Studies of Sol-Gel Evolution and Distribution of Eu3+ Ions in Glass–Ceramics Containing LaF3 Nanocrystals Depending on Initial Sols Composition
Abstract
1. Introduction
2. Results and Discussion
2.1. The Impact of Initial Composition on the Sol-Gel Transformation of Liquid Sols into Xerogels
2.2. The Evolution of Xerogels into Glass–Ceramics
- (1)
- La(CH3COO)3 + 3CF3COOH → La(CF3COO)3 + 3CH3COOH,
- (2)
- La(CF3COO)3 → LaF3 + MF3 + (CF3CO)2O + CO2 + CO.
2.3. Photoluminescence Studies of Prepared Sol-Gel Materials
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TEOS | tetraethoxysilane |
| DMF | N,N-dimethylformamide |
| EtOH | ethyl alcohol |
| AcOH | acetic acid |
| TFA | trifluoroacetic acid |
| PLE | photoluminescence excitation spectra |
| PL | photoluminescence spectra |
References
- Piotrowski, T.; Trejgis, K.; Maciejewska, K.; Ledwa, K.; Fond, B.; Marciniak, L. Thermochromic Luminescent Nanomaterials Based on Mn4+/Tb3+ Codoping for Temperature Imaging with Digital Cameras. ACS Appl. Mater. Interfaces 2020, 12, 44039–44048. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.; Jia, M.; Li, P.; Liu, G.; Sun, Z.; Fu, Z. Multifunctional Optical Thermometry Based on the Rare-Earth-Ions-Doped Up-/Down-Conversion Ba2TiGe2O8:Ln (Ln = Eu3+/Er3+/Ho3+/Yb3+) Phosphors. Inorg. Chem. 2019, 58, 7939–7946. [Google Scholar] [CrossRef] [PubMed]
- Rakov, N.; Xing, Y.; Maciel, G.S. Optical Thermometry Operation within All Three Biological Windows Using Nd3+:Er3+:Y2O3 Nanocomposite Phosphors. ACS Appl. Nano Mater. 2020, 3, 10479–10486. [Google Scholar] [CrossRef]
- Ahadi, K.; Gui, Z.; Porter, Z.; Lynn, J.W.; Xu, Z.; Wilson, S.D.; Janotti, A.; Stemmer, S. Carrier density control on magnetism and Berry phases in doped EuTiO3. APL Mater. 2018, 6, 056105. [Google Scholar] [CrossRef]
- Ahadi, K.; Stemmer, S. Novel Metal-Insulator Transition at the SmTiO3/SrTiO3 Interface. Phys. Rev. Lett. 2017, 118, 236803. [Google Scholar] [CrossRef]
- Ahadi, K.; Galletti, L.; Stemmer, S. Evidence of a topological Hall effect in Eu1-xSmxTiO3. Appl. Phys. Lett. 2017, 111, 172403. [Google Scholar] [CrossRef]
- Sandrini, M.; Muniz, R.F.; Zanuto, V.S.; Pedrochi, F.; Guyot, Y.; Bento, A.C.; Baesso, M.L.; Steimacher, A.; Neto, A.M. Enhanced and tunable white light emission from Ag nanoclusters and Eu3+-co-doped CaBAl glasses. RSC Adv. 2018, 8, 35263–35270. [Google Scholar] [CrossRef]
- Pavel, C.C.; Lacal-Arántegui, R.; Marmier, A.; Schüller, D.; Tzimas, E.; Buchert, M.; Jenseit, W.; Blagoeva, D. Substitution strategies for reducing the use of rare earths in wind turbines. Resour. Policy 2017, 52, 349–357. [Google Scholar] [CrossRef]
- Fedorov, P.P.; Luginina, A.A.; Popov, A.I. Transparent oxyfluoride glass ceramics. J. Fluor. Chem. 2015, 172, 22–50. [Google Scholar] [CrossRef]
- Pascual, M.J.; Garrido, C.; Durán, A.; Miguel, A.; Pascual, L.; de Pablos-Martín, A.; Fernández, J.; Balda, R. Optical Properties of Transparent Glass-Ceramics Containing Er3+-Doped Sodium Lutetium Fluoride Nanocrystals. Int. J. Appl. Glass Sci. 2016, 7, 27–40. [Google Scholar] [CrossRef]
- De Pablos-Martín, A.; Hémono, N.; Mather, G.C.; Bhattacharyya, S.; Höche, T.; Bornhöft, H.; Deubener, J.; Muñoz, F.; Durán, A.; Pascual, M.J. Crystallization Kinetics of LaF3 Nanocrystals in an Oxyfluoride Glass. J. Am. Ceram. Soc. 2011, 94, 2420–2428. [Google Scholar] [CrossRef]
- Wang, Q.; Ouyang, S.; Zhang, W.; Yang, B.; Zhang, Y.; Xia, H. Luminescent properties of Ce3+-doped transparent oxyfluoride glass ceramics containing BaGdF5 nanocrystals. J. Rare Earth 2015, 33, 13–19. [Google Scholar] [CrossRef]
- Liu, Q.; Tian, Y.; Tang, W.; Jing, X.; Zhang, J.; Xu, S. Comprehensive studies of the Ag+ effect on borosilicate glass ceramics containing Ag nanoparticles and Er-doped hexagonal NaYF4 nanocrystals: Morphology, structure, and 2.7 μm emission. Nanophotonics 2018, 7, 913–923. [Google Scholar] [CrossRef]
- Xu, W.; Hu, Y.; Jin, Z.; Zheng, L.; Zhang, Z.; Cao, W. Anti-Stokes excited Er3+/Yb3+ codoped oxyfluoride glass ceramic for luminescence thermometry. J. Lumin. 2018, 203, 401–408. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Pan, Y.; Chen, H.; Cao, Q.; Wei, X. Upconversion luminescence and optical thermometry properties of transparent oxyfluoride glass ceramics embedded with Ba4Y3F17:Yb3+,Er3+ nanocrystals. CrystEngComm 2020, 22, 6302–6309. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, K.; Liu, C.; Yin, Q.; Han, J.; Heo, J. Intense up-conversion emission from Er3+/Yb3+ ion co-doped transparent oxyfluoride glass-ceramics containing Y5O4F7 nanorods for optical thermometry. J. Mater. Chem. C 2019, 7, 6134–6143. [Google Scholar] [CrossRef]
- Fu, Y.; Zhao, L.; Guo, Y.; Yu, H. Highly sensitive optical thermometers based on unconventional thermometric coupled levels of Tm3+ following a Boltzmann-type distribution in oxyfluoride glass ceramics. New J. Chem. 2019, 43, 16664–16669. [Google Scholar] [CrossRef]
- Bae, S.-R.; Choi, Y.G.; Im, W.B.; Lee, K.S.; Chung, W.J. Rare earth doped silicate-oxyfluoride glass ceramics incorporating LaF3 nano-crystals for UV-LED color conversion. Opt. Mater. 2013, 35, 2034–2038. [Google Scholar] [CrossRef]
- Lee, S.H.; Bae, S.-R.; Choi, Y.G.; Chung, W.J. Eu2+/Eu3+-doped oxyfluoride glass ceramics with LaF3 for white LED color conversion. Opt. Mater. 2015, 41, 71–74. [Google Scholar] [CrossRef]
- Walas, M.; Lisowska, M.; Lewandowski, T.; Becerro, A.I.; Łapiński, M.; Synak, A.; Sadowski, W.; Kościelska, B. From structure to luminescence investigation of oxyfluoride transparent glasses and glass-ceramics doped with Eu3+/Dy3+ ions. J. Alloys Compd. 2019, 806, 1410–1418. [Google Scholar] [CrossRef]
- De Aldana, J.R.V.; Romero, C.; Fernández, J.; Gorni, G.; Pascual, M.J.; Durán, A.; Balda, R. Femtosecond laser direct inscription of 3D photonic devices in Er/Yb-doped oxyfluoride nano-glass ceramics. Opt. Mater. Express 2020, 10, 2695–2704. [Google Scholar] [CrossRef]
- Kang, S.; Huang, Z.; Lin, W.; Yang, D.; Zhao, J.; Qiao, X.; Xiao, X.; Xu, S.; Qiu, J.; Du, J.; et al. Enhanced single-mode fiber laser emission by nano-crystallization of oxyfluoride glass-ceramic cores. J. Mater. Chem. C 2019, 7, 5155–5162. [Google Scholar] [CrossRef]
- Del-Castillo, J.; Yanes, A.C.; Méndez-Ramos, J.; Tikhomirov, V.K.; Moshchalkov, V.V.; Rodríguez, V.D. Sol-gel preparation and white up-conversion luminescence in rare-earth doped PbF2 nanocrystals dissolved in silica glass. J. Sol-Gel Sci. Technol. 2010, 53, 509–514. [Google Scholar] [CrossRef]
- Biswas, K.; Balaji, S.; Karmakar, P.; Annapurna, K. Formation and spectra probing of transparent oxyfluoride glass-ceramics (Eu2+, Eu3+:BaGdF5) nanocrystals. Opt. Mater. 2015, 39, 153–159. [Google Scholar] [CrossRef]
- Zhang, Y.-X.; Wang, S.-X.; Di Lieto, A.; Yu, G.-L.; Yu, H.-H.; Zhang, H.-J.; Tonelli, M.; Xu, X.-G.; Wang, J.Y. Temperature-Dependent Fluorescence Properties and Diode-Pumped Deep Red Laser Performance of Pr:LiGdF4 Crystal. Chin. Phys. Lett. 2015, 32, 054210. [Google Scholar] [CrossRef]
- Li, M.; Bai, G.; Guo, Y.; Hu, L.; Zhang, J. Investigation on Tm3+-doped silicate glass for 1.8 μm emission. J. Lumin. 2012, 132, 1830–1835. [Google Scholar] [CrossRef]
- Rivera-López, F.; Babu, P.; Basavapoornima, C.; Jayasankar, C.K.; Lavín, V. Efficient Nd3+ → Yb3+ energy transfer processes in high phonon energy phosphate glasses for 1.0 µm Yb3+ ions. J. Appl. Phys. 2011, 109, 123514. [Google Scholar] [CrossRef]
- Cao, R.; Lu, Y.; Tian, Y.; Huang, F.; Xu, S.; Zhang, J. Spectroscopy of thulium and holmium co-doped silicate glasses. Opt. Mater. Express 2016, 6, 2252–2263. [Google Scholar] [CrossRef]
- De Pablos-Martín, A.; Pascual, M.J.; Durán, A. Transparent nano-glass-ceramic for photonic applications. Distribution of RE-doping elements in the fluoride nano-crystals analysed by XAS and HR-TEM. Adv. Sci. Technol. 2014, 90, 111–120. [Google Scholar] [CrossRef]
- Yu, H.; Guo, H.; Zhang, M.; Liu, Y.; Liu, M.; Zhao, L.-J. Distribution of Nd3+ ions in oxyfluoride glass ceramics. Nanoscale Res. Lett. 2012, 7, 275. [Google Scholar] [CrossRef]
- Ali, M.A.; Ren, J.; Liu, X.; Qiao, X.; Qiu, J. Understanding Enhanced Upconversion Luminescence in Oxyfluoride Glass-Ceramics Based on Local Structure Characterizations and Molecular Dynamics Simulations. J. Phys. Chem. C 2017, 121, 15384–15391. [Google Scholar] [CrossRef]
- Gorni, G.; Pascual, M.J.; Caballero, A.; Velázquez, J.J.; Mosa, J.; Castro, Y.; Durán, A. Crystallization mechanism in sol-gel oxyfluoride glass-ceramics. J. Non-Cryst. Solids 2018, 501, 145–152. [Google Scholar] [CrossRef]
- Lesniak, M.; Zmojda, J.; Kochanowicz, M.; Miluski, P.; Baranowska, A.; Mach, G.; Kuwik, M.; Pisarska, J.; Pisarski, W.A.; Dorosz, D. Spectroscopic Properties of Erbium-Doped Oxyfluoride Phospho-Tellurite Glass and Transparent Glass-Ceramic Containing BaF2 Nanocrystals. Materials 2019, 12, 3429. [Google Scholar] [CrossRef] [PubMed]
- Biswas, K.; Sontakke, A.D.; Annapurna, K. Synthesis and Structural Probing of Eu3+ Doped BaYF5 Nano-Crystals in Transparent Oxyfluoride Glass-Ceramics. Int. J. Appl. Glass Sci. 2012, 3, 154–162. [Google Scholar] [CrossRef]
- Zhao, Z.; Ai, B.; Liu, C.; Yin, Q.; Xia, M.; Zhao, X.; Jiang, Y. Er3+ Ions-Doped Germano-Gallate Oxyfluoride Glass-Ceramics Containing BaF2 Nanocrystals. J. Am. Ceram. Soc. 2015, 98, 2117–2121. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.Y.; Wang, W.C.; Zhang, W.J.; Zhang, Q.Y. 2.7 μm emission in Er3+ doped glass ceramics containing lutetium oxyfluoride nanocrystals. Opt. Mater. Express 2016, 6, 2759–2768. [Google Scholar] [CrossRef]
- Pan, J.; Xu, R.; Wang, M.; Gao, G.; Chen, J.; Hu, L.; Zhang, J. Enhanced 2.0 μm emission in Tm3+/Ho3+ codoped transparent oxyfluoride glass ceramics containing β-PbF2 nano-crystals. Solid State Commun. 2010, 150, 78–80. [Google Scholar] [CrossRef]
- Lin, Z.; Liang, X.; Ou, Y.; Fan, C.; Yuan, S.; Zeng, H.; Chen, G. Full color photoluminescence of Tb3+/Sm3+ codoped oxyfluoride aluminosilicate glasses and glass ceramics for white light emitting diodes. J. Alloys Compd. 2010, 496, L33–L37. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, S.; Fei, Y.; Huang, L.; Xu, S. Structure and optical properties of Dy3+/Tm3+ co-doped oxyfluoride glass ceramics containing β-NaGdF4 nanocrystals. Opt. Mater. 2014, 38, 92–96. [Google Scholar] [CrossRef]
- Zheng, B.; Cao, H.; Hu, J.; Gu, Z.; Zhang, Y. Structure and spectroscopic properties of Tb3+/Sm3+ co-doped oxyfluoride glass ceramics containing LiYF4 nanocrystals. J. Alloys Compd. 2018, 768, 150–156. [Google Scholar] [CrossRef]
- Danks, A.E.; Hall, S.R.; Schnepp, Z. The evolution of ‘sol-gel’ chemistry as a technique for materials synthesis. Mater. Horiz. 2016, 3, 91–112. [Google Scholar] [CrossRef]
- Vinogradov, A.V.; Vinogradov, V.V. Low-temperature sol-gel synthesis of crystalline materials. RSC Adv. 2014, 4, 45903–45919. [Google Scholar] [CrossRef]
- Prakashan, V.P.; Sajna, M.S.; Gejo, G.; Sanu, M.S.; Biju, P.R.; Cyriac, J.; Unnikrishnan, N.V. Perceiving impressive optical properties of ternary SiO2-TiO2-ZrO2:Eu3+ sol-gel glasses with high reluctance for concentration quenching: An experimental approach. J. Non-Cryst. Solids 2018, 482, 116–125. [Google Scholar] [CrossRef]
- Abo-Naf, A.M.; Abdel-Hameed, S.A.M.; Marzouk, M.A.; Hamdy, Y.M. Bifunctional ferromagnetic Eu-Gd-Bi-codoped hybrid organo-silica red emitting phosphors synthesized by a modified Pechini sol-gel method. Mater. Chem. Phys. 2017, 194, 198–205. [Google Scholar] [CrossRef]
- De Pablos-Martín, A.; Ristic, D.; Bhattacharyya, S.; Höche, T.; Mather, G.C.; Ramírez, M.O.; Soria, S.; Ferrari, M.; Righini, G.C.; Bausá, L.E.; et al. Effects of Tm3+ Additions on the Crystallization of LaF3 Nanocrystals in Oxyfluoride Glasses: Optical Characterization and Up-Conversion. J. Am. Ceram. Soc. 2013, 96, 447–457. [Google Scholar] [CrossRef]
- Secu, C.E.; Bartha, C.; Polosan, S.; Secu, M. Thermally activated conversion of a silicate gel to an oxyfluoride glass ceramics: Optical study using Eu3+ probe ion. J. Lumin. 2014, 146, 539–543. [Google Scholar] [CrossRef]
- Velázquez, J.J.; Mosa, J.; Gorni, G.; Balda, R.; Fernández, J.; Pascual, L.; Durán, A.; Castro, Y. Transparent SiO2-GdF3 sol-gel nano-glass ceramics for optical application. J. Sol-Gel Sci. Technol. 2019, 89, 322–332. [Google Scholar] [CrossRef]
- Kawamura, G.; Yoshimura, R.; Ota, K.; Oh, S.-Y.; Hakiri, N.; Muto, H.; Hayakawa, T.; Matsuda, A. A Unique Approach to Characterization of Sol-Gel-Derived Rare-Earth-Doped Oxyfluoride Glass-Ceramics. J. Am. Ceram. Soc. 2013, 96, 476–480. [Google Scholar] [CrossRef]
- Gorni, G.; Velázquez, J.J.; Mosa, J.; Mather, G.C.; Serrano, A.; Vila, M.; Castro, G.R.; Bravo, D.; Balda, R.; Fernández, J.; et al. Transparent Sol-Gel Oxyfluoride Glass-Ceramics with High Crystalline Fraction and Study of RE Incorporation. Nanomaterials 2019, 9, 530. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, N.; Szpikowska-Sroka, B.; Pietrasik, E.; Goryczka, T.; Pisarski, W.A. Structural and luminescence properties of silica powders and transparent glass-ceramics containing LaF3:Eu3+ nanocrystals. J. Am. Ceram. Soc. 2018, 101, 4654–4668. [Google Scholar] [CrossRef]
- Pawlik, N.; Szpikowska-Sroka, B.; Goryczka, T.; Zubko, M.; Lelątko, J.; Pisarski, W.A. Structure and luminescent properties of oxyfluoride glass-ceramics with YF3:Eu3+ nanocrystals derived by sol-gel method. J. Eur. Ceram. Soc. 2019, 39, 5010–5017. [Google Scholar] [CrossRef]
- Szpikowska-Sroka, B.; Pawlik, N.; Goryczka, T.; Pisarski, W.A. Influence of silicate sol-gel host matrices and catalyst agents on luminescence properties of Eu3+/Gd3+ under different excitation wavelength. RSC Adv. 2015, 5, 98773–98782. [Google Scholar] [CrossRef]
- Biswas, A.; Maciel, G.S.; Friend, C.S.; Prasad, P.N. Upconversion properties of a transparent Er3+-Yb3+ co-doped LaF3-SiO2 glass-ceramics prepared by sol-gel method. J. Non-Cryst. Solids 2003, 316, 393–397. [Google Scholar] [CrossRef]
- Suo, H.; Guo, C.; Zheng, J.; Zhou, B.; Ma, C.; Zhao, X.; Li, T.; Guo, P.; Goldys, E.M. Sensitivity Modulation of Upconverting Thermometry through Engineering Phonon Energy of a Matrix. ACS Appl. Mater. Interfaces 2016, 8, 30312–30319. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Cui, Z.; Jia, G.; Zhao, S.; Wang, H.; Deng, D.; Huang, L.; Xu, S. Optical spectroscopic studies on GdF3:Tb3+,Yb3+ nanocrystals. J. Lumin. 2014, 151, 201–205. [Google Scholar] [CrossRef]
- Haschke, J.M. Structural Correlations of the Lanthanide Halides and Related Compounds. Derivatives of the Anti-Nickel-Arsenide Structure. J. Solid State Chem. 1976, 18, 205–216. [Google Scholar] [CrossRef]
- Liu, H.-G.; Zheng, W.-C. Theoretical investigations of the optical and EPR spectra for trivalent cerium and ytterbium ions in orthorhombic YF3 crystal. Phys. B 2016, 496, 15–19. [Google Scholar] [CrossRef]
- Liu, S.; Chen, D.; Wan, Z.; Zhou, Y.; Huang, P.; Ji, Z. Phase structure control and optical spectroscopy of rare-earth activated GdF3 nanocrystal embedded glass ceramics via alkaline-earth/alkali-metal doping. RSC Adv. 2016, 6, 71176–71187. [Google Scholar] [CrossRef]
- He, S.; Huang, D.; Bi, H.; Zhi, L.; Hui, Y.; Cheng, X. Synthesis and characterization of silica aerogels dried under ambient pressure bed on water glass. J. Non-Cryst. Solids 2015, 410, 58–64. [Google Scholar] [CrossRef]
- Innocenzi, P. Infrared spectroscopy of sol-gel derived silica-based films: A spectra-microstructure overview. J. Non-Cryst. Solids 2003, 316, 309–319. [Google Scholar] [CrossRef]
- Osswald, J.; Fehr, K.T. FTIR spectroscopic study on liquid silica solutions and nanoscale particle size determination. J. Mater. Sci. 2006, 41, 1335–1339. [Google Scholar] [CrossRef]
- Kreienborg, N.M.; Merten, C. How to treat C-F stretching vibrations? A vibrational CD study on chiral fluorinated molecules. Phys. Chem. Chem. Phys. 2019, 21, 3506–3511. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, H.; Serra, J.; González, P.; León, B. Structural study of sol-gel silicate glasses by IR and Raman spectroscopies. J. Non-Cryst. Solids 2009, 355, 475–480. [Google Scholar] [CrossRef]
- Parrill, T.M. Heat treatment of spun-on acid-catalyzed sol-gel silica films. J. Mater. Res. 1994, 9, 723–730. [Google Scholar] [CrossRef]
- Kemnitz, E.; Noack, J. The non-aqueous fluorolytic sol-gel synthesis of nanoscaled metal fluorides. Dalton Trans. 2015, 44, 19411–19431. [Google Scholar] [CrossRef]
- Holder, C.F.; Schaak, R.E. Tutorial on Powder X-ray Diffraction for Characterizing Nanoscale Materials. ACS Nano 2019, 13, 7359–7365. [Google Scholar] [CrossRef]
- Yanes, A.C.; del-Castillo, J.; Méndez-Ramos, J.; Rodríguez, V.D.; Torres, M.E.; Arbiol, J. Luminescence and structural characterization of transparent nanostructures Eu3+-doped LaF3-SiO2 glass-ceramics prepared by sol-gel method. Opt Mater. 2007, 29, 999–1003. [Google Scholar] [CrossRef]
- Binnemans, K. Interpretation of europium(III) spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef]
- Kalinovskaya, I.V.; Zadorozhnaya, A.N.; Nikolenko, Y.M. Luminescent properties of europium carboxylates. J. Mol. Struct. 2015, 1100, 100–104. [Google Scholar] [CrossRef]
- Hehlen, M.P.; Cockroft, N.J.; Gosnell, T.R. Spectroscopic properties of Er3+- and Yb3+-doped soda-lime silicate and aluminosilicate glasses. Phys. Rev. B 1997, 56, 9302–9318. [Google Scholar] [CrossRef]
- Herrmann, A.; Rüssel, C. New Aluminosilicate Glasses as High-Power Laser Materials. Int. J. Appl. Glass Sci. 2015, 6, 210–219. [Google Scholar] [CrossRef]
- Van Dijk, J.M.F.; Schuurmans, M.F.H. On the nonradiative and radiative decay rates and a modified exponential energy gap law for 4f-4f transitions in rare-earth ions. J. Chem. Phys. 1983, 78, 5317–5323. [Google Scholar] [CrossRef]
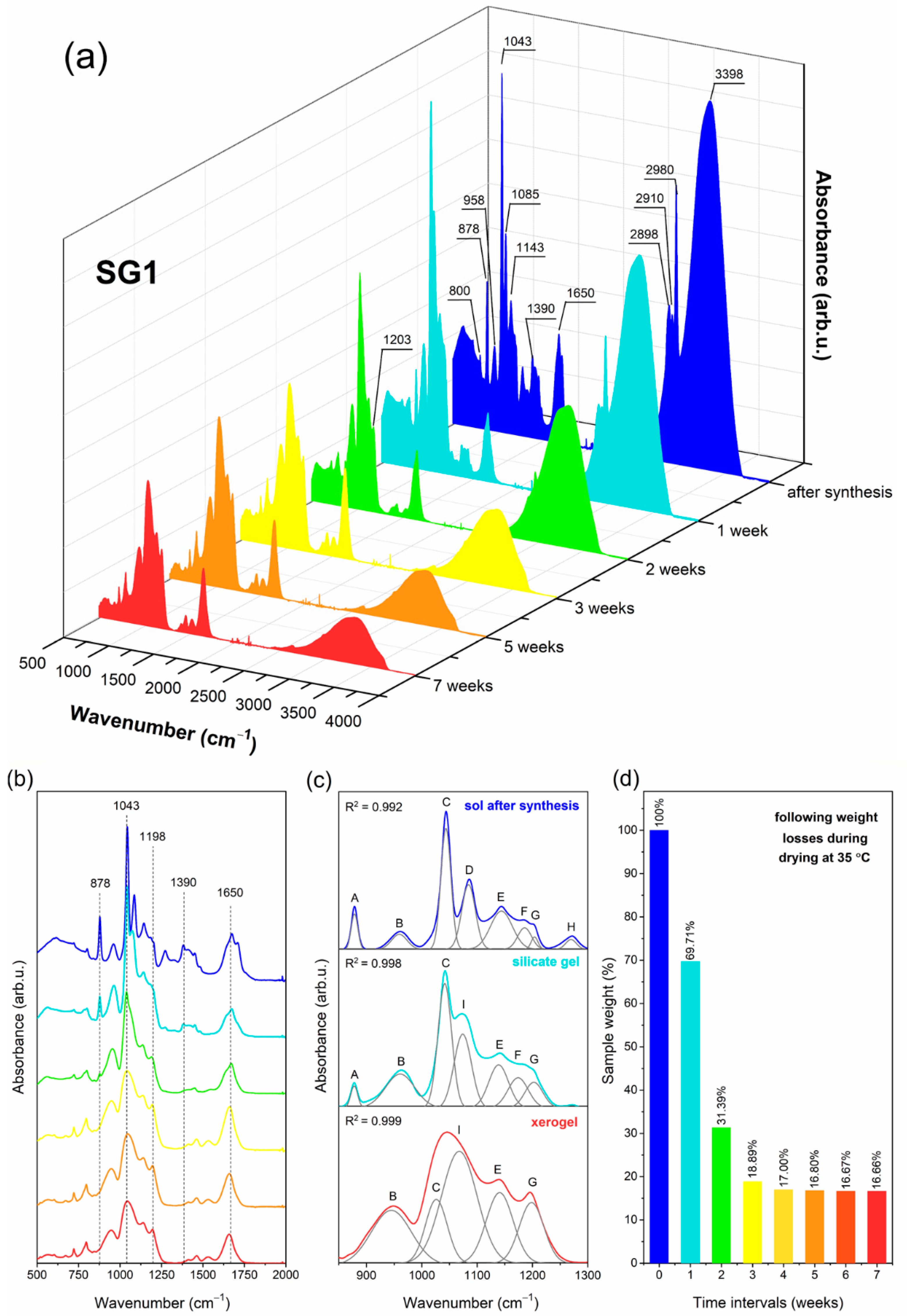

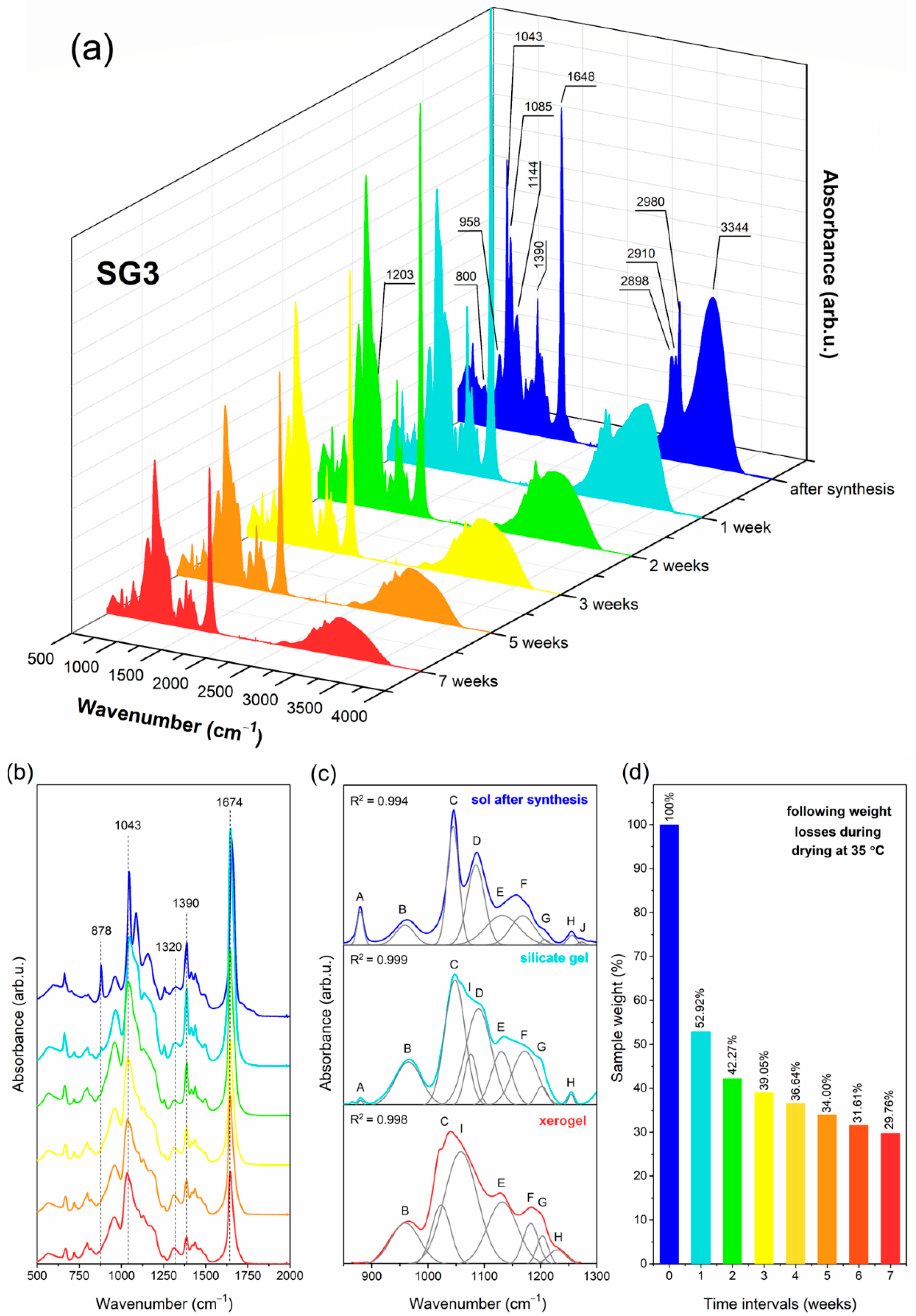
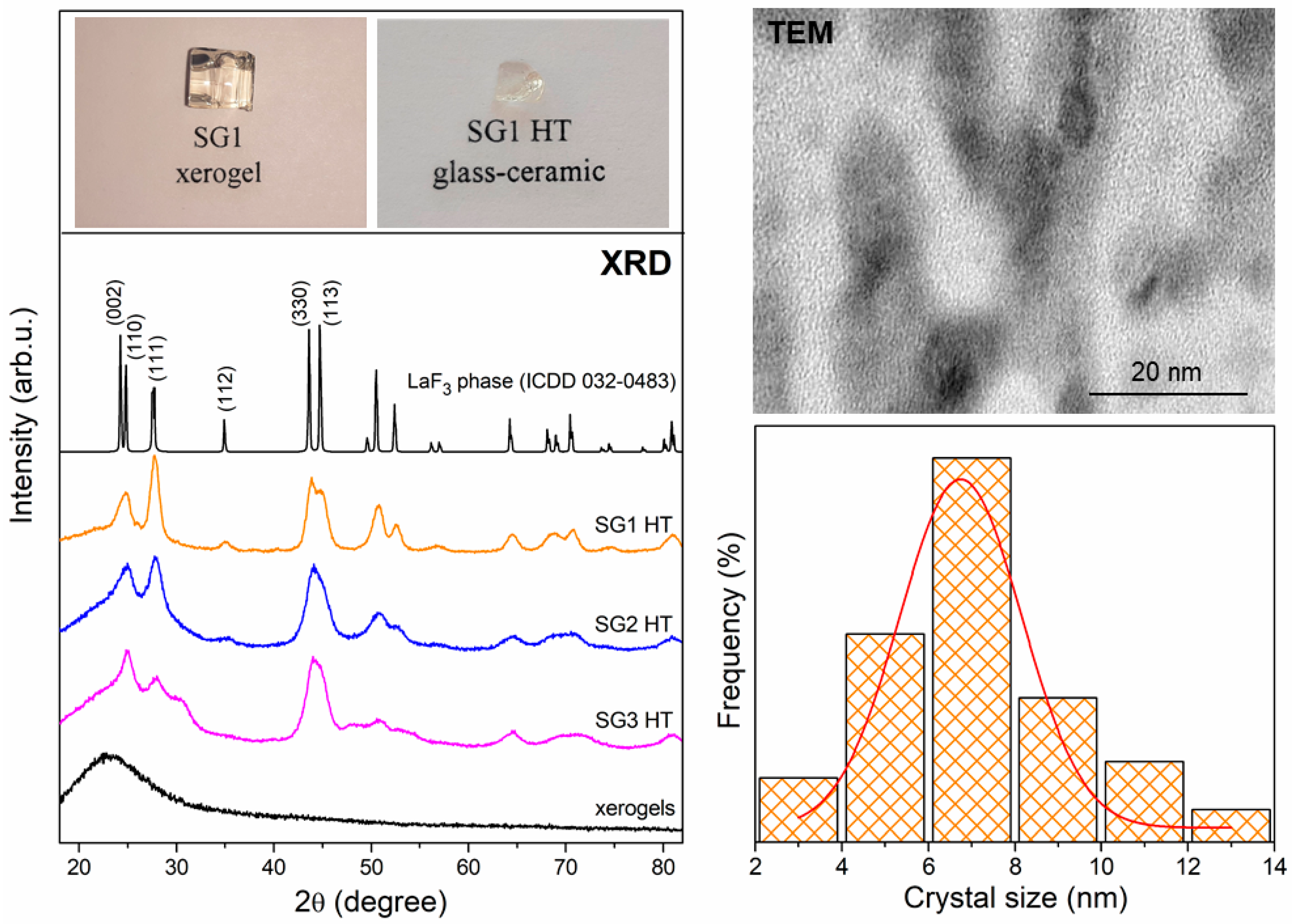
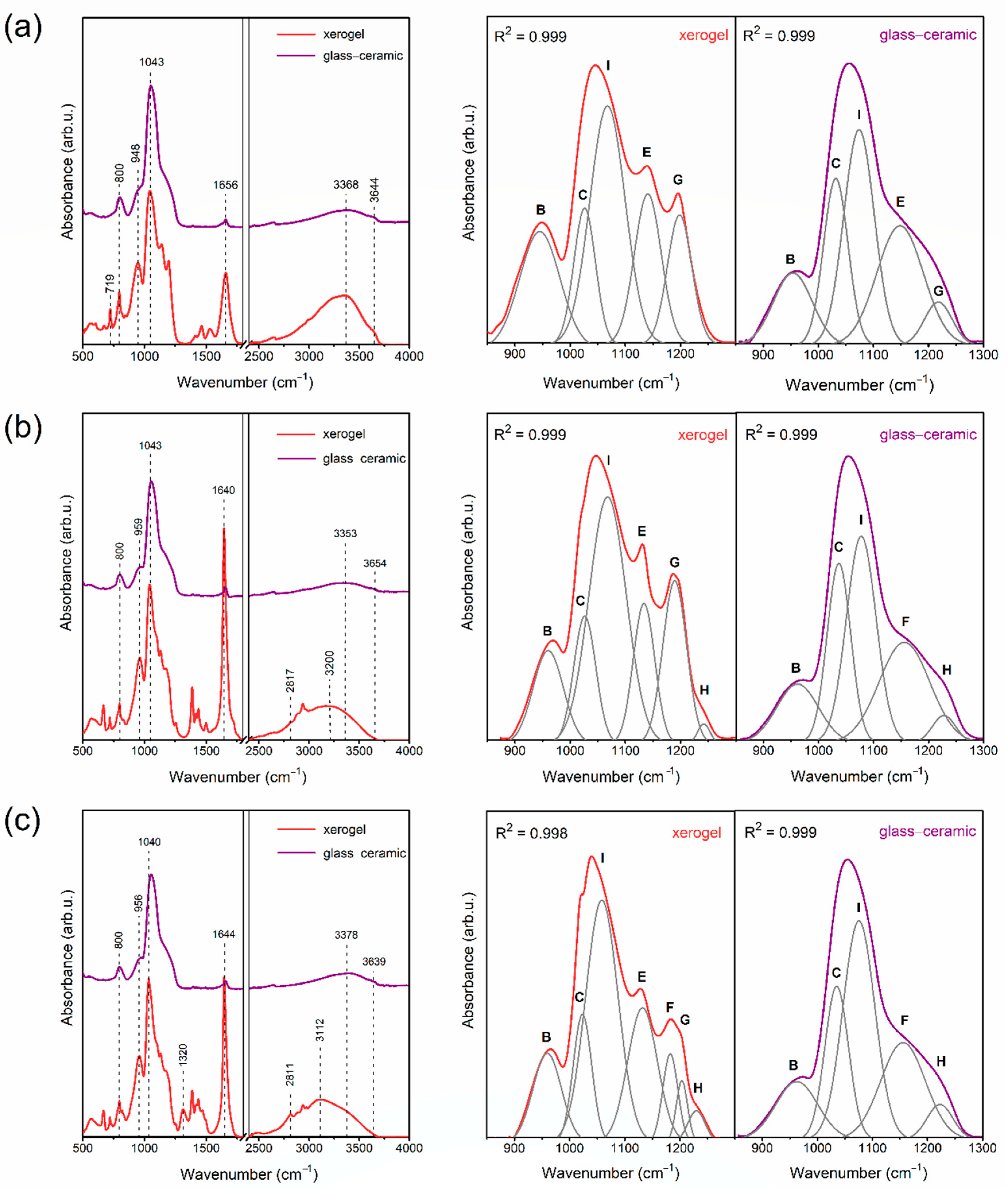

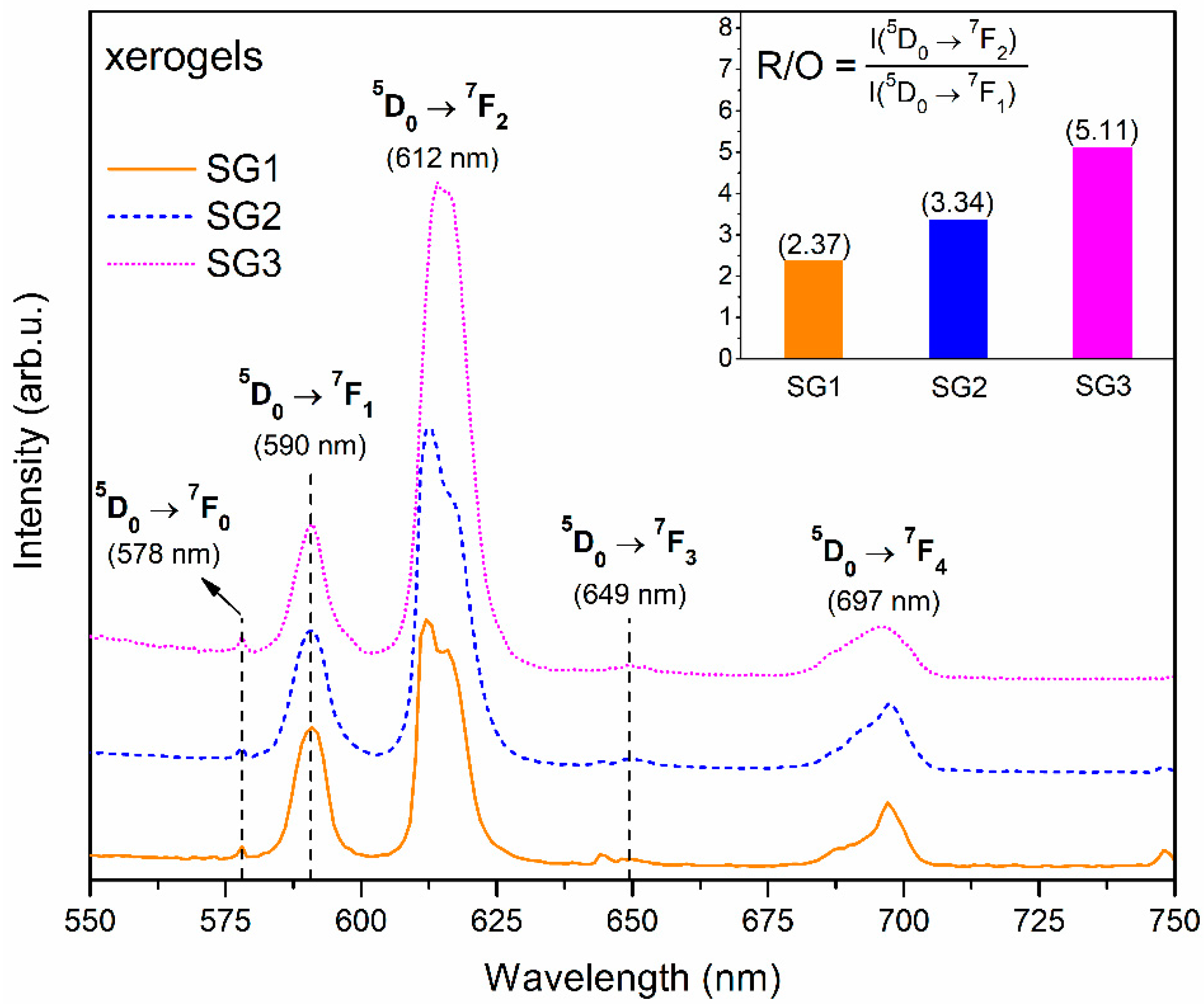

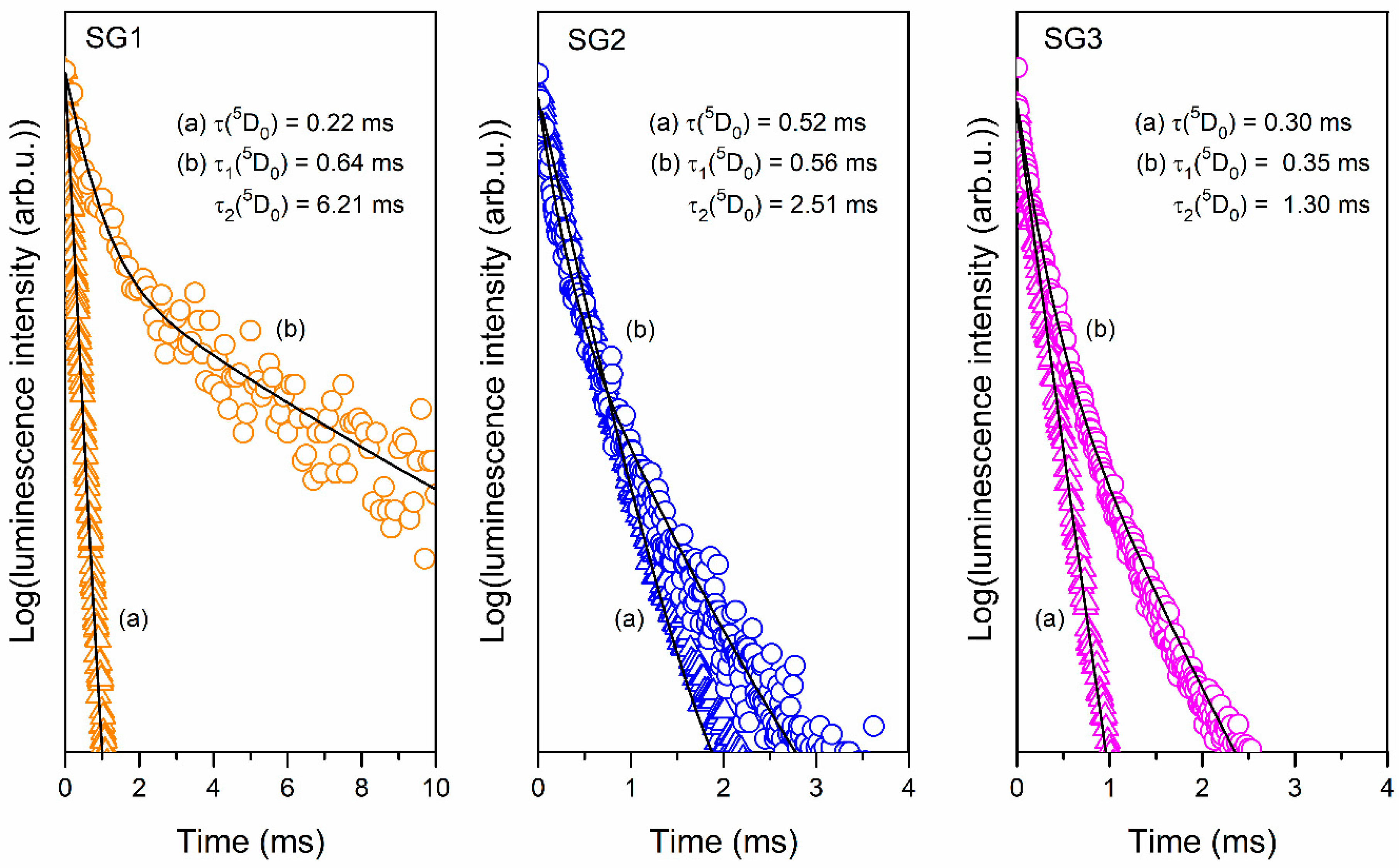
| IR Signal (cm−1) | Assignment |
|---|---|
| 561 | four-fold siloxane rings |
| 616 | six-fold siloxane rings |
| 719 | Si–O bond |
| 800 | TO2 mode of Si–O–Si siloxane bridges |
| 878 * (A) | two-fold rings, CH3CH2– groups, C=O groups |
| 958 * (B) | SiO4 tetrahedrons in Q2 units |
| 1043 * (C) | SiO4 tetrahedrons in Q3 units |
| 1065, 1074 * (I) | TO3 mode of Si–O–Si siloxane bridges |
| 1085 * (D) | non-hydrolyzed Si–O–C2H5 groups |
| 1144 * (E) | SiO4 tetrahedrons in Q4 units, C–F bond |
| 1186 * (F) | LO4 mode of Si–O–Si siloxane bridges |
| 1198, 1203 * (G) | TO4 mode of Si–O–Si siloxane bridges, C–F bond |
| 1255 * (H) | N–C bond, LO3 mode of Si–O–Si siloxane bridges |
| 1274 * (J) | CH3CH2– groups from ethyl alcohol |
| 1320 | NO3− anion |
| 1390, 1460, 2898, 2910, 2980 | C–H bond |
| 1648 | C=O groups, Si–OH groups, OH groups (from adsorbed water molecules) |
| 1712 | C=O groups |
| 2812 | C–N stretching |
| 3230 | hydrogen bonded OH moieties from residual water and organic compounds |
| 3398 | hydrogen bonded Si–OH groups |
| 3668 | non-hydrogen bonded surface Si–OH groups |
| Sample | Composition (in Molar Ratio) |
|---|---|
| SG1 | TEOS:EtOH:H2O:AcOH = 1:4:10:0.5 (90 wt.%) |
| TFA:La(CH3COO)3:Eu(CH3COO)3 = 5:1:0.05 (10 wt.%) | |
| SG2 | TEOS:EtOH:DMF:H2O:AcOH = 1:2:2:4:0.5 (90 wt.%) |
| TFA:La(CH3COO)3:Eu(CH3COO)3 = 5:1:0.05 (10 wt.%) | |
| SG3 | TEOS:EtOH:DMF:H2O:HNO3 = 1:2:2:4:0.4 (90 wt.%) |
| TFA:La(CH3COO)3:Eu(CH3COO)3 = 5:1:0.05 (10 wt.%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlik, N.; Szpikowska-Sroka, B.; Goryczka, T.; Pisarski, W.A. Studies of Sol-Gel Evolution and Distribution of Eu3+ Ions in Glass–Ceramics Containing LaF3 Nanocrystals Depending on Initial Sols Composition. Int. J. Mol. Sci. 2021, 22, 996. https://doi.org/10.3390/ijms22030996
Pawlik N, Szpikowska-Sroka B, Goryczka T, Pisarski WA. Studies of Sol-Gel Evolution and Distribution of Eu3+ Ions in Glass–Ceramics Containing LaF3 Nanocrystals Depending on Initial Sols Composition. International Journal of Molecular Sciences. 2021; 22(3):996. https://doi.org/10.3390/ijms22030996
Chicago/Turabian StylePawlik, Natalia, Barbara Szpikowska-Sroka, Tomasz Goryczka, and Wojciech A. Pisarski. 2021. "Studies of Sol-Gel Evolution and Distribution of Eu3+ Ions in Glass–Ceramics Containing LaF3 Nanocrystals Depending on Initial Sols Composition" International Journal of Molecular Sciences 22, no. 3: 996. https://doi.org/10.3390/ijms22030996
APA StylePawlik, N., Szpikowska-Sroka, B., Goryczka, T., & Pisarski, W. A. (2021). Studies of Sol-Gel Evolution and Distribution of Eu3+ Ions in Glass–Ceramics Containing LaF3 Nanocrystals Depending on Initial Sols Composition. International Journal of Molecular Sciences, 22(3), 996. https://doi.org/10.3390/ijms22030996







