HVCN1 but Not Potassium Channels Are Related to Mammalian Sperm Cryotolerance
Abstract
1. Introduction
2. Results
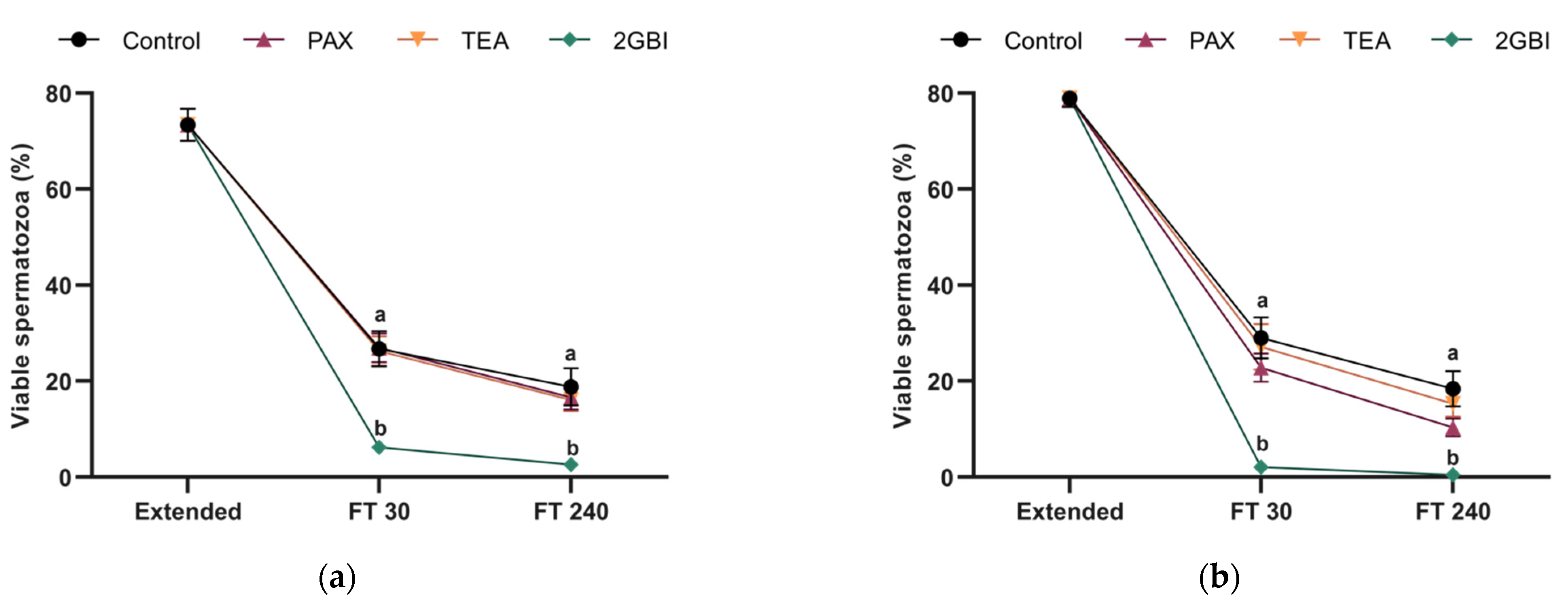
2.1. Sperm Viability
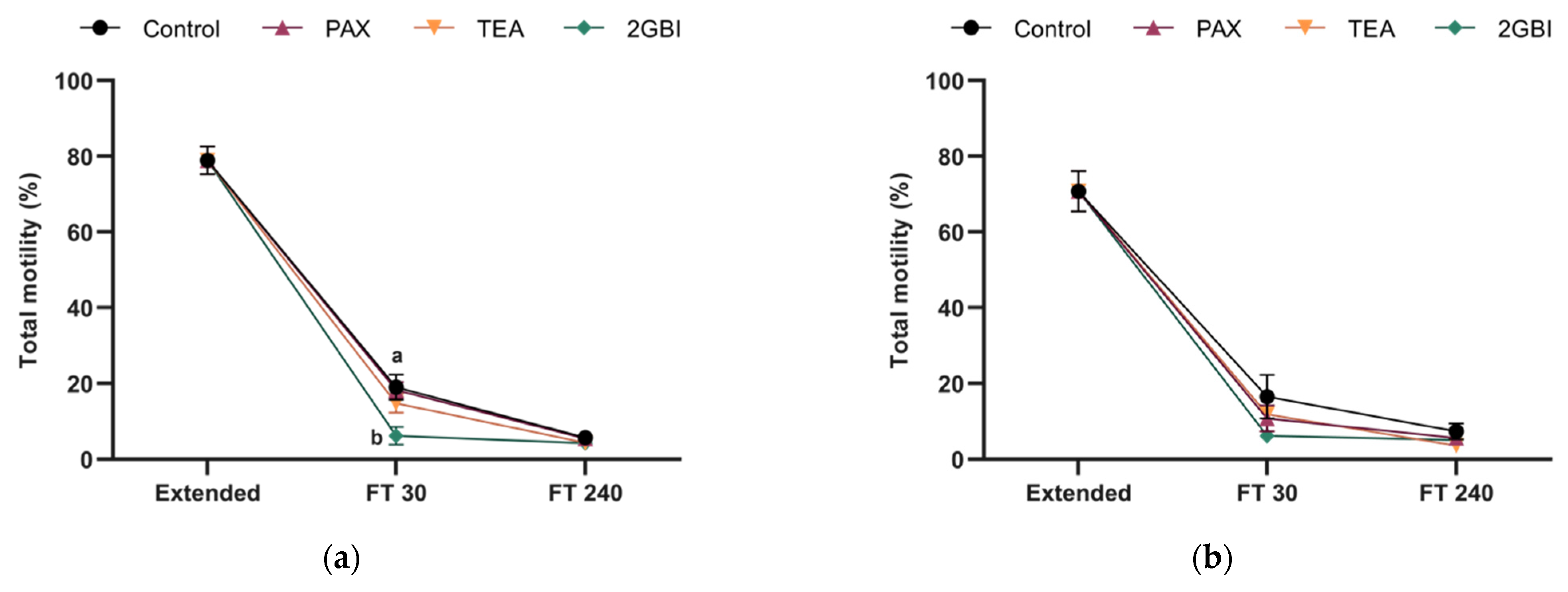
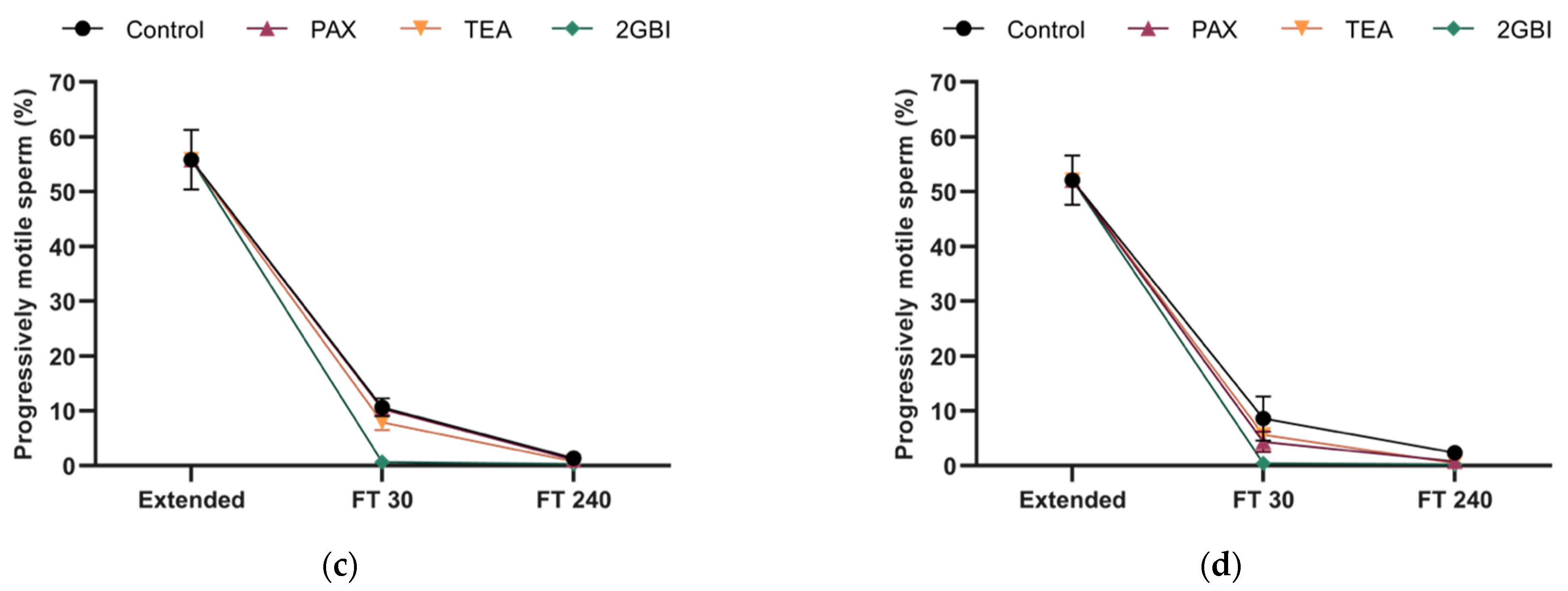
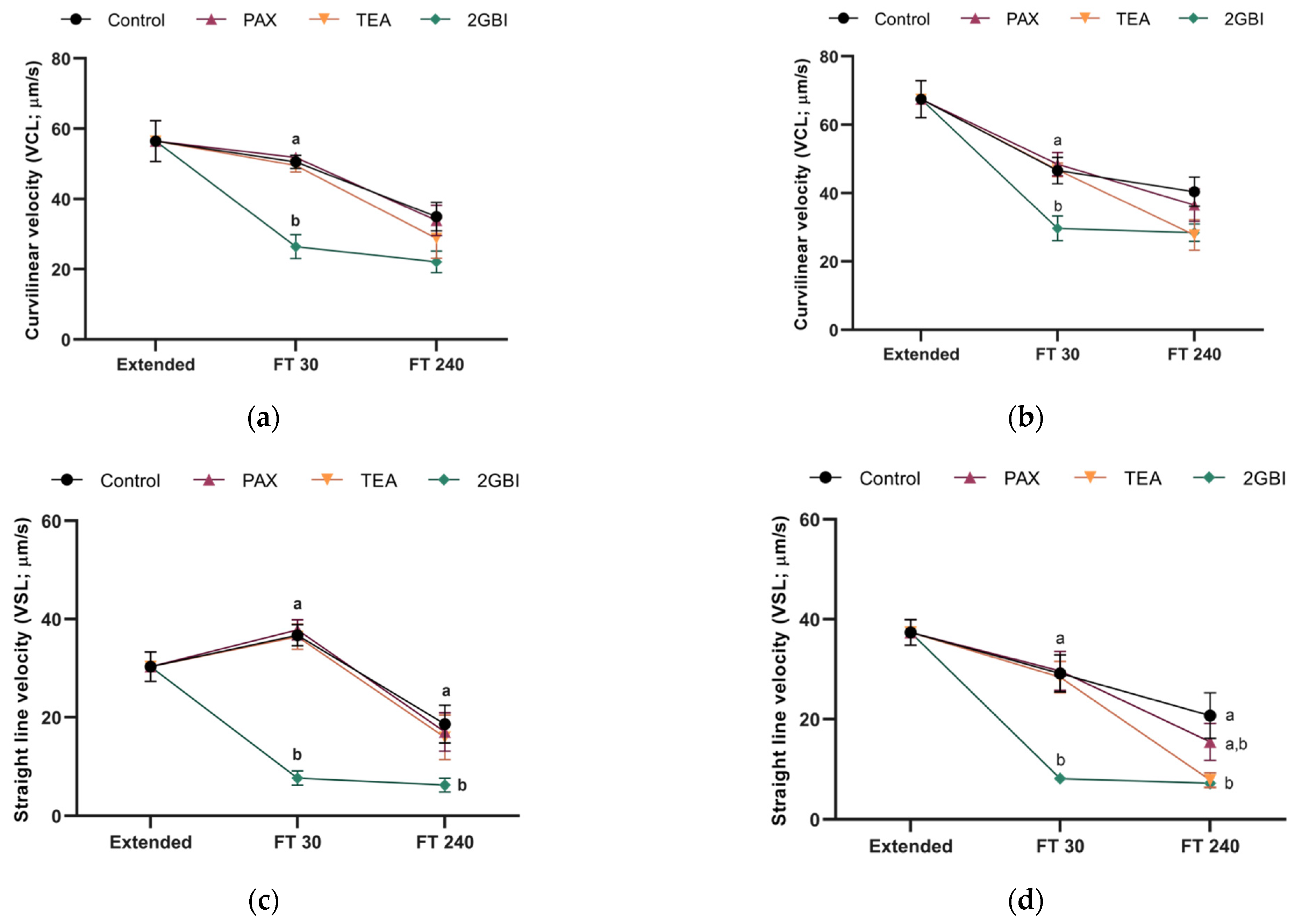
2.2. Sperm Motility
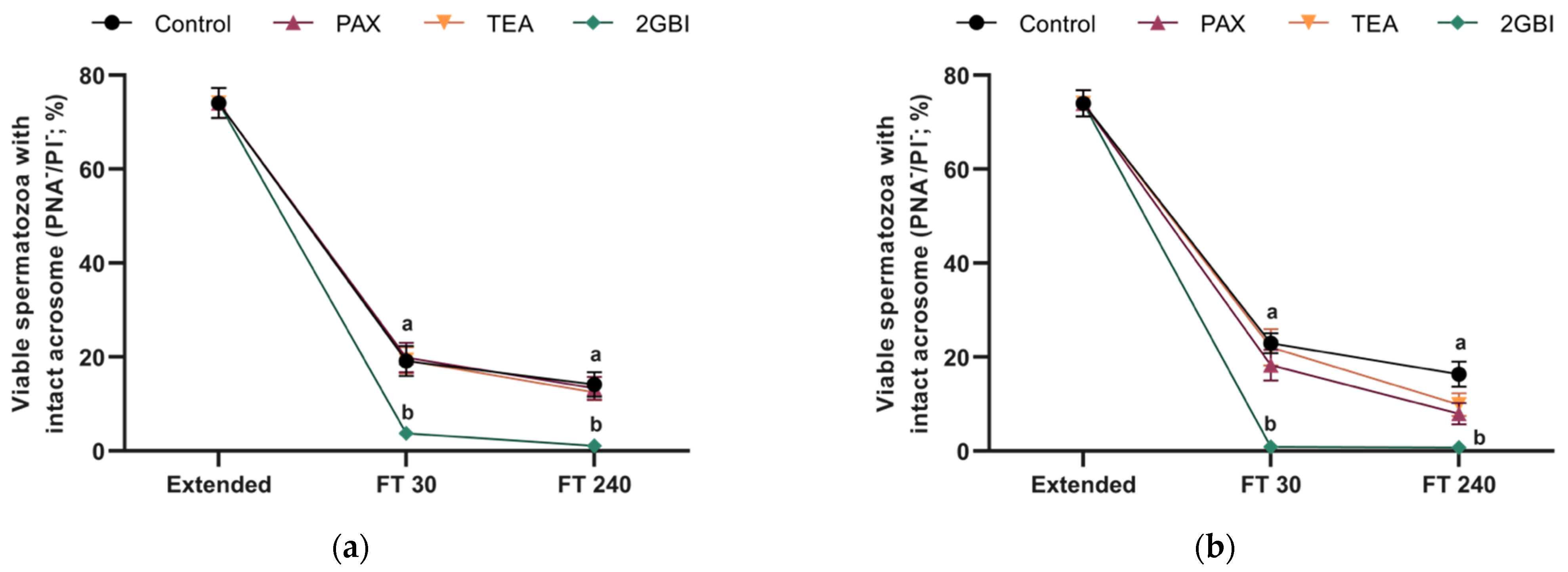
2.3. Acrosome Integrity
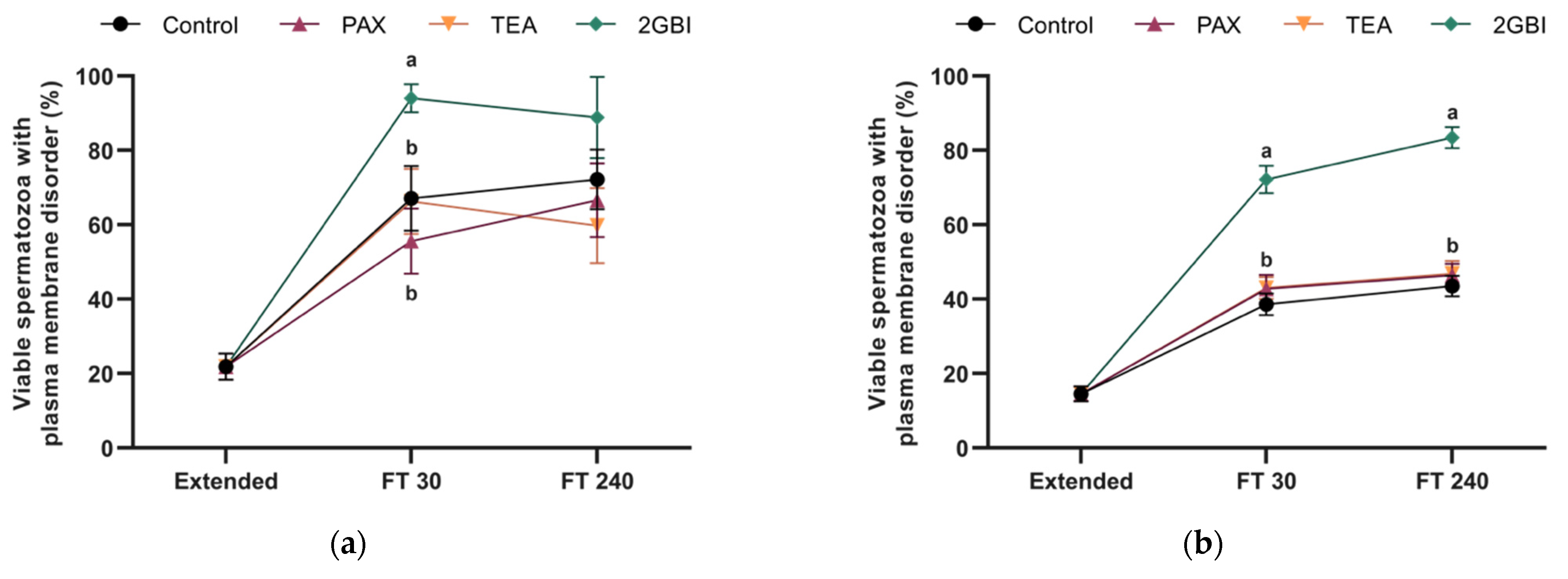
2.4. Membrane Lipid Disorder
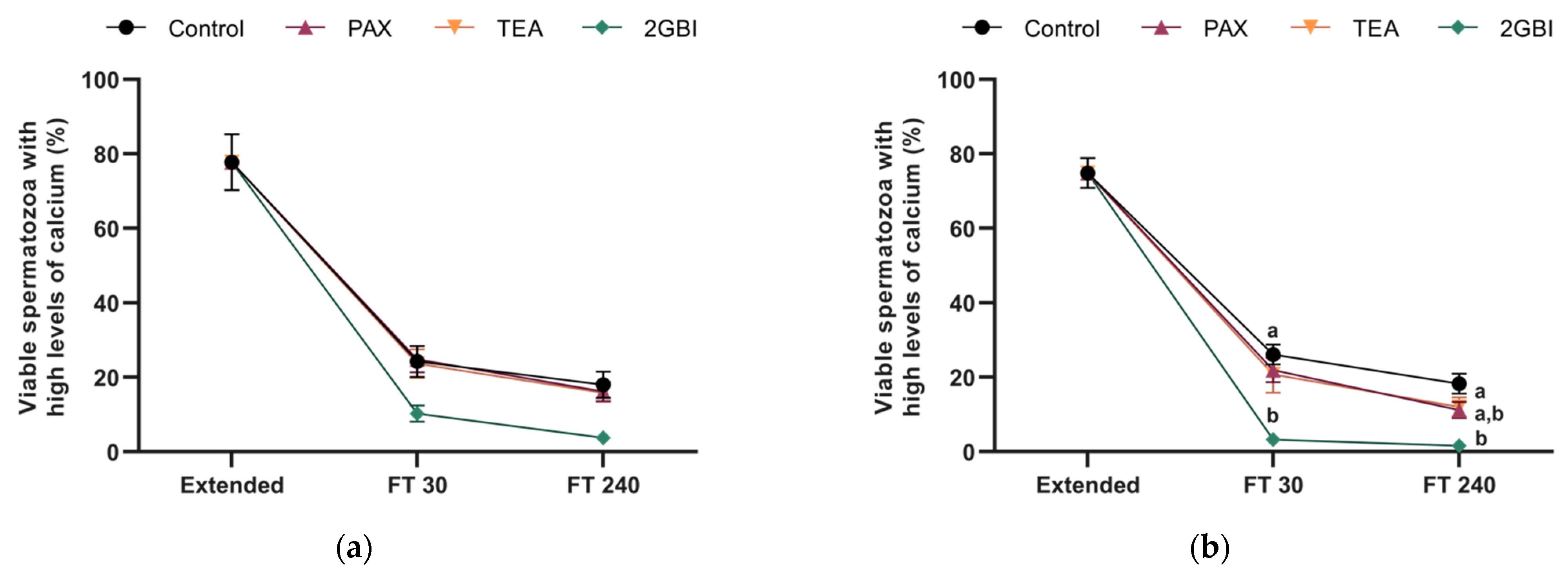
2.5. Intracellular Levels of Calcium
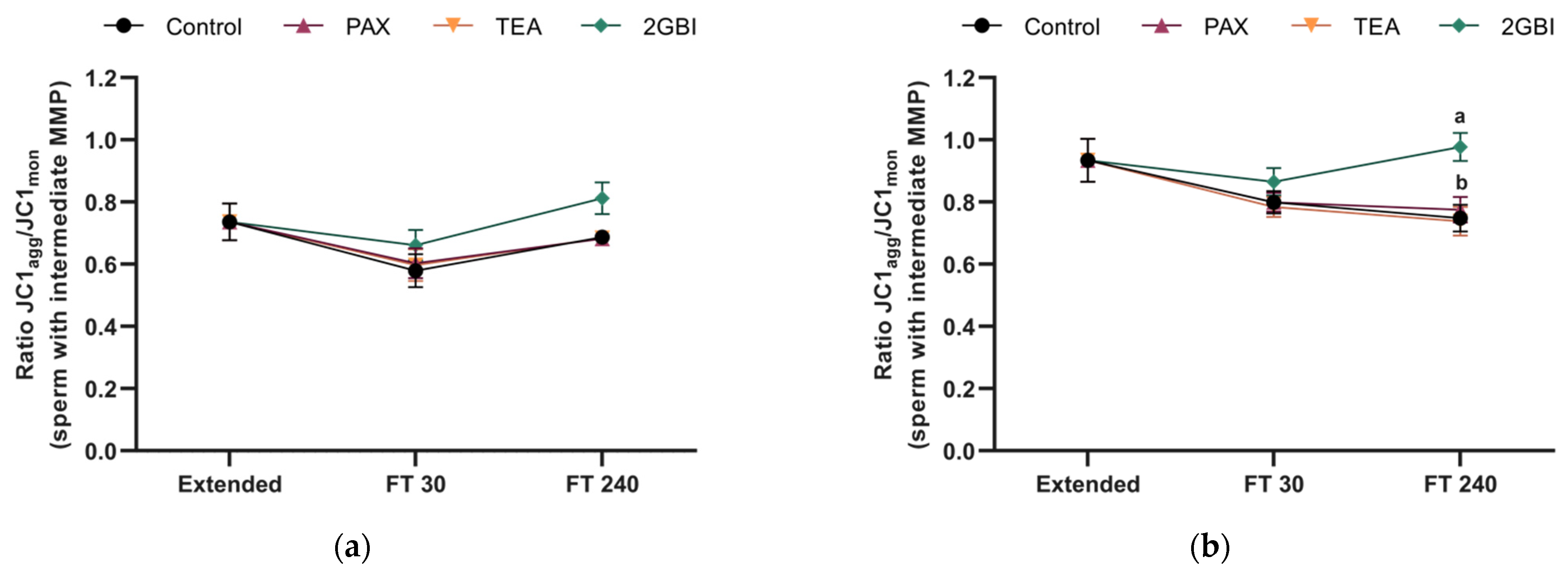
2.6. Mitochondrial Membrane Potential
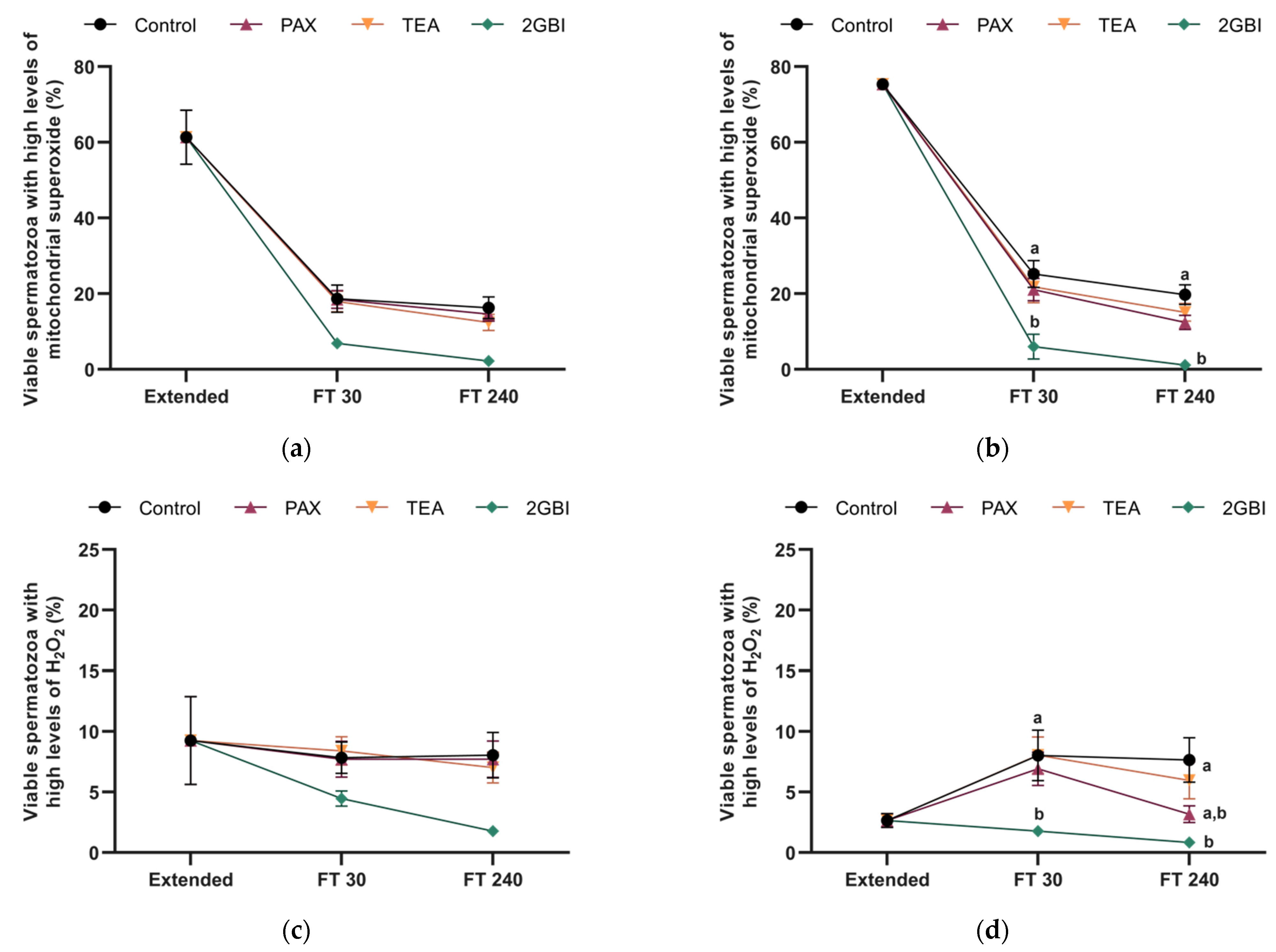
2.7. Intracellular Levels of Mitochondrial Superoxide (O2●−)
2.8. Intracellular Levels of Hydrogen Peroxide (H2O2)
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Semen samples
4.3. Sperm cryopreservation
4.4. Experimental Design
4.5. Sperm Motility
4.6. Flow Cytometry
4.6.1. Plasma and Acrosome Membrane Integrity
4.6.2. Plasma Membrane Lipid Disorder
4.6.3. Determination of Intracellular Calcium Levels
4.6.4. Mitochondrial Membrane Potential (MMP)
4.6.5. Intracellular Levels of Mitochondrial Superoxide (O2−⁻)
4.6.6. Intracellular Levels of Hydrogen Peroxide (H2O2)
4.7. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mishra, A.K.; Kumar, A.; Yadav, S.; Anand, M.; Yadav, B.; Nigam, R.; Garg, S.K.; Swain, D.K. Functional insights into voltage gated proton channel (Hv1) in bull spermatozoa. Theriogenology 2019, 136, 118–130. [Google Scholar] [CrossRef]
- Lishko, P.V.; Kirichok, Y.; Ren, D.; Navarro, B.; Chung, J.J.; Clapham, D.E. The control of male fertility by spermatozoan ion channels. Annu. Rev. Physiol. 2012, 74, 453–575. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.G.; Publicover, S.J.; Barrat, C.L.R.; Martins da Silva, S.J. Human sperm ion channel (dys)function: Implications for fertilization. Hum. Reprod. Update 2019, 25, 758–776. [Google Scholar] [CrossRef] [PubMed]
- Riva, N.S.; Ruhlmann, C.; Iaizzo, R.C.; Marcial López, C.A.; Martínez, A.G. Comparative analysis between slow freezing and ultra-rapid freezing for human sperm cryopreservation. JBRA Assist. Reprod. 2018, 22, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Najafi, L.; Halvaei, I.; Movahed, M. Canthaxanthin protects human sperm parameters during cryopreservation. Andrologia 2019, 51, e13389. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, R.; Fan, X.; Lv, Y.; Zheng, Y.; Hoque, M.S.A.; Wu, D.; Zeng, W. Resveratrol Improves Boar Sperm Quality via 5′AMP-Activated Protein Kinase Activation during Cryopreservation. Oxid. Med. Cell Longev. 2019, 2019, 5921503. [Google Scholar] [CrossRef]
- Pinart, E.; Puigmulé, M. Factors affecting boar reproduction, testis function and sperm quality. In Boar Reproduction. Fundamentals and New Biological Trends; Bonet, S., Casas, I., Holt, W.V., Yeste, M., Eds.; Springer: Berlin, Germany, 2013; pp. 109–202. [Google Scholar]
- Yeste, M. Sperm cryopreservation update: Cryodamage, markers, and factors affecting the sperm freezability in pigs. Theriogenology 2016, 85, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Bermúdez, A.; Llavanera, M.; Fernández-Bastit, L.; Recuero, S.; Mateo-Otero, Y.; Bonet, S.; Barranco, I.; Fernández-Fuertes, B.; Yeste, M. Aquaglyceroporins but not orthdox aquaporins are involved in the cryotolerance of pig spermatozoa. J. Anim. Sci. Biotech. 2019, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Llavanera, M.; Delgado-Bermúdez, A.; Fernández-Fuertes, B.; Recuero, S.; Mateo-Otero, Y.; Bonet, S.; Barranco, I.; Yeste, M. GSTM3, but not IZUMO1, is a cryotolerance marker of boar sperm. J. Anim. Sci. Biotechnol. 2019, 10, 61. [Google Scholar] [CrossRef]
- Yeste, M. Recent advances in boar sperm cryopreservation: State of the art and current perspectives. Reprod. Domest. Anim. 2015, 50, 71–79. [Google Scholar] [CrossRef]
- Yeste, M.; Rodríguez-Gil, J.E.; Bonet, S. Artificial insemination with frozen-thawed boar sperm. Mol. Reprod. Dev. 2017, 84, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Rath, D.; Bathgate, R.; Rodríguez-Martínez, H.; Roca, J.; Strzezek, J.; Waberski, D. Recent advances in boar semen cryopreservation. Soc. Reprod. Fertil. Suppl. 2009, 66, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Cerolini, S.; Maldjian, A.; Surai, P.; Noble, R. Viability, susceptibility to peroxidation and fatty acid composition of boar semen during liquid storage. Anim. Reprod. Sci. 2000, 58, 99–111. [Google Scholar] [CrossRef]
- Pinart, E.; Yeste, M.; Bonet, S. Acrosin activity is a good predictor of boar sperm freezability. Theriogenology 2015, 83, 1525–1533. [Google Scholar] [CrossRef]
- Sancho, S.; Casas, I.; Ekwall, H.; Rodríguez-Martínez, H.; Rodríguez-Gil, J.E.; Flores, E.; Pinart, E.; Briz, M.; Garcia-Gil, N.; Bassols, J.; et al. Effects of cryopreservation on semen quality and the expression of sperm membrane hexose transporters in the spermatozoa of Iberian pigs. Reproduction 2007, 134, 111–121. [Google Scholar] [CrossRef][Green Version]
- Casas, I.; Sancho, S.; Briz, M.; Pinart, E.; Bussalleu, E.; Yeste, M.; Bonet, S. Freezability prediction of boar ejaculates assessed by functional sperm parameters and sperm proteins. Theriogenology 2009, 72, 930–948. [Google Scholar] [CrossRef]
- Casas, I.; Sancho, S.; Ballester, J.; Briz, M.; Pinart, E.; Bussalleu, E.; Yeste, M.; Fàbrega, A.; Rodríguez-Gil, J.E.; Bonet, S. The HSP90AA1 sperm content and the prediction of the boar ejaculate freezability. Theriogenology 2010, 74, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, D.B.; Barros, T.B.; van Tilburg, M.F.; Martins, J.A.M.; Moura, A.A.; Moreno, F.B.; Monteiro-Moreira, A.C.; Moreira, R.A.; Toniolli, R. Sperm membrane proteins associated with the boar semen cryopreservation. Anim. Reprod. Sci. 2017, 183, 27–38. [Google Scholar] [CrossRef]
- Gómez-Fernández, J.; Gómez-Izquierdo, E.; Tomás, C.; Mocé, E.; de Mercado, E. Effect of different monosaccharides and disaccharides on boar sperm quality after cryopreservation. Anim. Reprod. Sci. 2012, 133, 109–116. [Google Scholar] [CrossRef]
- Prieto-Martínez, N.; Vilagran, I.; Morató, R.; Rivera del Álamo, M.M.; Rodríguez-Gil, J.E.; Bonet, S.; Yeste, M. Relationship of aquaporins 3 (AQP3), 7 (AQP7), and 11 (AQP11) with boar sperm resilience to withstand freeze-thawing procedures. Andrology 2017, 5, 1153–1164. [Google Scholar] [CrossRef]
- Lishko, P.V.; Botchkina, I.L.; Fedorenko, A.; Kirichok, Y. Acid Extrusion from Human Spermatozoa is Mediated by Flagellar Voltage-Gated Proton Channel. Cell 2010, 140, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Lishko, P.V.; Kirichok, Y. The role of Hv1 and CatSper channels in sperm activation. J. Physiol. 2010, 588, 4667–4672. [Google Scholar] [CrossRef] [PubMed]
- Berger, T.K.; Fußhöller, D.M.; Goodwin, N.; Bönigk, W.; Müller, A.; Dokani Khesroshahi, N.; Brenker, C.; Wachten, D.; Krause, E.; Kaupp, U.B.; et al. Post-translational cleavage of Hv1 in human sperm tunes pH- and voltage-dependent gating. J. Physiol. 2017, 595, 1533–1546. [Google Scholar] [CrossRef]
- Yeste, M.; Llavanera, M.; Pérez, G.; Scornik, F.; Puig-Parri, J.; Brugada, R.; Bonet, S.; Pinart, E. Elucidating the role of K+ channels during in vitro capacitation of boar spermatozoa: Do SLO1 channels play a crucial role? Int. J. Mol. Sci. 2019, 20, 6330. [Google Scholar] [CrossRef] [PubMed]
- Yeste, M.; Llavanera, M.; Mateo-Otero, Y.; Catalán, J.; Bonet, S.; Pinart, E. HVCN1 channels are relevant for the maintenance of sperm motility during in vitro capacitation of pig Spermatozoa. Int. J. Mol. Sci. 2020, 21, 3255. [Google Scholar] [CrossRef]
- Tang, Q.Y.; Zhang, Z.; Xia, X.M.; Lingle, C. Block mouse Slo1 and Slo3 K+ channels by CTX, IbTX, TEA, 4-AP and quinidine. Channels 2010, 4, 22–41. [Google Scholar] [CrossRef]
- Zhou, Y.; Lingle, C.J. Paxilline inhibits BK channels by an almost exclusively close-channel block mechanism. J. Gen. Physiol. 2014, 144, 415–440. [Google Scholar] [CrossRef]
- Hille, B. Ion Channels of Excitable Membranes, 3rd ed.; Oxford University Press: Oxford, UK, 2001; pp. 156–159. [Google Scholar]
- Rennhack, A.; Grahn, E.; Kaupp, U.B.; Berger, T.K. Photocontrol of the Hv1 proton channel. ACS Chem. Biol. 2017, 12, 2952–2957. [Google Scholar] [CrossRef]
- Hong, L.; Kim, I.H.; Tombola, F. Molecular determinants of Hv1 proton channel inhibition by guanidine derivatives. Proc. Natl. Acad. Sci. USA 2014, 111, 9971–9976. [Google Scholar] [CrossRef]
- Grötter, L.G.; Cattaneo, L.; Marini, P.E.; Kjelland, M.E.; Ferré, L.B. Recent advances in bovine sperm cryopreservation techniques with a focus on sperm post-thaw quality optimization. Reprod. Dom. Anim. 2019, 5, 655–665. [Google Scholar] [CrossRef]
- Vilagran, I.; Castillo, J.; Bonet, S.; Sancho, S.; Yeste, M.; Estanyol, J.M.; Oliva, R. Acrosin-binding protein (ACRBP) and triosephosphate isomerase (TPI) are good markers to predict boar sperm freezing capacity. Theriogenology 2013, 80, 443–450. [Google Scholar] [CrossRef]
- Vilagran, I.; Yeste, M.; Sancho, S.; Casas, I.; Rivera del Álamo, M.M.; Bonet, S. Relationship of sperm small heat-shock protein 10 and voltage-dependent anion channel 2 with semen freezability in boars. Theriogenology 2014, 82, 418–426. [Google Scholar] [CrossRef]
- Vilagran, I.; Yeste, M.; Sancho, S.; Castillo, J.; Oliva, R.; Bonet, S. Comparative analysis of boar seminal plasma proteome from different freezability ejaculates and identification of Fibronectin 1 as sperm freezability marker. Andrology 2015, 3, 345–356. [Google Scholar] [CrossRef]
- Chen, Q.; Peng, H.; Lei, L.; Zhang, Y.; Kuang, H.; Cao, Y.; Shi, Q.X.; Ma, T.; Duan, D. Aquaporin3 is a sperm water channel essential for postcopulatory sperm osmoadaptation and migration. Cell Res. 2011, 21, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Duan, E. Aquaporins in sperm osmoadaptation: An emerging role for volume regulation. Acta Pharmacol. Sin. 2011, 32, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.K.; Coger, R.N.; Schrum, L.W.; Lee, Y.C. The effects of over expressing aquaporins on the cryopreservation of hepatocytes. Cryobiology 2015, 71, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Barfield, J.P.; Yeung, C.H.; Cooper, T.G. Characterization of potassium channels involved in volume regulation of human spermatozoa. Mol. Hum. Reprod. 2005, 11, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Mannowetz, N.; Niadoo, N.M.; Choo, S.A.S.; Smith, J.F.; Lishko, P.V. Slo1 is the principal potassium channel of human spermatozoa. eLife 2013, 2, e01009. [Google Scholar] [CrossRef]
- Sun, X.H.; Zhu, Y.Y.; Wang, L.; Liu, H.L.; Ling, Y.; Li, Z.L.; Sun, L.B. The Catsper channel and its roles in male fertility: A systematic review. Reprod. Biol. Endocrinol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Santi, C.M.; Martínez-López, P.; De la Vega-Beltrán, J.L.; Butler, A.; Alisio, A.; Darszon, A.; Salkoff, L. The SLO3 sperm-specific potassium channel plays a vital role in male fertility. FEBS Lett. 2010, 584, 1041–1046. [Google Scholar] [CrossRef]
- Zeng, X.H.; Yang, C.; Kim, S.T.; Lingle, C.J.; Xia, X.M. Deletion of the Slo3 gene abolishes alkalization-activated K+ current in mouse spermatozoa. Proc. Natl. Acad. Sci. USA 2011, 108, 5879–5884. [Google Scholar] [CrossRef] [PubMed]
- De la Vega-Beltran, J.L.; Sánchez-Cárdenas, C.; Krapf, D.; Hernandez-González, E.O.; Wertheimer, E.; Treviño, C.L.; Visconti, P.E.; Darszon, A. Mouse sperm membrane potential hyperpolarization is necessary and sufficient to prepare sperm for the acrosome reaction. J. Biol. Chem. 2012, 287, 44384–44393. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Jerreira, J.J.; Dzikunu, V.; Butler, A.; Lybaert, P.; Yuan, P.; Magleby, K.L.; Salkoff, L.; Santi, C.M. A genetic variant of the sperm-specific SLO3 K+ channel has altered pH and Ca2+ sensitivities. J. Biol. Chem. 2017, 292, 8978–8987. [Google Scholar] [CrossRef] [PubMed]
- Brenker, C.; Zhou, Y.; Müller, A.; Echeverry, F.A.; Trötschel, C.; Poetsch, A.; Xia, X.M.; Bönigk, W.; Lingle, J.C.; Kaupp, U.B.; et al. The Ca2+-activated K+ current of human sperm is mediated by Slo3. eLife 2014, 3, e01438. [Google Scholar] [CrossRef]
- Yuan, P.; Leonetti, M.D.; Pico, A.R.; Hsiung, Y.; MacKinnon, R. Structure of the human BK channel Ca2+-activation apparatus at 3.0 resolution. Science 2010, 329, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Blässe, A.-K.; Oldenhof, H.; Ekhlasi-Hundrieser, M.; Wolkers, W.F.; Sieme, H.; Bollwein, H. Osmotic tolerance and intracellular ion concentrations of bovine sperm are affected by cryopreservation. Theriogenology 2012, 78, 1312–1320. [Google Scholar] [CrossRef]
- Mansell, S.A.; Publicover, S.J.; Barrat, C.L.R.; Wilson, S.M. Patch clamp studies of human sperm under physiological ionic conditions reveal three functionally and pharmacologically distinct cation channels. Mol. Hum. Reprod. 2014, 20, 392–408. [Google Scholar] [CrossRef]
- Zhao, R.; Kennedy, K.; De Blas, G.A.; Orta, G.; Pavarotti, M.A.; Arias, R.J.; de la Vega-Beltrán, J.L.; Li, Q.; Dai, H.; Perozo, E.; et al. Role of human Hv1 channels in sperm capacitation and white blood cell respiratory burst established by a designed peptide inhibitor. Proc. Natl. Acad. Sci. USA 2018, 115, E11847–E11856. [Google Scholar] [CrossRef]
- Chen, B.; Li, S.; Yan, Y.; Duan, Y.; Chang, S.; Wang, H.; Ji, W.; Wu, X.; Si, W. Cryopreservation of cynomolgus macaque (Macaca fascicularis) sperm with glycerol and ethylene glycol, and its effect on sperm-specific ion channels—CatSper and Hv1. Theriogenology 2017, 104, 37–42. [Google Scholar] [CrossRef]
- Keshtgar, S.; Ghanbari, H.; Ghani, E.; Shid Moosavi, S.M. Effect of CatSper and Hv1 channel inhibition on progesterone stimulated human sperm. J. Reprod. Infertil. 2018, 19, 133–139. [Google Scholar] [PubMed]
- Musset, B.; Clark, R.A.; DeCoursey, T.E.; Petheo, G.L.; Geiszt, M.; Chen, Y.; Cornell, J.E.; Eddy, C.A.; Brzyski, R.G.; El Jamali, A. NOX5 in human spermatozoa: Expression, function, and regulation. J. Biol. Chem. 2012, 287, 9376–9388. [Google Scholar] [CrossRef]
- Ghanbari, H.; Keshtgar, S.; Zare, H.R.; Gharesi-Fard, B. Inhibition of CatSper and Hv1 Channels and NOX5 Enzyme Affect Progesterone-Induced Increase of Intracellular Calcium Concentration and ROS Generation in Human Sperm. Iran. J. Med. Sci. 2019, 44, 127–134. [Google Scholar]
- Pursel, V.G.; Johnson, L.A. Freezing of boar spermatozoa: Fertilizing capacity with concentrated semen and a new thawing procedure. J. Anim. Sci. 1975, 40, 99–102. [Google Scholar] [CrossRef]
- Garner, D.L.; Johnson, L.A. Viability assessment of mammalian sperm using SYBR-14 and propidium iodide. Biol. Reprod. 1995, 53, 276–284. [Google Scholar] [CrossRef]
- Petrunkina, A.M.; Waberski, D.; Bollwein, H.; Sieme, H. Identifying non-sperm particles during flow cytometric physiological assessment: A simple approach. Theriogenology 2010, 73, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Nagy, S.; Jansen, J.; Topper, E.K.; Gadella, B.M. A Triple-Stain Flow Cytometric Method to Assess Plasma- and Acrosome-Membrane Integrity of Cryopreserved Bovine Sperm Immediately after Thawing in Presence of Egg-Yolk Particles. Biol. Reprod. 2003, 68, 1828–1835. [Google Scholar] [CrossRef] [PubMed]
- Rathi, R.; Colenbrander, B.; Bevers, M.M.; Gadella, B.M. Evaluation of in vitro capacitation of stallion spermatozoa. Biol. Reprod. 2001, 65, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Yeste, M.; Estrada, E.; Rivera Del Álamo, M.M.; Bonet, S.; Rigau, T.; Rodríguez-Gil, J.E. The increase in phosphorylation levels of serine residues of protein HSP70 during holding time at 17 °C is concomitant with a higher cryotolerance of boar spermatozoa. PLoS ONE 2014, 9, e90887. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pastor, F.; Mata-Campuzano, M.; Álvarez-Rodríguez, M.; Álvarez, M.; Anel, L.; De Paz, P. Probes and techniques for sperm evaluation by flow cytometry. Reprod. Domest. Anim. 2010, 45, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Ferrusola, C.; Sotillo-Galán, Y.; Varela-Fernández, E.; Gallardo-Bolaños, J.M.; Muriel, A.; González-Fernández, L.; Tapia, J.A.; Peña, F.J. Detection of “Apoptosis-Like” Changes During the Cryopreservation Process in Equine Sperm. J. Androl. 2008, 29, 213–221. [Google Scholar] [CrossRef]
- Guthrie, H.D.; Welch, G.R. Determination of intracellular reactive oxygen species and high mitochondrial membrane potential in Percoll-treated viable boar sperm using fluorescence-activated flow cytometry. J. Anim. Sci. 2006, 84, 2089–2100. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, M.E.; Kauffman, M.K.; Traore, K.; Zhu, H.; Trush, M.A.; Jia, Z.; Li, Y.R. MitoSOX-Based Flow Cytometry for Detecting Mitochondrial ROS. React. Oxyg. Species 2016, 2, 361–370. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado-Bermúdez, A.; Mateo-Otero, Y.; Llavanera, M.; Bonet, S.; Yeste, M.; Pinart, E. HVCN1 but Not Potassium Channels Are Related to Mammalian Sperm Cryotolerance. Int. J. Mol. Sci. 2021, 22, 1646. https://doi.org/10.3390/ijms22041646
Delgado-Bermúdez A, Mateo-Otero Y, Llavanera M, Bonet S, Yeste M, Pinart E. HVCN1 but Not Potassium Channels Are Related to Mammalian Sperm Cryotolerance. International Journal of Molecular Sciences. 2021; 22(4):1646. https://doi.org/10.3390/ijms22041646
Chicago/Turabian StyleDelgado-Bermúdez, Ariadna, Yentel Mateo-Otero, Marc Llavanera, Sergi Bonet, Marc Yeste, and Elisabeth Pinart. 2021. "HVCN1 but Not Potassium Channels Are Related to Mammalian Sperm Cryotolerance" International Journal of Molecular Sciences 22, no. 4: 1646. https://doi.org/10.3390/ijms22041646
APA StyleDelgado-Bermúdez, A., Mateo-Otero, Y., Llavanera, M., Bonet, S., Yeste, M., & Pinart, E. (2021). HVCN1 but Not Potassium Channels Are Related to Mammalian Sperm Cryotolerance. International Journal of Molecular Sciences, 22(4), 1646. https://doi.org/10.3390/ijms22041646








