In Vitro and In Vivo Efficacy of a Novel Glucose–Methotrexate Conjugate in Targeted Cancer Treatment
Abstract
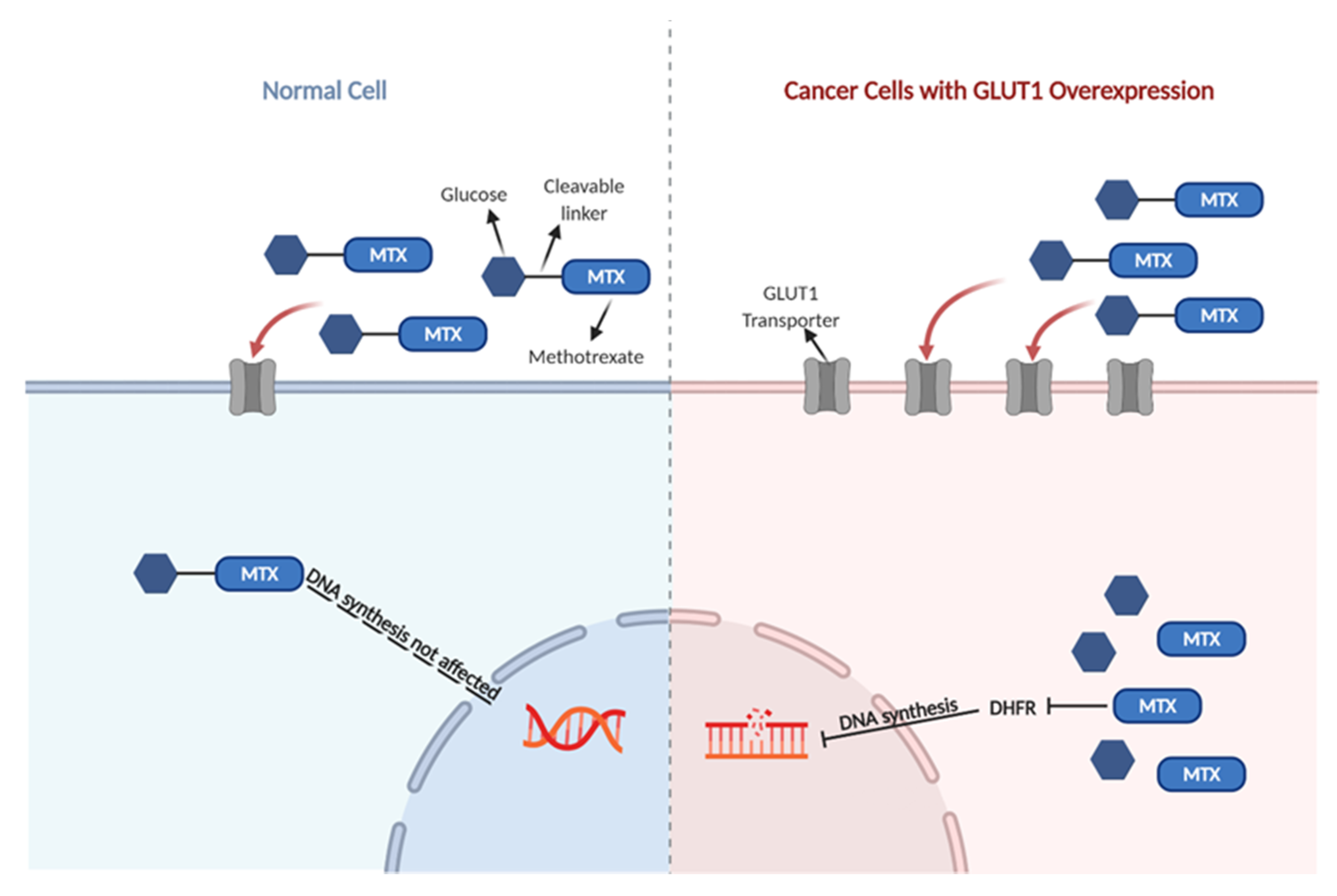
1. Introduction
2. Results
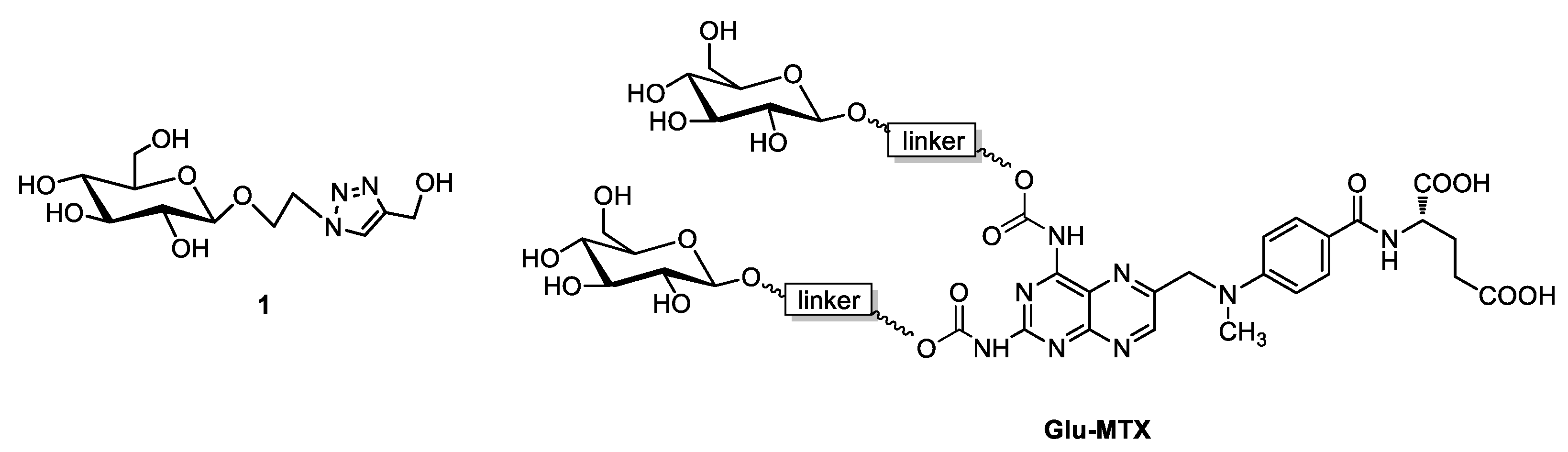
2.1. Synthesis of Sugar Derivative Emerging from GLU–MTX Conjugate Hydrolysis in Tumor Cells
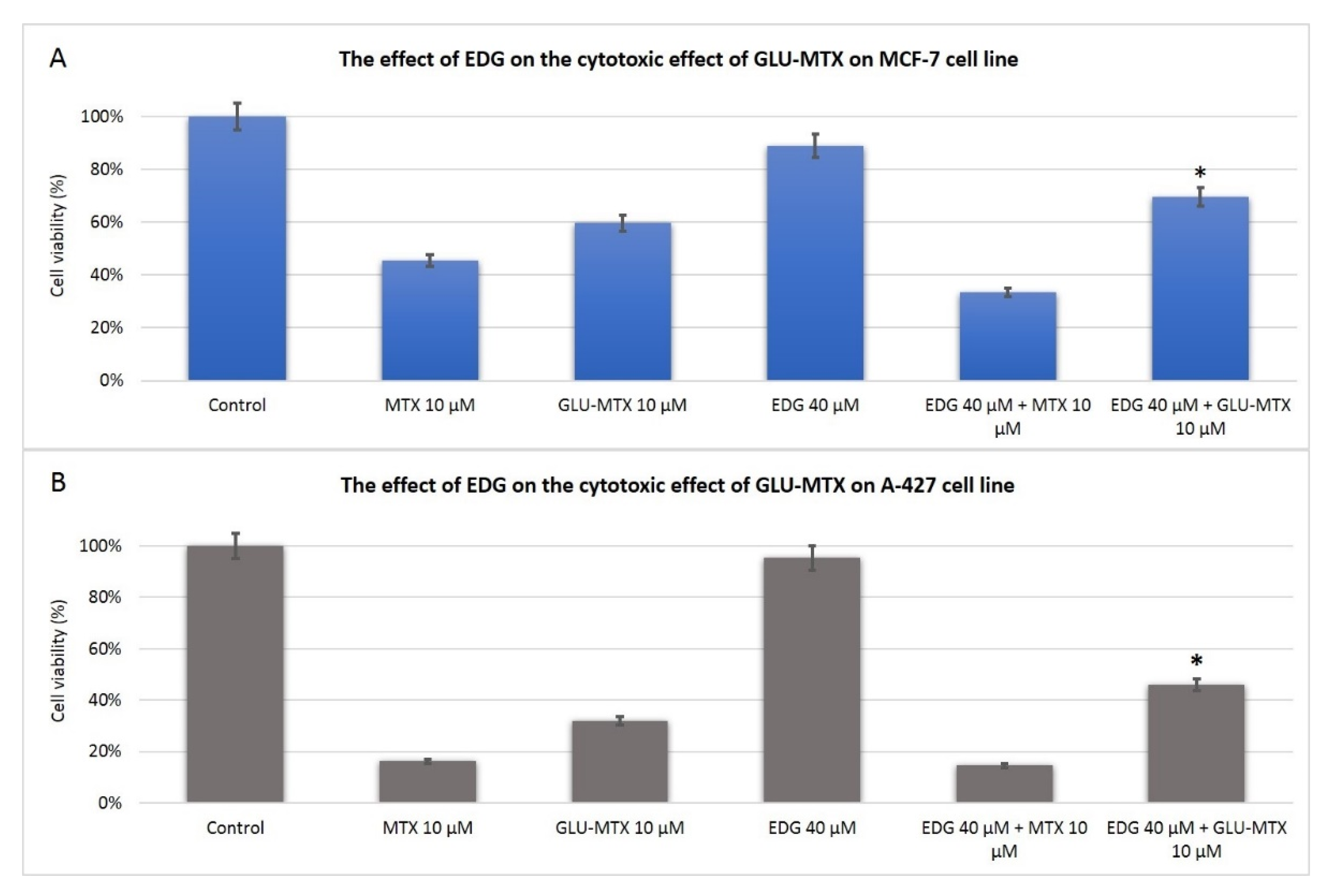
2.2. The Cytotoxic Effect of GLU–MTX Is Reversed by GLUT1 Inhibitor
2.3. Cellular Uptake of GLU–MTX Is Significantly Higher in SW-480 Colon Cancer Cells Compared to Free MTX
2.4. Both MTX and GLU–MTX Lead to Cell Cycle Arrest in S Phase
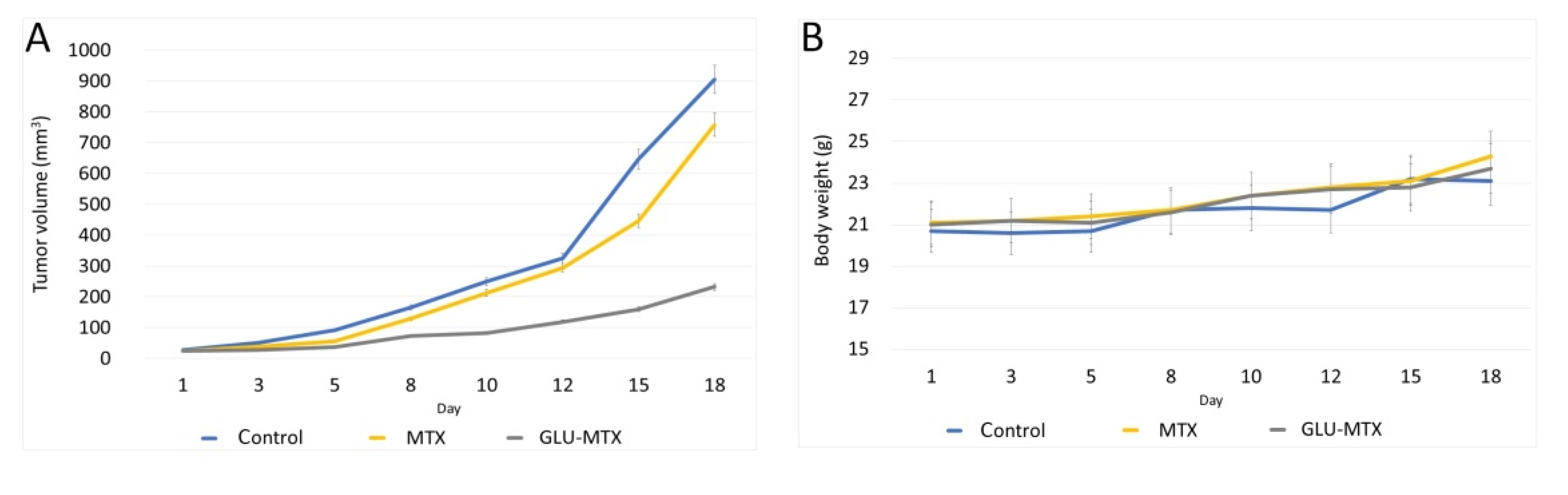
2.5. In Vivo Efficacy of GLU–MTX
3. Discussion
4. Materials and Methods
4.1. Chemistry
Synthesis of Glycoconjugate
4.2. Cell Culture
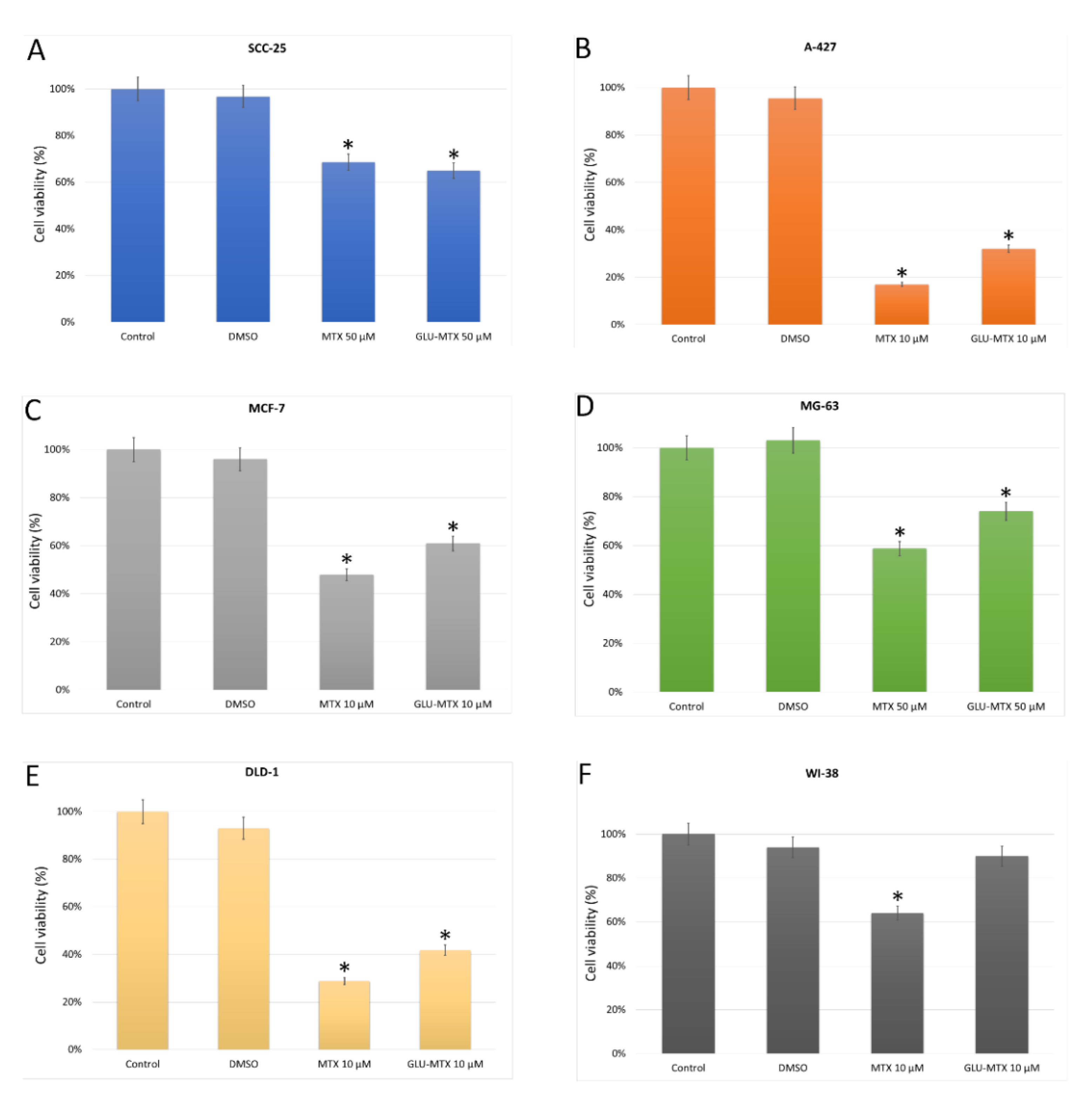
4.3. In Vitro Cytotoxicity of MTX and GLU–MTX
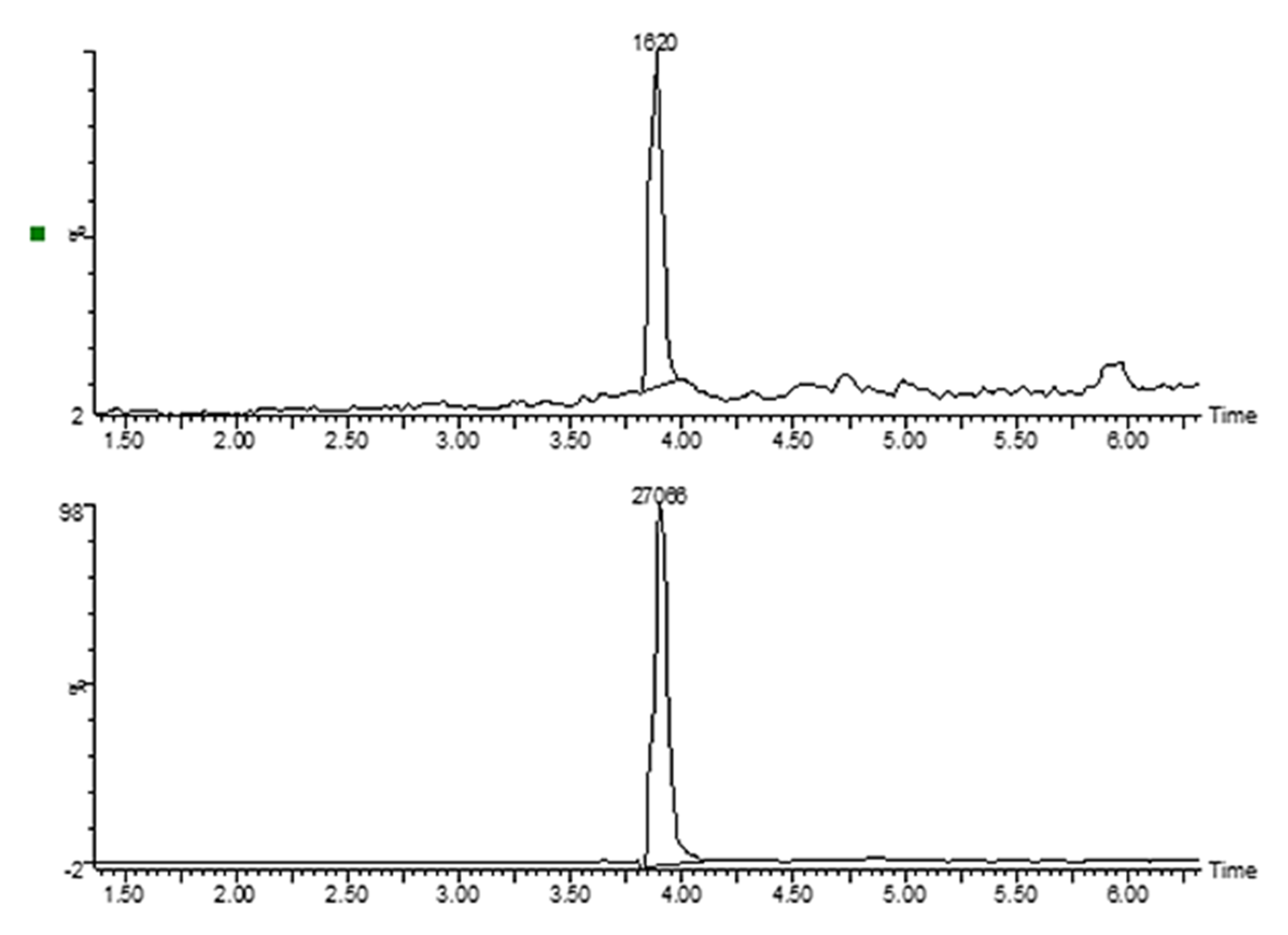
4.4. Measurement of Cellular Uptake of MTX and GLU–MTX by Mass Spectrometry
4.5. LC/MS Analysis
4.5.1. Equipment
4.5.2. LC Conditions
4.5.3. MS Conditions
4.6. Cell Cycle Analysis
4.7. Mouse Allograft Model of Human Breast Cancer
4.8. In Vivo Chemotherapy
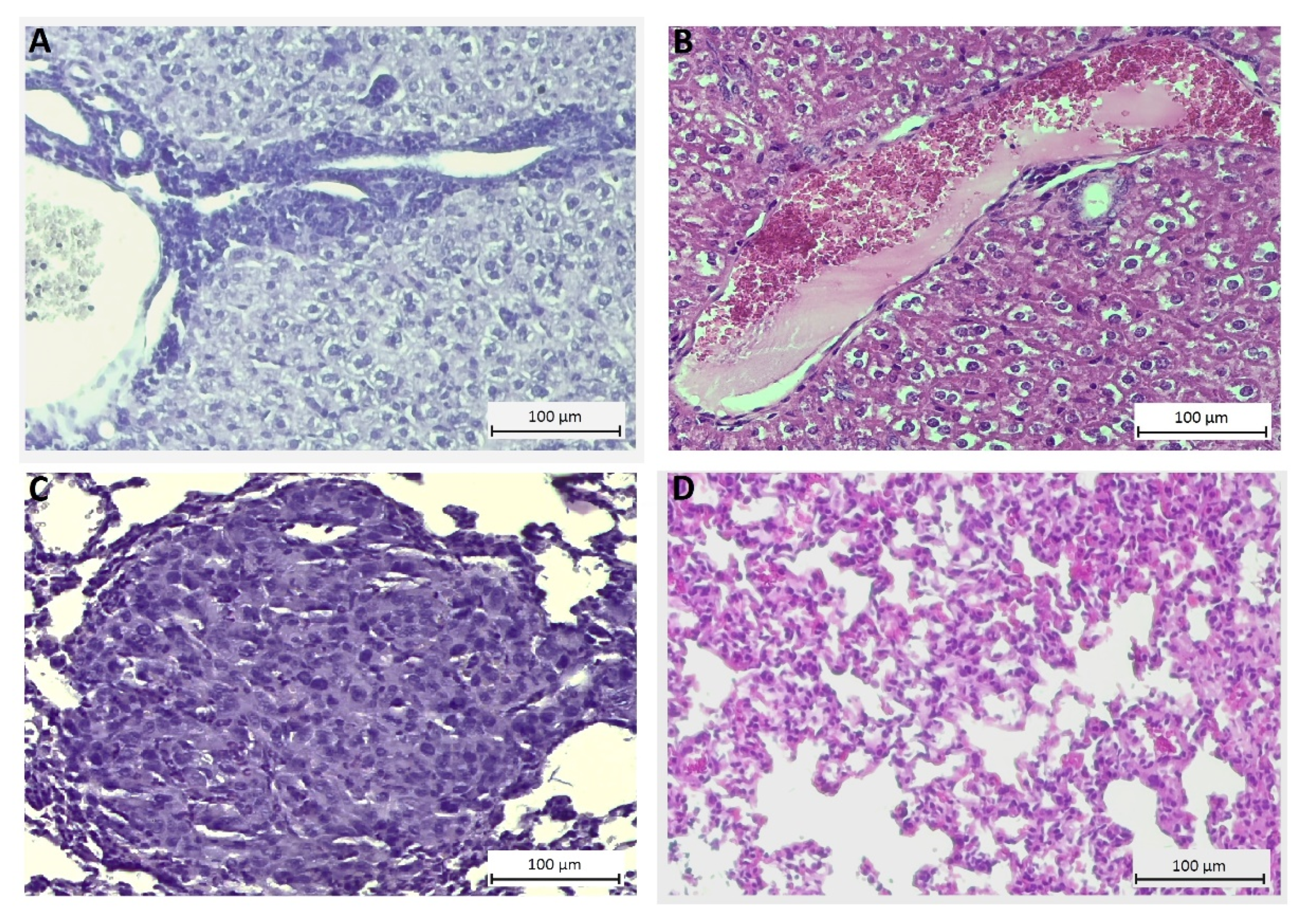
4.9. Histological Evaluation of Toxicity
4.10. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, Z.A.; Tripathi, R.; Mishra, B. Methotrexate: A detailed review on drug delivery and clinical aspects. Expert Opin. Drug Deliv. 2012, 9, 151–169. [Google Scholar] [CrossRef]
- Howard, S.C.; McCormick, J.; Pui, C.; Buddington, R.K.; Harvey, R.D. Preventing and Managing Toxicities of High-Dose Methotrexate. Oncologist 2016, 21, 1471–1482. [Google Scholar] [CrossRef] [PubMed]
- Świerkot, J. Toxicity of low dose methotrexate in rheumatoid arthritis. Adv. Clin. Exp. Med. 2007, 16, 287–295. [Google Scholar]
- Abolmaali, S.S.; Tamaddon, A.M.; Dinarvand, R. A review of therapeutic challenges and achievements of methotrexate delivery systems for treatment of cancer and rheumatoid arthritis. Cancer Chemother. Pharmacol. 2013, 71, 1115–1130. [Google Scholar] [CrossRef] [PubMed]
- Mahato, R.; Tai, W.; Cheng, K. Prodrugs for improving tumor targetability and efficiency. Adv. Drug Deliv. Rev. 2011, 63, 659–670. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Calvaresi, E.C.; Hergenrother, P.J. Glucose conjugation for the specific targeting and treatment of cancer. Chem. Sci. 2013, 4, 2319–2333. [Google Scholar] [CrossRef]
- Makuch, S.; Wozniak, M.; Krawczyk, M.; Pastuch-Gawołek, G.; Szeja, W.; Agrawal, S. Glycoconjugation as a promising treatment strategy for psoriasis. J. Pharmacol. Exp. Ther. 2020, 373, 204–212. [Google Scholar] [CrossRef]
- Patra, M.; Awuah, S.G.; Lippard, S.J. Chemical Approach to Positional Isomers of Glucose-Platinum Conjugates Reveals Specific Cancer Targeting through Glucose-Transporter-Mediated Uptake in Vitro and in Vivo. J. Am. Chem. Soc. 2016, 138, 12541–12551. [Google Scholar] [CrossRef]
- Agrawal, S.; Wozniak, M.; Luc, M.; Walaszek, K.; Pielka, E.; Szeja, W.; Pastuch-Gawolek, G.; Gamian, A.; Ziolkowski, P. Insulin and novel thioglycosides exert suppressive effect on human breast and colon carcinoma cells. Oncotarget 2017, 8, 114173–114182. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, W.; Idowu, M.O.; Oh, U.; Wang, X.Y.; Temkin, S.M.; Fang, X. Ovarian cancer relies on glucose transporter 1 to fuel glycolysis and growth: Anti-tumor activity of BAY-876. Cancers 2019, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Dyshlovoy, S.A.; Pelageev, D.N.; Hauschild, J.; Borisova, K.L.; Kaune, M.; Krisp, C.; Venz, S.; Sabutskii, Y.E.; Khmelevskaya, E.A.; Busenbender, T.; et al. Successful targeting of thewarburg effect in prostate cancer by glucose-conjugated 1,4-naphthoquinones. Cancers 2019, 11, 1609. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.M.; Martel, F. Targeting glucose transporters for breast cancer therapy: The effect of natural and synthetic compounds. Cancers 2020, 12, 154. [Google Scholar] [CrossRef] [PubMed]
- Tomaszowski, K.-H.; Hellmann, N.; Ponath, V.; Takatsu, H.; Shin, H.-W.; Kaina, B. Uptake of glucose-conjugated MGMT inhibitors in cancer cells: Role of flippases and type IV P-type ATPases. Sci. Rep. 2017, 7, 13925. [Google Scholar] [CrossRef]
- Pohl, J.; Bertram, B.; Hilgard, P.; Nowrousian, M.R.; Stüben, J.; Wießler, M. D-19575-a sugar-linked isophosphoramide mustard derivative exploiting transmembrane glucose transport. Cancer Chemother. Pharmacol. 1995, 35, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, M.; Pastuch-Gawołek, G.; Makuch, S.; Wiśniewski, J.; Ziółkowski, P.; Szeja, W.; Krawczyk, M.; Agrawal, S. Overcoming hypoxia-induced chemoresistance in cancer using a novel glycoconjugate of methotrexate. Pharmaceuticals 2021, 14, 13. [Google Scholar] [CrossRef]
- Patra, M.; Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. A Potent Glucose-Platinum Conjugate Exploits Glucose Transporters and Preferentially Accumulates in Cancer Cells. Angew. Chem. Int. Ed. 2016, 55, 2550–2554. [Google Scholar] [CrossRef]
- Young, C.D.; Lewis, A.S.; Rudolph, M.C.; Ruehle, M.D.; Jackman, M.R.; Yun, U.J.; Ilkun, O.; Pereira, R.; Abel, E.D.; Anderson, S.M. Modulation of glucose transporter 1 (GLUT1) expression levels alters mouse mammary tumor cell growth in vitro and in vivo. PLoS ONE 2011, 6, e23205. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.; Supuran, C.; Alfarouk, K. The Warburg Effect and the Hallmarks of Cancer. Anticancer Agents Med. Chem. 2017, 17, 164–170. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Yang, J.; Seeberger, P.H.; Yin, J. Glycoconjugates for glucose transporter-mediated cancer-specific targeting and treatment. Carbohydr. Res. 2020, 498, 108195. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Yan, N. GLUT, SGLT, and SWEET: Structural and mechanistic investigations of the glucose transporters. Protein Sci. 2016, 25, 546–558. [Google Scholar] [CrossRef] [PubMed]
- El Hilali, M.; Reux, B.; Debiton, E.; Leal, F.; Galmier, M.J.; Vivier, M.; Chezal, J.M.; Miot-Noirault, E.; Coudert, P.; Weber, V. Linker structure-activity relationships in fluorodeoxyglucose chlorambucil conjugates for tumor-targeted chemotherapy. Bioorg. Med. Chem. 2017, 25, 5692–5708. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, M.; Pastuch-Gawołek, G.; Pluta, A.; Erfurt, K.; Domiński, A.; Kurcok, P. 8-hydroxyquinoline glycoconjugates: Modifications in the linker structure and their effect on the cytotoxicity of the obtained compounds. Molecules 2019, 24, 4181. [Google Scholar] [CrossRef] [PubMed]
- Zemplén, G.; Pacsu, E. Über die Verseifung acetylierter Zucker und verwandter Substanzen. Ber. Dtsch. Chem. Ges. (A B Ser.) 1929, 62, 1613–1614. [Google Scholar] [CrossRef]

| G0/G1 | S | G2/M | |
|---|---|---|---|
| Control | 65.4 ± 2.1 | 22.99 ± 0.8 | 10.7 ± 0.6 |
| MTX | 23.05 ± 1.4 | 50.97 ± 2.3 | 17.17 ± 1.3 |
| GLU–MTX | 23.83 ± 1.7 | 50.61 ± 1.9 | 16.11 ± 1.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woźniak, M.; Pastuch-Gawołek, G.; Makuch, S.; Wiśniewski, J.; Krenács, T.; Hamar, P.; Gamian, A.; Szeja, W.; Szkudlarek, D.; Krawczyk, M.; et al. In Vitro and In Vivo Efficacy of a Novel Glucose–Methotrexate Conjugate in Targeted Cancer Treatment. Int. J. Mol. Sci. 2021, 22, 1748. https://doi.org/10.3390/ijms22041748
Woźniak M, Pastuch-Gawołek G, Makuch S, Wiśniewski J, Krenács T, Hamar P, Gamian A, Szeja W, Szkudlarek D, Krawczyk M, et al. In Vitro and In Vivo Efficacy of a Novel Glucose–Methotrexate Conjugate in Targeted Cancer Treatment. International Journal of Molecular Sciences. 2021; 22(4):1748. https://doi.org/10.3390/ijms22041748
Chicago/Turabian StyleWoźniak, Marta, Gabriela Pastuch-Gawołek, Sebastian Makuch, Jerzy Wiśniewski, Tibor Krenács, Peter Hamar, Andrzej Gamian, Wiesław Szeja, Danuta Szkudlarek, Monika Krawczyk, and et al. 2021. "In Vitro and In Vivo Efficacy of a Novel Glucose–Methotrexate Conjugate in Targeted Cancer Treatment" International Journal of Molecular Sciences 22, no. 4: 1748. https://doi.org/10.3390/ijms22041748
APA StyleWoźniak, M., Pastuch-Gawołek, G., Makuch, S., Wiśniewski, J., Krenács, T., Hamar, P., Gamian, A., Szeja, W., Szkudlarek, D., Krawczyk, M., & Agrawal, S. (2021). In Vitro and In Vivo Efficacy of a Novel Glucose–Methotrexate Conjugate in Targeted Cancer Treatment. International Journal of Molecular Sciences, 22(4), 1748. https://doi.org/10.3390/ijms22041748







