Trope, C.G.; Peltomäki, P.; Lothe, R.A. DNA Copy Number Changes in Malignant Ovarian Germ Cell Tumors. Cancer Res. 2000, 60, 3025–3030. [Google Scholar]
- Bussey, K.J.; Lawce, H.J.; Himoe, E.; Shu, X.O.; Suijkerbuijk, R.F.; Olson, S.B.; Magenis, R.E. Chromosomes 1 and 12 abnormalities in pediatric germ cell tumors by interphase fluorescence in situ hybridization. Cancer Genet. Cytogenet. 2001, 125, 112–118. [Google Scholar] [CrossRef]
- Cossu-Rocca, P.; Zhang, S.; Roth, L.M.; Eble, J.N.; Zheng, W.; Abdul Karim, F.W.; Michael, H.; Emerson, R.E.; Jones, T.D.; Hattab, E.M.; et al. Chromosome 12p abnormalities in dysgerminoma of the ovary: A FISH analysis. Mod. Pathol. 2006, 19, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Mayr, D.; Kaltz-Wittmer, C.; Arbogast, S.; Amann, G.; Aust, D.E.; Diebold, J. Characteristic Pattern of Genetic Aberrations in Ovarian Granulosa Cell Tumors. Mod. Pathol. 2002, 15, 951–957. [Google Scholar] [CrossRef]
- Lin, Y.S.; Eng, H.L.; Jan, Y.J.; Lee, H.S.; Ho, W.L.; Liou, C.P.; Lee, W.Y.; Tzeng, C.C. Molecular cytogenetics of ovarian granulosa cell tumors by comparative genomic hybridization. Gynecol. Oncol. 2005, 97, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Chen, X. Epigenetic Regulation of Germ Cells— Remember or Forget? Curr. Opin. Genet. Dev. 2015, 31, 20–27. [Google Scholar] [CrossRef]
- Cinalli, R.M.; Rangan, P.; Lehmann, R. Germ cells are forever. Cell 2008, 132, 559–562. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.C.; Wang, Y.Y.; Ge, W.; Cheng, S.F.; Dyce, P.W.; Shen, W. Epigenetic regulation during the differentiation of stem cells to germ cells. Oncotarget 2017, 8, 57836–57844. [Google Scholar] [CrossRef]
- Hajkova, P.; Ancelin, K.; Waldmann, T.; Lacoste, N.; Lange, U.C.; Cesari, F.; Lee, C.; Almouzni, G.; Schneider, R.; Surani, M.A. Chromatin dynamics during epigenetic reprogramming in the mouse germ line. Nature 2008, 452, 877–881. [Google Scholar] [CrossRef]
- Daujat, S.; Weiss, T.; Mohn, F.; Lange, U.C.; Ziegler-Birling, C.; Zeissler, U.; Lappe, M.; Schübeler, D.; Torres-Padilla, M.E.; Schneider, R. H3K64 trimethylation marks heterochromatin and is dynamically remodeled during developmental reprogramming. Nat. Struct. Mol. Biol. 2009, 16, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.C.; Ana, L.D.; Hazel, L.K.; Richard, A.A.; Angabin, M.; Marvin, L.M.; Gunapala, S. Fetal Cyclophosphamide Exposure Induces Testicular Cancer and Reduced Spermatogenesis and Ovarian Follicle Numbers in Mice. PLoS ONE 2014, 9, e93311. [Google Scholar]
- Zvi, R.; Alisa, K.E.; Dorit, K. Effect of environmental contamination on female and male gametes—A lesson from bovines. Anim. Reprod. 2020, 17, e20200041. [Google Scholar]
- Toft, G.; Jönsson, B.A.G.; Lindh, C.H.; Jensen, T.K.; Hjollund, N.H.; Vested, A.; Bonde, J.P. Association between Pregnancy Loss and Urinary Phthalate Levels around the Time of Conception. Environ. Health Perspect. 2012, 120, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Tranfo, G.; Caporossi, L.; Paci, E.; Aragona, C.; Romanzi, D.; De Carolis, C.; De Rosa, M.; Capanna, S.; Papaleo, B.; Pera, A. Urinary phthalate monoesters concentration in couples with infertility problems. Toxicol. Lett. 2012, 213, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Machtinger, R.; Gaskins, A.J.; Racowsky, C.; Mansur, A.; Adir, M.; Baccarelli, A.A.; Calafat, A.M.; Hauser, R. Urinary concentrations of biomarkers of phthalates and phthalate alternatives and IVF outcomes. Environ. Int. 2018, 111, 23–31. [Google Scholar] [CrossRef]
- Liu, J.C.; Yan, Z.H.; Li, B.; Yan, H.C.; Felici, M.D.; Shen, D. Di (2-ethylhexyl) phthalate impairs primordial follicle assembly by increasing PDE3A expression in oocytes. Environ. Pollut. 2020, 116088, online ahead of print. [Google Scholar]
- Gupta, R.K.; Singh, J.M.; Leslie, T.C.; Meachum, S.; Flaws, J.A.; Yao, H.H. C-(2-ethylhexyl) phthalate and mono-(2-ethylhexyl) phthalate inhibit growth and reduce estradiol levels of antral follicles in vitro. Toxicol. Appl. Pharmacol. 2010, 242, 224–230. [Google Scholar] [CrossRef]
- Hannon, P.R.; Niermann, S.; Flaws, J.A. Acute exposure to Di(2-Ethylhexyl) phthalate in adulthood causes adverse reproductive outcomes later in life and accelerates reproductive aging in female mice. Toxicol. Sci. 2016, 150, 97–108. [Google Scholar] [CrossRef]
- Kalo, D.; Roth, Z. Low level of mono(2-ethylhexyl) phthalate reduces oocyte developmental competence in association with impaired gene expression. Toxicology 2017, 377, 38–48. [Google Scholar] [CrossRef]
- Justin, D.V.; Catherine, A.V.; Craig, B.M.; Nicolas, R.L.; Alan, J.C. In vitro exposure to environmental tobacco smoke induces CYP1B1 expression in human luteinized granulosa cells. Reprod. Toxicol. 2006, 22, 731–737. [Google Scholar]
- Goodman, M.T.; McDuffie, K.; Kolonel, L.N.; Terada, K.; Donlon, T.A.; Wilkens, L.R.; Guo, C.; Marchand, L.L. Case-control study of ovarian cancer and polymorphisms in genes involved in catecholestrogen formation and metabolism. Cancer Epidemiol. Biomark. Prev. 2001, 10, 209–216. [Google Scholar]
- Alviggi, C.; Cariati, F.; Conforti, A.; De Rosa, P.; Vallone, R.S.I.; Pivonello, R.; De Placido, G. The effect of FT500 Plus® on ovarian stimulation in PCOS women. Reprod. Toxicol. 2016, 59, 40–44. [Google Scholar] [CrossRef]
- Budani, M.C.; Carletti, E.; Tiboni, G.M. Cigarette smoke is associated with altered expression of antioxidant enzymes in granulosa cells from women undergoing in vitro fertilization. Zygote 2017, 25, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Sinko, I.; Morocz, M.; Zadori, J.; Kokavszky, K.; Rasko, I. Effect of cigarette smoking on DNA damage of human cumulus cells analyzed by comet assay. Reprod. Toxicol. 2005, 20, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Shiloh, H.; Lahav-Baratz, S.; Koifman, M.; Ishai, D.; Bidder, D.; Weiner-Meganzi, Z.; Dirnfeld, M. The impact of cigarette smoking on zona pellucida thickness of oocytes and embryos prior to transfer into the uterine cavity. Hum. Reprod. 2004, 19, 157–159. [Google Scholar] [CrossRef]
- Sobinoff, A.P.; Beckett, E.L.; Jarnicki, A.G.; Sutherland, J.M.; McCluskey, A.; Hansbro, P.M.; McLaughlin, E.A. Scrambled and fried: Cigarette smoke exposure causes antral follicle destruction and oocyte dysfunction through oxidative stress. Toxicol. Appl. Pharmacol. 2013, 271, 156–167. [Google Scholar] [CrossRef]
- Jennings, P.C.; Merriman, J.A.; Beckett, E.L.; Hansbro, P.M.; Jones, K.T. Increased zona pellucida thickness and meiotic spindle disruption in oocytes from cigarette smoking mice. Hum. Reprod. 2011, 26, 878–884. [Google Scholar] [CrossRef]
- Paixao, L.L.; Gaspar-Reis, R.P.; Gonzalez, G.P.; Santos, A.S.; Santana, A.C.; Santos, R.M.; Spritzer, P.M.; Nascimento-Saba, C.C. Cigarette smoke impairs granulosa cell proliferation and oocyte growth after exposure cessation in young Swiss mice: An experimental study. J. Ovarian Res. 2012, 5, 25. [Google Scholar] [CrossRef]
- Mai, Z.; Lei, M.; Yu, B.; Du, H.; Liu, J. The effects of cigarette smoke extract on ovulation, oocyte morphology and ovarian gene expression in mice. PLoS ONE 2014, 9, e95945. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Kim, C.W.; Hwang, K.A.; Sung, J.H.; Lee, J.K.; Choi, K.C. Cigarette smoke impaired maturation of ovarian follicles and normal growth of uterus inner wall of female wild-type and hypertensive rats. Reprod. Toxicol. 2017, 73, 232–240. [Google Scholar] [CrossRef]
- Hakim, R.B.; Gray, R.H.; Zakur, H. Alcohol and caffeine consumption and decreased fertility. Fertil. Steril. 1998, 70, 632–637. [Google Scholar] [CrossRef]
- Eggert, J.; Theobald, H.; Engfeldt, H. Effects of alcohol consumption on female fertility during an 18-year period. Fertil. Steril. 2004, 81, 379–383. [Google Scholar] [CrossRef]
- Juhl, M.; Andersen, A.M.N.; Gronbaek, M.; Olsen, J. Moderate alcohol consumption and waiting time to pregnancy. Hum. Reprod. 2001, 16, 2705–2709. [Google Scholar] [CrossRef] [PubMed]
- Tolstrup, J.S.; Kjaer, S.K.; Holst, C.; Sharif, H.; Munk, C.; Osler, M.; Schmidt, L.; Andersen, A.M.N.; Gronbaek, M. Alcohol use as predictor for infertility in representative population of Danish women. Acta Obstet. Gynecol. Scand. 2003, 82, 744–749. [Google Scholar] [CrossRef]
- Chavarro, J.E.; Rich-Edwards, J.W.; Rosner, B.A.; Willett, W.C. Caffeinated and Alcoholic Beverage Intake in Relation to Ovulatory Disorder Infertility. Epidemiology 2009, 20, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Mueller, B.A.; Daling, J.R.; Weiss, N.S.; Moore, D.E. Recreational drug use and the risk of primary infertility. Epidemiology 1990, 1, 195–200. [Google Scholar] [CrossRef]
- Jukic, A.M.Z.; Weinberg, C.R.; Baird, D.D.; Wilcox, A.J. Lifestyle and reproductive factors associated with follicular phase length. J. Womens Health (Larchmt) 2007, 16, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Lammert, S.; Harrison, K.; Tosun, N.; Allen, S. Menstrual Cycle in Women Who Co-use Marijuana and Tobacco. J. Addict. Med. 2018, 12, 207–211. [Google Scholar] [CrossRef]
- Kraggerud, S.M.; Hoei-Hansen, C.E.; Alagaratnam, S.; Skotheim, R.I.; Abeler, V.M.; Meyts, E.R.D.; Lothe, R.A. Molecular Characteristics of Malignant Ovarian Germ Cell Tumors and Comparison With Testicular Counterparts: Implications for Pathogenesis. Endocr. Rev. 2013, 34, 339–376. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.R.; Skowron, M.A.; Albers, P.; Nettersheim, D. Molecular and epigenetic pathogenesis of germ cell tumors. Asian J. Urol. 2020, in press. [Google Scholar] [CrossRef]
- Ruark, E.; Seal, S.; McDonald, H.; Zhang, F.; Elliot, A.; Lau, K.W.; Perdeaux, E.; Rapley, E.; Eeles, R.; Peto, J.; et al. Identification of nine new susceptibility loci for testicular cancer, including variants near DAZL and PRDM14. Nat. Genet. 2013, 45, 686–689. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Günesdogan, U.; Zylicz, J.J.; Hackett, J.A.; Cougot, D.; Bao, S.; Lee, C.; Dietmann, S.; Allen, G.E.; Sengupta, R.; et al. PRMT5 protects genomic integrity during global DNA demethylation in primordial germ cells and preimplantation embryos. Mol. Cell 2014, 56, 564–579. [Google Scholar] [CrossRef]
- Lee, A.S.; Tang, C.; Rao, M.S.; Weissman, I.L.; Wu, J.C. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat. Med. 2013, 19, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.L.; Frazier, A.L.; Amatruda, J.F. Pediatric germ cell tumors: A developmental perspective. Adv. Urol. 2018, 2018, 9059382. [Google Scholar] [CrossRef]
- Boussios, S.; Mikropoulos, C.; Samartzis, E.; Karihtala, P.; Moschetta, M.; Sheriff, M.; Karathanasi, A.; Sadauskaite, A.; Rassy, E.; Pavlidis, N. Wise Management of Ovarian Cancer: On the Cutting Edge. J. Pers. Med. 2020, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zheng, T.; Hong, W.; Ye, H.; Hu, C.; Zheng, Y. Mechanism for the Decision of Ovarian Surface Epithelial Stem Cells to Undergo Neo-Oogenesis or Ovarian Tumorigenesis. Cell Physiol. Biochem. 2018, 50, 214–232. [Google Scholar] [CrossRef]
- Kurman, R.J.; Shih, I.M. The origin and pathogenesis of epithelial ovarian cancer: A proposed unifying theory. Am. J. Surg. Pathol. 2010, 34, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.W.; Pejovic, T.; Lawson, M.; Jurevic, L.; Hobbs, T.; Stouffer, R.L. Ovulation in the Absence of the Ovarian Surface Epithelium in the Primate. Biol. Reprod. 2010, 82, 599–605. [Google Scholar] [CrossRef][Green Version]
- Wright, J.W.; Pejovic, T.; Jurevic, L.; Bishop, C.V.; Hobbs, T.; Stouffer, R.L. Ovarian surface epitheliectomy in the non-human primate: Continued cyclic ovarian function and limited epithelial replacement. Hum. Reprod. 2011, 26, 1422–1430. [Google Scholar] [CrossRef]
- Suster, N.K.; Smrkolj, S.; Virant-Klun, I. Putative stem cells and epithelial-mesenchymal transition revealed in sections of ovarian tumor in patients with serous ovarian carcinoma using immunohistochemistry for Vimentin and pluripotency-related markers. J. Ovarian Res. 2017, 10, 11. [Google Scholar] [CrossRef]
- Zhu, Y.; Nilsson, M.; Sundfeldt, K. Phenotypic plasticity of the ovarian surface epithelium: TGF-beta 1 induction of epithelial to mesenchymal transition (EMT) in vitro. Endocrinology 2010, 151, 5497–5505. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sun, J.; Cai, B.; Xi, X.; Yang, L.; Zhang, Z.; Feng, Y.; Sun, Y. NANOG regulates epithelial-mesenchymal transition and chemo-resistance through activation of the STAT3 pathway in epithelial ovarian cancer. Tumour Biol. 2016, 37, 9671–9680. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, J.; Wang, X.; Li, X.; Liang, J.; Xing, H. Role of TWIST2, E-cadherin and Vimentin in epithelial ovarian carcinogenesis and prognosis and their interaction in cancer progression. Eur. J. Gynaecol. Oncol. 2016, 37, 100–108. [Google Scholar] [PubMed]
- Nogales, F.F.; Dulcey, I.; Preda, O. Germ cell tumors of the ovary: An update. Arch. Pathol. Lab. Med. 2014, 138, 351–362. [Google Scholar] [CrossRef]
- Chang, M.C.; Vargas, S.O.; Hornick, J.L.; Hirsch, M.S.; Crum, C.P.; Nucci, M.R. Embryonic stem cell transcription factors and D2-40 (podoplanin) as diagnostic immunohistochemical markers in ovarian germ cell tumors. Int. J. Gynecol. Pathol. 2009, 28, 347–355. [Google Scholar] [CrossRef]
- Murray, M.J.; Saini, H.K.; Siegler, C.A.; Hanning, J.E.; Barker, E.M.; Dongen, S.V.; Ward, D.M.; Raby, K.L.; Groves, I.J.; Scarpini, C.G.; et al. LIN28 expression in malignant germ cell tumors downregulates let-7 and increases oncogene levels. Cancer Res. 2013, 73, 4872–4884. [Google Scholar] [CrossRef]
- Schonberger, S.; Okpanyi, V.; Calaminus, G.; Heikaus, S.; Leuschner, I.; Nicholson, J.C.; Stoecklein, N.H.; Schneider, D.T.; Borkhardt, A. EPCAM-A novel molecular target for the treatment of pediatric and adult germ cell tumors. Genes Chromosomes Cancer 2013, 52, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Poynter, J.N.; Hooten, A.J.; Frazier, A.L.; Ross, J.A. Associations between variants in KITLG, SPRY4, BAK1, and DMRT1 and pediatric germ cell tumors. Genes Chromosomes Cancer 2012, 51, 266–271. [Google Scholar] [CrossRef]
- Molyneaux, K.A.; Zinszner, H.; Kunwar, P.S.; Schaible, K.; Stebler, J.; Sunshine, M.J.; O’Brien, W.; Raz, E.; Littman, D.; Wylie, C.; et al. The chemokine SDF1/CXCL12 and its receptor CXCR4 regulate mouse germ cell migration and survival. Development 2003, 130, 4279–4286. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, A.M.; Rezvani, M.; Elsayes, K.M.; Baskin, H., Jr.; Mourad, A.; Foster, B.R.; Jarboe, E.A.; Menias, C.O. Ovarian Malignant Germ Cell Tumors: Cellular Classification and Clinical and Imaging Features. Radiographics 2014, 34, 777–801. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, P.; Taxy, J.B. Educational Case: Yolk Sac (Endodermal Sinus) Tumor of the Ovary. Acad. Pathol. 2020, 7, 1–4. [Google Scholar] [CrossRef]
- Lakshmanan, M.; Gupta, S.; Kumar, V.; Akhtar, N.; Chaturvedi, A.; Misra, S.; Jain, K.; Garg, S. Germ Cell Tumor Ovary: An Institutional Experience of Treatment and Survival Outcomes. Indian J. Surg. Oncol. 2018, 9, 215–219. [Google Scholar] [CrossRef]
- Boussios, S.; Attygalle, A.; Hazell, S.; Moschetta, M.; Mclachlan, J.; Okines, A.; Banerjee, S. Malignant Ovarian Germ Cell Tumors in Postmenopausal Patients: The Royal Marsden Experience and Literature Review. Anticancer Res. 2015, 35, 6713–6722. [Google Scholar]
- Hong, R.; Suh, C.H.; Lee, M.J. Adenocarcinoma with Yolk Sac Tumor of the Stomach: Case Report with Review of the Literature and an Immunohistochemical Study. Korean J. Pathol. 2007, 41, 352–357. [Google Scholar]
- Cheng, L.; Zhang, S.; Talerman, A.; Roth, L.M. Morphologic, immunohistochemical, and fluorescence in situ hybridization study of ovarian embryonal carcinoma with comparison to solid variant of yolk sac tumor and immature teratoma. Hum. Pathol. 2010, 41, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, A.; Peng, Y.; Rakheja, D.; Wei, L.; Xue, D.; Allan, R.W.; Molberg, K.H.; Li, J.; Cao, D. Diagnostic utility of SALL4 in extragonadal yolk sac tumors: An immunohistochemical study of 59 cases with comparison to placental-like alkaline phosphatase, alpha-fetoprotein, and glypican-3. Am. J. Surg. Pathol. 2009, 33, 1529–1539. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Sarrado, L.; González-Ballano, I.; Herrero-Serrano, R.; Giménez-Molina, C.; Rodríguez-Solanilla, B.; Campillos-Maza, J.M. Hemoptysis as the first symptom in the diagnosis of metastatic choriocarcinoma in the third trimester of pregnancy: A case report. Case Rep. Womens Health 2020, 27, e00211. [Google Scholar] [CrossRef] [PubMed]
- Boussios, S.; Zarkavelis, G.; Seraj1, E.; Zerdes, I.; Tatsi, K.; Pentheroudakis, G. Non-epithelial Ovarian Cancer: Elucidating Uncommon Gynaecological Malignancies. Anticancer Res. 2016, 36, 5031–5042. [Google Scholar] [CrossRef]
- Gershenson, D.M. Current advances in the management of malignant germ cell and sex cord-stromal tumors of the ovary. Gynecol. Oncol. 2012, 125, 515–517. [Google Scholar] [CrossRef]
- Mangili, G.; Sigismondi, C.; Lorusso, D.; Cormio, G.; Candiani, M.; Scarfone, G.; Mascilini, F.; Gadducci, A.; Mosconi, A.M.; Scollo, P.; et al. The role of staging and adjuvant chemotherapy in stage I malignant ovarian germ cell tumors (MOGTs): The MITO-9 study. Ann. Oncol. 2017, 28, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Azambuja, A.A.; Engroff, P.; Silva, B.T.; Zorzetti, R.C.S.; Morrone, F.B. Evaluation of nuclear NF-κB, transglutaminase2, and ERCC1 as predictors of platinum resistance in testicular tumors. Int. Braz. J. Urol. 2020, 46, 353–362. [Google Scholar] [CrossRef] [PubMed]
- AlDubayan, S.H.; Pyle, L.C.; Gamulin, M.; Kulis, T.; Moore, N.D.; Taylor-Weiner, A.; Hmaid, A.A.; Reardon, B.; Wubbenhorst, B.; Godse, R.; et al. Association of Inherited Pathogenic Variants in Checkpoint Kinase 2 (CHEK2) With Susceptibility to Testicular Germ Cell Tumors. JAMA Oncol. 2019, 5, 514–522. [Google Scholar] [CrossRef]
- Cavallo, F.; Feldman, D.R.; Barchi, M. Revisiting DNA damage repair, p53-mediated apoptosis and cisplatin sensitivity in germ cell tumors. Int. J. Dev. Biol. 2013, 57, 273–280. [Google Scholar] [CrossRef]
- Lobo, J.; Constancio, V.; Guimaraes-Teixeira, C.; Leite-Silva, P.; Miranda-Goncalves, V.; Sequeira, J.P.; Pistoni, L.; Guimaraes, R.; Cantante, M.; Braga, I.; et al. Promoter methylation of DNA homologous recombination genes is predictive of the responsiveness to PARP inhibitor treatment in testicular germ cell tumors. Mol. Oncol. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Koster, R.; Di Pietro, A.; Timmer-Bosscha, H.; Gibcus, J.H.; Van Den Berg, A.; Suurmeijer, A.J.; Bischoff, R.; Gietema, J.A.; De Jong, S. Cytoplasmic p21 expression levels determine cisplatin resistance in human testicular cancer. J. Clin. Investig. 2010, 120, 3594–3605. [Google Scholar] [CrossRef] [PubMed]
- Koster, R.; van Vugt, M.A.T.M.; Timmer-Bosscha, H.; Gietema, J.A.; de Jong, S. Unravelling mechanisms of cisplatin sensitivity and resistance in testicular cancer. Expert Rev. Mol. Med. 2013, 15, e12. [Google Scholar] [CrossRef] [PubMed]
- Gutekunst, M.; Mueller, T.; Weilbacher, A.; Dengler, M.A.; Bedke, J.; Kruck, S.; Oren, M.; Aulitzky, W.E.; Van Der Kuip, H. Cisplatin hypersensitivity of testicular germ cell tumors is determined by high constitutive Noxa levels mediated by Oct-4. Cancer Res. 2013, 73, 1460–1469. [Google Scholar] [CrossRef]
- Pawinski, A.; Favalli, G.; Ploch, E.; Sahmoud, T.; Van Oosterom, A.T.; Pecorelli, S. PVB chemotherapy in patients with recurrent or advanced dysgerminoma: A phase II study of the EORTC Gynaecological Cancer Cooperative Group. Clin. Oncol. 1998, 10, 301–305. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Morice, P.; Lorusso, D.; Prat, J.; Oaknin, A.; Pautier, P.; Colombo, N.; ESMO Guidelines Committee. Non-epithelial ovarian cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv1–iv18. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhuo, X.; Yang, J.; Cao, D.; Shen, K.; Huang, H.; Wu, M.; Pan, L.; Xiang, Y.; Guo, L. Outcomes and Prognostic Factors of Patients With Recurrent and Persistent Malignant Ovarian Germ Cell Tumors. Arch. Gynecol. Obstet. 2020, 301, 1021–1026. [Google Scholar] [CrossRef]
- Uccello, M.; Boussios, S.; Samartzis, E.P.; Moschetta, M. Systemic anti-cancer treatment in malignant ovarian germ cell tumours (MOGCTs): Current management and promising approaches. Ann. Transl. Med. 2020, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
| Types | Prevalence Among OGCTs | Targeted Age Groups | Diagnostic Markers |
|---|---|---|---|
| Dysgerminomas | Most common type | Mostly occur in adolescence and early childhood, rarely old age | LDH, β-hCG, AP |
| Teratomas | Second most common type | Mostly occur during young age, rarely old age | AFP, β-hCG |
| Yolk-sac tumors | Third most common type | Mostly occur in women with 20–30 years age, rarely old age | Schiller Duval bodies, AFP, LDH, α1-antitrypsin |
| Choriocarcinomas | Rare form | During or after pregnancy | AFP, β-hCG |
| Mixed germ cell tumors | Common form | Different age groups | LDH, AFP, β-hCG |
| Embryonal carcinomas | Rare form | Predominantly occurs in children and adolescents | LDH, AFP, β-hCG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bharti, D.; Tikka, M.; Lee, S.-Y.; Bok, E.-Y.; Lee, H.-J.; Rho, G.-J. Female Germ Cell Development, Functioning and Associated Adversities under Unfavorable Circumstances. Int. J. Mol. Sci. 2021, 22, 1979. https://doi.org/10.3390/ijms22041979
Bharti D, Tikka M, Lee S-Y, Bok E-Y, Lee H-J, Rho G-J. Female Germ Cell Development, Functioning and Associated Adversities under Unfavorable Circumstances. International Journal of Molecular Sciences. 2021; 22(4):1979. https://doi.org/10.3390/ijms22041979
Chicago/Turabian StyleBharti, Dinesh, Manisha Tikka, Sang-Yun Lee, Eun-Yeong Bok, Hyeon-Jeong Lee, and Gyu-Jin Rho. 2021. "Female Germ Cell Development, Functioning and Associated Adversities under Unfavorable Circumstances" International Journal of Molecular Sciences 22, no. 4: 1979. https://doi.org/10.3390/ijms22041979
APA StyleBharti, D., Tikka, M., Lee, S.-Y., Bok, E.-Y., Lee, H.-J., & Rho, G.-J. (2021). Female Germ Cell Development, Functioning and Associated Adversities under Unfavorable Circumstances. International Journal of Molecular Sciences, 22(4), 1979. https://doi.org/10.3390/ijms22041979






