The Anticancer Effect of Natural Plant Alkaloid Isoquinolines
Abstract
1. Introduction
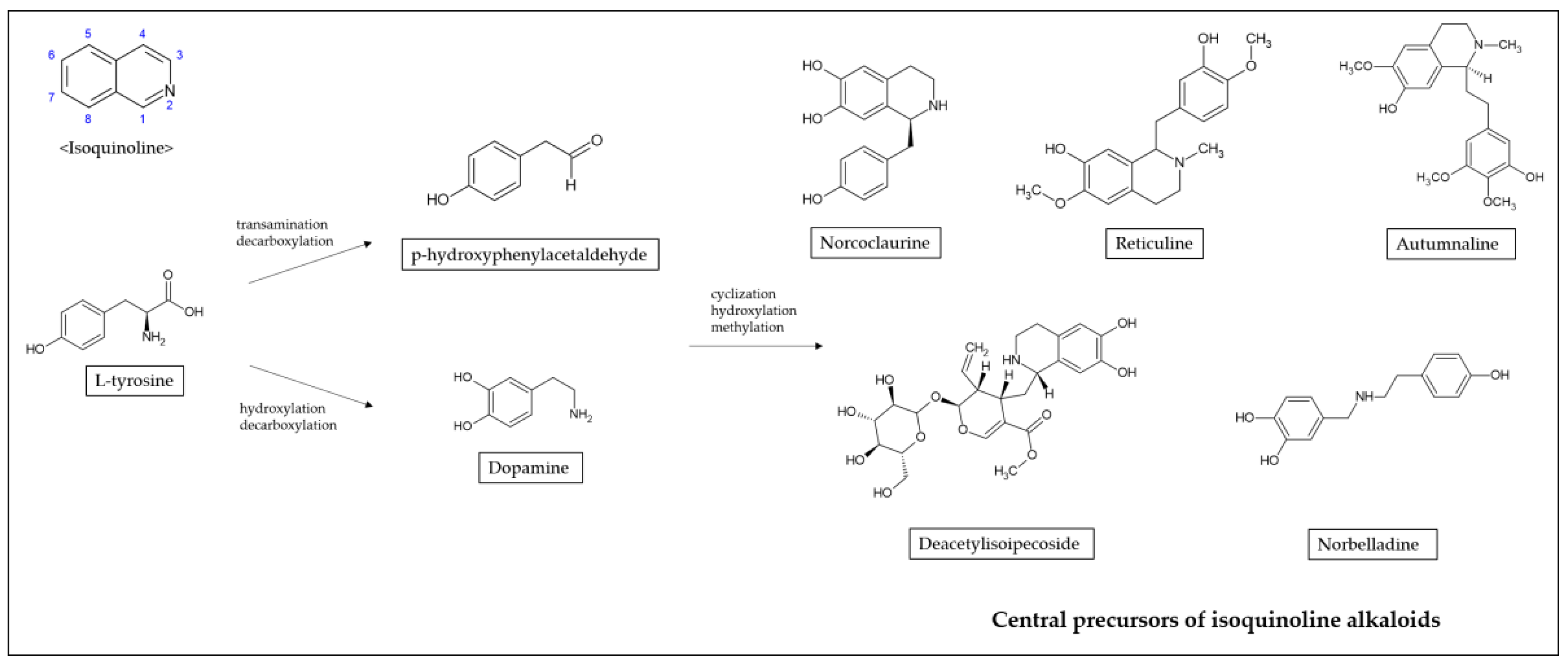
2. Isoquinoline Alkaloids Derived from Various Herb Extracts
3. Biological Functions
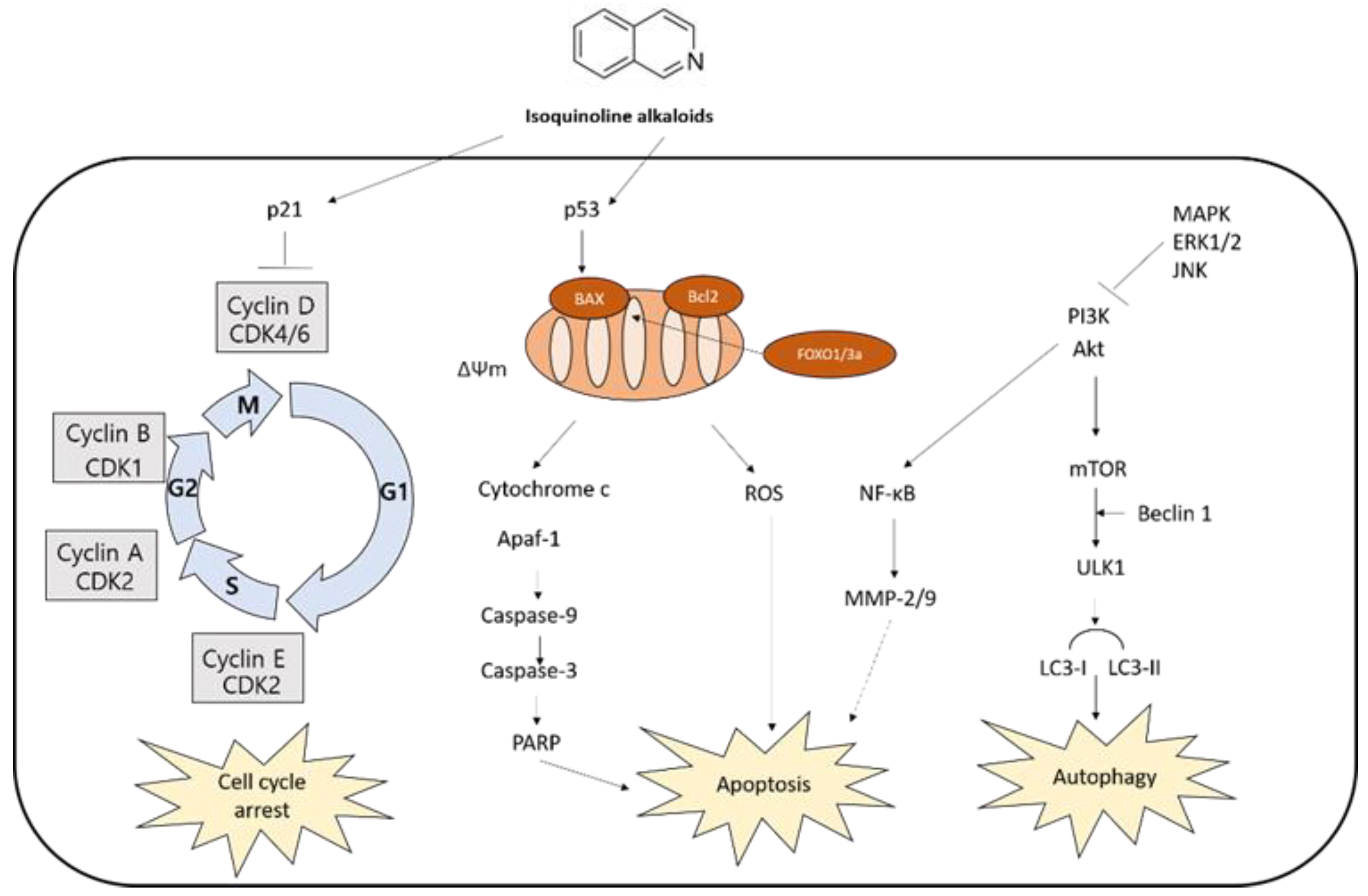
4. Anticancer Effects of Isoquinoline Alkaloids
4.1. Apoptosis-Mediated Cell Death
4.1.1. Caspase-Dependent Apoptosis
4.1.2. MAPK-Mediated Apoptosis
4.2. Cell Cycle Arrest
4.3. Autophagy-Mediated Cell Death
| Mechanisms | Cancer Type | Effect | Compounds | Reference |
|---|---|---|---|---|
| Apoptosis | Colorectal cancer | Accumulation of cells in sub G0 phase Increase in Bax expression | Berberine | [73] |
| Breast cancer | Condensed chromatin with fragmented nuclei Accumulation of cells in sub G0 phase | Noscapine | [83] | |
| Gastric cancer | Decrease of mitochondrial membrane potential Increased release of cytochrome c Activation of caspase-3/8/9 and PARP Decrease in Bcl-2 expression Increase in Bax expression Apoptotic DNA fragmentation | Chelerythrine | [59] | |
| Breast cancer Liver cancer Lung cancer Prostate cancer | Berberine | [66,67,68,84,85,86] | ||
| Leukemia | Berberine Scoulerine | [87,88] | ||
| Colorectal cancer | Noscapine Sanguinarine | [41,63] | ||
| Breast cancer | Decrease of mitochondrial membrane potential Increased phosphorylation of JNK Increased release of cytochrome c and AIF Activation of caspase-3 Decrease in Bcl-2 expression Increase in Bax expression | Berberine | [89] | |
| Colorectal cancer | Liensinine | [90] | ||
| Lung cancer | Increased phosphorylation of p38 MAPK Increase in transcriptional activity of FoxO3a | Berberine | [72] | |
| Liver cancer | Suppressed PI3K/Akt/mTOR pathway Increased phosphorylation of JNK Reactive oxygen species (ROS) generation Increase in Bim expression and transcriptional activity of FoxO | Berberine | [91] | |
| Prostate cancer | Decrease of mitochondrial membrane potential Decrease in Bcl-2, Bcl-XL, and XIAP expression Increase in Bax, Bad, and Apaf-1 expression Increased cytochrome c and AIF release Activation of caspase-3 and PARP Suppression of PI3K/Akt pathway | Sinomenine | [92] | |
| Liver cancer | Tetrandrine | [93] | ||
| Lung cancer | Reactive oxygen species (ROS) generation Activation of caspase-3/8/9 and PARP Endoplasmic reticulum (ER) stress activation Increased phosphorylation of JNK Suppression of PI3K/Akt pathway | Chelerythrine | [64] | |
| Liver cancer Colorectal cancer | Coptisine | [94,95] | ||
| Colorectal cancer | Scoulerine | [96] | ||
| Renal cancer | Decreased phosphorylation of ERK and Akt Decrease in Bcl-2 expression Increase in Bax and p53 expression | Chelerythrine | [97] | |
| Oral cancer | Increase in FasL expression Decrease in Bcl-2 and Bcl-xL expression Increase in Bax, Bad, and Apaf-1 expression Activation of caspase-3/8/9 and PARP Increased phosphorylation of p38 MAPK | Berberine | [98] | |
| Cell cycle arrest | Breast cancer Colorectal cancer Gastric cancer Pancreatic cancer Prostate cancer | G1 phase cell cycle arrest | Berberine Sanguinarine Chelerythrine | [57,60,61,86,99] |
| Colorectal cancer Glioblastoma Lung cancer | G1 phase cell cycle arrest induction of p21 inhibition of cyclin D1 | Tetrandrine Berberine | [59,76,100,101] | |
| Gastric cancer Ovarian cancer | S phase cell cycle arrest | Chelerythrine Liriodenine | [59] | |
| Glioblastoma | G2/M phase cell arrest Enhanced cyclin dependent kinase 1 (Cdk1)/cyclin B1 complex activity | Chelidonine | [102] | |
| Colorectal cancer | Liensinine Noscapine Berberine | [41,58,92] | ||
| Leukemia | Scoulerine | [88] | ||
| Breast cancer | Noscapine | [83] | ||
| Prostate cancer | Protopine | [103] | ||
| Autophagy | Breast cancer Gastric cancer Glioblastoma cancer Liver cancer Lungcancer | Enhanced expression of LC3-II Increase of AMPK activity Downregulated expression of PI3K, Akt, and mTOR Activation of Beclin-1 | Berberine Neferine Sanguinarine Chelerythrine | [64,80,81,82,86,99,100] |
5. Molecular Mechanisms of Anticancer Effects
5.1. Binding to Polynucleic Acids
5.2. Binding to Microtubules
5.3. Inhibition of Enzyme Activity
5.4. Epigenetic Modulation
| Tumor Type | Compounds | Effect | Reference |
|---|---|---|---|
| Liver cancer | Berberine | Reduced DNA methylation level in promoter regions of CYP2B6 and CYP3A4 genes | [127] |
| Myeloma | Berberine | Increased the level of Set9 (lysine methyltransferase) Increased the level of methylation of the RelA subunit Inhibited NF-κB nuclear translocation and miR-21 transcription Hypomethylation of p53 promoter | [129,132] |
| Colorectal cancer | Berberine | Increased the level of DNMT1, DNMT3A, DNMT3B Increased the level of miR-152, miR-429, miR-29a | [130] |
| Lung cancer | Berberine | Decrease of HDAC activity Hyperacetylated histones H3 and H4 Decreased level of tumor necrosis factor-α (TNF-α), COX-2, MMP-2, and MMP-9 Increased the level of p21 and p53 | [128] |
| Cervical cancer | Sanguinarine | Reduced H3K9, H3K4, and H3R17 methylation | [131] |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviation
| AChE | Acetylcholinesterase |
| AD | Alzheimer’s disease |
| AMPK | AMP-activated protein kinase |
| ATF3 | Activating transcription factor 3 |
| BuChE | Butylcholinesterase |
| CAM | Complementary and alternative medicine |
| CDK1 | Cyclin Dependent Kinase 1 |
| COX-2 | Cyclooxygenase-2 |
| CYP2B6 | Cytochrome P450 2B6 |
| CYP3A4 | Cytochrome P450 3A4 |
| DISC | Death-inducing signaling complex |
| DNMT | DNA methyltransferase |
| DR | Death receptor |
| EGFR | Epidermal growth factor receptor |
| ERK | Extracellular signal-regulated kinase |
| FADD | Fas-associated death domain |
| FOXO3a | Forkhead box class O 3a |
| GSK-3β | Glycogen synthase kinase 3 beta |
| HDAC | Histone deacetylase |
| IKK | I kappa B kinase |
| JNK | Jun N-terminal kinase |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinase |
| mTOR | Mechanistic target of rapamycin |
| NAG-1 | (NSAID) activated gene-1 |
| NF-κB | Nuclear factor-kappa B |
| PARP | Poly ADP-ribose polymerase |
| PI3K | Phosphoinositide 3-kinase |
| PKC | Protein kinase C |
| ROS | Reactive oxygen species |
| SAR | Structure–activity relationship |
| TLR4 | Toll-like receptor 4 |
| TNFR1 | Tumor necrosis factor receptor 1 |
| TNF-α | Tumor necrosis factor-α |
| TRADD | TNFR1-associated death domain protein |
| ULK1 | Unc-51 Like Autophagy Activating Kinase 1 |
References
- Sener, S.F.; Grey, N. The global burden of cancer. J. Surg. Oncol. 2005, 92, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Cassileth, B. Complementary or alternative medicine in cancer care—myths and realities. Nat. Rev. Clin. Oncol. 2013, 10, 656. [Google Scholar] [CrossRef] [PubMed]
- Jermini, M.; Dubois, J.; Rodondi, P.-Y.; Zaman, K.; Buclin, T.; Csajka, C.; Orcurto, A.; Rothuizen, L.E. Complementary medicine use during cancer treatment and potential herb-drug interactions from a cross-sectional study in an academic centre. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Choi, B.Y.; Joo, J.-C.; Lee, Y.K.; Jang, I.-S.; Park, S.J.; Park, Y.J. Anti-cancer effect of Scutellaria baicalensis in combination with cisplatin in human ovarian cancer cell. BMC Complement. Altern. Med. 2017, 17, 277. [Google Scholar] [CrossRef]
- Lee, Y.K.; Lim, J.; Yoon, S.Y.; Joo, J.-C.; Park, S.J.; Park, Y.J. Promotion of Cell Death in Cisplatin-Resistant Ovarian Cancer Cells through KDM1B-DCLRE1B Modulation. Int. J. Mol. Sci. 2019, 20, 2443. [Google Scholar] [CrossRef]
- Mukherjee, A.; Basu, S.; Sarkar, N.; Ghosh, A. Advances in cancer therapy with plant based natural products. Curr. Med. Chem. 2001, 8, 1467–1486. [Google Scholar] [CrossRef]
- Gerenčer, M.; Turecek, P.L.; Kistner, O.; Mitterer, A.; Savidis-Dacho, H.; Barrett, N.P. In vitro and in vivo anti-retroviral activity of the substance purified from the aqueous extract of Chelidonium majus L. Antivir. Res. 2006, 72, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Lee, K.; Lee, M.-H.; Kim, S.-H.; Ham, I.; Choi, H.-Y. Inhibitory effects of Chelidonium majus extract on atopic dermatitis-like skin lesions in NC/Nga mice. J. Ethnopharmacol. 2011, 138, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.J.; Bhattacharjee, N.; Khuda-Bukhsh, A.R. Efficacy of a plant extract (Chelidonium majus L.) in combating induced hepatocarcinogenesis in mice. Food Chem. Toxicol. 2008, 46, 1474–1487. [Google Scholar] [CrossRef]
- Nadova, S.; Miadokova, E.; Alfoldiova, L.; Kopaskova, M.; Hasplova, K.; Hudecova, A.; Vaculcikova, D.; Gregan, F.; Cipak, L. Potential antioxidant activity, cytotoxic and apoptosis-inducing effects of Chelidonium majus L. extract on leukemia cells. Neuro Endocrinol. Lett. 2008, 29, 649–652. [Google Scholar]
- Mikołajczak, P.Ł.; Kedzia, B.; Ożarowski, M.; Kujawski, R.; Bogacz, A.; Bartkowiak-Wieczorek, J.; Białas, W.; Gryszczyńska, A.; Buchwald, W.; Szulc, M.; et al. Evaluation of anti-inflammatory and analgesic activities of extracts from herb of Chelidonium majus L. Cent. Eur. J. Immunol. 2015, 40, 400. [Google Scholar] [CrossRef] [PubMed]
- Satyajit, D.; Lutfun, N. Chemistry for Pharmacy Students General, Organic and Natural Product Chemistry; John Wiley & Sons Ltd.: Chichester, UK, 2007. [Google Scholar]
- Chrzanowska, M.; Grajewska, A.; Rozwadowska, M.D. Asymmetric synthesis of isoquinoline alkaloids: 2004–2015. Chem. Rev. 2016, 116, 12369–12465. [Google Scholar] [CrossRef]
- Diamond, A.; Desgagné-Penix, I. Metabolic engineering for the production of plant isoquinoline alkaloids. Plant Biotechnol. J. 2016, 14, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Civjan, N. Natural Products in Chemical Biology; Wiley Online Library: Hoboken, NJ, USA, 2012. [Google Scholar]
- Brahmachari, G. Bioactive Natural Products; Wiley Online Library: Hoboken, NJ, USA, 2015. [Google Scholar]
- Weid, M.; Ziegler, J.; Kutchan, T.M. The roles of latex and the vascular bundle in morphine biosynthesis in the opium poppy, Papaver somniferum. Proc. Natl. Acad. Sci. USA 2004, 101, 13957–13962. [Google Scholar] [CrossRef] [PubMed]
- Preininger, V. Chemotaxonomy of the Papaveraceae Alkaloids. In The Chemistry and Biology of Isoquinoline Alkaloids; Springer: New York, NY, USA, 1985; pp. 23–37. [Google Scholar]
- Sárközi, Á.; Janicsák, G.; Kursinszki, L.; Kéry, Á. Alkaloid composition of Chelidonium majus L. studied by different chromatographic techniques. Chromatographia 2006, 63, S81–S86. [Google Scholar]
- Hostalkova, A.; Marikova, J.; Opletal, L.; Korabecny, J.; Hulcova, D.; Kunes, J.; Novakova, L.; Perez, D.I.; Jun, D.; Kucera, T.; et al. Isoquinoline alkaloids from Berberis vulgaris as potential lead compounds for the treatment of Alzheimer’s disease. J. Nat. Prod. 2019, 82, 239–248. [Google Scholar] [CrossRef]
- Steglich, W.; Fugmann, B.; Lang-Fugmann, S. Römpp Encyclopedia Natural Products; Georg Thieme: Stuttgart, Germany, 2000. [Google Scholar]
- Bhadra, K.; Kumar, G.S. Therapeutic potential of nucleic acid-binding isoquinoline alkaloids: Binding aspects and implications for drug design. Med. Res. Rev. 2011, 31, 821–862. [Google Scholar] [CrossRef]
- Maiti, M.; Kumar, G.S. Polymorphic nucleic acid binding of bioactive isoquinoline alkaloids and their role in cancer. J. Nucleic Acids 2010, 2010, 593408. [Google Scholar] [CrossRef]
- Das, S.; Kumar, G.S.; Ray, A.; Maiti, M. Spectroscopic and thermodynamic studies on the binding of sanguinarine and berberine to triple and double helical DNA and RNA structures. J. Biomol. Struct. Dyn. 2003, 20, 703–713. [Google Scholar] [CrossRef]
- Hossain, M.; Kabir, A.; Suresh Kumar, G. Binding of the anticancer alkaloid sanguinarine with tRNAphe: Spectroscopic and calorimetric studies. J. Biomol. Struct. Dyn. 2012, 30, 223–234. [Google Scholar] [CrossRef]
- Basu, P.; Suresh Kumar, G. A comparative study on the interaction of the putative anticancer alkaloids, sanguinarine and chelerythrine, with single-and double-stranded, and heat-denatured DNAs. J. Biomol. Struct. Dyn. 2015, 33, 2594–2605. [Google Scholar] [CrossRef]
- Pagliosa, L.; Monteiro, S.; Silva, K.; De Andrade, J.; Dutilh, J.; Bastida, J.; Cammarota, M.; Zuanazzi, J. Effect of isoquinoline alkaloids from two Hippeastrum species on in vitro acetylcholinesterase activity. Phytomedicine 2010, 17, 698–701. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.; Liu, A.; Li, T.-K.; Wu, H.-Y.; Desai, S.; Mao, Y.; Rubin, E.; Lavoie, E.; Makhey, D.; Liu, L.F. Selective cytotoxicity of topoisomerase-directed protoberberines against glioblastoma cells. Biochem. Pharmacol. 1998, 56, 1157–1166. [Google Scholar] [CrossRef]
- Gatto, B.; Sanders, M.M.; Yu, C.; Wu, H.Y.; Makhey, D.; Lavoie, E.J.; Liu, L.F. Identification of topoisomerase I as the cytotoxic target of the protoberberine alkaloid coralyne. Cancer Res. 1996, 56, 2795–2800. [Google Scholar] [PubMed]
- Salminen, K.A.; Meyer, A.; Jerabkova, L.; Korhonen, L.E.; Rahnasto, M.; Juvonen, R.O.; Imming, P.; Raunio, H. Inhibition of human drug metabolizing cytochrome P450 enzymes by plant isoquinoline alkaloids. Phytomedicine 2011, 18, 533–538. [Google Scholar] [CrossRef]
- El-Readi, M.Z.; Eid, S.; Ashour, M.L.; Tahrani, A.; Wink, M. Modulation of multidrug resistance in cancer cells by chelidonine and Chelidonium majus alkaloids. Phytomedicine 2013, 20, 282–294. [Google Scholar] [CrossRef]
- Pandey, M.K.; Sung, B.; Kunnumakkara, A.B.; Sethi, G.; Chaturvedi, M.M.; Aggarwal, B.B. Berberine modifies cysteine 179 of IκBα kinase, suppresses nuclear factor-κB–regulated antiapoptotic gene products, and potentiates apoptosis. Cancer Res. 2008, 68, 5370–5379. [Google Scholar] [CrossRef]
- Fukuda, K.; Hibiya, Y.; Mutoh, M.; Koshiji, M.; Akao, S.; Fujiwara, H. Inhibition by berberine of cyclooxygenase-2 transcriptional activity in human colon cancer cells. J. Ethnopharmacol. 1999, 66, 227–233. [Google Scholar] [CrossRef]
- Dighe, S.N.; Deora, G.S.; De La Mora, E.; Nachon, F.; Chan, S.; Parat, M.-O.; Brazzolotto, X.; Ross, B.P. Discovery and structure–activity relationships of a highly selective butyrylcholinesterase inhibitor by structure-based virtual screening. J. Med. Chem. 2016, 59, 7683–7689. [Google Scholar] [CrossRef]
- Cahlíková, L.; Opletal, L.; Kurfürst, M.; Macáková, K.; Kulhánková, A.; Hošt’álková, A. Acetylcholinesterase and butyrylcholinesterase inhibitory compounds from Chelidonium majus (Papaveraceae). Nat. Prod. Commun. 2010, 5. [Google Scholar]
- Pilch, D.S.; Yu, C.; Makhey, D.; Lavoie, E.J.; Srinivasan, A.R.; Olson, W.K.; Sauers, R.R.; Breslauer, K.J.; Geacintov, N.E.; Liu, L.F. Minor groove-directed and intercalative ligand− DNA interactions in the poisoning of human DNA topoisomerase I by protoberberine analogs. Biochemistry 1997, 36, 12542–12553. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Hua, G.; Meng, Z.; Gao, P. Antibacterial mechanisms of berberine and reasons for little resistance of bacteria. Chin. Herb. Med. 2010, 3, 27–35. [Google Scholar]
- Tan, G.T.; Pezzuto, J.M.; Kinghorn, A.D.; Hughes, S.H. Evaluation of natural products as inhibitors of human immunodeficiency virus type 1 (HIV-1) reverse transcriptase. J. Nat. Prod. 1991, 54, 143–154. [Google Scholar] [CrossRef]
- Sethi, M.L. Enzyme inhibition VI: Inhibition of reverse transcriptase activity by protoberberine alkaloids and structure–activity relationships. J. Pharm. Sci. 1983, 72, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-R.; Liu, M.; Peng, X.-L.; Lei, X.-F.; Zhang, J.-X.; Dong, W.-G. Noscapine induces mitochondria-mediated apoptosis in human colon cancer cells in vivo and in vitro. Biochem. Biophys. Res. Commun. 2012, 421, 627–633. [Google Scholar] [CrossRef]
- Gottshall, R.Y.; Lucas, E.H.; Lickfeldt, A.; Roberts, J.M. The occurrence of antibacterial substances active against Mycobacterium tuberculosis in seed plants. J. Clin. Investig. 1949, 28, 920–923. [Google Scholar] [CrossRef]
- Dermarderosian, A. Medicinal teas-boon or bane. Drug Ther. 1977, 7, 178. [Google Scholar]
- Vollekova, A.; Košťálová, D.; Sochorova, R. Isoquinoline alkaloids from Mahonia aquifolium stem bark are active against Malassezia spp. Folia Microbiol. 2001, 46, 107. [Google Scholar] [CrossRef]
- Cecil, C.E.; Davis, J.M.; Cech, N.B.; Laster, S.M. Inhibition of H1N1 influenza A virus growth and induction of inflammatory mediators by the isoquinoline alkaloid berberine and extracts of goldenseal (Hydrastis canadensis). Int. Immunopharmacol. 2011, 11, 1706–1714. [Google Scholar] [CrossRef]
- Varghese, F.S.; Thaa, B.; Amrun, S.N.; Simarmata, D.; Rausalu, K.; Nyman, T.A.; Merits, A.; McInerney, G.M.; Ng, L.F.P.; Ahola, T. The antiviral alkaloid berberine reduces chikungunya virus-induced mitogen-activated protein kinase signaling. J. Virol. 2016, 90, 9743–9757. [Google Scholar] [CrossRef]
- Wang, X.; Feng, S.; Ding, N.; He, Y.; Li, C.; Li, M.; Ding, X.; Ding, H.; Li, J.; Wu, J.; et al. Anti-inflammatory effects of berberine hydrochloride in an LPS-induced murine model of mastitis. Evid. Based Complement. Altern. Med. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.-H.; Ahn, I.-S.; Kim, Y.-H.; Park, J.-W.; Lee, S.-Y.; Hyun, C.-K.; Do, M.-S. Berberine reduces the expression of adipogenic enzymes and inflammatory molecules of 3T3-L1 adipocyte. Exp. Mol. Med. 2006, 38, 599–605. [Google Scholar] [CrossRef]
- Liao, W.; He, X.; Yi, Z.; Xiang, W.; Ding, Y. Chelidonine suppresses LPS-Induced production of inflammatory mediators through the inhibitory of the TLR4/NF-κB signaling pathway in RAW264. 7 macrophages. Biomed. Pharmacother. 2018, 107, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Aldulaimi, A.K.O.; Abd-Azziz, S.S.S.; Bakri, Y.M.; Nafiah, M.A.; Aowda, S.; Awang, K.; Litaudon, M. Two New isoquinoline alkaloids from the bark of Alphonsea cylindrica King and their antioxidant activity. Phytochem. Lett. 2019, 29, 110–114. [Google Scholar] [CrossRef]
- Maiza-benabdesselam, F.; Khentache, S.; Bougoffa, K.; Chibane, M.; Adach, S.; Chapeleur, Y.; Max, H.; Laurain-Mattar, D. Antioxidant activities of alkaloid extracts of two Algerian species of Fumaria: Fumaria capreolata and Fumaria bastardii. Biol. Chem. 2007, 1, 28. [Google Scholar]
- Havelek, R.; Seifrtova, M.; Královec, K.; Krocova, E.; Tejkalova, V.; Novotny, I.; Cahlíková, L.; Safratova, M.; Opletal, L.; Bilkova, Z.; et al. Comparative cytotoxicity of chelidonine and homochelidonine, the dimethoxy analogues isolated from Chelidonium majus L.(Papaveraceae), against human leukemic and lung carcinoma cells. Phytomedicine 2016, 23, 253–266. [Google Scholar] [CrossRef]
- Choi, S.U.; Baek, N.-L.; Kim, S.-H.; Yang, J.H.; Eun, J.S.; Shin, T.Y.; Lim, J.P.; Lee, J.H.; Jeon, H.; Yun, M.-Y.; et al. Cytotoxic isoquinoline alkaloids from the aerial parts ofCorydalis incisa. Arch. Pharmacal Res. 2007, 30, 151–154. [Google Scholar] [CrossRef]
- Al-ghazzawi, A.M. Anti-cancer activity of new benzyl isoquinoline alkaloid from Saudi plant Annona squamosa. BMC Chem. 2019, 13, 1–6. [Google Scholar] [CrossRef]
- Iizuka, N. Inhibitory effect of Coptidis Rhizoma and berberine on the proliferation of human esophageal cancer cell lines. Cancer Lett. 2000, 148, 19–25. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Fulda, S.; Debatin, K.M. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 2006, 25, 4798–4811. [Google Scholar] [CrossRef]
- Shi, Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol. Cell 2002, 9, 459–470. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, Y.; Zhang, L.; Zhang, J.; Wei, X. Chelerythrine chloride from Macleaya cordata induces growth inhibition and apoptosis in human gastric cancer BGC-823 cells. Acta Pharm. Sin. B 2012, 2, 464–471. [Google Scholar] [CrossRef]
- Sun, M.; Lou, W.; Chun, J.Y.; Cho, D.S.; Nadiminty, N.; Evans, C.P.; Chen, J.; Yue, J.; Zhou, Q.; Gao, A.C. Sanguinarine suppresses prostate tumor growth and inhibits survivin expression. Genes Cancer 2010, 1, 283–292. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Venkat, P.S.; Gu, C.; Meng, Y. Sanguinarine exhibits potent efficacy against cervical cancer cells through inhibiting the STAT3 pathway in vitro and in vivo. Cancer Manag. Res. 2019, 11, 7557. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, H.; Reagan-Shaw, S.; Breur, J.; Ahmad, N. Sanguinarine induces apoptosis of human pancreatic carcinoma AsPC-1 and BxPC-3 cells via modulations in Bcl-2 family proteins. Cancer Lett. 2007, 249, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Chen, Z.; Han, Q.; Chen, C.; Jing, L.; Liu, Y.; Zhao, L.; Yao, X.; Sun, X. Sanguinarine triggers intrinsic apoptosis to suppress colorectal cancer growth through disassociation between STRAP and MELK. BMC Cancer 2018, 18, 578. [Google Scholar] [CrossRef]
- Tang, Z.-H.; Cao, W.-X.; Wang, Z.-Y.; Lu, J.-H.; Liu, B.; Chen, X.; Lu, J.-J. Induction of reactive oxygen species-stimulated distinctive autophagy by chelerythrine in non-small cell lung cancer cells. Redox Biol. 2017, 12, 367–376. [Google Scholar] [CrossRef]
- Xie, Y.-J.; Gao, W.-N.; Wu, Q.-B.; Yao, X.-J.; Jiang, Z.-B.; Wang, Y.-W.; Wang, W.-J.; Li, W.; Hussain, S.; Liu, L.; et al. Chelidonine Selectively Inhibits the Growth of Gefitinib-resistant Non-small Cell Lung Cancer Cells through the EGFR-AMPK Pathway. Pharmacol. Res. 2020, 159, 104934. [Google Scholar] [CrossRef]
- Xiao, Y.; Tian, C.; Huang, T.; Han, B.; Wang, M.; Ma, H.; Li, Z.; Ye, X.; Li, X. 8-Cetylberberine inhibits growth of lung cancer in vitro and in vivo. Life Sci. 2018, 192, 259–269. [Google Scholar] [CrossRef]
- Katiyar, S.K.; Meeran, S.M.; Katiyar, N.; Akhtar, S. p53 cooperates berberine-induced growth inhibition and apoptosis of non-small cell human lung cancer cells in vitro and tumor xenograft growth in vivo. Mol. Carcinog. 2009, 48, 24–37. [Google Scholar] [CrossRef]
- Zhao, Y.; Jing, Z.; Lv, J.; Zhang, Z.; Lin, J.; Cao, X.; Zhao, Z.; Liu, P.; Mao, W. Berberine activates caspase-9/cytochrome c-mediated apoptosis to suppress triple-negative breast cancer cells in vitro and in vivo. Biomed. Pharmacother. 2017, 95, 18–24. [Google Scholar] [CrossRef]
- Seger, R.; Krebs, E.G. The MAPK signaling cascade. FASEB J. 1995, 9, 726–735. [Google Scholar] [CrossRef]
- Wada, T.; Penninger, J.M. Mitogen-activated protein kinases in apoptosis regulation. Oncogene 2004, 23, 2838–2849. [Google Scholar] [CrossRef] [PubMed]
- Schönwasser, D.C.; Marais, R.M.; Marshall, C.J.; Parker, P.J. Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol. Cell Biol. 1998, 18, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Tang, Q.; Wu, J.; Zhao, S.; Liang, Z.; Li, L.; Wu, W.; Hann, S.S. p38α MAPK-mediated induction and interaction of FOXO3a and p53 contribute to the inhibited-growth and induced-apoptosis of human lung adenocarcinoma cells by berberine. J. Exp. Clin. Cancer Res. 2014, 33, 36. [Google Scholar] [CrossRef]
- Piyanuch, R.; Sukhthankar, M.; Wandee, G.; Baek, S.J. Berberine, a natural isoquinoline alkaloid, induces NAG-1 and ATF3 expression in human colorectal cancer cells. Cancer lett. 2007, 258, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Otto, T.; Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer 2017, 17, 93. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.C.; Xia, Q.; Luo, R.Z.; Huang, P.Y.; Sun, Y.L.; Shi, Y.X.; Jiang, W.Q. Berberine inhibits the growth of human colorectal adenocarcinoma in vitro and in vivo. J. Nat. Med. 2014, 68, 53–62. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Y.; Gao, H.; Xu, W.; Zhang, C.; Lai, J.; Liu, X.; Huang, H. Berberine Inhibits Cell Proliferation by Interfering with Wild-Type and Mutant P53 in Human Glioma Cells. OncoTargets Ther. 2020, 13, 12151–12162. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.-L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Tanida, I.; Ueno, T.; Kominami, E. LC3 and Autophagy. Methods Mol. Biol. 2008, 445, 77–88. [Google Scholar] [PubMed]
- Mah, L.Y.; Ryan, K.M. Autophagy and cancer. Cold Spring Harb Perspect Biol. 2012, 4, a008821. [Google Scholar] [CrossRef]
- Wang, J.; Qi, Q.; Feng, Z.; Zhang, X.; Huang, B.; Chen, A.; Prestegarden, L.; Li, X.; Wang, J. Berberine induces autophagy in glioblastoma by targeting the AMPK/mTOR/ULK1-pathway. Oncotarget 2016, 7, 66944–66958. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, X.; Cao, S.; Sun, Y.; He, X.; Jiang, B.; Yu, Y.; Duan, J.; Qiu, F.; Kang, N. Berberine represses human gastric cancer cell growth in vitro and in vivo by inducing cytostatic autophagy via inhibition of MAPK/mTOR/p70S6K and Akt signaling pathways. Biomed. Pharmacother. 2020, 128, 110245. [Google Scholar] [CrossRef]
- Poornima, P.; Weng, C.F.; Padma, V.V. Neferine from Nelumbo nucifera induces autophagy through the inhibition of PI3K/Akt/mTOR pathway and ROS hyper generation in A549 cells. Food Chem. 2013, 141, 3598–3605. [Google Scholar] [CrossRef]
- Aneja, R.; Vangapandu, S.N.; Lopus, M.; Viswesarappa, V.G.; Dhiman, N.; Verma, A.; Chandra, R.; Panda, D.; Joshi, H.C. Synthesis of microtubule-interfering halogenated noscapine analogs that perturb mitosis in cancer cells followed by cell death. Biochem. Pharmacol. 2006, 72, 415–426. [Google Scholar] [CrossRef]
- Meeran, S.M.; Katiyar, S.; Katiyar, S.K. Berberine-induced apoptosis in human prostate cancer cells is initiated by reactive oxygen species generation. Toxicol. Appl. Pharmacol. 2008, 229, 33–43. [Google Scholar] [CrossRef]
- Mantena, S.K.; Sharma, S.D.; Katiyar, S.K. Berberine, a natural product, induces G1-phase cell cycle arrest and caspase-3-dependent apoptosis in human prostate carcinoma cells. Mol. Cancer Ther. 2006, 5, 296–308. [Google Scholar] [CrossRef]
- Wang, N.; Feng, Y.; Zhu, M.; Tsang, C.-M.; Man, K.; Tong, Y.; Tsao, S.-W. Berberine induces autophagic cell death and mitochondrial apoptosis in liver cancer cells: The cellular mechanism. J. Cell. Biochem. 2010, 111, 1426–1436. [Google Scholar] [CrossRef]
- Jantova, S.; Cipak, L.; Letasiova, S. Berberine induces apoptosis through a mitochondrial/caspase pathway in human promonocytic U937 cells. Toxicol. In Vitro 2007, 21, 25–31. [Google Scholar] [CrossRef]
- Habartova, K.; Havelek, R.; Seifrtova, M.; Kralovec, K.; Cahlikova, L.; Chlebek, J.; Cermakova, E.; Mazankova, N.; Marikova, J.; Kunes, J.; et al. Scoulerine affects microtubule structure, inhibits proliferation, arrests cell cycle and thus culminates in the apoptotic death of cancer cells. Sci. Rep. 2018, 8, 4829. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Xu, Y.; Huang, X.; Chen, Y.; Fu, J.; Xi, M.; Wang, L. Berberine-induced apoptosis in human breast cancer cells is mediated by reactive oxygen species generation and mitochondrial-related apoptotic pathway. Tumor Biol. 2015, 36, 1279–1288. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.-J.; Huang, X.-H.; Zheng, C.-C.; Yin, X.-F.; Li, B.; He, Q.-Y. Liensinine perchlorate inhibits colorectal cancer tumorigenesis by inducing mitochondrial dysfunction and apoptosis. Food Funct. 2018, 9, 5536–5546. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Rizvi, F.; Raisuddin, S.; Kakkar, P. FoxO proteins′ nuclear retention and BH3-only protein Bim induction evoke mitochondrial dysfunction-mediated apoptosis in berberine-treated HepG2 cells. Free Radic. Biol. Med. 2014, 76, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Li, Q.; Wang, Z.; Cao, X. Sinomenine inhibits proliferation, migration, invasion and promotes apoptosis of prostate cancer cells by regulation of miR-23a. Biomed. Pharmacother. 2019, 112, 108592. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gong, K.; Mao, X.; Li, W. Tetrandrine induces apoptosis by activating reactive oxygen species and repressing Akt activity in human hepatocellular carcinoma. Int. J. Cancer 2011, 129, 1519–1531. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Hwangbo, H.; Lee, H.; Park, C.; Kim, G.-Y.; Moon, S.-K.; Yun, S.J.; Kim, W.-J.; Cheong, J.; Choi, Y.H. Induction of apoptosis by coptisine in Hep3B hepatocellular carcinoma cells through activation of the ROS-mediated JNK signaling pathway. Int. J. Mol. Sci. 2020, 21, 5502. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Jiang, P.; Li, Z.; Yü, Y.; Huang, T.; Ye, X.; Li, X. Coptisine-induced apoptosis in human colon cancer cells (HCT-116) is mediated by PI3K/Akt and mitochondrial-associated apoptotic pathway. Phytomedicine 2018, 48, 152–160. [Google Scholar] [CrossRef]
- Tian, J.; Mo, J.; Xu, L.; Zhang, R.; Qiao, Y.; Liu, B.; Jiang, L.; Ma, S.; Shi, G. Scoulerine promotes cell viability reduction and apoptosis by activating ROS-dependent endoplasmic reticulum stress in colorectal cancer cells. Chem. Biol. Interact. 2020, 327, 109184. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-M.; Zhang, M.; Fan, P.-L.; Qin, Y.-H.; Zhao, H.-W. Chelerythrine chloride induces apoptosis in renal cancer HEK-293 and SW-839 cell lines. Oncol. Lett. 2016, 11, 3917–3924. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Oh, D.; Yim, M.-J.; Park, J.-J.; Kang, K.-R.; Cho, I.-A.; Moon, S.-M.; Oh, J.-S.; You, J.-S.; Kim, C.-S.; et al. Berberine induces FasL-related apoptosis through p38 activation in KB human oral cancer cells. Oncol. Rep. 2015, 33, 1775–1782. [Google Scholar] [CrossRef]
- Si, Y.; Wang, J.; Liu, X.; Zhou, T.; Xiang, Y.; Zhang, T.; Wang, X.; Feng, T.; Xu, L.; Yu, Q.; et al. Ethoxysanguinarine, a Novel Direct Activator of AMP-Activated Protein Kinase, Induces Autophagy and Exhibits Therapeutic Potential in Breast Cancer Cells. Front. Pharmacol. 2020, 10, 1503. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Zhang, Z.-Q.; Wang, B.; Jiang, H.-X.; Cheng, L.; Shen, L.-M. Berberine-induced apoptotic and autophagic death of HepG2 cells requires AMPK activation. Cancer Cell Int. 2014, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Stiborová, M.; Šimánek, V.; Frei, E.; Hobza, P.; Ulrichová, J. DNA adduct formation from quaternary benzo [c] phenanthridine alkaloids sanguinarine and chelerythrine as revealed by the 32P-postlabeling technique. Chem. Biol. Interact. 2002, 140, 231–242. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Lee, K.W.; Kim, M.; Lee, Y.; Yoo, J.; Hwangbo, C.; Park, K.H.; Kim, K.D. Chelidonine induces caspase-dependent and caspase-independent cell death through G2/M arrest in the T98G human glioblastoma cell line. Evid. Based Complement. Altern. Med. 2019, 2019, 6318179. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Liao, C.-H.; Chang, Y.-L.; Guh, J.-H.; Pan, S.-L.; Teng, C.-M. Protopine, a novel microtubule-stabilizing agent, causes mitotic arrest and apoptotic cell death in human hormone-refractory prostate cancer cell lines. Cancer Lett. 2012, 315, 1–11. [Google Scholar] [CrossRef]
- Jordan, M.A.; Wilson, L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 2004, 4, 253–265. [Google Scholar] [CrossRef]
- Lopus, M.; Panda, D. The benzophenanthridine alkaloid sanguinarine perturbs microtubule assembly dynamics through tubulin binding: A possible mechanism for its antiproliferative activity. FEBS J. 2006, 273, 2139–2150. [Google Scholar] [CrossRef]
- Cheriyamundath, S.; Mahaddalkar, T.; Nagireddy, P.K.R.; Sridhar, B.; Kantevari, S.; Lopus, M. Insights into the structure and tubulin-targeted anticancer potential of N-(3-bromobenzyl) noscapine. Pharmacol. Rep. 2019, 71, 48–53. [Google Scholar] [CrossRef]
- Panzer, A.; Joubert, A.M.; Bianchi, P.C.; Hamel, E.; Seegers, J.C. The effects of chelidonine on tubulin polymerisation, cell cycle progression and selected signal transmission pathways. Eur. J. Cell Biol. 2001, 80, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Goldbrunner, M.; Loidl, G.; Polossek, T.; Mannschreck, A.; von Angerer, E. Inhibition of tubulin polymerization by 5, 6-dihydroindolo [2, 1-a] isoquinoline derivatives. J. Med. Chem. 1997, 40, 3524–3533. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Kwon, Y.; Kim, J.H.; Muller, M.T.; Chung, I.K. Induction of topoisomerase II-mediated DNA cleavage by a protoberberine alkaloid, berberrubine. Biochemistry 1998, 37, 16316–16324. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chowdhury, S.R.; Sarkar, T.; Chakrabarti, T.; Majumder, H.K.; Jha, T.; Mukhopadhyay, S. A new bisbenzylisoquinoline alkaloid isolated from Thalictrum foliolosum, as a potent inhibitor of DNA topoisomerase IB of Leishmania donovani. Fitoterapia 2016, 109, 25–30. [Google Scholar] [CrossRef]
- Kazemi Noureini, S.; Fatemi, L.; Wink, M. Telomere shortening in breast cancer cells (MCF7) under treatment with low doses of the benzylisoquinoline alkaloid chelidonine. PLoS ONE 2018, 13, e0204901. [Google Scholar] [CrossRef]
- Ma, Y.; Ou, T.-M.; Tan, J.-H.; Hou, J.-Q.; Huang, S.-L.; Gu, L.-Q.; Huang, Z.-S. Synthesis and evaluation of 9-O-substituted berberine derivatives containing aza-aromatic terminal group as highly selective telomeric G-quadruplex stabilizing ligands. Bioorganic Med. Chem. Lett. 2009, 19, 3414–3417. [Google Scholar] [CrossRef]
- Zhang, W.-J.; Ou, T.-M.; Lu, Y.-J.; Huang, Y.-Y.; Wu, W.-B.; Huang, Z.-S.; Zhou, J.-L.; Wong, K.-Y.; Gu, L.-Q. 9-Substituted berberine derivatives as G-quadruplex stabilizing ligands in telomeric DNA. Bioorganic Med. Chem. 2007, 15, 5493–5501. [Google Scholar] [CrossRef]
- Wu, H.L.; Hsu, C.Y.; Liu, W.H.; Yung, B.Y.M. Berberine-induced apoptosis of human leukemia HL-60 cells is associated with down-regulation of nucleophosmin/B23 and telomerase activity. Int. J. Cancer 1999, 81, 923–929. [Google Scholar] [CrossRef]
- Chan, S.-L.; Lee, M.C.; Tan, K.O.; Yang, L.-K.; Lee, A.S.Y.; Flotow, H.; Fu, N.Y.; Butler, M.S.; Soejarto, D.D.; Buss, A.D.; et al. Identification of chelerythrine as an inhibitor of BclXL function. J. Biol. Chem. 2003, 278, 20453–20456. [Google Scholar] [CrossRef] [PubMed]
- Lauf, P.K.; Heiny, J.; Meller, J.; Lepera, M.A.; Koikov, L.; Alter, G.M.; Brown, T.L.; Adragna, N.C. Canonical Bcl-2 motifs of the Na+/K+ pump revealed by the BH3 mimetic chelerythrine: Early signal transducers of apoptosis? Cell. Physiol. Biochem. 2013, 31, 257–276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Yang, L.; Zhao, Q.; Caen, J.P.; He, H.Y.; Jin, Q.H.; Guo, L.H.; Alemany, M.; Zhang, L.Y.; Shi, Y. Induction of acetylcholinesterase expression during apoptosis in various cell types. Cell Death Differ. 2002, 9, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Giacobini, E. Cholinergic function and Alzheimer′s disease. Int. J. Geriatr. Psychiatry 2003, 18, S1–S5. [Google Scholar] [CrossRef] [PubMed]
- Greig, N.H.; Utsuki, T.; Yu, Q.S.; Zhu, X.; Holloway, H.W.; Perry, T.; Lee, B.; Ingram, D.K.; Lahiri, D.K. A new therapeutic target in Alzheimer’s disease treatment: Attention to butyrylcholinesterase. Curr. Med. Res. Opin. 2001, 17, 159–165. [Google Scholar] [CrossRef]
- Morgan, H.D.; Santos, F.; Green, K.; Dean, W.; Reik, W. Epigenetic reprogramming in mammals. Hum. Mol. Genet. 2005, 14, R47–R58. [Google Scholar] [CrossRef]
- Feinberg, A.P.; Ohlsson, R.; Henikoff, S. The epigenetic progenitor origin of human cancer. Nat. Rev. Genet. 2006, 7, 21–33. [Google Scholar] [CrossRef]
- Jones, P.A.; Baylin, S.B. The fundamental role of epigenetic events in cancer. Nat. Rev. Genet. 2002, 3, 415–428. [Google Scholar] [CrossRef]
- Esteller, M. Cancer epigenomics: DNA methylomes and histone-modification maps. Nat. Rev. Genet. 2007, 8, 286–298. [Google Scholar] [CrossRef]
- Allis, C.D.; Jenuwein, T. The molecular hallmarks of epigenetic control. Nat. Rev. Genet. 2016, 17, 487. [Google Scholar] [CrossRef]
- Riggs, M.; Whittaker, R.G.; Neumann, J.R.; Ingram, V.M. n-Butyrate causes histone modification in HeLa and Friend erythroleukaemia cells. Nature 1977, 268, 462–464. [Google Scholar] [CrossRef]
- Berghe, W.V. Epigenetic impact of dietary polyphenols in cancer chemoprevention: Lifelong remodeling of our epigenomes. Pharmacol. Res. 2012, 65, 565–576. [Google Scholar] [CrossRef]
- Zhang, L.; Miao, X.-J.; Wang, X.; Pan, H.-H.; Li, P.; Ren, H.; Jia, Y.-R.; Lu, C.; Wang, H.-B.; Yuan, L.; et al. Antiproliferation of berberine is mediated by epigenetic modification of constitutive androstane receptor (CAR) metabolic pathway in hepatoma cells. Sci. Rep. 2016, 6, 28116. [Google Scholar] [CrossRef]
- Kalaiarasi, A.; Anusha, C.; Sankar, R.; Rajasekaran, S.; Marshal, J.J.; Muthusamy, K.; Ravikumar, V. Plant isoquinoline alkaloid berberine exhibits chromatin remodeling by modulation of histone deacetylase to induce growth arrest and apoptosis in the A549 cell line. J. Agric. Food Chem. 2016, 64, 9542–9550. [Google Scholar] [CrossRef]
- Hu, H.-Y.; Li, K.-P.; Wang, X.-J.; Liu, Y.; Lu, Z.-G.; Dong, R.-H.; Guo, H.-B.; Zhang, M.-X. Set9, NF-κB, and microRNA-21 mediate berberine-induced apoptosis of human multiple myeloma cells. Acta Pharmacol. Sin. 2013, 34, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Liu, H.; Gong, X.-L.; Wu, L.-Y.; Wen, B. Effect of evodiamine and berberine on the interaction between DNMTs and target microRNAs during malignant transformation of the coln by TGF-β1. Oncol. Rep. 2017, 37, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Selvi, R.; Pradhan, S.K.; Shandilya, J.; Das, C.; Sailaja, B.S.; Gadad, S.S.; Reddy, A.; Dasgupta, D.; Kundu, T.K. Sanguinarine interacts with chromatin, modulates epigenetic modifications, and transcription in the context of chromatin. Chem. Biol. 2009, 16, 203–216. [Google Scholar] [CrossRef]
- Qing, Y.; Hu, H.; Liu, Y.; Feng, T.; Meng, W.; Jiang, L.; Sun, Y.; Yao, Y. Berberine induces apoptosis in human multiple myeloma cell line U266 through hypomethylation of p53 promoter. Cell Biol. Int. 2014, 38, 563–570. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, D.; Yoon, S.Y.; Park, S.J.; Park, Y.J. The Anticancer Effect of Natural Plant Alkaloid Isoquinolines. Int. J. Mol. Sci. 2021, 22, 1653. https://doi.org/10.3390/ijms22041653
Yun D, Yoon SY, Park SJ, Park YJ. The Anticancer Effect of Natural Plant Alkaloid Isoquinolines. International Journal of Molecular Sciences. 2021; 22(4):1653. https://doi.org/10.3390/ijms22041653
Chicago/Turabian StyleYun, Dahye, So Young Yoon, Soo Jung Park, and Yoon Jung Park. 2021. "The Anticancer Effect of Natural Plant Alkaloid Isoquinolines" International Journal of Molecular Sciences 22, no. 4: 1653. https://doi.org/10.3390/ijms22041653
APA StyleYun, D., Yoon, S. Y., Park, S. J., & Park, Y. J. (2021). The Anticancer Effect of Natural Plant Alkaloid Isoquinolines. International Journal of Molecular Sciences, 22(4), 1653. https://doi.org/10.3390/ijms22041653






