Maternal Low-Grade Chronic Inflammation and Intrauterine Programming of Health and Disease
Abstract
1. Introduction
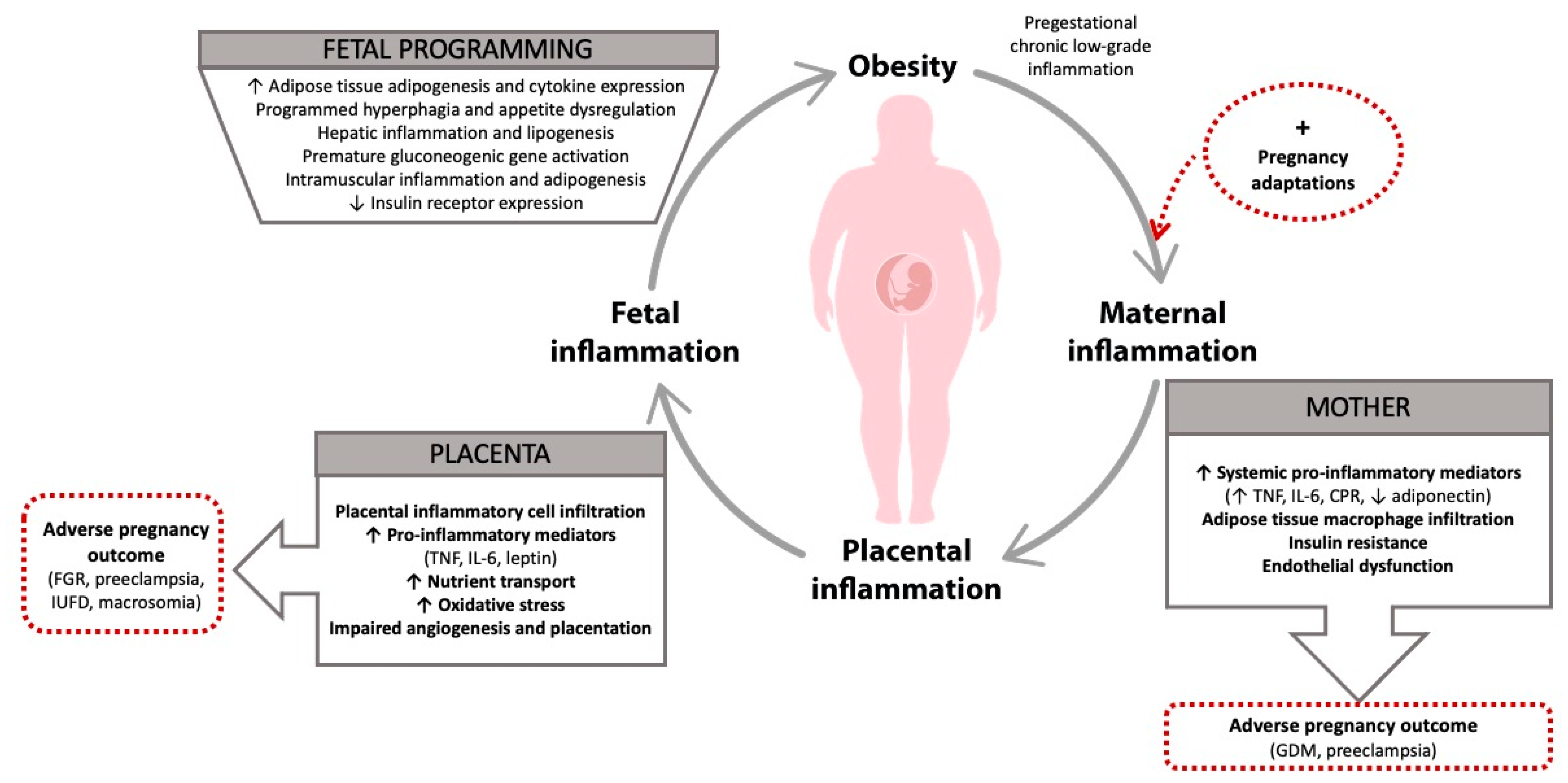
2. Pathobiology of Maternal Obesity
2.1. Maternal Systemic Low-Grade Inflammation
2.2. Placental Inflammation
2.3. Fetal Inflammation
3. Maternal Obesity-Related Inflammation and Developmental Programming
3.1. Evidence from Animal Models
3.2. Evidence from Human Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mor, G.; Cardenas, I.; Abrahams, V.; Guller, S. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann. N. Y. Acad. Sci. 2011, 1221, 80–87. [Google Scholar] [CrossRef] [PubMed]
- St-Germain, L.E.; Castellana, B.; Baltayeva, J.; Beristain, A.G. Maternal Obesity and the Uterine Immune Cell Landscape: The Shaping Role of Inflammation. Int. J. Mol. Sci. 2020, 21, 3776. [Google Scholar] [CrossRef] [PubMed]
- Aluvihare, V.R.; Kallikourdis, M.; Betz, A.G. Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol. 2004, 5, 266–271. [Google Scholar] [CrossRef]
- Williams, P.J.; Searle, R.F.; Robson, S.C.; Innes, B.A.; Bulmer, J.N. Decidual leucocyte populations in early to late gestation normal human pregnancy. J. Reprod. Immunol. 2009, 82, 24–31. [Google Scholar] [CrossRef]
- Hanna, J.; Goldman-Wohl, D.; Hamani, Y.; Avraham, I.; Greenfield, C.; Natanson-Yaron, S.; Prus, D.; Cohen-Daniel, L.; Arnon, T.I.; Manaster, I.; et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat. Med. 2006, 12, 1065–1074. [Google Scholar] [CrossRef]
- Plaks, V.; Birnberg, T.; Berkutzki, T.; Sela, S.; BenYashar, A.; Kalchenko, V.; Mor, G.; Keshet, E.; Dekel, N.; Neeman, M.; et al. Uterine DCs are crucial for decidua formation during embryo implantation in mice. J. Clin. Investig. 2008, 118, 3954–3965. [Google Scholar] [CrossRef]
- Schumacher, A.; Wafula, P.O.; Bertoja, A.Z.; Sollwedel, A.; Thuere, C.; Wollenberg, I.; Yagita, H.; Volk, H.D.; Zenclussen, A.C. Mechanisms of action of regulatory T cells specific for paternal antigens during pregnancy. Obs. Gynecol. 2007, 110, 1137–1145. [Google Scholar] [CrossRef]
- Chudnovets, A.; Liu, J.; Narasimhan, H.; Liu, Y.; Burd, I. Role of inflammation in virus pathogenesis during pregnancy. J. Virol. 2020, 28, 01381-19. [Google Scholar] [CrossRef]
- WHO Data. Available online: https://www.euro.who.int/en/healthtopics/noncommunicable-diseases/obesity/data-and-statistics (accessed on 14 December 2020).
- Lisonkova, S.; Muraca, G.M.; Potts, J. Association between Prepregnancy Body Mass Index and Severe Maternal Morbidity. JAMA 2017, 318, 1777. [Google Scholar] [CrossRef]
- Santos, S.; Voerman, E.; Amiano, P.; Barros, H.; Beilin, L.J.; Bergström, A.; Charles, M.A.; Chatzi, L.; Chevrier, C.; Chrousos, G.P.; et al. Impact of maternal body mass index and gestational weight gain on pregnancy complications: An individual participant data meta-analysis of European, North American and Australian cohorts. BJOG 2019, 126, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef]
- Odegaard, J.I.; Ricardo-Gonzalez, R.R.; Goforth, M.H.; Morel, C.R.; Subramanian, V.; Mukundan, L.; Red Eagle, A.; Vats, D.; Brombacher, F.; Ferrante, A.W.; et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature 2007, 447, 1116–1120. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Maury, E.; Brichard, S.M. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol. Cell Endocrinol. 2010, 314, 1–16. [Google Scholar] [CrossRef]
- Denison, F.C.; Roberts, K.A.; Barr, S.M.; Norman, J.E. Obesity, pregnancy, inflammation, and vascular function. Reproduction 2010, 140, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Cancello, R.; Tordjman, J.; Poitou, C.; Guilhem, G.; Bouillot, J.L.; Hugol, D.; Coussieu, C.; Basdevant, A.; Bar Hen, A.; Bedossa, P.; et al. Increased infiltration of macrophages in omental adipose tissue is associated with marked hepatic lesions in morbid human obesity. Diabetes 2006, 55, 1554–1561. [Google Scholar] [CrossRef]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Ann. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef]
- Romero, R.; Gotsch, F.; Pineles, B.; Kusanovic, J.P. Inflammation in pregnancy: Its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr. Rev. 2007, 65, 194–202. [Google Scholar] [CrossRef]
- Basu, S.; Haghiac, M.; Surace, P.; Challier, J.C.; Guerre-Millo, M.; Singh, K.; Waters, T.; Minium, J.; Presley, L.; Catalano, P.M.; et al. Pregravid obesity associates with increased maternal endotoxemia and metabolic inflammation. Obesity 2011, 19, 476–482. [Google Scholar] [CrossRef]
- Segovia, S.A.; Vickers, M.H.; Reynolds, C.M. The impact of maternal obesity on inflammatory processes and consequences for later offspring health outcomes. J. Dev. Orig. Health Dis. 2017, 8, 529–540. [Google Scholar] [CrossRef]
- Zambon, M.; Mandò, C.; Lissoni, A.; Anelli, G.M.; Novielli, C.; Cardellicchio, M.; Leone, R.; Monari, M.N.; Massari, M.; Cetin, I.; et al. Inflammatory and Oxidative Responses in Pregnancies with Obesity and Periodontal Disease. Reprod. Sci. 2018, 25, 1474–1484. [Google Scholar] [CrossRef]
- Radaelli, T.; Uvena-Celebrezze, J.; Minium, J.; Huston-Presley, L.; Catalano, P.; Hauguel-de Mouzon, S. Maternal interleukin-6: Marker of fetal growth and adiposity. J. Soc. Gynecol. Investig. 2006, 13, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, J.E.; Ferrell, W.R.; Crawford, L.; Wallace, A.M.; Greer, I.A.; Sattar, N. Maternal obesity is associated with dysregulation of metabolic, vascular, and inflammatory pathways. J. Clin. Endocrinol. Metab. 2002, 87, 4231–4237. [Google Scholar] [CrossRef]
- Stewart, F.M.; Freeman, D.J.; Ramsay, J.E.; Greer, I.A.; Caslake, M.; Ferrell, W.R. Longitudinal assessment of maternal endothelial function and markers of inflammation and placental function throughout pregnancy in lean and obese mothers. J. Clin. Endocrinol. Metab. 2007, 92, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Challier, J.C.; Basu, S.; Bintein, T.; Minium, J.; Hotmire, K.; Catalano, P.M.; Hauguel-de Mouzon, S. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta 2008, 29, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Christian, L.M.; Porter, K. Longitudinal changes in serum proinflammatory markers across pregnancy and postpartum: Effects of maternal body mass index. Cytokine 2014, 70, 134–140. [Google Scholar] [CrossRef]
- Kelly, A.C.; Powell, T.L.; Jansson, T. Placental function in maternal obesity. Clin. Sci. 2020, 134, 961–984. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.M.; Horgan, G.W.; Bhattacharya, S. Placental weight and efficiency in relation to maternal body mass index and the risk of pregnancy complications in women delivering singleton babies. Placenta 2012, 33, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, L.; Franx, A.; Vogelvang, T.E.; Houben, M.L.; van Rijn, B.B.; Nikkels, P.G. Association of Maternal Prepregnancy Body Mass Index with Placental Histopathological Characteristics in Uncomplicated Term Pregnancies. Pediatr. Dev. Pathol. 2019, 22, 45–52. [Google Scholar] [CrossRef]
- Bianchi, C.; Taricco, E.; Cardellicchio, M.; Mandò, C.; Massari, M.; Savasi, V.; Cetin, I. The role of obesity and gestational diabetes on placental size and fetal oxygenation. Placenta 2020, 103, 59–63. [Google Scholar] [CrossRef]
- Castellana, B.; Perdu, S.; Kim, Y.; Chan, K.; Atif, J.; Marziali, M.; Beristain, A.G. Maternal obesity alters uterine NK activity through a functional KIR2DL1/S1 imbalance. Immunol. Cell Biol. 2018, 96, 805–819. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Tian, M.; Hu, X.; Wang, L.; Ji, J.; Liao, A. The altered PD-1/PD-L1 pathway delivers the ’one-two punch’ effects to promote the Treg/Th17 imbalance in pre-eclampsia. Cell Mol. Immunol. 2018, 15, 710–723. [Google Scholar] [CrossRef] [PubMed]
- Varastehpour, A.; Radaelli, T.; Minium, J.; Ortega, H.; Herrera, E.; Catalano, P.; Hauguel-de Mouzon, S. Activation of phospholipase A2 is associated with generation of placental lipid signals and fetal obesity. J. Clin. Endocrinol. Metab. 2006, 91, 248–255. [Google Scholar] [CrossRef]
- Jones, H.N.; Jansson, T.; Powell, T.L. IL-6 stimulates system a amino acid transporter activity in trophoblast cells through STAT3 and increased expression of SNAT2. Am. J. Physiol Cell Physiol. 2009, 297, 1228–1235. [Google Scholar] [CrossRef]
- Jansson, N.; Greenwood, S.L.; Johansson, B.R.; Powell, T.L.; Jansson, T. Leptin stimulates the activity of the system A amino acid transporter in human placental villous fragments. J. Clin. Endocrinol. Metab. 2003, 88, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Cameo, P.; Bischof, P.; Calvo, J.C. Effect of leptin on progesterone, human chorionic gonadotropin, and interleukin-6 secretion by human term trophoblast cells in culture. Biol. Reprod. 2003, 68, 472–477. [Google Scholar] [CrossRef]
- Jones, H.N.; Jansson, T.; Powell, T.L. Full-length adiponectin attenuates insulin signaling and inhibits insulin-stimulated amino Acid transport in human primary trophoblast cells. Diabetes 2010, 59, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Duval, F.; Santos, E.D.; Poidatz, D.; Sérazin, V.; Gronier, H.; Vialard, F.; Dieudonné, M.N. Adiponectin Inhibits Nutrient Transporters and Promotes Apoptosis in Human Villous Cytotrophoblasts: Involvement in the Control of Fetal Growth. Biol. Reprod. 2016, 94, 111. [Google Scholar] [CrossRef]
- Rhee, J.S.; Saben, J.L.; Mayer, A.L.; Schulte, M.B.; Asghar, Z.; Stephens, C.; Chi, M.M.; Moley, K.H. Diet-induced obesity impairs endometrial stromal cell decidualization: A potential role for impaired autophagy. Hum. Reprod. 2016, 31, 1315–1326. [Google Scholar] [CrossRef] [PubMed]
- Stuart, T.J.; O’Neill, K.; Condon, D.; Sasson, I.; Sen, P.; Xia, Y.; Simmons, R.A. Diet-induced obesity alters the maternal metabolome and early placenta transcriptome and decreases placenta vascularity in the mouse. Biol. Reprod. 2018, 98, 795–809. [Google Scholar] [CrossRef] [PubMed]
- Islami, D.; Bischof, P.; Chardonnens, D. Modulation of placental vascular endothelial growth factor by leptin and hCG. Mol. Hum. Reprod. 2003, 9, 395–398. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pérez-Pérez, A.; Maymó, J.; Gambino, Y.; Dueñas, J.L.; Goberna, R.; Varone, C.; Sánchez-Margalet, V. Leptin stimulates protein synthesis-activating translation machinery in human trophoblastic cells. Biol. Reprod. 2009, 81, 826–832. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saben, J.; Lindsey, F.; Zhong, Y.; Thakali, K.; Badger, T.M.; Andres, A.; Gomez-Acevedo, H.; Shankar, K. Maternal obesity is associated with a lipotoxic placental environment. Placenta 2014, 35, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Fattuoni, C.; Mandò, C.; Palmas, F.; Anelli, G.M.; Novielli, C.; Parejo Laudicina, E.; Savasi, V.M.; Barberini, L.; Dessì, A.; Pintus, R.; et al. Preliminary metabolomics analysis of placenta in maternal obesity. Placenta 2018, 61, 89–95. [Google Scholar] [CrossRef]
- Mandò, C.; Anelli, G.M.; Novielli, C.; Panina-Bordignon, P.; Massari, M.; Mazzocco, M.I.; Cetin, I. Impact of Obesity and Hyperglycemia on Placental Mitochondria. Oxid Med. Cell Longev. 2018, 14, 2378189. [Google Scholar] [CrossRef]
- Ballesteros-Guzmán, A.K.; Carrasco-Legleu, C.E.; Levario-Carrillo, M.; Chávez-Corral, D.V.; Sánchez-Ramírez, B.; Mariñelarena-Carrillo, E.O.; Guerrero-Salgado, F.; Reza-López, S.A. Prepregnancy Obesity, Maternal Dietary Intake, and Oxidative Stress Biomarkers in the Fetomaternal Unit. Biomed. Res. Int. 2019, 5070453. [Google Scholar] [CrossRef]
- Roberts, K.A.; Riley, S.C.; Reynolds, R.M.; Barr, S.; Evans, M.; Statham, A.; Hor, K.; Jabbour, H.N.; Norman, J.E.; Denison, F.C. Placental structure and inflammation in pregnancies associated with obesity. Placenta 2011, 32, 247–254. [Google Scholar] [CrossRef]
- Ingvorsen, C.; Thysen, A.H.; Fernandez-Twinn, D.; Nordby, P.; Nielsen, K.F.; Ozanne, S.E.; Brix, S.; Hellgren, L.I. Effects of pregnancy on obesity-induced inflammation in a mouse model of fetal programming. Int. J. Obes. 2014, 38, 1282–1289. [Google Scholar] [CrossRef]
- Nogues, P.; Dos Santos, E.; Couturier-Tarrade, A.; Berveiller, P.; Arnould, L.; Lamy, E.; Grassin-Delyle, S.; Vialard, F.; Dieudonne, M.N. Maternal obesity influences placental nutrient transport, inflammatory status and morphology in human term placenta. J. Clin. Endocrinol. Metab. 2020, 16, 660. [Google Scholar] [CrossRef]
- Santos-Rosendo, C.; Bugatto, F.; González-Domínguez, A.; Lechuga-Sancho, A.M.; Mateos, M.R.; Visiedo, F. Placental Adaptive Changes to Protect Function and Decrease Oxidative Damage in Metabolically Healthy Maternal Obesity. Antioxidants 2020, 9, 794. [Google Scholar] [CrossRef]
- Atègbo, J.M.; Grissa, O.; Yessoufou, A.; Hichami, A.; Dramane, K.L.; Moutairou, K.; Miled, A.; Grissa, A.; Jerbi, M.; Tabka, Z.; et al. Modulation of adipokines and cytokines in gestational diabetes and macrosomia. J. Clin. Endocrinol. Metab. 2006, 91, 4137–4143. [Google Scholar] [CrossRef]
- Aye, I.L.; Lager, S.; Ramirez, V.I.; Gaccioli, F.; Dudley, D.J.; Jansson, T.; Powell, T.L. Increasing maternal body mass index is associated with systemic inflammation in the mother and the activation of distinct placental inflammatory pathways. Biol. Reprod. 2014, 90, 129. [Google Scholar] [CrossRef] [PubMed]
- Mandò, C.; Calabrese, S.; Mazzocco, M.I.; Novielli, C.; Anelli, G.M.; Antonazzo, P.; Cetin, I. Sex specific adaptations in placental biometry of overweight and obese women. Placenta 2016, 38, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Leon-Garcia, S.M.; Roeder, H.A.; Nelson, K.K.; Liao, X.; Pizzo, D.P.; Laurent, L.C.; Parast, M.M.; LaCoursiere, D.Y. Maternal obesity and sex-specific differences in placental pathology. Placenta 2016, 38, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Barke, T.L.; Money, K.M.; Du, L.; Serezani, A.; Gannon, M.; Mirnics, K.; Aronoff, D.M. Sex modifies placental gene expression in response to metabolic and inflammatory stress. Placenta 2019, 78, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Muralimanoharan, S.; Guo, C.; Myatt, L.; Maloyan, A. Sexual dimorphism in miR-210 expression and mitochondrial dysfunction in the placenta with maternal obesity. Int. J. Obes. 2015, 39, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Ingvorsen, C.; Brix, S.; Ozanne, S.E.; Hellgren, L.I. The effect of maternal Inflammation on foetal programming of metabolic disease. Acta Physiol. 2015, 214, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.A.; Chin, P.Y.; Femia, J.G.; Brown, H.M. Embryotoxic cytokines-Potential roles in embryo loss and fetal programming. J. Reprod Immunol. 2018, 125, 80–88. [Google Scholar] [CrossRef]
- Cetin, I.; Parisi, F.; Berti, C.; Mandò, C.; Desoye, G. Placental fatty acid transport in maternal obesity. J. Dev. Orig. Health Dis. 2012, 3, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.J.; Du, M.; Nathanielsz, P.W.; Ford, S.P. Maternal obesity up-regulates inflammatory signaling pathways and enhances cytokine expression in the mid-gestation sheep placenta. Placenta 2010, 31, 387–391. [Google Scholar] [CrossRef]
- Lieb, W.; Pencina, M.J.; Lanier, K.J.; Tofler, G.H.; Levy, D.; Fox, C.S.; Wang, T.J.; D’Agostino, R.B.; Vasan, R.S. Association of parental obesity with concentrations of select systemic biomarkers in nonobese offspring: The Framingham Heart Study. Diabetes 2009, 58, 134–137. [Google Scholar] [CrossRef]
- Leibowitz, K.L.; Moore, R.H.; Ahima, R.S.; Stunkard, A.J.; Stallings, V.A.; Berkowitz, R.I.; Chittams, J.L.; Faith, M.S.; Stettler, N. Maternal obesity associated with inflammation in their children. World J. Pediatr. 2012, 8, 76–79. [Google Scholar] [CrossRef]
- Ashdown, H.; Dumont, Y.; Ng, M.; Poole, S.; Boksa, P.; Luheshi, G.N. The role of cytokines in mediating effects of prenatal infection on the fetus: Implications for schizophrenia. Mol. Psychiatry 2006, 11, 47–55. [Google Scholar] [CrossRef]
- Oskvig, D.B.; Elkahloun, A.G.; Johnson, K.R.; Phillips, T.M.; Herkenham, M. Maternal immune activation by LPS selectively alters specific gene expression profiles of interneuron migration and oxidative stress in the fetus without triggering a fetal immune response. Brain Behav. Immun. 2012, 26, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Wienecke, J.; Hebel, K.; Hegel, K.J.; Pierau, M.; Brune, T.; Reinhold, D.; Pethe, A.; Brunner-Weinzierl, M.C. Pro-inflammatory effector Th cells transmigrate through anti-inflammatory environments into the murine fetus. Placenta 2012, 33, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Berti, C.; Cetin, I.; Agostoni, C.; Desoye, G.; Devlieger, R.; Emmett, P.M.; Ensenauer, R.; Hauner, H.; Herrera, E.; Hoesli, I.; et al. Pregnancy and infants’ outcome: Nutritional and metabolic implications. Crit. Rev. Food Sci. Nutr. 2016, 56, 82–91. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Barker, D.J. Fetal nutrition and adult disease. Am. J. Clin. Nutr. 2000, 71, 1344S–1352S. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A. Living with the past: Evolution, development, and patterns of disease. Science 2004, 305, 1733–1736. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A.; Beedle, A.S.; Spencer, H.G. Predictive adaptive responses in perspective. Trends Endocrinol. Metab 2008, 19, 109–110. [Google Scholar] [CrossRef] [PubMed]
- Vonnahme, K.A.; Hess, B.W.; Hansen, T.R.; McCormick, R.J.; Rule, D.C.; Moss, G.E.; Murdoch, W.J.; Nijland, M.J.; Skinner, D.C.; Nathanielsz, P.W.; et al. Maternal undernutrition from early- to mid-gestation leads to growth retardation, cardiac ventricular hypertrophy, and increased liver weight in the fetal sheep. Biol. Reprod. 2003, 69, 133–140. [Google Scholar] [CrossRef]
- Long, N.M.; George, L.A.; Uthlaut, A.B.; Smith, D.T.; Nijland, M.J.; Nathanielsz, P.W.; Ford, S.P. Maternal obesity and increased nutrient intake before and during gestation in the ewe results in altered growth, adiposity, and glucose tolerance in adult offspring. J. Anim. Sci. 2010, 88, 3546–3553. [Google Scholar] [CrossRef] [PubMed]
- Tarry-Adkins, J.L.; Ozanne, S.E. Mechanisms of early life programming: Current knowledge and future directions. Am. J. Clin. Nutr. 2011, 94, 1765S–1771S. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Kubota, N.; Tobe, K.; Terauchi, Y.; Miki, H.; Komeda, K.; Tamemoto, H.; Yamauchi, T.; Hagura, R.; Ito, C.; et al. The role of PPARgamma as a thrifty gene both in mice and humans. Br. J. Nutr. 2000, 84, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, C.; Larsson, B.M.; Jennische, E.; Eriksson, E.; Björntorp, P.; York, D.A.; Holmäng, A. Maternal endotoxemia results in obesity and insulin resistance in adult male offspring. Endocrinology 2001, 142, 2622–2630. [Google Scholar] [CrossRef][Green Version]
- Wei, Y.L.; Li, X.H.; Zhou, J.Z. Prenatal exposure to lipopolysaccharide results in increases in blood pressure and body weight in rats. Acta Pharm. Sin. 2007, 28, 651–656. [Google Scholar] [CrossRef]
- Pérez-Echarri, N.; Pérez-Matute, P.; Marcos-Gómez, B.; Baena, M.J.; Marti, A.; Martínez, J.A.; Moreno-Aliaga, M.J. Differential inflammatory status in rats susceptible or resistant to diet-induced obesity: Effects of EPA ethyl ester treatment. Eur. J. Nutr. 2008, 47, 380–386. [Google Scholar] [CrossRef]
- Samuelsson, A.M.; Matthews, P.A.; Argenton, M.; Christie, M.R.; McConnell, J.M.; Jansen, E.H.; Piersma, A.H.; Ozanne, S.E.; Twinn, D.F.; Remacle, C.; et al. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: A novel murine model of developmental programming. Hypertension 2008, 51, 383–392. [Google Scholar] [CrossRef]
- Gregorio, B.M.; Souza-Mello, V.; Carvalho, J.J.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. Maternal high-fat intake predisposes nonalcoholic fatty liver disease in C57BL/6 offspring. Am. J. Obstet. Gynecol. 2010, 203, e1–e8. [Google Scholar] [CrossRef]
- Yan, X.; Zhu, M.J.; Xu, W.; Tong, J.F.; Ford, S.P.; Nathanielsz, P.W.; Du, M. Up-regulation of Toll-like receptor 4/nuclear factor-kappaB signaling is associated with enhanced adipogenesis and insulin resistance in fetal skeletal muscle of obese sheep at late gestation. Endocrinology 2010, 151, 380–387. [Google Scholar] [CrossRef]
- Park, E.J.; Lee, J.H.; Yu, G.Y.; He, G.; Ali, S.R.; Holzer, R.G.; Osterreicher, C.H.; Takahashi, H.; Karin, M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell 2010, 140, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Rattanatray, L.; MacLaughlin, S.M.; Kleemann, D.O.; Walker, S.K.; Muhlhausler, B.S.; McMillen, I.C. Impact of maternal periconceptional overnutrition on fat mass and expression of adipogenic and lipogenic genes in visceral and subcutaneous fat depots in the postnatal lamb. Endocrinology 2010, 151, 5195–5205. [Google Scholar] [CrossRef] [PubMed]
- Kirsten, T.B.; Lippi, L.L.; Bevilacqua, E.; Bernardi, M.M. LPS exposure increases maternal corticosterone levels, causes placental injury and increases IL-1Β levels in adult rat offspring: Relevance to autism. PLoS ONE 2013, 8, 82244. [Google Scholar] [CrossRef] [PubMed]
- Murabayashi, N.; Sugiyama, T.; Zhang, L.; Kamimoto, Y.; Umekawa, T.; Ma, N.; Sagawa, N. Maternal high-fat diets cause insulin resistance through inflammatory changes in fetal adipose tissue. Eur. J. Obs. Gynecol. Reprod. Biol. 2013, 169, 39–44. [Google Scholar] [CrossRef]
- Desai, M.; Jellyman, J.K.; Han, G.; Beall, M.; Lane, R.H.; Ross, M.G. Maternal obesity and high-fat diet program offspring metabolic syndrome. Am. J. Obs. Gynecol. 2014, 211, e1–e237. [Google Scholar] [CrossRef]
- Fink, L.N.; Costford, S.R.; Lee, Y.S.; Jensen, T.E.; Bilan, P.J.; Oberbach, A.; Blüher, M.; Olefsky, J.M.; Sams, A.; Klip, A. Pro-inflammatory macrophages increase in skeletal muscle of high fat-fed mice and correlate with metabolic risk markers in humans. Obesity 2014, 22, 747–757. [Google Scholar] [CrossRef]
- Desai, M.; Han, G.; Ross, M.G. Programmed hyperphagia in offspring of obese dams: Altered expression of hypothalamic nutrient sensors, neurogenic factors and epigenetic modulators. Appetite 2016, 99, 193–199. [Google Scholar] [CrossRef]
- Thompson, M.D.; Cismowski, M.J.; Trask, A.J.; Lallier, S.W.; Graf, A.E.; Rogers, L.K.; Lucchesi, P.A.; Brigstock, D.R. Enhanced Steatosis and Fibrosis in Liver of Adult Offspring Exposed to Maternal High-Fat Diet. Gene Expr. 2016, 17, 47–59. [Google Scholar] [CrossRef]
- Cadaret, C.N.; Merrick, E.M.; Barnes, T.L.; Beede, K.A.; Posont, R.J.; Petersen, J.L.; Yates, D.T. Sustained maternal inflammation during the early third trimester yields fetal adaptations that impair subsequent skeletal muscle growth and glucose metabolism in sheep. Transl. Anim. Sci. 2018, 2, S14–S18. [Google Scholar] [CrossRef]
- Adams, R.C.M.; Smith, C. Chronic Gestational Inflammation: Transfer of Maternal Adaptation over Two Generations of Progeny. Mediat. Inflamm. 2019, 2019, 9160941. [Google Scholar] [CrossRef]
- Adams, R.C.M.; Smith, C. In utero Exposure to Maternal Chronic Inflammation Transfers a Pro-Inflammatory Profile to Generation F2 via Sex-Specific Mechanisms. Front Immunol. 2020, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Litzenburger, T.; Huber, E.K.; Dinger, K.; Wilke, R.; Vohlen, C.; Selle, J.; Kadah, M.; Persigehl, T.; Heneweer, C.; Dötsch, J.; et al. Maternal high-fat diet induces long-term obesity with sex-dependent metabolic programming of adipocyte differentiation, hypertrophy and dysfunction in the offspring. Clin. Sci. 2020, 134, 921–939. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Sloboda, D.M.; Vickers, M.H. Maternal obesity and developmental programming of metabolic disorders in offspring: Evidence from animal models. Exp. Diabetes Res. 2011, 2011, 592408. [Google Scholar] [CrossRef]
- Ornoy, A. Prenatal origin of obesity and their complications: Gestational diabetes, maternal overweight and the paradoxical effects of fetal growth restriction and macrosomia. Reprod. Toxicol. 2011, 32, 205–212. [Google Scholar] [CrossRef]
- Poon, K. Behavioral Feeding Circuit: Dietary Fat-Induced Effects of Inflammatory Mediators in the Hypothalamus. Front. Endocrinol. 2020, 11, 591559. [Google Scholar] [CrossRef]
- Du, M.; Yan, X.; Tong, J.F.; Zhao, J.; Zhu, M.J. Maternal obesity, inflammation, and fetal skeletal muscle development. Biol Reprod. 2010, 82, 4–12. [Google Scholar] [CrossRef]
- Segovia, S.A.; Vickers, M.H.; Gray, C.; Reynolds, C.M. Maternal obesity, inflammation, and developmental programming. Biomed. Res. Int. 2014, 2014, 418975. [Google Scholar] [CrossRef]
- Draper, E.; Reynolds, C.M.; Canavan, M.; Mills, K.H.; Loscher, C.E.; Roche, H.M. Omega-3 fatty acids attenuate dendritic cell function via NF-κB independent of PPARγ. J. Nutr. Biochem. 2011, 22, 784–790. [Google Scholar] [CrossRef]
- LeMieux, M.J.; Kalupahana, N.S.; Scoggin, S.; Moustaid-Moussa, N. Eicosapentaenoic acid reduces adipocyte hypertrophy and inflammation in diet-induced obese mice in an adiposity-independent manner. J. Nutr. 2015, 145, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Satokar, V.V.; Cutfield, W.S.; Cameron-Smith, D.; Albert, B.B. Omega-3 fats in pregnancy: Could a targeted approach lead to better metabolic health for children? Nutr. Rev. 2020, 24, 071. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.M.; Shankar, K. Obesity and pregnancy: Mechanisms of short term and long term adverse consequences for mother and child. BMJ 2017, 356, 1. [Google Scholar] [CrossRef]
- Kislal, S.; Shook, L.L.; Edlow, A.G. Perinatal exposure to maternal obesity: Lasting cardiometabolic impact on offspring. Prenat Diagn. 2020, 40, 1109–1125. [Google Scholar] [CrossRef] [PubMed]
- Gillman, M.W.; Ludwig, D.S. How early should obesity prevention start? N. Engl. J. Med. 2013, 369, 2173–2175. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.A.; Lichtenstein, P.; Långström, N. Association of maternal diabetes mellitus in pregnancy with offspring adiposity into early adulthood: Sibling study in a prospective cohort of 280,866 men from 248,293 families. Circulation 2011, 123, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.F.; Lazdam, M.; Lewandowski, A.J.; Worton, S.A.; Kelly, B.; Kenworthy, Y.; Adwani, S.; Wilkinson, A.R.; McCormick, K.; Sargent, I.; et al. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: A systematic review. Pediatrics 2012, 129, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- Efstathiou, S.P.; Skeva, I.I.; Zorbala, E.; Georgiou, E.; Mountokalakis, T.D. Metabolic syndrome in adolescence: Can it be predicted from natal and parental profile? The Prediction of Metabolic Syndrome in Adolescence (PREMA) study. Circulation 2012, 125, 902–910. [Google Scholar] [CrossRef]
- Ravelli, A.C.; van Der Meulen, J.H.; Osmond, C.; Barker, D.J.; Bleker, O.P. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am. J. Clin. Nutr. 1999, 70, 811–816. [Google Scholar] [CrossRef]
- Roseboom, T.; de Rooij, S.; Painter, R. The Dutch famine and its long-term consequences for adult health. Early Hum. Dev. 2006, 82, 485–491. [Google Scholar] [CrossRef]
- Van Abeelen, A.F.; Veenendaal, M.V.; Painter, R.C.; de Rooij, S.R.; Dijkgraaf, M.G.; Bossuyt, P.M.; Elias, S.G.; Grobbee, D.E.; Uiterwaal, C.S.; Roseboom, T.J. Survival effects of prenatal famine exposure. Am. J. Clin. Nutr. 2012, 95, 179–183. [Google Scholar] [CrossRef]
- Deierlein, A.L.; Siega-Riz, A.M.; Adair, L.S.; Herring, A.H. Effects of pre-pregnancy body mass index and gestational weight gain on infant anthropometric outcomes. J. Pediatr. 2011, 158, 221–226. [Google Scholar] [CrossRef]
- Voerman, E.; Santos, S.; Patro Golab, B.; Amiano, P.; Ballester, F.; Barros, H.; Bergström, A.; Charles, M.A.; Chatzi, L.; Chevrier, C.; et al. Maternal body mass index, gestational weight gain, and the risk of overweight and obesity across childhood: An individual participant data meta-analysis. PLoS Med. 2019, 16, e1002744. [Google Scholar] [CrossRef]
- Eshriqui, I.; Valente, A.M.M.; Folchetti, L.D.; Almeida-Pititto, B.; Ferreira, S.R.G. Pre-pregnancy body mass index is associated with offspring body composition in adulthood before adiposity-related disorders: A retrospective cohort. Public Health Nutr. 2020, 1–17. [Google Scholar] [CrossRef]
- Kral, J.G.; Biron, S.; Simard, S.; Hould, F.S.; Lebel, S.; Marceau, S.; Marceau, P. Large maternal weight loss from obesity surgery prevents transmission of obesity to children who were followed for 2 to 18 years. Pediatrics 2006, 118, 1644–1649. [Google Scholar] [CrossRef] [PubMed]
- Margerison-Zilko, C.E.; Shrimali, B.P.; Eskenazi, B.; Lahiff, M.; Lindquist, A.R.; Abrams, B.F. Trimester of maternal gestational weight gain and offspring body weight at birth and age five. Matern Child. Health J. 2012, 16, 1215–1223. [Google Scholar] [CrossRef]
- Brumbaugh, D.E.; Tearse, P.; Cree-Green, M.; Fenton, L.Z.; Brown, M.; Scherzinger, A.; Reynolds, R.; Alston, M.; Hoffman, C.; Pan, Z.; et al. Intrahepatic fat is increased in the neonatal offspring of obese women with gestational diabetes. J. Pediatr. 2013, 162, 930–936.e1. [Google Scholar] [CrossRef]
- Boyle, K.E.; Patinkin, Z.W.; Shapiro, A.L.; Baker, P.R., II; Dabelea, D.; Friedman, J.E. Mesenchymal Stem Cells from Infants Born to Obese Mothers Exhibit Greater Potential for Adipogenesis: The Healthy Start BabyBUMP Project. Diabetes 2016, 65, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Murrin, C.M.; Kelly, G.E.; Tremblay, R.E.; Kelleher, C.C. Body mass index and height over three generations: Evidence from the Lifeways cross-generational cohort study. BMC Public Health 2012, 12, 81. [Google Scholar] [CrossRef]
- Simeoni, U.; Armengaud, J.B.; Siddeek, B.; Tolsa, J.F. Perinatal Origins of Adult Disease. Neonatology 2018, 113, 393–399. [Google Scholar] [CrossRef]
- Fernandez-Twinn, D.S.; Hjort, L.; Novakovic, B.; Ozanne, S.E.; Saffery, R. Intrauterine programming of obesity and type 2 diabetes. Diabetologia 2019, 62, 1789–1801. [Google Scholar] [CrossRef] [PubMed]
- Hjort, L.; Martino, D.; Grunnet, L.G.; Naeem, H.; Maksimovic, J.; Olsson, A.H.; Zhang, C.; Ling, C.; Olsen, S.F.; Saffery, R.; et al. Gestational diabetes and maternal obesity are associated with epigenome-wide methylation changes in children. JCI Insight 2018, 3, e122572. [Google Scholar] [CrossRef]
- Martin, C.L.; Jima, D.; Sharp, G.C.; McCullough, L.E.; Park, S.S.; Gowdy, K.M.; Skaar, D.; Cowley, M.; Maguire, R.L.; Fuemmeler, B.; et al. Maternal pre-pregnancy obesity, offspring cord blood DNA methylation, and offspring cardiometabolic health in early childhood: An epigenome-wide association study. Epigenetics 2019, 14, 325–340. [Google Scholar] [CrossRef]
- Ruebel, M.L.; Cotter, M.; Sims, C.R.; Moutos, D.M.; Badger, T.M.; Cleves, M.A.; Shankar, K.; Andres, A. Obesity Modulates Inflammation and Lipid Metabolism Oocyte Gene Expression: A Single-Cell Transcriptome Perspective. J. Clin. Endocrinol. Metab. 2017, 102, 2029–2038. [Google Scholar] [CrossRef]
- Song, J.; Xiang, S.; Pang, C.; Guo, J.; Sun, Z. Metabolomic alternations of follicular fluid of obese women undergoing in-vitro fertilization treatment. Sci. Rep. 2020, 10, 5968. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, J.; Heslehurst, N.; Hall, J.; Schoenaker, D.A.J.M.; Hutchinson, J.; Cade, J.E.; Poston, L.; Barrett, G.; Crozier, S.R.; Barker, M.; et al. Before the beginning: Nutrition and lifestyle in the preconception period and its importance for future health. Lancet 2018, 391, 830–1841. [Google Scholar] [CrossRef]
- Yang, X.; Wu, L.L.; Chura, L.R.; Liang, X.; Lane, M.; Norman, R.J.; Robker, R.L. Exposure to lipid-rich follicular fluid is associated with endoplasmic reticulum stress and impaired oocyte maturation in cumulus-oocyte complexes. Fertil. Steril. 2012, 97, 1438–1443. [Google Scholar] [CrossRef]
- Gonzalez, M.B.; Lane, M.; Knight, E.J.; Robker, R.L. Inflammatory markers in human follicular fluid correlate with lipid levels and Body Mass Index. J. Reprod. Immunol. 2018, 130, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, M.A.; Fleming, T.P.; Watkins, A.J. Periconceptional environment and the developmental origins of disease. J. Endocrinol. 2019, 242, T33–T49. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, E.R.; Karmon, A.E.; Gold, J.; Petrozza, J.C.; Styer, A.K. Reproductive outcomes in oocyte donation cycles are associated with donor BMI. Hum. Reprod. 2016, 31, 385–392. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jordaens, L.; Van Hoeck, V.; Maillo, V.; Gutierrez-Adan, A.; Marei, W.F.A.; Vlaeminck, B.; Thys, S.; Sturmey, R.G.; Bols, P.E.J.; Leroy, J.L.M.R. Maternal metabolic stress may affect oviduct gatekeeper function. Reproduction 2017, 153, 759–773. [Google Scholar] [CrossRef] [PubMed]
- Snider, A.P.; Wood, J.R. Obesity induces ovarian inflammation and reduces oocyte quality. Reproduction 2019, 158, R79–R90. [Google Scholar] [CrossRef] [PubMed]
- Davey Smith, G.; Steer, C.; Leary, S.; Ness, A. Is there an intrauterine influence on obesity? Evidence from parent child associations in the Avon Longitudinal Study of Parents and Children (ALSPAC). Arch. Dis. Child. 2007, 92, 876–880. [Google Scholar] [CrossRef]
- Xu, Y.; Nisenblat, V.; Lu, C.; Li, R.; Qiao, J.; Zhen, X.; Wang, S. Pretreatment with coenzyme Q10 improves ovarian response and embryo quality in low-prognosis young women with decreased ovarian reserve: A randomized controlled trial. Reprod. Biol. Endocrinol. 2018, 16, 29. [Google Scholar] [CrossRef]
- Tamura, H.; Jozaki, M.; Tanabe, M.; Shirafuta, Y.; Mihara, Y.; Shinagawa, M.; Tamura, I.; Maekawa, R.; Sato, S.; Taketani, T.; et al. Importance of Melatonin in Assisted Reproductive Technology and Ovarian Aging. Int. J. Mol. Sci. 2020, 21, 1135. [Google Scholar] [CrossRef]
- Tesarik, J.; Mendoza-Tesarik, R. Melatonin: The First Noninvasive Causal Therapy for Both Endometriosis and Adenomyosis? J. Gynecol. Women’s Health 2018, 12, 555829. [Google Scholar] [CrossRef]
| Animal Model | Inflammation Pathway | Offspring Outcomes | Maternal Outcomes | |
|---|---|---|---|---|
| Hara et al. (2000) [75] | Rodent/Human Pro12Ala PPARgamma2 polymorphism | Diet-induced |
↑ leptin |
↓ Ala12 in the diabetic group |
| Nilsson et al. (2001) [76] | Rodent | LPS injection-induced |
↑ circulating leptin, 17beta-estradiol and progesterone ↑ hippocampal glucocorticoid receptor expression ↓ insulin sensitivity, corticosterone response to stress
heart and adrenals enlargement | |
| Wei et al. (2007) [77] | Rodent | LPS injection-induced | ↑ systemic arterial blood pressure, body weight, food intake, adipose tissue weight ↑ circulating leptin | |
| Perez-Echarri et al. (2008) [78] | Rodent | Diet-induced Eicosapentaenoic (EPA) omega-3 fatty acid treatment | ↑ TNF, IL-6 and haptoglobin in white adipose tissue ↓ haptoglobin serum levels
Reversal of serum haptoglobin increase; Prevention of obesity-associated inflammation in adipose tissue | |
| Samuelsson et al. (2008) [79] | Rodent | Diet-induced | ↑ body weight, blood pressure, adiposity with adipocyte hypertrophy, hyperphagia; ↓ locomotor activity and skeletal muscle mass ↓ adipocyte β 2- and β 3-adrenoreceptor and PPAR-γ expression; Systemic artery endothelial dysfunction and hypertension ↑ fasting insulin and glucose levels | |
| Gregorio et al. (2010) [80] | Rodent | Diet-induced | Liver modifications: Insulin resistance and lower GLUT-2 expression ↑ sterol regulatory element-binding protein-1c expression; Hepatic steatosis | |
| Yan et al. (2010) [81] | Sheep | Diet-induced | Skeletal muscle modifications: ↑ PPAR-γ, TLR2–4, NF-κB, and TNFα gene expression ↑ intramuscolar adipogenesis and macrophage infiltration ↑ circulating insulin concentrations ↓ insulin receptor mRNA expression and insulin sensitivity | |
| Park et al. (2010) [82] | Rodent | Diet-induced | ↑ tumor-promoting cytokines IL-6 and TNF which cause hepatic inflammation and activation of the oncogenic transcription factors → hepatocellular carcinoma development | |
| Rattanatray et al. (2010) [83] | Sheep | Diet-induced | ↑ body fat mass in female offspring, reversible by maternal dietary restriction; No effect on PPAR-γ, G3PDH, lipoprotein lipase, leptin and adiponectin mRNA expression | |
| Kirsten et al. (2013) [84] | Rodent | LPS injection-induced | ↑ IL-1β serum levels
| ↑ maternal serum corticosterone levels, higher postimplantation loss |
| Murabayashi et al. (2013) [85] | Rodent | Diet-induced | = fetal weight ↑ plasma glucose and insulin levels adipocyte hypertrophy ↑ adipocyte expression of chemokine receptor-2 and TNFα mRNA ↓ adipocyte GLUT-4 expression | ↑ bodyweight, glucose intolerance and insulin resistance |
| Desai et al. (2014) [86] | Rodent | Diet-induced (during pregnancy and/or lactation) | ↑ adiposity ↑ body weight only when overnutrition was prolonged during lactation; Hyperglycemia ↑ systolic blood pressure ↑ plasma corticosterone levels in case of maternal gestational overnutrition | ↑ body fat and plasma corticosterone levels |
| Fink et al. (2014) [87] | Rodent Human | Diet-induced | Glucose intolerance
Dysregulated muscle inflammatory gene expression | |
| Desai et al. (2016) [88] | Rodent | Diet-induced | ↑ adiposity despite unaffected body weight in males ↓ energy sensors (DNA methylase) ↑ appetite and ↓ satiety neuropeptides; Altered development, neuronal abnormal differentiation and appetite dysregulation in hypothalamus and adult arcuate nucleus | |
| Thompson et al. (2016) [89] | Rodent | Diet-induced | ↑ hepatocyte proliferation and stellate cell activation; Hepatosteatosis ↑ susceptibility to development of steatosis and rapid disease progression with sustained fibrotic phenotype | |
| Cadaret et al. (2018) [90] | Sheep | LPS injection-induced | Altered muscle metabolic capacity with ↓ glucose oxidation capacity FGR fetuses with –22% in body weight ↓ β cell function | ↑ circulating inflammatory cells |
| Adams et al. (2019; 2020) [91,92] | Rodent (F1 and F2 generations) | LPS injection-induced | Glucocorticoid hypersensitivity in F1 offspring with elevated corticosterone and increased leukocyte glucocorticoid receptor level, further transmitted to the F2 offspring without additional insults (>male offspring) ↑ IL-1β cytokine responses in female offspring only | |
| Litzenburger et al. (2020) [93] | Rodent | Diet-induced | ↑ white adipose tissue (female > male) ↑ adipocyte size Sex-dependent metabolic programming of white adipose tissue dysfunction with dysregulation of lipolytic, adipogenic and stemness-related markers |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parisi, F.; Milazzo, R.; Savasi, V.M.; Cetin, I. Maternal Low-Grade Chronic Inflammation and Intrauterine Programming of Health and Disease. Int. J. Mol. Sci. 2021, 22, 1732. https://doi.org/10.3390/ijms22041732
Parisi F, Milazzo R, Savasi VM, Cetin I. Maternal Low-Grade Chronic Inflammation and Intrauterine Programming of Health and Disease. International Journal of Molecular Sciences. 2021; 22(4):1732. https://doi.org/10.3390/ijms22041732
Chicago/Turabian StyleParisi, Francesca, Roberta Milazzo, Valeria M. Savasi, and Irene Cetin. 2021. "Maternal Low-Grade Chronic Inflammation and Intrauterine Programming of Health and Disease" International Journal of Molecular Sciences 22, no. 4: 1732. https://doi.org/10.3390/ijms22041732
APA StyleParisi, F., Milazzo, R., Savasi, V. M., & Cetin, I. (2021). Maternal Low-Grade Chronic Inflammation and Intrauterine Programming of Health and Disease. International Journal of Molecular Sciences, 22(4), 1732. https://doi.org/10.3390/ijms22041732






