Altered mRNA and Protein Expression of Monocarboxylate Transporter MCT1 in the Cerebral Cortex and Cerebellum of Prion Protein Knockout Mice
Abstract
1. Introduction
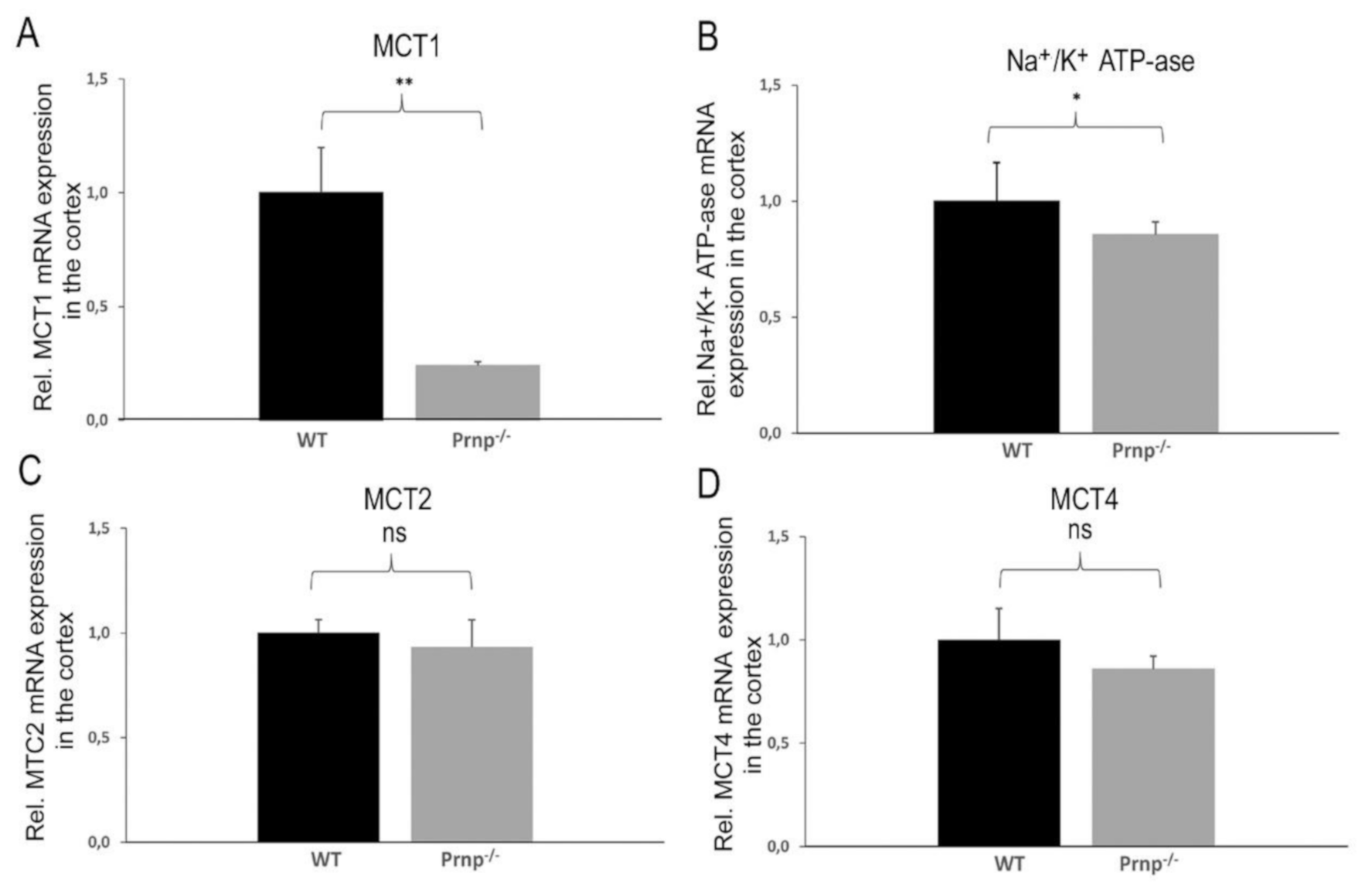
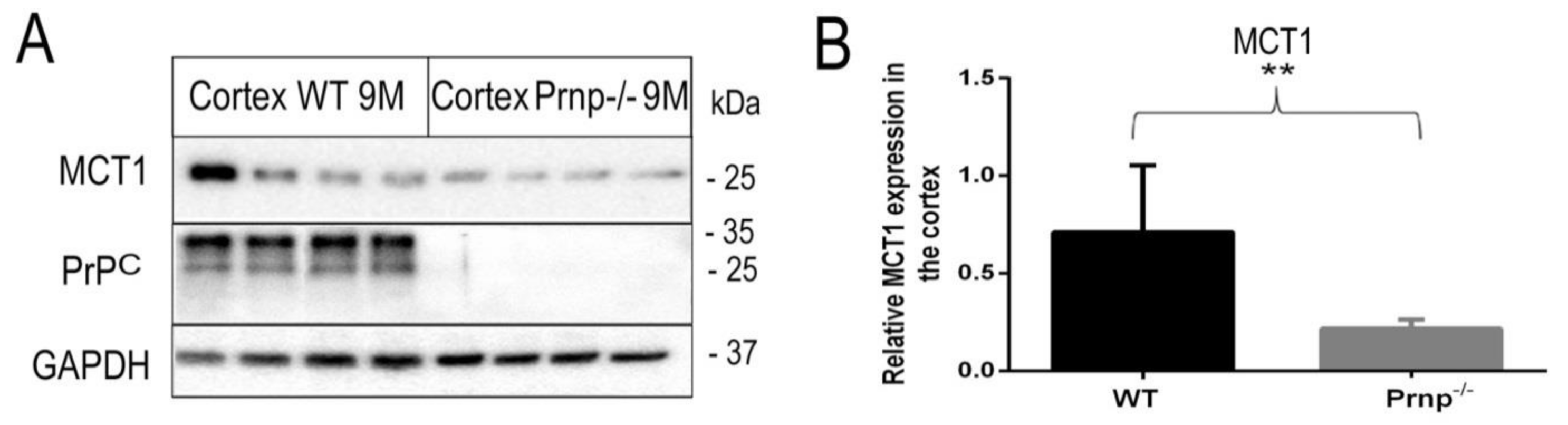
2. Results
3. Discussion
4. Material and Methods
4.1. Animals
4.2. Brain Homogenates for RNA Preparation
4.3. Primer Sequence
4.4. Protein Analysis
Brain Homogenates
4.5. Western Blotting
4.6. Ethics Approval
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ashok, A.; Singh, N. Prion protein modulates glucose homeostasis by altering intracellular iron. Sci. Rep. 2018, 8, 6556. [Google Scholar] [CrossRef] [PubMed]
- Bourgognon, J.M.; Spiers, J.G.; Scheiblich, H.; Antonov, A.; Bradley, S.J.; Tobin, A.B.; Steinert, J.R. Alterations in neuronal metabolism contribute to the pathogenesis of prion disease. Cell Death Differ. 2018, 25, 1408–1425. [Google Scholar] [CrossRef]
- Brito, G.; Roffe, M.; Lupinacci, F.; Santos, T.; Beraldo, F.; Martins, V.R.; Hajj, G.N. The role of cellular prion protein in the regulation of insulin signaling. Mol. Biol. Cell 2013, 24, 3775. [Google Scholar] [CrossRef]
- Strom, A.; Wang, G.S.; Reimer, R.; Finegood, D.T.; Scott, F.W. Pronounced cytosolic aggregation of cellular prion protein in pancreatic beta-cells in response to hyperglycemia. Lab. Investig. 2007, 87, 139–149. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Strom, A.; Wang, G.S.; Scott, F.W. Impaired glucose tolerance in mice lacking cellular prion protein. Pancreas 2011, 40, 229–232. [Google Scholar] [CrossRef]
- Gawinecka, J.; Dieks, J.; Asif, A.R.; Carimalo, J.; Heinemann, U.; Streich, J.H.; Dihazi, H.; Schulz-Schaeffer, W.; Zerr, I. Codon 129 polymorphism specific cerebrospinal fluid proteome pattern in sporadic Creutzfeldt-Jakob disease and the implication of glycolytic enzymes in prion-induced pathology. J. Proteome Res. 2010, 9, 5646–5657. [Google Scholar] [CrossRef]
- Li, Q.Q.; Sun, Y.P.; Ruan, C.P.; Xu, X.Y.; Ge, J.H.; He, J.; Xu, Z.D.; Wang, Q.; Gao, W.C. Cellular prion protein promotes glucose uptake through the Fyn-HIF-2α-Glut1 pathway to support colorectal cancer cell survival. Cancer Sci. 2011, 102, 400–406. [Google Scholar] [CrossRef]
- Ramljak, S.; Herlyn, H.; Zerr, I. Cellular Prion Protein (PrPc) and hypoxia: True to each other in good times and in bad, in sickness, and in health. Front. Cell. Neurosci. 2016, 10, 292. [Google Scholar] [CrossRef]
- Ansoleaga, B.; Garcia-Esparcia, P.; Llorens, F.; Hernández-Ortega, K.; Carmona Tech, M.; Antonio Del Rio, J.; Zerr, I.; Ferrer, I. Altered mitochondria, protein synthesis machinery, and purine metabolism are molecular contributors to the pathogenesis of Creutzfeldt-Jakob Disease. J. Neuropathol. Exp. Neurol. 2016, 75, 755–769. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Sancheti, H.; Patil, I.; Cadenas, E. Energy metabolism and inflammation in brain aging and Alzheimer’s disease. Free Radic. Biol. Med. 2016, 100, 108–122. [Google Scholar] [CrossRef]
- Fu, W.; Shi, D.; Westaway, D.; Jhamandas, J.H. Bioenergetic mechanisms in astrocytes may contribute to amyloid plaque deposition and toxicity. J. Biol. Chem. 2015, 290, 12504–12513. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cheng, X.; Dang, R.; Zhang, W.; Zhang, J.; Yao, Z. Lactate deficit in an Alzheimer disease mouse model: The relationship to neuronal damage. J. Neuropathol. Exp. Neurol. 2018, 77, 1163–1176. [Google Scholar] [CrossRef] [PubMed]
- Ramljak, S.; Schmitz, M.; Zafar, S.; Wrede, A.; Schenkel, S.; Asif, A.R.; Carimalo, J.; Doeppner, T.R.; Schulz-Schaeffer, W.J.; Weise, J.; et al. Cellular prion protein directly interacts with and enhances lactate dehydrogenase expression under hypoxic conditions. Exp. Neurol. 2015, 271, 155–167. [Google Scholar] [CrossRef] [PubMed]
- McLennan, N.F.; Brennan, P.M.; McNeill, A.; Davies, I.; Fotheringham, A.; Rennison, K.A.; Ritchie, D.; Brannan, F.; Head, M.W.; Ironside, J.W.; et al. Prion protein accumulation and neuroprotection in hypoxic brain damage. Am. J. Pathol. 2004, 165, 227–235. [Google Scholar] [CrossRef]
- Mitteregger, G.; Vosko, M.; Krebs, B.; Xiang, W.; Kohlmannsperger, V.; Nölting, S.; Hamann, G.F.; Kretzschmar, H.A. The role of the octarepeat region in neuroprotective function of the cellular prion protein. Brain Pathol. 2007, 17, 174–183. [Google Scholar] [CrossRef]
- Andres-Benito, P.; Dominguez-Gonzalez, M.; Ferrer, I. Altered gene transcription linked to astrocytes and oligodendrocytes in frontal cortex in Creutzfeldt-Jakob disease. Prion 2018, 12, 216–225. [Google Scholar] [CrossRef]
- Ferrer, I.; Puig, B.; Blanco, R.; Martí, E. Prion protein deposition and abnormal synaptic protein expression in the cerebellum in Creutzfeldt-Jakob disease. Neuroscience 2000, 97, 715–726. [Google Scholar] [CrossRef]
- Tschampa, H.J.; Kallenberg, K.; Kretzschmar, H.A.; Meissner, B.; Knauth, M.; Urbach, H.; Zerr, I. Pattern of cortical changes in sporadic Creutzfeldt-Jakob disease. AJNR Am. J. Neuroradiol. 2007, 28, 1114–1118. [Google Scholar] [CrossRef]
- Meissner, B.; Kallenberg, K.; Sanchez-Juan, P.; Ramljak, S.; Krasnianski, A.; Heinemann, U.; Eigenbrod, S.; Gelpi, E.; Barsic, B.; Kretzschmar, H.A.; et al. MRI and clinical syndrome in dura mater-related Creutzfeldt-Jakob disease. J. Neurol. 2009, 256, 355–363. [Google Scholar] [CrossRef]
- Maekawa, F.; Minehira, K.; Kadomatsu, K.; Pellerin, L. Basal and stimulated lactate fluxes in primary cultures of astrocytes are differentially controlled by distinct proteins. J. Neurochem. 2008, 107, 789–798. [Google Scholar] [CrossRef]
- Schmitz, M.; Greis, C.; Ottis, P.; Silva, C.J.; Schulz-Schaeffer, W.J.; Wrede, A.; Koppe, K.; Onisko, B.; Requena, J.R.; Govindarajan, N.; et al. Loss of prion protein leads to age-dependent behavioral abnormalities and changes in cytoskeletal protein expression. Mol. Neurobiol. 2014, 50, 923–936. [Google Scholar] [CrossRef]
- Llorens, F.; Ansoleaga, B.; Garcia-Esparcia, P.; Zafar, S.; Grau-Rivera, O.; López-González, I.; Blanco, R.; Carmona, M.; Yagüe, J.; Nos, C.; et al. PrP mRNA and protein expression in brain and PrPc in CSF in Creutzfeldt-Jakob disease MM1 and VV2. Prion 2013, 7, 383–393. [Google Scholar] [CrossRef]
- Fragoso, D.C.; Goncalves Filho, A.L.; Pacheco, F.T.; Barros, B.R.; Aguiar Littig, I.; Nunes, R.H.; Maia Júnior, A.C.; da Rocha, A.J. Imaging of Creutzfeldt-Jakob disease: Imaging patterns and their differential diagnosis. Radiographics 2017, 37, 234–257. [Google Scholar] [CrossRef]
- Miranda-Gonçalves, V.; Granja, S.; Martinho, O.; Honavar, M.; Pojo, M.; Costa, B.M.; Pires, M.M.; Pinheiro, C.; Cordeiro, M.; Bebiano, G.; et al. Hypoxia-mediated upregulation of MCT1 expression supports the glycolytic phenotype of glioblastomas. Oncotarget 2016, 7, 46335–46353. [Google Scholar] [CrossRef]
- Lee, Y.; Morrison, B.M.; Li, Y.; Lengacher, S.; Farah, M.H.; Hoffman, P.N.; Liu, Y.; Tsingalia, A.; Jin, L.; Zhang, P.W.; et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 2012, 487, 443–448. [Google Scholar] [CrossRef]
- Cudalbu, C.; Craveiro, M.; Mlynárik, V.; Bremer, J.; Aguzzi, A.; Gruetter, R. In Vivo longitudinal (1)H MRS study of transgenic mouse models of prion disease in the hippocampus and cerebellum at 14.1 T. Neurochem. Res. 2015, 40, 2639–2646. [Google Scholar] [CrossRef]
- Kleene, R.; Loers, G.; Langer, J.; Frobert, Y.; Buck, F.; Schachner, M. Prion protein regulates glutamate-dependent lactate transport of astrocytes. J. Neurosci. 2007, 27, 12331–12340. [Google Scholar] [CrossRef]
- Pellerin, L.; Bouzier-Sore, A.K.; Aubert, A.; Serres, S.; Merle, M.; Costalat, R.; Magistretti, P.J. Activity-dependent regulation of energy metabolism by astrocytes: An update. Glia 2007, 55, 1251–1262. [Google Scholar] [CrossRef]
- Guitart, K.; Loers, G.; Schachner, M.; Kleene, R. Prion protein regulates glutathione metabolism and neural glutamate and cysteine uptake via excitatory amino acid transporter 3. J. Neurochem. 2015, 133, 558–571. [Google Scholar] [CrossRef]
- Suzuki, A.; Stern, S.A.; Bozdagi, O.; Huntley, G.W.; Walker, R.H.; Magistretti, P.J.; Alberini, C.M. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 2011, 144, 810–823. [Google Scholar] [CrossRef]
- Steele, A.D.; Lindquist, S.; Aguzzi, A. The prion protein knockout mouse: A phenotype under challenge. Prion 2007, 1, 83–93. [Google Scholar] [CrossRef]
- Pérez-Escuredo, J.; Van Hée, V.F.; Sboarina, M.; Falces, J.; Payen, V.L.; Pellerin, L.; Sonveaux, P. Monocarboxylate transporters in the brain and in cancer. Biochim. Biophys. Acta 2016, 1863, 2481–2497. [Google Scholar] [CrossRef]
- Yu, W.; Krook-Magnuson, E. Cognitive Collaborations: Bidirectional functional connectivity between the cerebellum and the hippocampus. Front. Syst. Neurosci. 2015, 9, 177. [Google Scholar] [CrossRef]
- Bohne, P.; Schwarz, M.K.; Herlitze, S.; Mark, M.D. A new projection from the deep cerebellar nuclei to the hippocampus via the ventrolateral and laterodorsal thalamus in mice. Front. Neural Circuits 2019, 13, 51. [Google Scholar] [CrossRef]
- Watson, T.C.; Becker, N.; Apps, R.; Jones, M.W. Back to front: Cerebellar connections and interactions with the prefrontal cortex. Front. Syst. Neurosci. 2014, 8, 4. [Google Scholar] [CrossRef]
- Bueler, H.; Fischer, M.; Lang, Y.; Bluethmann, H.; Lipp, H.P.; DeArmond, S.J.; Prusiner, S.B.; Aguet, M.; Weissmann, C. Normal development and behavior of mice lacking the neuronal cell-surface PrP protein. Nature 1992, 356, 577–582. [Google Scholar] [CrossRef]
- Schmitz, M.; Zafar, S.; Silva, C.J.; Zerr, I. Behavioral abnormalities in prion protein knockout mice and the potential relevance of PrPC for the cytoskeleton. Prion 2014, 8, 381–386. [Google Scholar] [CrossRef]


| Name | Forward Sequence | Reverse Sequence |
|---|---|---|
| LDH-A | CAGTGGCTTTGCCAAAAACCGAGT | CCATCAGGTAACGGAACCGCG |
| LDH-B | CCTGCTGACTTTGCAGTGGCTCC | TCGCCGCGGCAGCCTCATCAT |
| Basigin | CAAGGTACTGCAGGAGGACACTCT | TCAGGAAGGAAGATGCAGGAATATT |
| Na+/K+-ATPase α2 | GAGACGCGCAATATCTGTTTCTT | ACCTGTGGCAATCACAATGC |
| MCT1 | TTGGACCCCAGAGGTTCTCC | AGGCGGCCTAAAAGTGGTG |
| MCT2 | CAGCAACAGCGTGATAGAGCTT | TGGTTGCAGGTTGAATGCTAAT |
| MCT4 | CGGCTGGCGGTAACAGAGTA | CGGCCTCGGACCTGAGTATT |
| β-Actin | GCTTCTTTGCAGCTCCTTCGT | ATATCGTCATCCATGGCGAAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramljak, S.; Schmitz, M.; Repond, C.; Zerr, I.; Pellerin, L. Altered mRNA and Protein Expression of Monocarboxylate Transporter MCT1 in the Cerebral Cortex and Cerebellum of Prion Protein Knockout Mice. Int. J. Mol. Sci. 2021, 22, 1566. https://doi.org/10.3390/ijms22041566
Ramljak S, Schmitz M, Repond C, Zerr I, Pellerin L. Altered mRNA and Protein Expression of Monocarboxylate Transporter MCT1 in the Cerebral Cortex and Cerebellum of Prion Protein Knockout Mice. International Journal of Molecular Sciences. 2021; 22(4):1566. https://doi.org/10.3390/ijms22041566
Chicago/Turabian StyleRamljak, Sanja, Matthias Schmitz, Cendrine Repond, Inga Zerr, and Luc Pellerin. 2021. "Altered mRNA and Protein Expression of Monocarboxylate Transporter MCT1 in the Cerebral Cortex and Cerebellum of Prion Protein Knockout Mice" International Journal of Molecular Sciences 22, no. 4: 1566. https://doi.org/10.3390/ijms22041566
APA StyleRamljak, S., Schmitz, M., Repond, C., Zerr, I., & Pellerin, L. (2021). Altered mRNA and Protein Expression of Monocarboxylate Transporter MCT1 in the Cerebral Cortex and Cerebellum of Prion Protein Knockout Mice. International Journal of Molecular Sciences, 22(4), 1566. https://doi.org/10.3390/ijms22041566







