Prenatal THC Does Not Affect Female Mesolimbic Dopaminergic System in Preadolescent Rats
Abstract
1. Introduction
2. Results
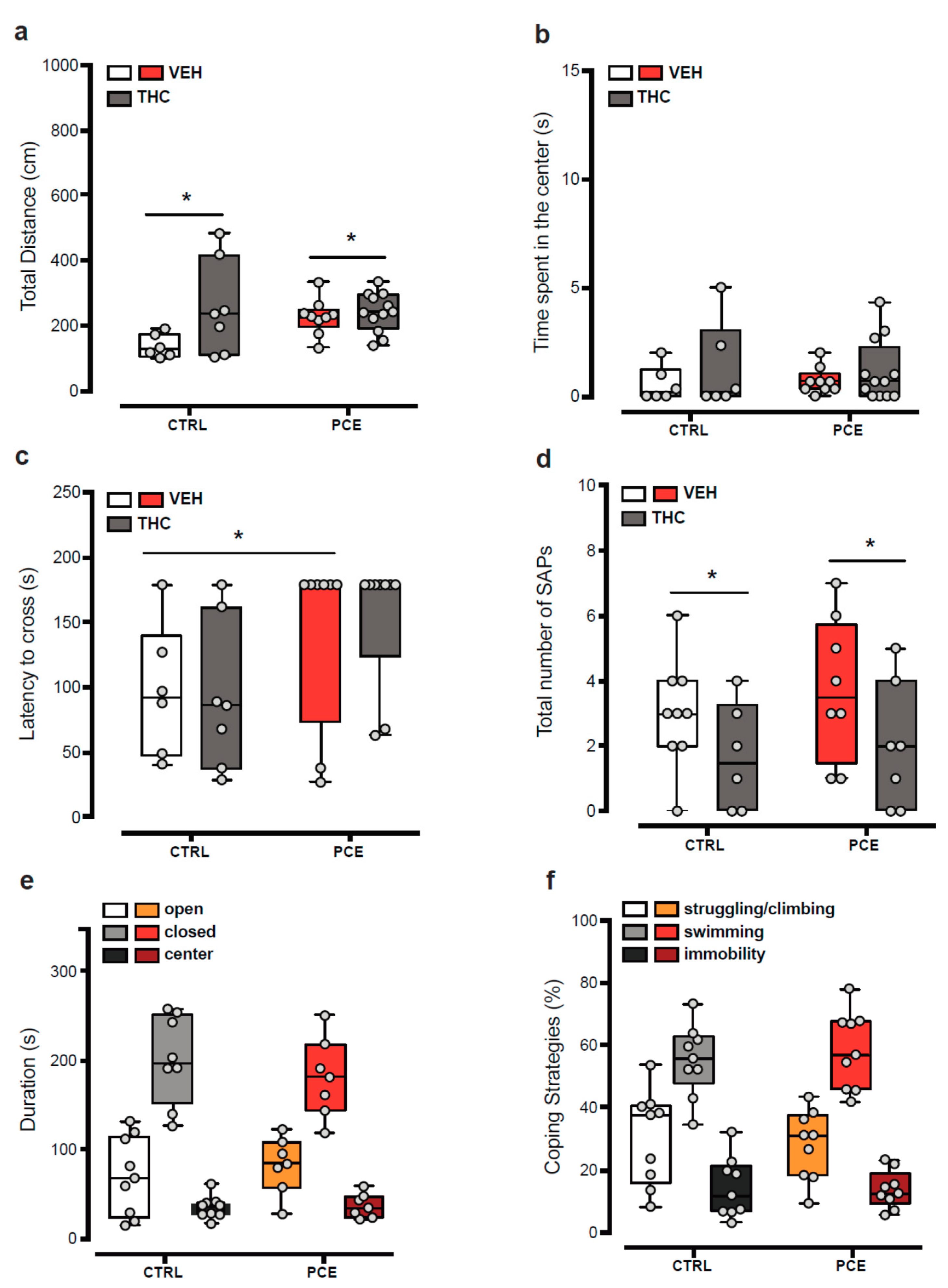
2.1. Impact of PCE on Behavior in Female Preadolescent Rats
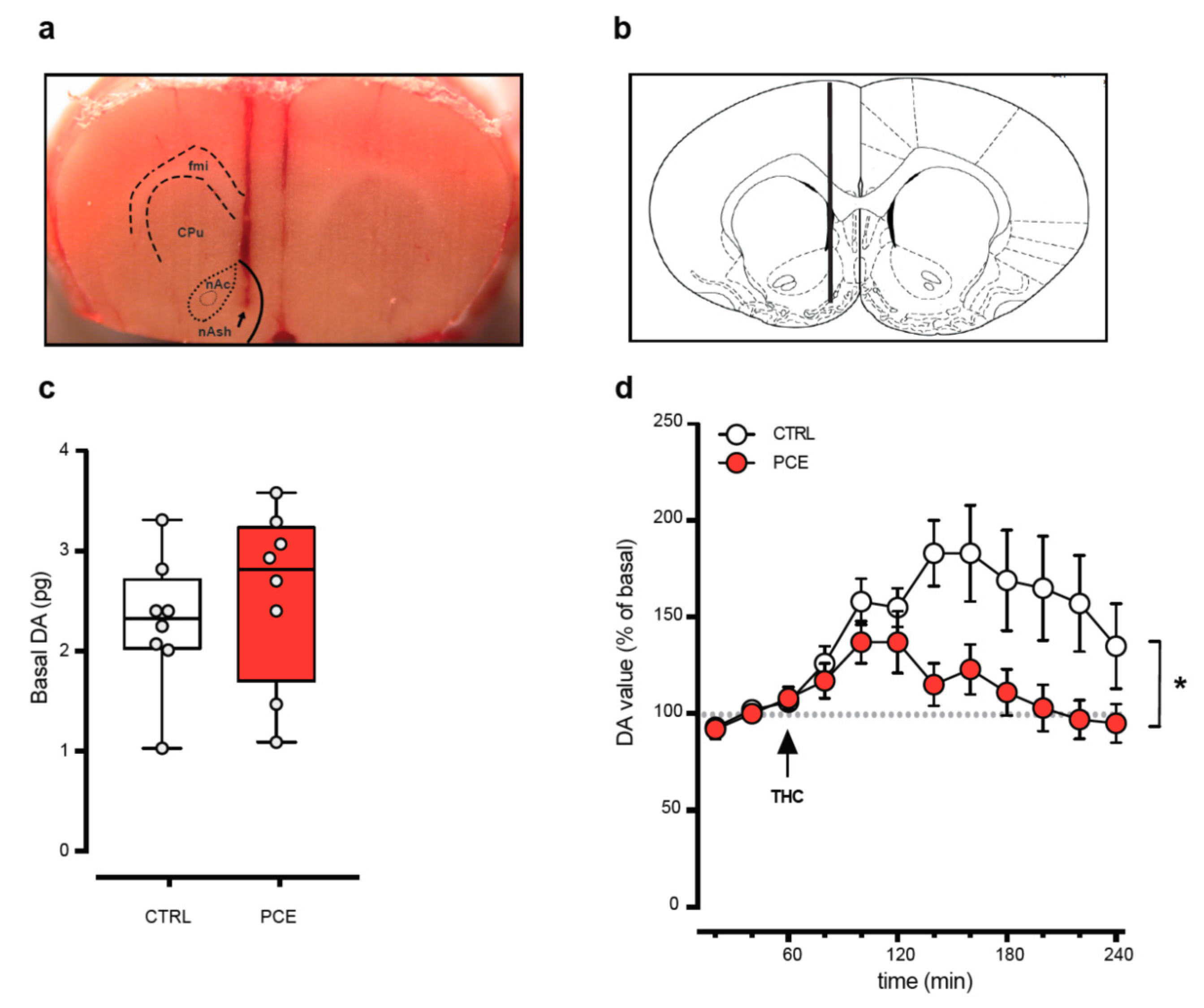
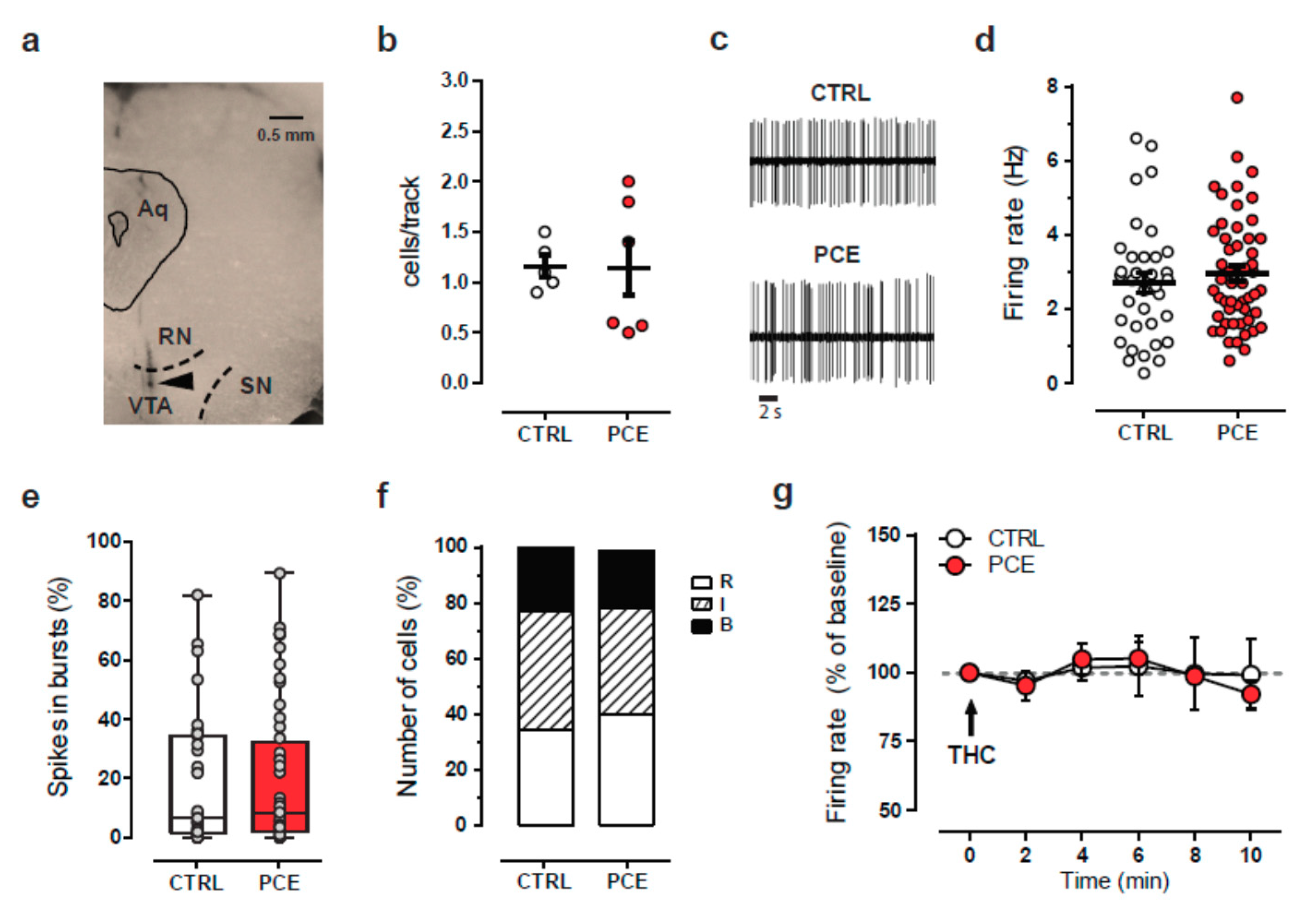
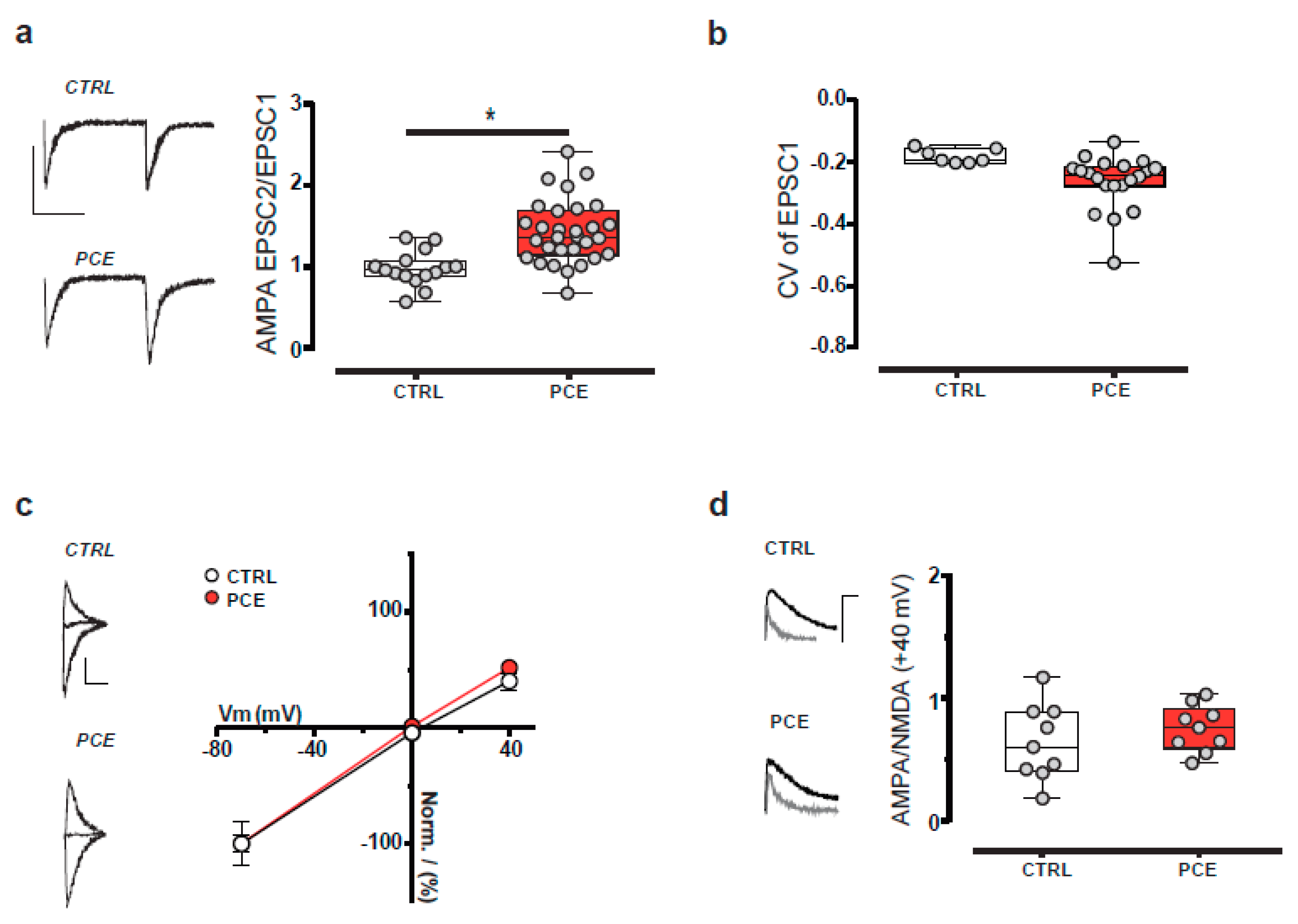
2.2. PCE Effect on Mesolimbic Dopamine Transmission
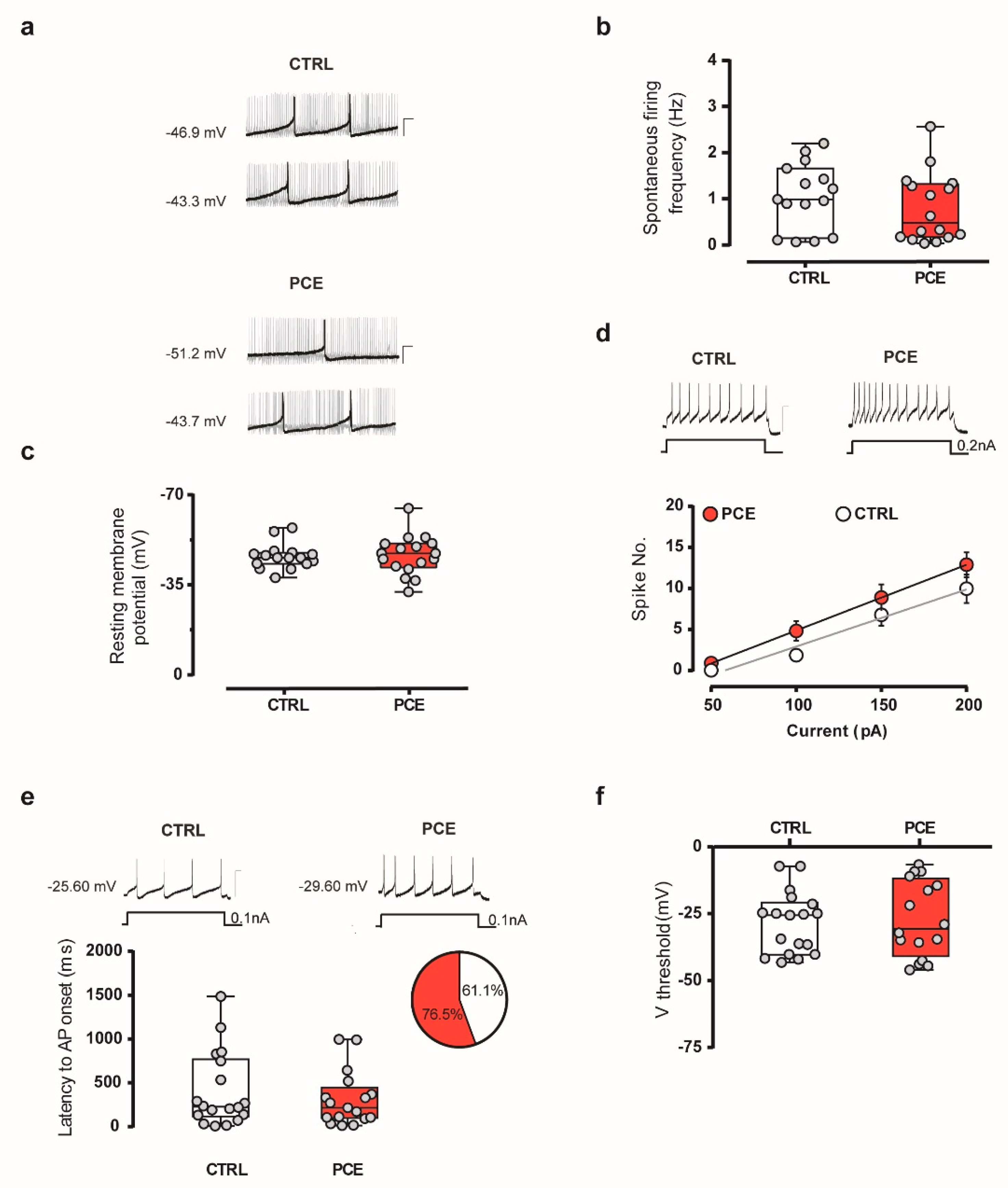
2.3. Intrinsic and Synaptic Properties of Putative VTA Dopamine Neurons in PCE Females
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Drugs and Treatments
4.3. Behavioral Tests
4.3.1. Locomotor Activity
4.3.2. Wire-Beam Bridge Test
4.3.3. Elevated Plus Maze
4.3.4. Forced Swim Test
4.3.5. Passive Avoidance
4.3.6. Social Interaction Test
4.3.7. Sucrose Preference Test
4.4. Cerebral Microdialysis
4.5. In Vivo Single-Unit Electrophysiological Recordings
4.6. Ex Vivo Electrophysiological Recordings
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kessler, R.C.; Angermeyer, M.; Anthony, J.C.; Ron, D.G.; Demyttenaere, K.; Gasquet, I.; Giovanni, D.G.; Gluzman, S.; Gureje, O.; Haro, J.M.; et al. Lifetime prevalence and age of onset distributions of mental disorders in the World Health Organization’s World Mental Health Survey Initiative. World Psychatry 2007, 6, 168–176. [Google Scholar]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Wang, P.S.; Berglund, P.A.; Olfson, M.; Kessler, R.C. Delays in Initial Treatment Contact after First Onset of a Mental Disorder. Health Serv. Res. 2004, 39, 393–416. [Google Scholar] [CrossRef]
- Schlüter-Müller, S. Children of Mentally Ill Parents. A High Risk Population. Psychiatr Danub 2020, 32, 346–348. [Google Scholar] [PubMed]
- Stracke, M.; Gilbert, K.; Kieser, M.; Klose, C.; Krisam, J.; Ebert, D.D.; Buntrock, C.; Christiansen, H. COMPARE Family (Children of Mentally Ill Parents at Risk Evaluation): A Study Protocol for a Preventive Intervention for Children of Mentally Ill Parents (Triple P, Evidence-Based Program That Enhances Parentings Skills, in Addition to Gold-Standard CBT With the Mentally Ill Parent) in a Multicenter RCT—Part II. Front. Psychiatry 2019, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, J.T.; Lopez-Rodriguez, A.B.; Fonseca, F.; Farré, M.; Torrens, M.; Viveros, M.-P. Effects of cannabis exposure in the prenatal and adolescent periods: Preclinical and clinical studies in both sexes. Front. Neuroendocr. 2020, 57, 100841. [Google Scholar] [CrossRef] [PubMed]
- Scheyer, A.F.; Melis, M.; Trezza, V.; Manzoni, O.J. Consequences of Perinatal Cannabis Exposure. Trends Neurosci. 2019, 42, 871–884. [Google Scholar] [CrossRef]
- Hurd, Y.L.; Manzoni, O.J.; Pletnikov, M.V.; Lee, F.S.; Bhattacharyya, S.; Melis, M. Cannabis and the Developing Brain: Insights into Its Long-Lasting Effects. J. Neurosci. 2019, 39, 8250–8258. [Google Scholar] [CrossRef]
- El Marroun, H.; Bolhuis, K.; Franken, I.H.A.; Jaddoe, V.W.V.; Hillegers, M.H.; Lahey, B.B.; Tiemeier, H. Preconception and prenatal cannabis use and the risk of behavioural and emotional problems in the offspring; a multi-informant prospective longitudinal study. Int. J. Epidemiol. 2018, 48, 287–296. [Google Scholar] [CrossRef]
- Roncero, C.; Valriberas-Herrero, I.; Mezzatesta-Gava, M.; Villegas, J.L.; Aguilar, L.; Grau-Lopez, L. Cannabis use during pregnancy and its relationship with fetal developmental outcomes and psychiatric disorders. A systematic review. Reprod. Health 2020, 17, 25. [Google Scholar] [CrossRef]
- Paul, S.E.; Hatoum, A.S.; Fine, J.D.; Johnson, E.C.; Hansen, I.; Karcher, N.R.; Moreau, A.L.; Bondy, E.; Qu, Y.; Carter, E.B.; et al. Associations Between Prenatal Cannabis Exposure and Childhood Outcomes: Results from the ABCD Study. JAMA Psychiatry 2020, 78, 64–76. [Google Scholar] [CrossRef]
- Singh, S.; Filion, K.B.; Abenhaim, H.A.; Eisenberg, M.J. Prevalence and outcomes of prenatal recreational cannabis use in high-income countries: A scoping review. BJOG 2020, 127, 8–16. [Google Scholar] [CrossRef]
- Volkow, N.D.; Han, B.; Compton, W.M.; McCance-Katz, E.F. Self-Reported Medical and Nonmedical Cannabis Use among Pregnant Women in the United States. JAMA 2019, 322, 167–169. [Google Scholar] [CrossRef]
- Hasin, D. US Epidemiology of Cannabis Use and Associated Problems. Neuropsychopharmacology 2018, 43, 195–212. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.S. Pediatric Concerns Due to Expanded Cannabis Use: Unintended Consequences of Legalization. J. Med. Toxicol. 2017, 13, 99–105. [Google Scholar] [CrossRef]
- Corsi, D.J.; Donelle, J.; Sucha, E.; Hawken, S.; Hsu, H.; El-Chaâr, D.; Bisnaire, L.; Fell, D.; Wen, S.W.; Walker, M. Maternal cannabis use in pregnancy and child neurodevelopmental outcomes. Nat. Med. 2020, 26, 1536–1540. [Google Scholar] [CrossRef]
- Gunn, J.K.L.; Rosales, C.B.; Center, K.E.; Nuñez, A.; Gibson, S.J.; Christ, C.; Ehiri, J. Prenatal exposure to cannabis and maternal and child health outcomes: A systematic review and meta-analysis. BMJ Open 2016, 6, e009986. [Google Scholar] [CrossRef] [PubMed]
- Jutras-Aswad, D.; DiNieri, J.A.; Harkany, T.; Hurd, Y.L. Neurobiological consequences of maternal cannabis on human fetal development and its neuropsychiatric outcome. Eur. Arch. Psychiatry Clin. Neurosci. 2009, 259, 395–412. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.V.; DiNieri, J.A.; Szutorisz, H.; Hurd, Y.L. Molecular mechanisms of maternal cannabis and cigarette use on human neurodevelopment. Eur. J. Neurosci. 2011, 34, 1574–1583. [Google Scholar] [CrossRef]
- Basavarajappa, B.S.; Nixon, R.A.; Arancio, O. Endocannabinoid system: Emerging role from neurodevelopment to Neuro-Degeneration. Mini Rev. Med. Chem. 2009, 9, 448–462. [Google Scholar] [CrossRef]
- Alpár, A.; Di Marzo, V.; Harkany, T. At the Tip of an Iceberg: Prenatal Marijuana and Its Possible Relation to Neuropsychiatric Outcome in the Offspring. Biol. Psychiatry 2016, 79, e33–e45. [Google Scholar] [CrossRef]
- Fride, E.; Gobshtis, N.; Dahan, H.; Weller, A.; Giuffrida, A.; Ben-Shabat, S. Chapter 6 The Endocannabinoid System During Development: Emphasis on Perinatal Events and Delayed Effects. Vitam. Horm. 2009, 81, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Ruiz, J.; Berrendero, F.; Hernandez, M.L.; Ramos, J.A. The endogenous cannabinoid system and brain development. Trends Neurosci. 2000, 23, 1420. [Google Scholar] [CrossRef]
- Richardson, K.A.; Hester, A.K.; McLemore, G.L. Prenatal cannabis exposure–The “first hit” to the endocannabinoid system. Neurotoxicol. Teratol. 2016, 58, 5–14. [Google Scholar] [CrossRef]
- Traccis, F.; Frau, R.; Melis, M. Gender Differences in the Outcome of Offspring Prenatally Exposed to Drugs of Abuse. Front. Behav. Neurosci. 2020, 14, 72. [Google Scholar] [CrossRef]
- De Salas-Quiroga, A.; Díaz-Alonso, J.; García-Rincón, D.; Remmers, F.; Vega, D.; Gómez-Cañas, M.; Lutz, B.; Guzmán, M.; Galve-Roperh, I. Prenatal exposure to cannabinoids evokes long-lasting functional alterations by targeting CB1 receptors on developing cortical neurons. Proc. Natl. Acad. Sci. USA 2015, 112, 13693–13698. [Google Scholar] [CrossRef]
- Gómez, M.; Hernandez, M.L.; Johansson, B.; De Miguel, R.; Ramos, J.A.; Fernández-Ruiz, J. Prenatal cannabinoid exposure and gene expression for neural adhesion molecule L1 in the fetal rat brain. Dev. Brain Res. 2003, 147, 201–207. [Google Scholar] [CrossRef]
- Saez, T.M.M.; Aronne, M.P.; Caltana, L.; Brusco, A. Prenatal exposure to the CB1 and CB2 cannabinoid receptor agonist WIN 55,212-2 alters migration of early-born glutamatergic neurons and GABAergic interneurons in the rat cerebral cortex. J. Neurochem. 2014, 129, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Calvigioni, D.; Hurd, Y.L.; Harkany, T.; Keimpema, E. Neuronal substrates and functional consequences of prenatal cannabis exposure. Eur. Child Adolesc. Psychiatry 2014, 23, 931–941. [Google Scholar] [CrossRef]
- Spano, M.S.; Ellgren, M.; Wang, X.; Hurd, Y.L. Prenatal Cannabis Exposure Increases Heroin Seeking with Allostatic Changes in Limbic Enkephalin Systems in Adulthood. Biol. Psychiatry 2007, 61, 554–563. [Google Scholar] [CrossRef]
- DiNieri, J.A.; Wang, X.; Szutorisz, H.; Spano, S.M.; Kaur, J.; Casaccia, P.; Dow-Edwards, D.; Hurd, Y.L. Maternal Cannabis Use Alters Ventral Striatal Dopamine D2 Gene Regulation in the Offspring. Biol. Psychiatry 2011, 70, 763–769. [Google Scholar] [CrossRef]
- Bara, A.; Manduca, A.; Bernabeu, A.; Borsoi, M.; Serviado, M.; Lassalle, O.; Murphy, M.N.; Wager-Miller, J.; Mackie, K.; Pelissier-Alicot, A.-L.; et al. Sex-Dependent effects of in utero cannabinoid exposure on cortical function. eLife 2018, 7. [Google Scholar] [CrossRef]
- Manduca, A.; Servadio, M.; Melancia, F.; Schiavi, S.; Manzoni, O.J.; Trezza, V. Sex-Specific behavioural deficits induced at early life by prenatal exposure to the cannabinoid receptor agonist WIN55, 212–2 depend on mGlu5 receptor signalling. Br. J. Pharmacol. 2020, 177, 449–463. [Google Scholar] [CrossRef]
- Frau, R.; Miczán, V.; Traccis, F.; Aroni, S.; Pongor, C.I.; Saba, P.; Serra, V.; Sagheddu, C.; Fanni, S.; Congiu, M.; et al. Prenatal THC exposure produces a hyperdopaminergic phenotype rescued by pregnenolone. Nat. Neurosci. 2019, 22, 1975–1985. [Google Scholar] [CrossRef]
- De Salas-Quiroga, A.; García-Rincón, D.; Gómez-Domínguez, D.; Valero, M.; Simón-Sánchez, S.; Paraíso-Luna, J.; Aguareles, J.; Pujadas, M.; Muguruza, C.; Callado, L.F.; et al. Long-Term hippocampal interneuronopathy drives Sex-Dimorphic spatial memory impairment induced by prenatal THC exposure. Neuropsychopharmacology 2020, 45, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Sagheddu, C.; Traccis, F.; Serra, V.; Congiu, M.; Frau, R.; Cheer, J.F.; Melis, M. Mesolimbic dopamine dysregulation as a signature of information processing deficits imposed by prenatal THC exposure. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2021, 105, 110128. [Google Scholar] [CrossRef]
- Navarro, M.; Rubio, P.; Rodriguez de Fonseca, F. Sex-Dimorphic psychomotor activation after perinatal exposure to (-)-delta 9-Tetrahydrocannabinol. An ontogenic study in Wistar rats. Psychopharmacology 1994, 116, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Vela, G.; Martin, S.; Garcia-Gil, L.; Crespo, J.A.; Ruiz-Gayo, M.; Fernandez-Ruiz, J.J.; Garcia-Lecumberri, C.; Pelaprat, D.; Fuentes, J.A.; Ramos, J.A.; et al. Maternal exposure to delta9-Tetrahydrocannabinol facilitates morphine Self-Administration behavior and changes regional binding to central mu opioid receptors in adult offspring female rats. Brain Res. 1998, 807, 101–109. [Google Scholar] [CrossRef]
- Fernández-Ruiz, J.; De Miguel, R.; Hernandez, M.L.; Cebeira, M.; Ramos, J.A. Comparisons between brain dopaminergic neurons of juvenile and aged rats: Sex-Related differences. Mech. Ageing Dev. 1992, 63, 45–55. [Google Scholar] [CrossRef]
- González, B.; De Miguel, R.; Martín, S.; Pérez-Rosado, A.; Romero, J.; García-Lecumberri, C.; Fernández-Ruiz, J.; Ramos, J.A.; Ambrosio, E. Effects of perinatal exposure to delta 9-Tetrahydrocannabinol on operant Morphine-Reinforced behavior. Pharmacol. Biochem. Behav. 2003, 75, 577–584. [Google Scholar] [CrossRef]
- Navarro, M.; De Miguel, R.; Rodriguez de Fonseca, F.; Ramos, J.A.; Fernandez-Ruiz, J.J. Perinatal cannabinoid exposure modifies the sociosexual approach behavior and the mesolimbic dopaminergic activity of adult male rats. Behav. Brain Res. 1996, 75, 91–98. [Google Scholar] [CrossRef]
- Goldstein, D.S. Biomarkers, Mechanisms, and Potential Prevention of Catecholamine Neuron Loss in Parkinson Disease. Stud. Surf. Sci. Catal. 2013, 68, 235–272. [Google Scholar] [CrossRef]
- Cachope, R.; Mateo, Y.; Mathur, B.N.; Irving, J.; Wang, H.-L.; Morales, M.; Lovinger, D.M.; Cheer, J.F. Selective Activation of Cholinergic Interneurons Enhances Accumbal Phasic Dopamine Release: Setting the Tone for Reward Processing. Cell Rep. 2012, 2, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Mateo, Y.; Johnson, K.A.; Covey, D.P.; Atwood, B.K.; Wang, H.-L.; Zhang, S.; Gildish, I.; Cachope, R.; Bellocchio, L.; Guzmán, M.; et al. Endocannabinoid Actions on Cortical Terminals Orchestrate Local Modulation of Dopamine Release in the Nucleus Accumbens. Neuron 2017, 96, 1112–1126. [Google Scholar] [CrossRef]
- Liu, C.; Kaeser, P.S. Mechanisms and regulation of dopamine release. Curr. Opin. Neurobiol. 2019, 57, 46–53. [Google Scholar] [CrossRef]
- Melis, M.; De Felice, M.; Lecca, S.; Fattore, L.; Pistis, M. Sex-Specific tonic 2-Arachidonoylglycerol signaling at inhibitory inputs onto dopamine neurons of Lister Hooded rats. Front. Integr. Neurosci. 2013, 7, 93. [Google Scholar] [CrossRef][Green Version]
- Meyer, H.C.; Lee, F.S.; Gee, D.G. The Role of the Endocannabinoid System and Genetic Variation in Adolescent Brain De-velopment. Neuropsychopharmacology 2018, 43, 21–33. [Google Scholar] [CrossRef]
- Mitra, I.; Tsang, K.; Ladd-Acosta, C.; Croen, L.A.; Aldinger, K.A.; Hendren, R.L.; Traglia, M.; Lavillaureix, A.; Zaitlen, N.; Oldham, M.C.; et al. Pleiotropic Mechanisms Indicated for Sex Differences in Autism. PLoS Genet 2016, 12, e1006425. [Google Scholar] [CrossRef] [PubMed]
- Werling, D.M.; Geschwind, D.H. Sex differences in autism spectrum disorders. Curr. Opin. Neurol. 2013, 26, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Baron-Cohen, S.; Lombardo, M.V.; Auyeung, B.; Ashwin, E.; Chakrabarti, B.; Knickmeyer, R. Why Are Autism Spectrum Conditions More Prevalent in Males? PLoS Biol. 2011, 9, e1001081. [Google Scholar] [CrossRef]
- Riecher-Rössler, A. Sex and gender differences in mental disorders. Lancet Psychiatry 2017, 4, 8–9. [Google Scholar] [CrossRef]
- De Felice, M.; Melis, M.; Aroni, S.; Muntoni, A.L.; Fanni, S.; Frau, R.; Devoto, P.; Pistis, M. The PPARalpha agonist fenofibrate attenuates disruption of dopamine function in a maternal immune activation rat model of schizophrenia. CNS Neurosci. Ther. 2019, 25, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.; Megens, A.; Peek, M.; Wallace, E.M. Sexual origins of placental dysfunction. Lancet 2000, 355, 203–204. [Google Scholar] [CrossRef]
- Binder, N.K.; Beard, S.A.; Kaitu’U-Lino, T.J.; Tong, S.; Hannan, N.J.; Gardner, D.K. Paternal obesity in a rodent model affects placental gene expression in a Sex-Specific manner. Reproduction 2015, 149, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-Y.; Ganguly, A.; Rubbi, L.; Orozco, L.D.; Morselli, M.; Ashraf, D.; Jaroszewicz, A.; Feng, S.; Jacobsen, S.E.; Nakano, A.; et al. Intrauterine calorie restriction affects placental DNA methylation and gene expression. Physiol. Genom. 2013, 45, 565–576. [Google Scholar] [CrossRef]
- Natale, B.V.; Gustin, K.N.; Lee, K.; Holloway, A.C.; Laviolette, S.R.; Natale, D.R.C.; Hardy, D.B. Δ9-Tetrahydrocannabinol exposure during rat pregnancy leads to symmetrical fetal growth restriction and labyrinth-specific vascular defects in the placenta. Sci. Rep. 2020, 10, 544. [Google Scholar] [CrossRef]
- Guneykaya, D.; Ivanov, A.; Hernandez, D.P.; Haage, V.; Wojtas, B.; Meyer, N.; Maricos, M.; Jordan, P.; Buonfiglioli, A.; Gielniewski, B.; et al. Transcriptional and Translational Differences of Microglia from Male and Female Brains. Cell Rep. 2018, 24, 2773–2783. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Della Torre, S.; Maggi, A. Sexual differentiation of microglia. Front. Neuroendocr. 2019, 52, 156–164. [Google Scholar] [CrossRef]
- Yasuda, K.; Maki, T.; Kinoshita, H.; Kaji, S.; Toyokawa, M.; Nishigori, R.; Kinoshita, Y.; Ono, Y.; Kinoshita, A.; Takahashi, R. Sex-Specific differences in transcriptomic profiles and cellular characteristics of oligodendrocyte precursor cells. Stem Cell Res. 2020, 46, 101866. [Google Scholar] [CrossRef] [PubMed]
- Spiro, A.S.; Wong, A.; Boucher, A.A.; Arnold, J.C. Enhanced brain disposition and effects of Delta9-Tetrahydrocannabinol in P-Glycoprotein and breast cancer resistance protein knockout mice. PLoS ONE 2012, 7, e35937. [Google Scholar] [CrossRef][Green Version]
- Tanaka, Y.; Slitt, A.L.; Leazer, T.M.; Maher, J.M.; Klaassen, C.D. Tissue distribution and hormonal regulation of the breast cancer resistance protein (Bcrp/Abcg2) in rats and mice. Biochem. Biophys. Res. Commun. 2004, 326, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Hahnova-Cygalova, L.; Ceckova, M.; Staud, F. Fetoprotective activity of breast cancer resistance protein (BCRP, ABCG2): Expression and function throughout pregnancy. Drug Metab. Rev. 2010, 43, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Myllynen, P.; Kummu, M.; Sieppi, E. ABCB1 and ABCG2 expression in the placenta and fetus: An interspecies comparison. Expert Opin. Drug Metab. Toxicol. 2010, 6, 1385–1398. [Google Scholar] [CrossRef]
- Holland, M.L.; Lau, D.T.T.; Allen, J.D.; Arnold, J.C. The multidrug transporter ABCG2 (BCRP) is inhibited by Plant-Derived cannabinoids. Br. J. Pharmacol. 2007, 152, 815–824. [Google Scholar] [CrossRef]
- Wiley, J.L.; O’Connell, M.M.; Tokarz, M.E.; Wright, M.J., Jr. Pharmacological effects of acute and repeated administration of Delta(9)-Tetrahydrocannabinol in adolescent and adult rats. J. Pharmacol. Exp. Ther. 2007, 320, 1097–1105. [Google Scholar] [CrossRef]
- Mehmedic, Z.; Chandra, S.; Slade, D.; Denham, H.; Foster, S.; Patel, A.S.; Ross, S.A.; Khan, I.A.; ElSohly, M.A. Potency trends of Delta9-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. J. Forensic Sci. 2010, 55, 1209–1217. [Google Scholar] [CrossRef]
- Huber, J.; Darling, S.; Park, K.; Soliman, K.F.A. Altered responsiveness to stress and NMDA following prenatal exposure to cocaine. Physiol. Behav. 2001, 72, 181–188. [Google Scholar] [CrossRef]
- Henarejos, A.B.M.; Popović, N.; Bokonjić, D.; Morales-Delgado, N.; Alonso, A.; Bleda, M.C.; Popović, M. Sex and Time of Day Impact on Anxiety and Passive Avoidance Memory Strategies in Mice. Front. Behav. Neurosci. 2020, 14, 68. [Google Scholar] [CrossRef] [PubMed]
- Trezza, V.; Campolongo, P.; Cassano, T.; Macheda, T.; Dipasquale, P.; Carratu, M.R.; Gaetani, S.; Cuomo, V. Effects of Per-Inatal exposure to Delta-9-Tetrahydrocannabinol on the emotional reactivity of the offspring: A longitudinal behavioral study in Wistar rats. Psychopharmacology 2008, 198, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Bortolato, M.; Godar, S.C.; Alzghoul, L.; Zhang, J.; Darling, R.D.; Simpson, K.L.; Bini, V.; Chen, K.; Wellman, C.L.; Lin, R.C.S.; et al. Monoamine oxidase A and A/B knockout mice display autistic like features. Int. J. Neuropsychopharmacol. 2013, 16, 869–888. [Google Scholar] [CrossRef]
- Frey, A.; Popp, S.; Post, A.; Langer, S.; Lehmann, M.; Hofmann, U.; Siren, A.-L.; Hommers, L.; Schmitt, A.; Strekalova, T.; et al. Experimental heart failure causes depression-like behavior together with differential regulation of inflammatory and structural genes in the brain. Front. Behav. Neurosci. 2014, 8, 376. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: San Diego, CA, USA, 2007. [Google Scholar]
- Devoto, P.; Flore, G.; Longu, G.; Pira, L.; Gessa, G.L. Origin of extracellular dopamine from dopamine and noradrenaline neurons in the medial prefrontal and occipital cortex. Synapse 2003, 50, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.W.; North, R.A. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J. Physiol. 1992, 450, 455–468. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Traccis, F.; Serra, V.; Sagheddu, C.; Congiu, M.; Saba, P.; Giua, G.; Devoto, P.; Frau, R.; Cheer, J.F.; Melis, M. Prenatal THC Does Not Affect Female Mesolimbic Dopaminergic System in Preadolescent Rats. Int. J. Mol. Sci. 2021, 22, 1666. https://doi.org/10.3390/ijms22041666
Traccis F, Serra V, Sagheddu C, Congiu M, Saba P, Giua G, Devoto P, Frau R, Cheer JF, Melis M. Prenatal THC Does Not Affect Female Mesolimbic Dopaminergic System in Preadolescent Rats. International Journal of Molecular Sciences. 2021; 22(4):1666. https://doi.org/10.3390/ijms22041666
Chicago/Turabian StyleTraccis, Francesco, Valeria Serra, Claudia Sagheddu, Mauro Congiu, Pierluigi Saba, Gabriele Giua, Paola Devoto, Roberto Frau, Joseph Francois Cheer, and Miriam Melis. 2021. "Prenatal THC Does Not Affect Female Mesolimbic Dopaminergic System in Preadolescent Rats" International Journal of Molecular Sciences 22, no. 4: 1666. https://doi.org/10.3390/ijms22041666
APA StyleTraccis, F., Serra, V., Sagheddu, C., Congiu, M., Saba, P., Giua, G., Devoto, P., Frau, R., Cheer, J. F., & Melis, M. (2021). Prenatal THC Does Not Affect Female Mesolimbic Dopaminergic System in Preadolescent Rats. International Journal of Molecular Sciences, 22(4), 1666. https://doi.org/10.3390/ijms22041666






