The Effect of Lead Exposure on Autism Development
Abstract
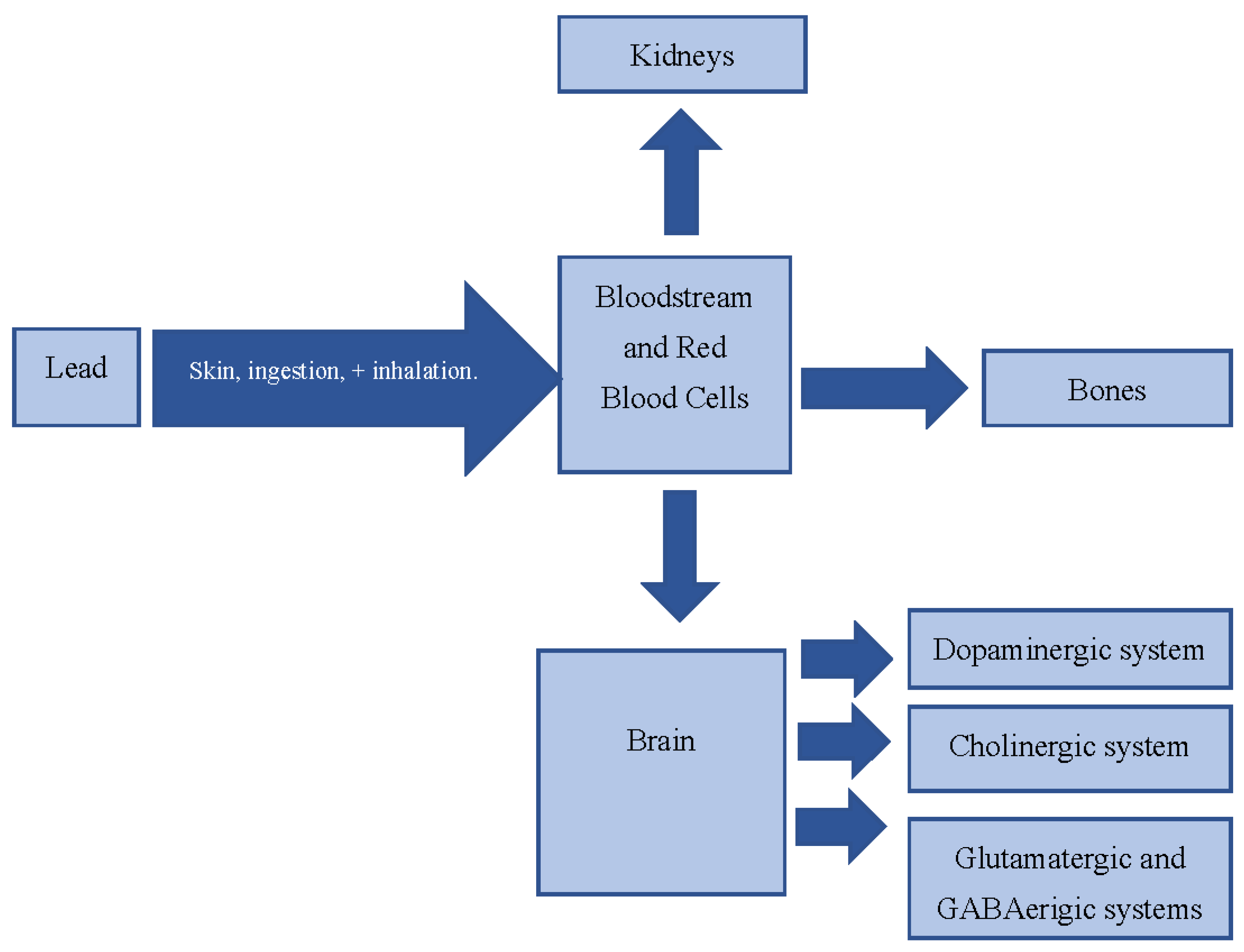
1. Introduction
2. Methods
3. The Association between Lead Exposure and the Comorbidities of Autism
3.1. Intelligence Scores
3.2. Memory
3.3. Language
3.4. Social Withdrawal
4. Mechanisms of Action
4.1. Impact on Cholinergic System and Energy Metabolism
4.2. Impact on the Dopaminergic System
4.3. Impact on the Glutamatergic and GABAergic Systems
4.4. Influence on the NMDA Receptor
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centre for Disease Control and Prevention. Data and Statistics on Autism Spectrum Disorder. Available online: https://www.cdc.gov/ncbddd/autism/data.html (accessed on 21 September 2020).
- Centre for Disease Control and Prevention. Prevalence of Autism Spectrum Disorders—Autism and Developmental Disabilities Monitoring Network, 14 Sites, United States. 2008. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/ss6103a1.htm (accessed on 21 September 2020).
- National Institute for Mental Health. Autism Spectrum Disorder. Available online: https://www.nimh.nih.gov/health/topics/autism-spectrum-disorders-asd/index.shtml (accessed on 12 September 2020).
- World Health Organization. Lead Poisoning and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/lead-poisoning-and-health (accessed on 12 September 2020).
- Ou, J.-J.; Shi, L.-J.; Xun, G.-L.; Chen, C.; Wu, R.-R.; Luo, X.-R.; Zhang, F.-Y.; Zhao, J.-P. Employment and financial burden of families with preschool children diagnosed with autism spectrum disorders in urban China: Results from a descriptive study. BMC Psychiatry 2015, 15. [Google Scholar] [CrossRef]
- Dickerson, A.S.; Rahbar, M.H.; Han, I.; Bakian, A.V.; Bilder, D.A.; Harrington, R.A.; Pettygrove, S.; Durkin, M.; Kirby, R.S.; Wingate, M.S.; et al. Autism spectrum disorder prevalence and proximity to industrial facilities releasing arsenic, lead or mercury. Sci. Total. Environ. 2015, 536, 245–251. [Google Scholar] [CrossRef]
- Mason, L.H.; Harp, J.P.; Han, D.Y. Pb neurotoxicity: Neuropsychological Effects of lead toxicity. Biomed. Res. Int. 2014, 2014, 840547. [Google Scholar] [CrossRef]
- Mayo Clinic. Lead Poisoning—Diagnosis and Treatment—Mayo Clinic. Available online: https://www.mayoclinic.org/diseases-conditions/lead-poisoning/diagnosis-treatment/drc-20354723 (accessed on 31 January 2021).
- Rădulescu, A.; Lundgren, S. Pharmacokinetic model of lead absorption and calcium competitive dynamics. Sci. Rep. 2019, 9, 1–27. [Google Scholar] [CrossRef]
- Centre for Disease Control and Prevention. Blood Lead Levels in Children. Available online: https://www.cdc.gov/nceh/lead/prevention/blood-lead-levels.htm (accessed on 21 September 2020).
- Listos, J.; Baranowska-Bosiacka, I.; Talarek, S.; Listos, P.; Orzelska, J.; Fidecka, S.; Gutowska, I.; Kolasa, A.; Rybicka, M.; Chlubek, D. The effect of perinatal lead exposure on dopamine receptor d2 expression in morphine dependent rats. Toxicology 2013, 310, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Reuben, A.; Caspi, A.; Belsky, D.W.; Broadbent, J.; Harrington, H.; Sugden, K.; Houts, R.M.; Ramrakha, S.; Poulton, R.; Moffitt, T.E. Association of childhood blood lead levels with cognitive function and socioeconomic status at age 38 years and with IQ Change and socioeconomic mobility between childhood and adulthood. JAMA 2017, 317, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Baghurst, P.; McMichael, A.; Sawyer, M.; Mudge, J. Lifetime exposure to environmental lead and children’s intelligence at 11–13 years: The Port Pirie cohort study. BMJ 1996, 312, 1569–1575. [Google Scholar] [CrossRef]
- Canfield, R.L.; Henderson, C.R.; Cory-Slechta, D.A.; Cox, C.; Jusko, T.A.; Lanphear, B.P. Intellectual Impairment in children with blood lead concentrations below 10 Μg per deciliter. N. Engl. J. Med. 2003, 348, 1517–1526. [Google Scholar] [CrossRef]
- Koller, K.; Brown, T.; Spurgeon, A.; Levy, L. Recent Developments in low-level lead exposure and intellectual impairment in children. Environ. Health Perspect. 2004, 112, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Bleecker, M.L.; Ford, D.P.; Lindgren, K.N.; Hoese, V.M.; Walsh, K.S.; Vaughan, C.G. Differential Effects of lead exposure on components of verbal memory. Occup. Environ. Med. 2005, 62, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, D.; Leviton, A.; Needleman, H.L.; Waternaux, C.; Rabinowitz, M. Low-Level lead exposure and infant development in the first year. Neurobehav. Toxicol. Teratol. 1986, 8, 151–161. [Google Scholar] [PubMed]
- Yuan, W.; Holland, S.K.; Cecil, K.M.; Dietrich, K.N.; Wessel, S.D.; Altaye, M.; Hornung, R.W.; Ris, M.D.; Egelhoff, J.C.; Lanphear, B.P. The Impact of early childhood lead exposure on brain organization: A functional magnetic resonance imaging study of language function. Pediatrics 2006, 118, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Stollery, B.T.; Broadbent, D.E.; Banks, H.A.; Lee, W.R. Short Term prospective study of cognitive functioning in lead workers. Br. J. Ind. Med. 1991, 48, 739–749. [Google Scholar] [CrossRef]
- Needleman, H.L.; Schell, A.; Bellinger, D.; Leviton, A.; Allred, E.N. The Long-term effects of exposure to low doses of lead in childhood. N. Engl. J. Med. 1990, 322, 83–88. [Google Scholar] [CrossRef]
- Lanphear, B.P.; Dietrich, K.; Auinger, P.; Cox, C. Cognitive deficits associated with blood lead concentrations <10 Microg/DL in U.S. Children and adolescents. Public Health Rep. 2000, 115, 521–529. [Google Scholar] [CrossRef]
- Khalil, N.; Morrow, L.A.; Needleman, H.; Talbott, E.O.; Wilson, J.W.; Cauley, J.A. Association of cumulative lead and neurocognitive function in an occupational cohort. Neuropsychology 2009, 23, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Geier, D.A.; Kern, J.K.; Geier, M.R. Blood lead levels and learning disabilities: A Cross-sectional study of the 2003–2004 National health and nutrition examination survey (NHANES). Int. J. Environ. Res. Public Health 2017, 14, 1202. [Google Scholar] [CrossRef]
- Fenga, C.; Gangemi, S.; Alibrandi, A.; Costa, C.; Micali, E. Relationship between Lead exposure and mild cognitive impairment. J. Prev. Med. Hyg. 2016, 57, E205–E210. [Google Scholar]
- Sciarillo, W.G.; Alexander, G.; Farrell, K.P. Lead Exposure and child behavior. Am. J. Public Health 1992, 82, 1356–1360. [Google Scholar] [CrossRef]
- Roy, A.; Bellinger, D.; Hu, H.; Schwartz, J.; Ettinger, A.S.; Wright, R.O.; Bouchard, M.; Palaniappan, K.; Balakrishnan, K. Lead Exposure and behavior among young children in Chennai, India. Environ. Health Perspect. 2009, 117, 1607–1611. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, A.L.; Dreyer, B.P.; Fierman, A.H.; Rosen, C.M.; Legano, L.A.; Kruger, H.A.; Lim, S.W.; Courtlandt, C.D. Low-Level lead exposure and behavior in early childhood. Pediatrics 1998, 101, e10. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kang, J.-C. Effects of sub-chronic exposure to lead (Pb) and Ascorbic acid in juvenile rockfish: Antioxidant responses, MT Gene expression, and neurotransmitters. Chemosphere 2017, 171, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, M.T.; Naghizadeh, B.; López-Larrubia, P.; Cauli, O. Behavioral Deficits induced by lead exposure are accompanied by serotonergic and cholinergic alterations in the prefrontal cortex. Neurochem. Int. 2013, 62, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Akinyemi, A.J.; Miah, M.R.; Ijomone, O.M.; Tsatsakis, A.; Soares, F.A.A.; Tinkov, A.A.; Skalny, A.V.; Venkataramani, V.; Aschner, M. Lead (Pb) Exposure induces dopaminergic neurotoxicity in caenorhabditis elegans: Involvement of the dopamine transporter. Toxicol. Rep. 2019, 6, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Dua, R.; Gill, K.D. Impaired Energy metabolism after co-exposure to lead and ethanol. Basic Clin. Pharmacol. Toxicol. 2005, 96, 475–479. [Google Scholar] [CrossRef]
- Zhao, Q.; Slavkovich, V.; Zheng, W. Lead Exposure promotes translocation of protein kinase c activities in rat choroid plexus in vitro, but not in vivo. Toxicol. Appl. Pharmacol. 1998, 149, 99–106. [Google Scholar] [CrossRef]
- Hwang, K.-Y.; Lee, B.-K.; Bressler, J.P.; Bolla, K.I.; Stewart, W.F.; Schwartz, B.S. Protein kinase C Activity and the relations between blood lead and neurobehavioral function in lead workers. Environ. Health Perspect. 2002, 110, 133–138. [Google Scholar] [CrossRef]
- Kursula, P.; Majava, V. A Structural insight into lead neurotoxicity and calmodulin activation by heavy metals. Acta Cryst. Sect. F Struct. Biol. Cryst. Commun. 2007, 63, 653–656. [Google Scholar] [CrossRef]
- Chin, K.; Ryu, J.H.; Cheong, J.H.; Ko, K.H.; Kuroiwa, Y. Selective Effect of chronic lead ingestion on tyrosine hydroxylase activity in brain regions of rats. J. Toxicol. Sci. 1992, 17, 197–210. [Google Scholar] [CrossRef]
- Gandal, M.J.; Haney, J.R.; Parikshak, N.N.; Leppa, V.; Ramaswami, G.; Hartl, C.; Schork, A.J.; Appadurai, V.; Buil, A.; Werge, T.M.; et al. Shared Molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Science 2018, 359, 693–697. [Google Scholar] [CrossRef]
- Chao, O.Y.; Pathak, S.S.; Zhang, H.; Dunaway, N.; Li, J.-S.; Mattern, C.; Nikolaus, S.; Huston, J.P.; Yang, Y.-M. Altered Dopaminergic pathways and therapeutic effects of intranasal dopamine in two distinct mouse models of autism. Mol. Brain 2020, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- ScienceDirect. Na+/K+-ATPase—an Overview. Available online: https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/na-k-atpase (accessed on 21 September 2020).
- Blatt, G.J.; Fatemi, S.H. Alterations in GABAergic Biomarkers in the autism brain: Research findings and Clinical implications. Anat. Rec. 2011, 294, 1646–1652. [Google Scholar] [CrossRef]
- Haam, J.; Yakel, J.L. Cholinergic modulation of the hippocampal region and memory function. J. Neurochem. 2017, 142, 111–121. [Google Scholar] [CrossRef]
- Harry, G.J.; Schmitt, T.J.; Gong, Z.; Brown, H.; Zawia, N.; Evans, H.L. Lead-Induced alterations of glial fibrillary acidic protein (GFAP) in the developing rat brain. Toxicol. Appl. Pharmacol. 1996, 139, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, A.; Peña de Ortiz, S. Lead (Pb+2) Impairs long-term memory and blocks learning-induced increases in hippocampal protein kinase C activity. Toxicol. Appl. Pharmacol. 2004, 200, 27–39. [Google Scholar] [CrossRef]
- Markovac, J.; Goldstein, G.W. Lead Activates protein kinase C in Immature rat brain microvessels. Toxicol. Appl. Pharmacol. 1988, 96, 14–23. [Google Scholar] [CrossRef]
- Quaak, I.; Brouns, M.R.; de Bor, M.V. The dynamics of autism spectrum disorders: How Neurotoxic compounds and neurotransmitters interact. Int. J. Environ. Res. Public Health 2013, 10, 3384–3408. [Google Scholar] [CrossRef] [PubMed]
- Hovde, M.J.; Larson, G.H.; Vaughan, R.A.; Foster, J.D. Model Systems for analysis of dopamine transporter function and regulation. Neurochem. Int. 2019, 123, 13–21. [Google Scholar] [CrossRef]
- Horder, J.; Petrinovic, M.M.; Mendez, M.A.; Bruns, A.; Takumi, T.; Spooren, W.; Barker, G.J.; Künnecke, B.; Murphy, D.G. Glutamate and GABA in Autism spectrum disorder—A Translational magnetic resonance spectroscopy study in man and rodent models. Transl. Psychiatry 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Parpura, V.; Haydon, P.G. Physiological Astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons. Proc. Natl. Acad. Sci. USA 2000, 97, 8629–8634. Available online: https://europepmc.org/article/pmc/pmc26999 (accessed on 21 September 2020). [CrossRef]
- Guariglia, S.R.; Stansfield, K.H.; McGlothan, J.; Guilarte, T.R. Chronic early life lead (Pb2+) Exposure alters presynaptic vesicle pools in hippocampal synapses. BMC Pharmacol. Toxicol. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Neal, A.P.; Stansfield, K.H.; Worley, P.F.; Thompson, R.E.; Guilarte, T.R. Lead Exposure during synaptogenesis alters vesicular proteins and impairs vesicular release: Potential Role of NMDA Receptor-dependent BDNF signaling. Toxicol. Sci. 2010, 116, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Neal, A.P.; Guilarte, T.R. Molecular neurobiology of lead (Pb2+): Effects on synaptic function. Mol. Neurobiol. 2010, 42, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.A. Long-Term Potentiation and Memory. Physiol. Rev. 2004, 81, 87–136. Available online: https://journals.physiology.org/doi/full/10.1152/physrev.00014.2003 (accessed on 21 September 2020).
- Tarabeux, J.; Kebir, O.; Gauthier, J.; Hamdan, F.F.; Xiong, L.; Piton, A.; Spiegelman, D.; Henrion, É.; Millet, B.; Fathalli, F.; et al. Rare Mutations in N-methyl-D-Aspartate glutamate receptors in autism spectrum disorders and schizophrenia. Transl. Psychiatry 2011, 1, e55. [Google Scholar] [CrossRef]
- Eadie, B.D.; Cushman, J.; Kannangara, T.S.; Fanselow, M.S.; Christie, B.R. NMDA Receptor hypofunction in the dentate gyrus and impaired context discrimination in adult Fmr1 knockout mice. Hippocampus 2012, 22, 241–254. [Google Scholar] [CrossRef]
- Jett, D.A.; Kuhlmann, A.C.; Guilarte, T.R. Intrahippocampal Administration of lead (Pb) Impairs performance of rats in the morris water maze. Pharmacol. Biochem. Behav. 1997, 57, 263–269. [Google Scholar] [CrossRef]
- Neal, A.P.; Worley, P.F.; Guilarte, T.R. Lead Exposure during synaptogenesis alters NMDA receptor targeting via NMDA Receptor inhibition. Neurotoxicology 2011, 32, 281–289. [Google Scholar] [CrossRef]
- Nihei, M.K.; Desmond, N.L.; McGlothan, J.L.; Kuhlmann, A.C.; Guilarte, T.R. N-Methyl-d-aspartate Receptor subunit changes are associated with lead-induced deficits of long-term potentiation and spatial Learning. Neuroscience 2000, 99, 233–242. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goel, A.; Aschner, M. The Effect of Lead Exposure on Autism Development. Int. J. Mol. Sci. 2021, 22, 1637. https://doi.org/10.3390/ijms22041637
Goel A, Aschner M. The Effect of Lead Exposure on Autism Development. International Journal of Molecular Sciences. 2021; 22(4):1637. https://doi.org/10.3390/ijms22041637
Chicago/Turabian StyleGoel, Aanya, and Michael Aschner. 2021. "The Effect of Lead Exposure on Autism Development" International Journal of Molecular Sciences 22, no. 4: 1637. https://doi.org/10.3390/ijms22041637
APA StyleGoel, A., & Aschner, M. (2021). The Effect of Lead Exposure on Autism Development. International Journal of Molecular Sciences, 22(4), 1637. https://doi.org/10.3390/ijms22041637




