Single Amino Acid Exchange in ACTIN2 Confers Increased Tolerance to Oxidative Stress in Arabidopsis der1–3 Mutant
Abstract
1. Introduction
2. Results
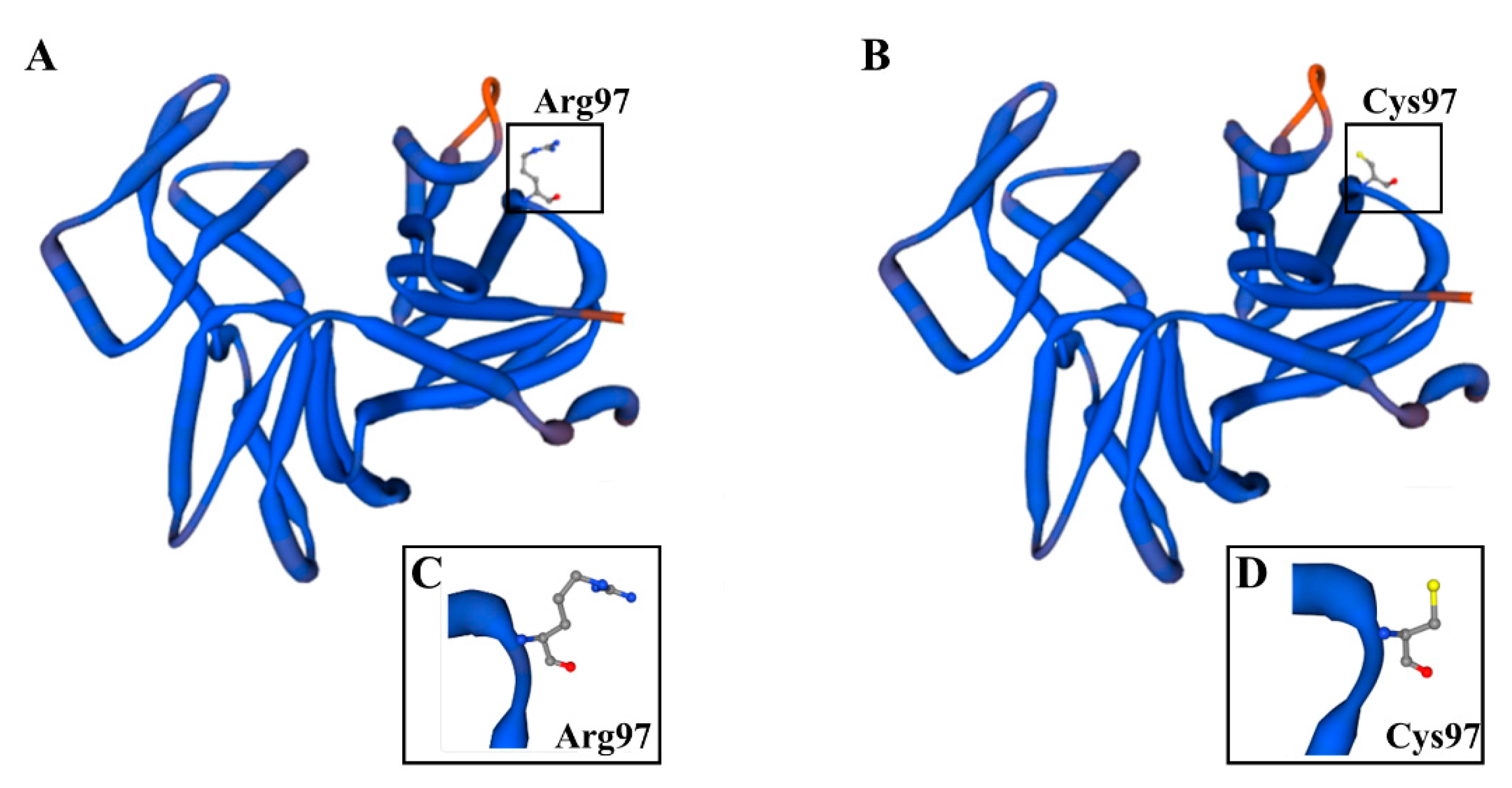
2.1. Impact of the der1–3 Mutation and Its Topology on Protein Tertiary Structure
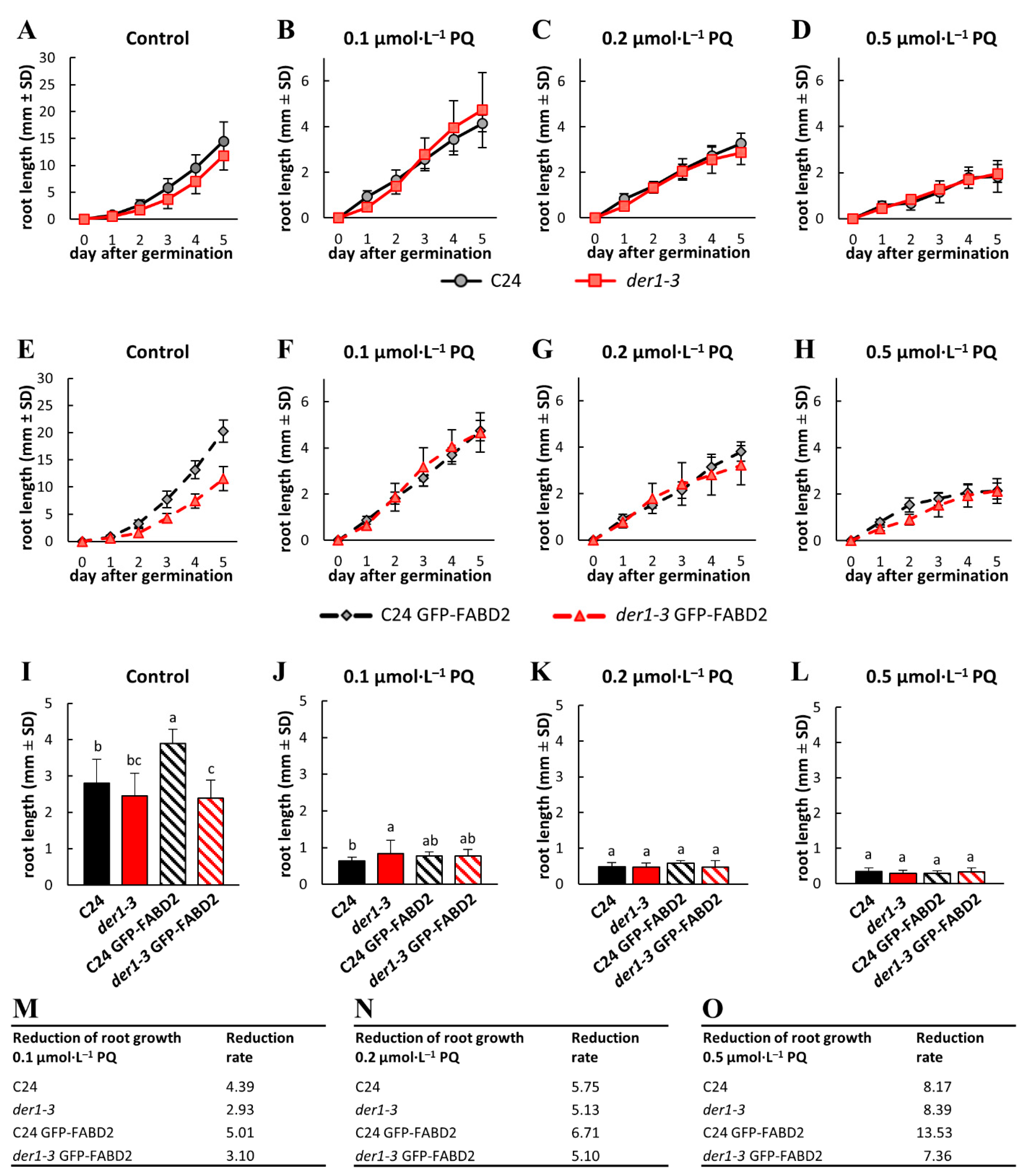
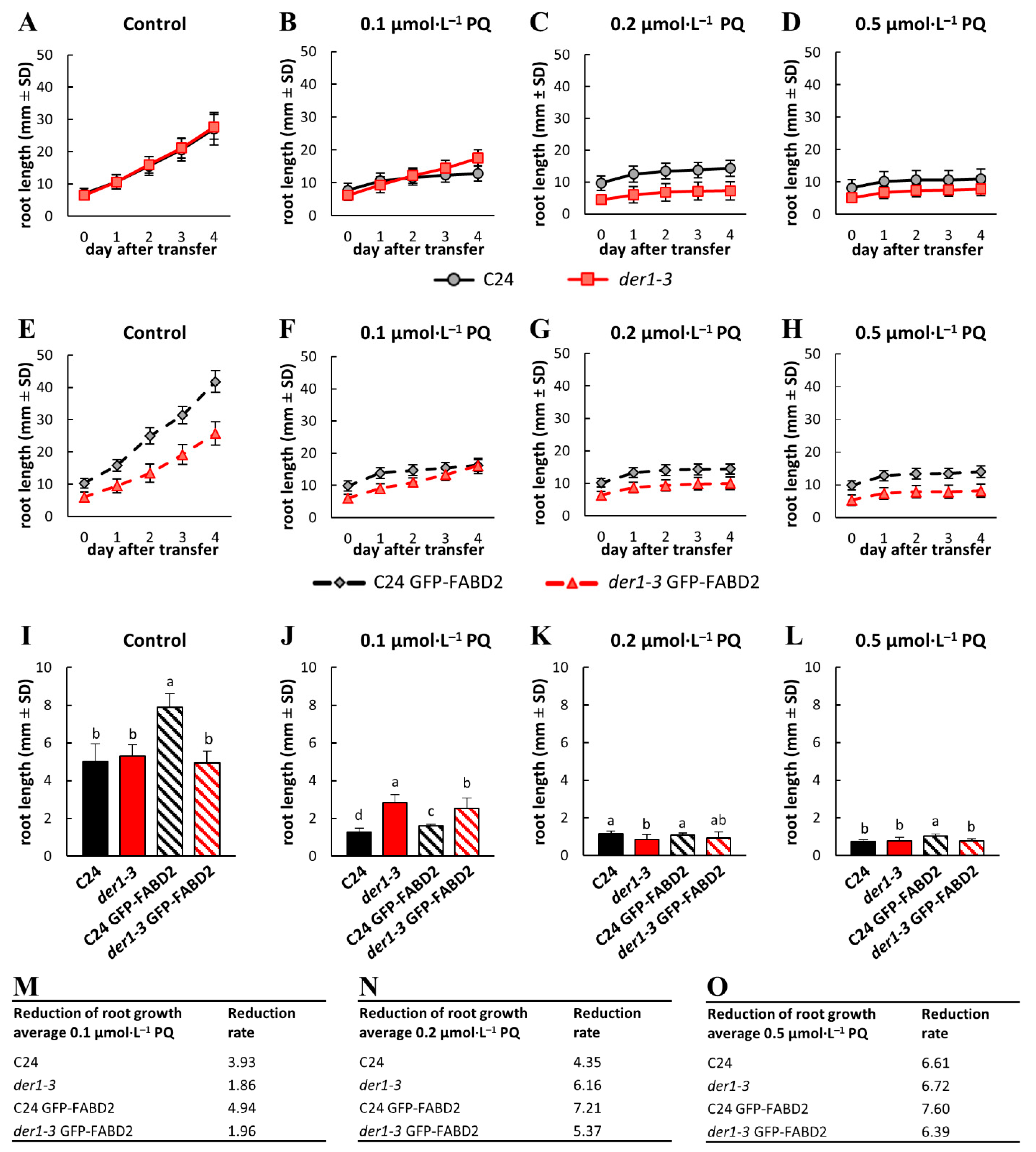
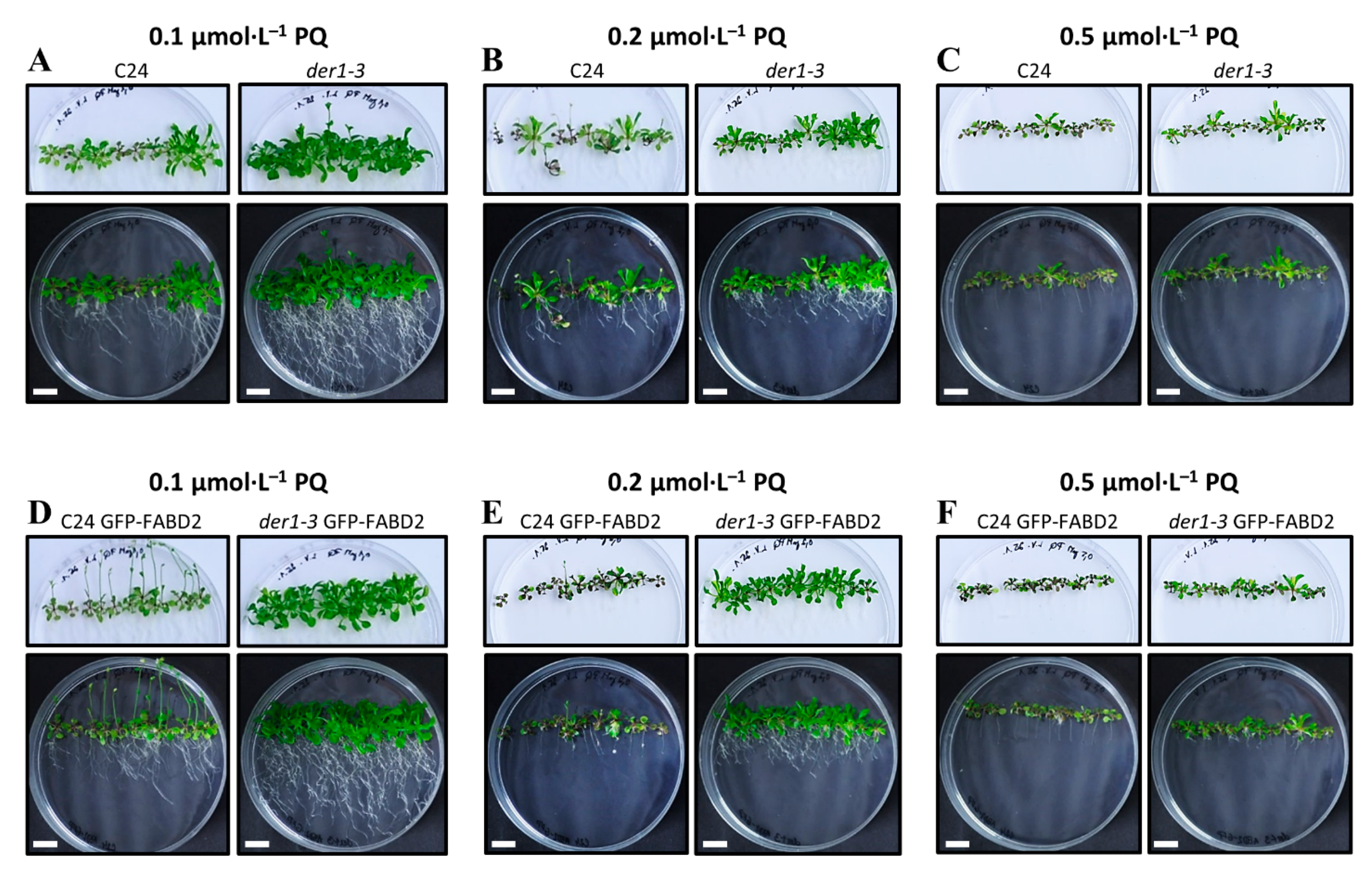
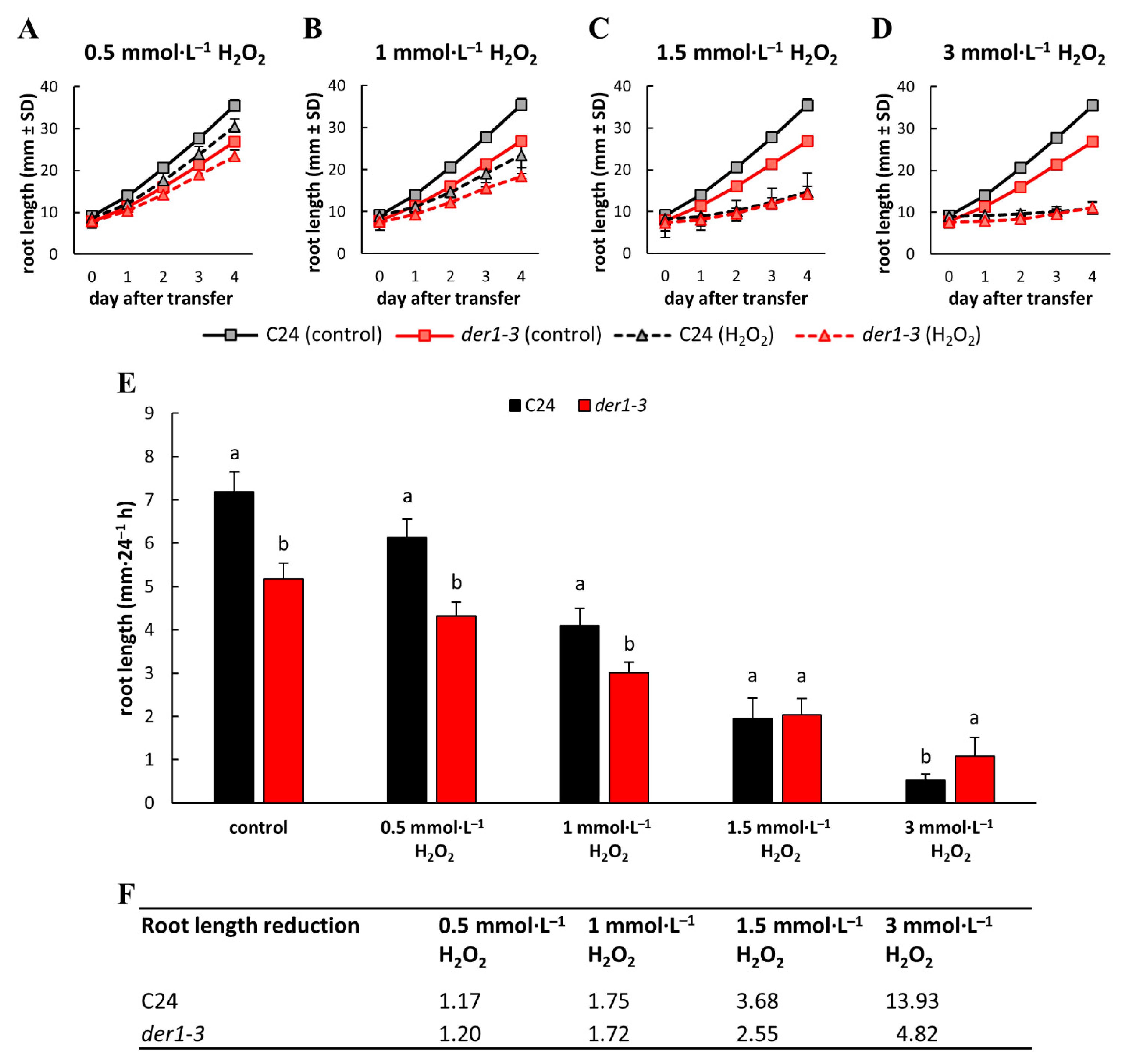
2.2. Influence of Oxidative Stress on Post-Germination Root Growth
2.3. Biomass Production Affected by Oxidative Stress
2.4. Plant Developmental Responses to Oxidative Stress
2.5. Oxidative Stress and Response of the Actin Cytoskeleton
2.6. ROS Production, Lipid Peroxidation and Antioxidant Activity
3. Discussion
4. Materials and Methods
4.1. Plant Material and Cultivation In Vitro
4.2. Transgenic Lines and Transformation Method
4.3. Application of Stress Factors
4.4. Phenotypical Analysis
4.5. Sample Preparation and Microscopic Analysis
4.6. Histochemical Detection of O2•− and H2O2 Production
4.7. Analysis of Enzymatic Activity and Immunoblotting
4.8. TBARS Assay
4.9. Modelling of ACTIN2 Protein Structure
4.10. Data Acquisition and Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van Breusegem, F. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 1360–1385. [Google Scholar] [CrossRef]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as key players in plant stress signalling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef]
- Mhamdi, A.; Van Breusegem, F. Reactive oxygen species in plant development. Development 2018, 145, dev164376. [Google Scholar] [CrossRef]
- Konig, J.; Muthuramalingam, M.; Dietz, K.-J. Mechanisms and dynamics in the thiol/disulfide redox regulatory network: Transmitters, sensors and tar-gets. Curr. Opin. Plant Biol. 2012, 15, 261–268. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox signaling in plants. Antioxid. Redox Signal. 2013, 18, 2087–2090. [Google Scholar] [CrossRef] [PubMed]
- Vaahtera, L.; Brosché, M.; Wrzaczek, M.; Kangasjärvi, J. Specificity in ROS signaling and transcript signatures. Antioxid. Redox Signal. 2014, 21, 1422–1441. [Google Scholar] [CrossRef]
- Mignolet-Spruyt, L.; Xu, E.; Idänheimo, N.; Hoeberichts, F.A.; Mühlenbock, P.; Brosché, M.; Van Breusegem, F.; Kangasjärvi, J. Spreading the news: Subcellular and organellar reactive oxygen species production and signalling. J. Exp. Bot. 2016, 67, 3831–3844. [Google Scholar] [CrossRef]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Vanderauwera, S.; Suzuki, N.; Miller, G.; van de Cotte, B.; Morsa, S.; Ravanat, J.-L.; Hegie, A.; Triantaphylidès, C.; Shulaev, V.; Van Montagu, M.C.E.; et al. Extranuclear protection of chromosomal DNA from oxidative stress. Proc. Natl. Acad. Sci. USA 2011, 108, 1711–1716. [Google Scholar] [CrossRef] [PubMed]
- Dvořák, P.; Krasylenko, Y.; Zeiner, A.; Šamaj, J.; Takáč, T. Signaling Toward Reactive Oxygen Species-Scavenging Enzymes in Plants. Front. Plant Sci. 2021, 11, 618835. [Google Scholar] [CrossRef]
- Riley, D.; Wilkinson, W.; Tucker, B.V. Biological unavailability of bound paraquat residues in soil. In Bound and Conjugated Pesticide Residues; Kaufamn, D., Still, G.G., Paulson, G.D., Bandal, S.K., Eds.; American Chemical Society: Washington, DC, USA, 1976; Volume 29, pp. 301–353. [Google Scholar]
- Hawkes, T.R. Mechanisms of resistance to paraquat in plants. Pest Manag. Sci. 2014, 70, 1316–1323. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A.; Kós, P.B.; Hideg, E. Superoxide anion radicals generated by methylviologen in photosystem I damage photosystem II. Physiol. Plant. 2011, 142, 17–25. [Google Scholar] [CrossRef]
- Farrington, J.A.; Ebert, M.; Land, E.J.; Fletcher, K. Bipyridylium quaternary salts and related compounds. V. Pulse radiolysis studies of the reaction of paraquat radical with oxygen. Implications for the mode of action of bipyridyl herbicides. Biochim. Biophys. Acta Bioenerg. 1973, 314, 372–381. [Google Scholar] [CrossRef]
- Bus, J.S.; Aust, S.D.; Gibson, J.E. Superoxide- and singlet oxygen-catalysed lipid peroxidation as a possible mechanism for paraquat (methyl viologen) toxicity. Biochem. Biophys. Res. Commun. 1974, 58, 749–755. [Google Scholar] [CrossRef]
- Kunert, K.J.; Dodge, A.D. Herbicide-induced radical damage and antioxidative systems. In Target Sites of Herbicide Action, 1st ed.; Boger, P., Sandmann, G., Eds.; CRC Press: Boca Raton, FL, USA, 1989; pp. 49–63. [Google Scholar]
- Han, H.J.; Peng, R.H.; Zhu, B.; Fu, X.Y.; Zhao, W.; Shi, B.; Yao, Q.H. Gene expression profiles of Arabidopsis under the stress of methyl viologen: A microarray analysis. Mol. Biol. Rep. 2014, 41, 7089–7102. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Contento, A.L.; Nguyen, P.Q.; Bassham, D.C. Degradation of oxidized proteins by autophagy during oxidative stress in Arabidopsis. Plant Physiol. 2007, 143, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Maurino, V.G.; Flügge, U.-I. Experimental systems to assess the effects of reactive oxygen species in plant tissues. Plant Signal. Behav. 2008, 3, 923–928. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Levine, A.; Tenhaken, R.; Dixon, R.; Lamb, C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 1994, 79, 583–593. [Google Scholar] [CrossRef]
- Willekens, H.; Chamnongpol, S.; Davey, M.; Schraudner, M.; Langebartels, C.; Van Montagu, M.; Inzé, D.; Van Camp, V. Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. EMBO J. 1997, 16, 4806–4816. [Google Scholar] [CrossRef]
- Dat, J.; Vandenabeele, S.; Vranová, E.; Van Montagu, M.; Inzé, D.; Breusegem, F. Dual action of the active oxygen species during plant stress responses. Cell. Mol. Life Sci. 2000, 57, 779–795. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.W.; Dickinson, B.C.; Chang, C.J. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 15681–15686. [Google Scholar] [CrossRef]
- O’Brien, I.E.W.; Baguley, B.C.; Murray, B.G.; Morris, B.A.M.; Ferguson, I.B. Early stages of the apoptotic pathway in plant cells are reversible. Plant J. 1998, 13, 803–814. [Google Scholar] [CrossRef]
- Yao, N.; Tada, Y.; Park, P.; Nakayashiki, H.; Tosa, Y.; Mayama, S. Novel evidence for apoptotic cell response and differential signals in chromatin condensation and DNA cleavage in victorin-treated oats. Plant J. 2001, 28, 13–26. [Google Scholar] [CrossRef]
- Takáč, T.; Obert, B.; Rolčík, J.; Šamaj, J. Improvement of adventitious root formation in flax using hydrogen peroxide. New Biotechnol. 2016, 33, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Douglas, C.J. Phenylpropanoid metabolism and lignin biosynthesis: From weeds to trees. Trends Plant Sci. 1996, 1, 171–178. [Google Scholar] [CrossRef]
- Mauch-Mani, B.; Slusarenko, A.J. Production of salicylic acid precursors is a major function of phenylalanine ammonia-lyase in the resistance of Arabidopsis to Peronospora parasitica. Plant Cell 1996, 8, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Pickett, C.B.; Lu, A.Y.H. Glutathione S-transferases: Gene structure, regulation, and biological function. Annu. Rev. Biochem. 1989, 58, 743–764. [Google Scholar] [CrossRef]
- Desikan, R.; Reynolds, A.; Hancock, J.T.; Neill, S.J. Harpin and hydrogen peroxide both initiate programmed cell death but have differential effects on defence gene expression in Arabidopsis suspension cultures. Biochem. J. 1998, 330, 115–120. [Google Scholar] [CrossRef]
- Park, H.-J.; Miura, Y.; Kawakita, K.; Yoshioka, H.; Doke, N. Physiological mechanisms of a sub-systemic oxidative burst triggered by elicitor-induced local oxidative burst in potato tuber slices. Plant Cell Physiol. 1998, 39, 1218–1225. [Google Scholar]
- Rao, M.V.; Davis, K.R. Ozone-induced cell death occurs via two distinct mechanisms in Arabidopsis: The role of salicylic acid. Plant J. 1999, 17, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Wasteneys, G.O.; Galway, M.E. Remodeling the cytoskeleton for growth and form: An overview with some new views. Annu. Rev. Plant Biol. 2003, 54, 691–722. [Google Scholar] [CrossRef]
- Staiger, C.J.; Blanchoin, L. Actin dynamics: Old friends with new stories. Curr. Opin. Plant Biol. 2006, 9, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, Z.; Guo, G.; Guo, Y. Microfilament dynamics is required for root growth under alkaline stress in Arabidopsis. J. Integr. Plant Biol. 2010, 52, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Gapper, C.; Kaya, H.; Bell, E.; Kuchitsu, K.; Dolan, L. Local positive feedback regulation determines cell shape in root hair cells. Science 2008, 319, 1241–1244. [Google Scholar] [CrossRef] [PubMed]
- Wallström, S.V.; Aidemarka, M.; Escobar, M.A.; Rasmusson, A.G. An alternatively spliced domain of the NDC1 NAD(P)H dehydrogenase gene strongly influences the expression of the ACTIN2 reference gene in Arabidopsis thaliana. Plant Sci. 2012, 183, 190–196. [Google Scholar] [CrossRef]
- Liu, S.G.; Zhu, D.Z.; Chen, G.H.; Gao, X.-Q.; Zhang, X.S. Disrupted actin dynamics trigger an increment in the reactive oxygen species levels in the Arabidopsis root under salt stress. Plant Cell Rep. 2012, 31, 1219–1226. [Google Scholar] [CrossRef]
- Zwiewka, M.; Bielach, A.; Tamizhselvan, P.; Madhavan, S.; Ryad, E.E.; Tan, S.; Hrtyan, M.; Dobrev, P.; Vanková, R.; Friml, J.; et al. Root adaptation to H2O2-induced oxidative stress by ARF-GEF BEN1- and cytoskeleton-mediated PIN2 trafficking. Plant Cell Physiol. 2019, 60, 255–273. [Google Scholar] [CrossRef]
- Baluška, F.; Salaj, J.; Mathur, J.; Braun, M.; Jasper, F.; Šamaj, J.; Chua, N.H.; Barlow, P.W.; Volkmann, D. Root hair formation: F-actin-dependent tip growth is initiated by local assembly of profilin-supported F-actin meshworks accumulated within expansin-enriched bulges. Dev. Biol. 2000, 227, 618–632. [Google Scholar] [CrossRef]
- Gilliland, L.U.; Kandasamy, M.K.; Pawloski, L.C.; Meagher, R.B. Both vegetative and reproductive actin isovariants complement the stunted root hair phenotype of the Arabidopsis act2-1 mutation. Plant Physiol. 2002, 130, 2199–2209. [Google Scholar] [CrossRef]
- Ringli, C.; Baumberger, N.; Diet, A.; Frey, B.; Keller, B. ACTIN2 is essential for bulge site selection and tip growth during root hair development of Arabidopsis. Plant Physiol. 2002, 129, 1464–1472. [Google Scholar] [CrossRef]
- McDowell, J.M.; Huang, S.R.; McKinney, E.C.; An, Y.Q.; Meagher, R.B. Structure and evolution of the actin gene family in Arabidopsis thaliana. Genetics 1996, 142, 587–602. [Google Scholar] [CrossRef] [PubMed]
- Meagher, R.B.; McKinney, E.C.; Vitale, A.V. The evolution of new structures: Clues from plant cytoskeletal genes. Trends Genet. 1999, 15, 278–284. [Google Scholar] [CrossRef]
- Vaškebová, L.; Šamaj, J.; Ovečka, M. Single-point ACT2 gene mutation in the Arabidopsis root hair mutant der1-3 affects overall actin organization, root growth and plant development. Ann. Bot. 2018, 122, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Ringli, C.; Baumberger, N.; Keller, B. The Arabidopsis root hair mutants der2–der9 are affected at different stages of root hair development. Plant Cell Physiol. 2005, 46, 1046–1053. [Google Scholar] [CrossRef]
- Sunkar, R.; Kapoor, A.; Zhu, J.-K. Posttranscriptional induction of two Cu/Zn superoxide dismutase gene in Arabidopsis is mediated by downregulation of miR398 and important for oxidative stress tolerance. Plant Cell 2006, 18, 2051–2065. [Google Scholar] [CrossRef]
- Dvořák, P.; Krasylenko, Y.; Ovečka, M.; Basheer, J.; Zapletalová, V.; Šamaj, J.; Takáč, T. In vivo light-sheet microscopy resolves localisation patterns of FSD1, a superoxide dismutase with function in root development and osmoprotection. Plant Cell Environ. 2021, 44, 68–87. [Google Scholar]
- Lamkemeyer, P.; Laxa, M.; Collin, V.; Li, W.; Finkemeier, I.; Schöttler, M.A.; Holtkamp, V.; Tognetii, V.B.; Issakidis-Bourguet, E.; Kandlbinder, A.; et al. Peroxiredoxin Q of Arabidopsis thaliana is attached to the thylakoids and functions in context of photosynthesis. Plant J. 2006, 45, 968–981. [Google Scholar] [CrossRef]
- Yoshida, K.; Hara, S.; Hisabori, T. Thioredoxin selectivity for thiol-based redox regulation of target proteins in chloroplasts. J. Biol. Chem. 2015, 290, 14278–14288. [Google Scholar] [CrossRef]
- Dietz, K.-J.; Jacob, S.; Oelze, M.-L.; Laxa, M.; Tognetti, V.; Nunes de Miranda, S.M.; Baier, M.; Finkemeier, I. The function of peroxiredoxins in plant organelle redox metabolism. J. Exp. Bot. 2006, 57, 1697–1709. [Google Scholar] [CrossRef]
- Pandya-Kumar, N.; Shema, R.; Kumar, M.; Mayzlish-Gati, E.; Levy, D.; Zemach, H.; Belausov, E.; Wininger, S.; Abu-Abied, M.; Kapulnik, Y.; et al. Strigolactone analog GR24 triggers changes in PIN2 polarity, vesicle trafficking and actin filament architecture. New Phytol. 2014, 202, 1184–1196. [Google Scholar] [CrossRef]
- Diet, A.; Brunner, S.; Ringli, C. The enl mutants enhance the lrx1 root hair mutant phenotype of Arabidopsis thaliana. Plant Cell Physiol. 2004, 45, 734–741. [Google Scholar] [CrossRef]
- Kabsch, W.; Mannherz, H.G.; Suck, D.; Pai, E.F.; Holmes, K.C. Atomic structure of the actin: DNAse-I complex. Nature 1990, 347, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Mouratou, B.; Biou, V.; Joubert, A.; Cohen, J.; Shields, D.J.; Geldner, N.; Jürgens, G.; Melançon, P.; Cherfils, J. The domain architecture of large guanine nucleotide exchange factors for the small GTP-binding protein Arf. BMC Genom. 2005, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, S.; Lu, Y.; Alvarez, S.; Hicks, L.M.; Ge, X.; Xia, Y. Proteomic analysis of early-responsive redox-sensitive proteins in Arabidopsis. J. Proteome Res. 2012, 11, 412–424. [Google Scholar] [CrossRef]
- Lian, N.; Wang, X.; Jing, Y.; Lin, J. Regulation of Cytoskeleton-associated Protein Activities: Linking Cellular Signals to Plant Cytoskeletal Function. J. Integr. Plant Biol. 2021, 63, 241–250. [Google Scholar] [CrossRef]
- Matoušková, J.; Janda, M.; Fišer, R.; Šašek, V.; Kocourková, D.; Burketová, L.; Dušková, J.; Martinec, J.; Valentová, O. Changes in Actin Dynamics Are Involved in Salicylic Acid Signaling Pathway. Plant Sci. 2014, 223, 36–44. [Google Scholar] [CrossRef]
- Takáč, T.; Bekešová, S.; Šamaj, J. Actin Depolymerization-Induced Changes in Proteome of Arabidopsis Roots. J. Proteom. 2017, 153, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, J.-H.; Wang, W.; Chen, N.-Z.; Ma, T.-S.; Xi, Y.-N.; Zhang, X.-L.; Lin, H.-F.; Bai, Y.; Huang, S.-J.; et al. ARP2/3 Complex-Mediated Actin Dynamics Is Required for Hydrogen Peroxide-Induced Stomatal Closure in Arabidopsis: H2O2 and Actin Dynamics in ABA Signalling. Plant Cell Environ. 2014, 37, 1548–1560. [Google Scholar] [CrossRef]
- Li, J.; Cao, L.; Staiger, C.J. Capping Protein Modulates Actin Remodeling in Response to Reactive Oxygen Species during Plant Innate Immunity. Plant Physiol. 2017, 173, 1125–1136. [Google Scholar] [CrossRef]
- Frémont, S.; Hammich, H.; Bai, J.; Wioland, H.; Klinkert, K.; Rocancourt, M.; Kikuti, C.; Stroebel, D.; Romet-Lemonne, G.; Pylypenko, O.; et al. Oxidation of F-Actin Controls the Terminal Steps of Cytokinesis. Nat. Commun. 2017, 8, 14528. [Google Scholar] [CrossRef] [PubMed]
- Sakai, J.; Li, J.; Subramanian, K.K.; Mondal, S.; Bajrami, B.; Hattori, H.; Jia, Y.; Dickinson, B.C.; Zhong, J.; Ye, K.; et al. Reactive Oxygen Species-Induced Actin Glutathionylation Controls Actin Dynamics in Neutrophils. Immunity 2012, 37, 1037–1049. [Google Scholar] [CrossRef]
- Mustafa, A.K.; Gadalla, M.M.; Sen, N.; Kim, S.; Mu, W.; Gazi, S.K.; Barrow, R.K.; Yang, G.; Wang, R.; Snyder, S.H. H2S Signals Through Protein S-Sulfhydration. Sci. Signal. 2009, 2, ra72. [Google Scholar] [CrossRef] [PubMed]
- Stojkov, D.; Amini, P.; Oberson, K.; Sokollik, C.; Duppenthaler, A.; Simon, H.-U.; Yousefi, S. ROS and Glutathionylation Balance Cytoskeletal Dynamics in Neutrophil Extracellular Trap Formation. J. Cell Biol. 2017, 216, 4073–4090. [Google Scholar] [CrossRef] [PubMed]
- Gellert, M.; Hanschmann, E.-M.; Lepka, K.; Berndt, C.; Lillig, C.H. Redox Regulation of Cytoskeletal Dynamics during Differentiation and De-Differentiation. Biochim. Biophys. Acta (BBA) Gen. Subj. 2015, 1850, 1575–1587. [Google Scholar] [CrossRef]
- Mashima, T.; Naito, M.; Noguchi, K.; Miller, D.K.; Nicholson, D.W.; Tsuruo, T. Actin Cleavage by CPP-32/Apopain during the Development of Apoptosis. Oncogene 1997, 14, 1007–1012. [Google Scholar] [CrossRef]
- Mashima, T.; Naito, M.; Tsuruo, T. Caspase-Mediated Cleavage of Cytoskeletal Actin Plays a Positive Role in the Process of Morphological Apoptosis. Oncogene 1999, 18, 2423–2430. [Google Scholar] [CrossRef] [PubMed]
- Beemster, G.T.S.; De Vusser, K.; De Tavernier, E.; De Bock, K.; Inze, D. Variation in growth rate between Arabidopsis ecotypes is correlated with cell division and A-type cyclin-dependent kinase activity1. Plant Physiol. 2002, 129, 854–864. [Google Scholar] [CrossRef]
- Voigt, B.; Timmers, A.C.J.; Šamaj, J.; Müller, J.; Baluška, F.; Menzel, D. GFP-FABD2 fusion construct allows in vivo visualization of the dynamic actin cytoskeleton in all cells of Arabidopsis seedlings. Eur. J. Cell Biol. 2005, 84, 595–608. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Ovečka, M.; Lang, I.; Baluška, F.; Ismail, A.; Illeš, P.; Lichtscheidl, I.K. Endocytosis and vesicle trafficking during tip growth of root hairs. Protoplasma 2005, 226, 39–54. [Google Scholar] [CrossRef]
- Ramel, F.; Sulmon, C.; Bogard, M.; Couée, I.; Gouesbet, G. Differential patterns of reactive oxygen species and antioxidative mechanisms during atrazine injury and sucrose-induced tolerance in Arabidopsis thaliana plantlets. BMC Plant Biol. 2009, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Daudi, A.; Cheng, Z.; O’Brien, J.A.; Mammarella, N.; Khan, S.; Ausubel, F.M.; Bolwell, G.P. The apoplastic oxidative burst peroxidase in Arabidopsis is a major component of pattern-triggered immunity. Plant Cell 2012, 24, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Takáč, T.; Šamajová, O.; Vadovič, P.; Pechan, T.; Košútová, P.; Ovečka, M.; Husičková, A.; Komis, G.; Šamaj, J. Proteomic and biochemical analyses show a functional network of proteins involved in antioxidant defense of the Arabidopsis anp2anp3 double mutant. J. Proteome Res. 2014, 13, 5347–5361. [Google Scholar]
- Yoshida, K.; Hisabori, T. Two Distinct Redox Cascades Cooperatively Regulate Chloroplast Functions and Sustain Plant Viability. Proc. Natl. Acad. Sci. USA 2016, 113, E3967–E3976. [Google Scholar] [CrossRef]
- Takáč, T.; Šamajová, O.; Luptovčiak, I.; Pechan, T.; Šamaj, J. Feedback microtubule control and microtubule-actin cross-talk in Arabidopsis revealed by integrative proteomic and cell biology analysis of KATANIN 1 mutants. Mol. Cell. Proteom. 2017, 16, 1591–1609. [Google Scholar] [CrossRef]
- Larkindale, J.; Knight, M.R. Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol. 2002, 128, 682–695. [Google Scholar] [CrossRef]
- Pallotta, M.A.; Graham, R.D.; Langridge, P.; Sparrow, D.H.B.; Barker, S.J. RFLP mapping of manganese efficiency in barley. Theor. Appl. Genet. 2000, 101, 1100–1108. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuběnová, L.; Takáč, T.; Šamaj, J.; Ovečka, M. Single Amino Acid Exchange in ACTIN2 Confers Increased Tolerance to Oxidative Stress in Arabidopsis der1–3 Mutant. Int. J. Mol. Sci. 2021, 22, 1879. https://doi.org/10.3390/ijms22041879
Kuběnová L, Takáč T, Šamaj J, Ovečka M. Single Amino Acid Exchange in ACTIN2 Confers Increased Tolerance to Oxidative Stress in Arabidopsis der1–3 Mutant. International Journal of Molecular Sciences. 2021; 22(4):1879. https://doi.org/10.3390/ijms22041879
Chicago/Turabian StyleKuběnová, Lenka, Tomáš Takáč, Jozef Šamaj, and Miroslav Ovečka. 2021. "Single Amino Acid Exchange in ACTIN2 Confers Increased Tolerance to Oxidative Stress in Arabidopsis der1–3 Mutant" International Journal of Molecular Sciences 22, no. 4: 1879. https://doi.org/10.3390/ijms22041879
APA StyleKuběnová, L., Takáč, T., Šamaj, J., & Ovečka, M. (2021). Single Amino Acid Exchange in ACTIN2 Confers Increased Tolerance to Oxidative Stress in Arabidopsis der1–3 Mutant. International Journal of Molecular Sciences, 22(4), 1879. https://doi.org/10.3390/ijms22041879







