Engineering and Assessing Cardiac Tissue Complexity
Abstract
1. Introduction
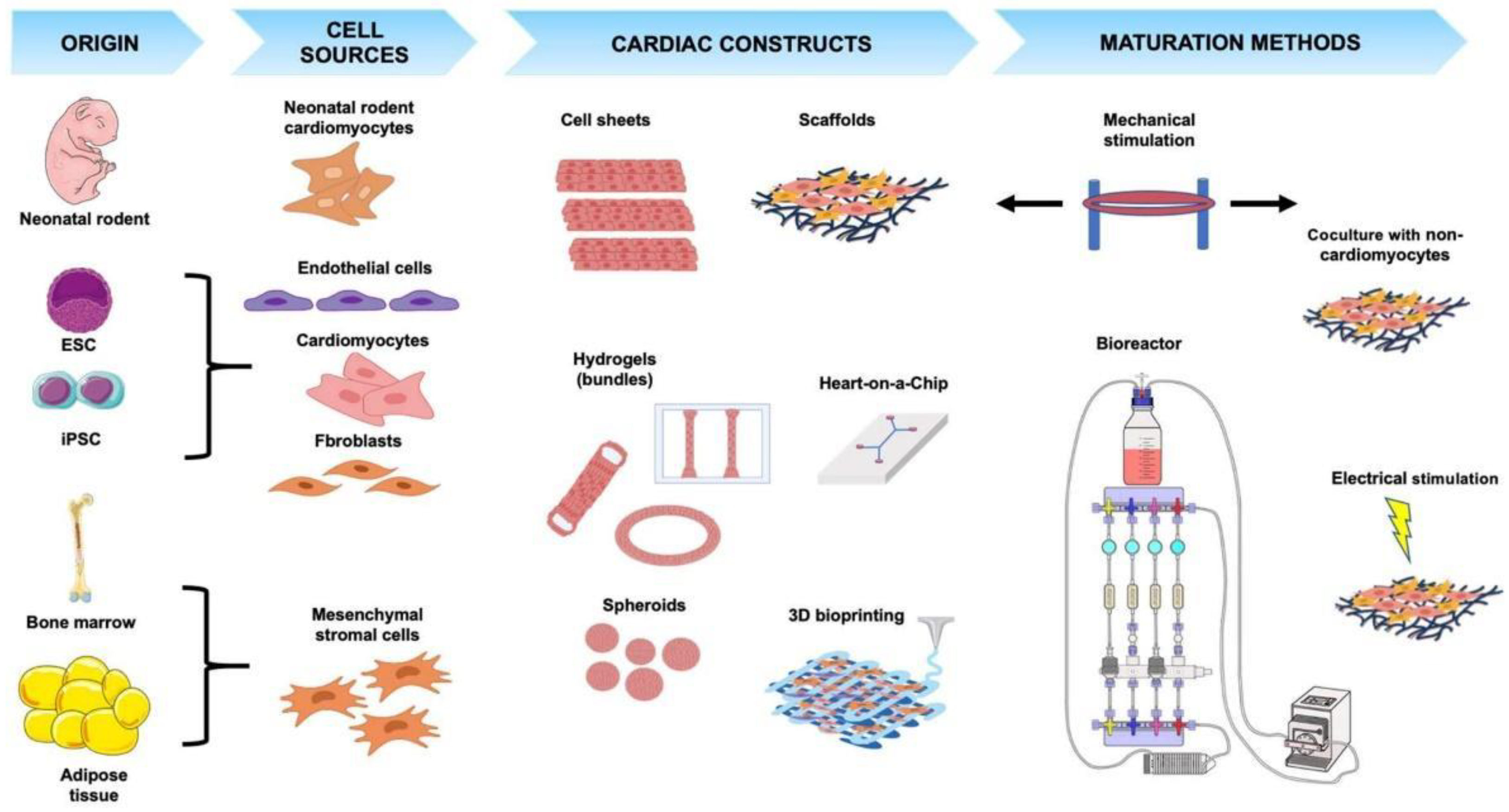
2. Cell Sources
2.1. Neonatal Rodent Myocytes
2.2. Pluripotent Stem Cells
2.3. Mesenchymal Stromal Cells
2.4. Non-Myocytes
3. Cardiac Tissue Engineering Systems
3.1. Cell Sheets
3.2. Scaffolds
3.3. Hydrogels
3.4. Cardiac Spheroids (and ‘Organoids’)
3.5. Heart-on-a-Chip
3.6. 3D Bioprinting
3.7. Other Structures
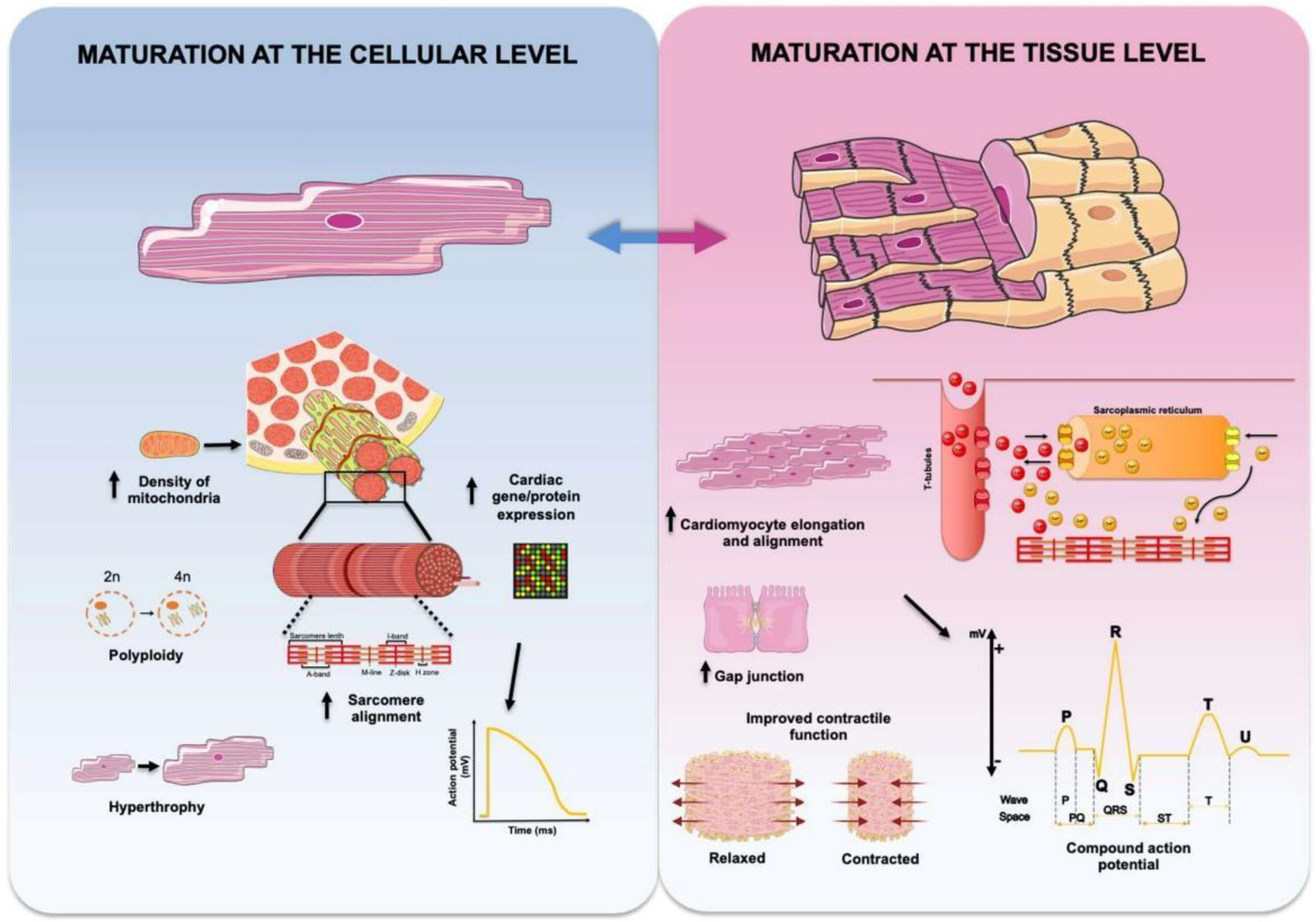
4. Maturation of Engineered Cardiac Tissues
4.1. Mechanical Stimulation
4.2. Electrical Stimulation
4.3. Coculture with Non-CMs
5. Functional Assessment of Engineered Cardiac Constructs
5.1. Patch-Clamp and Microelectrodes
5.2. Multielectrode Arrays
5.3. Optical Mapping
5.4. Force Transducers
6. Summary and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Després, J.-P.; Fullerton, H.J.; et al. Heart Disease and Stroke Statistics-2016 Update: A Report from the American Heart Association. Circulation 2016, 133, e38–e360. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, O.; Zdunek, S.; Felker, A.; Salehpour, M.; Alkass, K.; Bernard, S.; Sjostrom, S.L.; Szewczykowska, M.; Jackowska, T.; dos Remedios, C.; et al. Dynamics of Cell Generation and Turnover in the Human Heart. Cell 2015, 161, 1566–1575. [Google Scholar] [CrossRef] [PubMed]
- See, F.; Kompa, A.; Martin, J.; Lewis, D.; Krum, H. Fibrosis as a Therapeutic Target Post-Myocardial Infarction. Curr. Pharm. Design 2005, 11, 477–487. [Google Scholar] [CrossRef]
- Yacoub, M. Cardiac Donation after Circulatory Death: A Time to Reflect. Lancet 2015, 385, 2554–2556. [Google Scholar] [CrossRef]
- Müller, P.; Lemcke, H.; David, R. Stem Cell Therapy in Heart Diseases–Cell Types, Mechanisms and Improvement Strategies. Cell. Physiol. Biochem. 2018, 48, 2607–2655. [Google Scholar] [CrossRef]
- Menasché, P. Cell Therapy Trials for Heart Regeneration-Lessons Learned and Future Directions. Nat. Rev. Cardiol. 2018, 15, 659–671. [Google Scholar] [CrossRef]
- Mirotsou, M.; Jayawardena, T.M.; Schmeckpeper, J.; Gnecchi, M.; Dzau, V.J. Paracrine Mechanisms of Stem Cell Reparative and Regenerative Actions in The Heart. J. Mol. Cell. Cardiol. 2011, 50, 280–289. [Google Scholar] [CrossRef]
- Nguyen, P.K.; Rhee, J.-W.; Wu, J.C. Adult Stem Cell Therapy and Heart Failure, 2000 to 2016: A Systematic Review. JAMA Cardiol 2016, 1, 831–841. [Google Scholar] [CrossRef]
- Jackman, C.; Li, H.; Bursac, N. Long-Term Contractile Activity and Thyroid Hormone Supplementation Produce Engineered Rat Myocardium with Adult-Like Structure and Function. Acta Biomater. 2018, 78, 98–110. [Google Scholar] [CrossRef]
- Shadrin, I.Y.; Allen, B.W.; Qian, Y.; Jackman, C.P.; Carlson, A.L.; Juhas, M.E.; Bursac, N. Cardiopatch Platform Enables Maturation and Scale-Up of Human Pluripotent Stem Cell-Derived Engineered Heart Tissues. Nat. Commun. 2017, 8, 1825. [Google Scholar] [CrossRef] [PubMed]
- Benam, K.H.; Dauth, S.; Hassell, B.; Herland, A.; Jain, A.; Jang, K.-J.; Karalis, K.; Kim, H.J.; MacQueen, L.; Mahmoodian, R.; et al. Engineered In Vitro Disease Models. Annu. Rev. Pathol. Mech. Dis. 2015, 10, 195–262. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.H.; Shin, S.R.; Deddens, J.C.; Fratta, G.; Mandla, S.; Yazdi, I.K.; Prakash, G.; Antona, S.; Demarchi, D.; Buijsrogge, M.P.; et al. Engineered 3D Cardiac Fibrotic Tissue to Study Fibrotic Remodeling. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, I.C.; Mayourian, J.; Murphy, J.F.; Stillitano, F.; Ceholski, D.K.; Costa, K.D. Cardiac Tissue Engineering Models of Inherited and Acquired Cardiomyopathies. Methods Mol. Biol. 2018, 1816, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; McCain, M.L.; Yang, L.; He, A.; Pasqualini, F.S.; Agarwal, A.; Yuan, H.; Jiang, D.; Zhang, D.; Zangi, L.; et al. Modeling the Mitochondrial Cardiomyopathy of Barth Syndrome with Induced Pluripotent Stem Cell and Heart-On-Chip Technologies. Nat. Med. 2014, 20, 616–623. [Google Scholar] [CrossRef]
- Hansen, A.; Eder, A.; Bönstrup, M.; Flato, M.; Mewe, M.; Schaaf, S.; Aksehirlioglu, B.; Schwörer, A.; Uebeler, J.; Eschenhagen, T. Development of a Drug Screening Platform Based on Engineered Heart Tissue. Circ. Res. 2010, 107, 35–44. [Google Scholar] [CrossRef]
- Veldhuizen, J.; Migrino, R.Q.; Nikkhah, M. Three-Dimensional Microengineered Models of Human Cardiac Diseases. J. Biol. Eng. 2019, 13, 29. [Google Scholar] [CrossRef]
- Hulsmans, M.; Clauss, S.; Xiao, L.; Aguirre, A.D.; King, K.R.; Hanley, A.; Hucker, W.J.; Wülfers, E.M.; Seemann, G.; Courties, G.; et al. Macrophages Facilitate Electrical Conduction in the Heart. Cell 2017, 169, 510–522.e20. [Google Scholar] [CrossRef]
- Pinto, A.R.; Ilinykh, A.; Ivey, M.J.; Kuwabara, J.T.; D’Antoni, M.L.; Debuque, R.; Chandran, A.; Wang, L.; Arora, K.; Rosenthal, N.A.; et al. Revisiting Cardiac Cellular Composition. Circ. Res. 2016, 118, 400–409. [Google Scholar] [CrossRef]
- Zhou, P.; Pu, W.T. Recounting Cardiac Cellular Composition. Circ. Res. 2016, 118, 368–370. [Google Scholar] [CrossRef]
- Carrier, R.L.; Rupnick, M.; Langer, R.; Schoen, F.J.; Freed, L.E.; Vunjak-Novakovic, G. Perfusion Improves Tissue Architecture of Engineered Cardiac Muscle. Tissue Eng. 2002, 8, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Radisic, M.; Yang, L.; Boublik, J.; Cohen, R.J.; Langer, R.; Freed, L.E.; Vunjak-Novakovic, G. Medium Perfusion Enables Engineering of Compact and Contractile Cardiac Tissue. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H507–H516. [Google Scholar] [CrossRef] [PubMed]
- Radisic, M.; Park, H.; Shing, H.; Consi, T.; Schoen, F.J.; Langer, R.; Freed, L.E.; Vunjak-Novakovic, G. Functional Assembly of Engineered Myocardium by Electrical Stimulation of Cardiac Myocytes Cultured on Scaffolds. Proc. Natl. Acad. Sci. USA 2004, 101, 18129–18134. [Google Scholar] [CrossRef] [PubMed]
- Radisic, M.; Marsano, A.; Maidhof, R.; Wang, Y.; Vunjak-Novakovic, G. Cardiac Tissue Engineering Using Perfusion Bioreactor Systems. Nat. Protoc. 2008, 3, 719–738. [Google Scholar] [CrossRef] [PubMed]
- Tandon, N.; Cannizzaro, C.; Chao, P.-H.G.; Maidhof, R.; Marsano, A.; Au, H.T.H.; Radisic, M.; Vunjak-Novakovic, G. Electrical Stimulation Systems for Cardiac Tissue Engineering. Nat. Protoc. 2009, 4, 155–173. [Google Scholar] [CrossRef] [PubMed]
- Barash, Y.; Dvir, T.; Tandeitnik, P.; Ruvinov, E.; Guterman, H.; Cohen, S. Electric Field Stimulation Integrated into Perfusion Bioreactor for Cardiac Tissue Engineering. Tissue Eng. Part C Methods 2010, 16, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
- Maidhof, R.; Tandon, N.; Lee, E.J.; Luo, J.; Duan, Y.; Yeager, K.; Konofagou, E.; Vunjak-Novakovic, G. Biomimetic Perfusion and Electrical Stimulation Applied in Concert Improved the Assembly of Engineered Cardiac Tissue. J. Tissue Eng. Regen. Med. 2012, 6, e12–e23. [Google Scholar] [CrossRef] [PubMed]
- Shachar, M.; Benishti, N.; Cohen, S. Effects of Mechanical Stimulation Induced by Compression and Medium Perfusion on Cardiac Tissue Engineering. Biotechnol. Prog. 2012, 28, 1551–1559. [Google Scholar] [CrossRef]
- Fleischer, S.; Shapira, A.; Feiner, R.; Dvir, T. Modular Assembly of Thick Multifunctional Cardiac Patches. Proc. Natl. Acad. Sci. USA 2017, 114, 1898–1903. [Google Scholar] [CrossRef]
- Riegler, J.; Tiburcy, M.; Ebert, A.; Tzatzalos, E.; Raaz, U.; Abilez, O.J.; Shen, Q.; Kooreman, N.G.; Neofytou, E.; Chen, V.C.; et al. Human Engineered Heart Muscles Engraft and Survive Long Term in a Rodent Myocardial Infarction Model. Circ. Res. 2015, 117, 720–730. [Google Scholar] [CrossRef]
- Mihic, A.; Li, J.; Miyagi, Y.; Gagliardi, M.; Li, S.-H.; Zu, J.; Weisel, R.D.; Keller, G.; Li, R.-K. The Effect of Cyclic Stretch on Maturation and 3D Tissue Formation of Human Embryonic Stem Cell-Derived Cardiomyocytes. Biomaterials 2014, 35, 2798–2808. [Google Scholar] [CrossRef] [PubMed]
- Valls-Margarit, M.; Iglesias-García, O.; Di Guglielmo, C.; Sarlabous, L.; Tadevosyan, K.; Paoli, R.; Comelles, J.; Blanco-Almazán, D.; Jiménez-Delgado, S.; Castillo-Fernández, O.; et al. Engineered Macroscale Cardiac Constructs Elicit Human Myocardial Tissue-like Functionality. Stem Cell Rep. 2019, 13, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, W.-H.; Didié, M.; Wasmeier, G.H.; Nixdorff, U.; Hess, A.; Melnychenko, I.; Boy, O.; Neuhuber, W.L.; Weyand, M.; Eschenhagen, T. Cardiac Grafting of Engineered Heart Tissue in Syngenic Rats. Circulation 2002, 106, I151–I157. [Google Scholar] [PubMed]
- Kensah, G.; Gruh, I.; Viering, J.; Schumann, H.; Dahlmann, J.; Meyer, H.; Skvorc, D.; Bär, A.; Akhyari, P.; Heisterkamp, A.; et al. A Novel Miniaturized Multimodal Bioreactor for Continuous In Situ Assessment of Bioartificial Cardiac Tissue During Stimulation and Maturation. Tissue Eng. Part C Methods 2011, 17, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Godier-Furnémont, A.F.G.; Tiburcy, M.; Wagner, E.; Dewenter, M.; Lämmle, S.; El-Armouche, A.; Lehnart, S.E.; Vunjak-Novakovic, G.; Zimmermann, W.-H. Physiologic Force-Frequency Response in Engineered Heart Muscle by Electromechanical Stimulation. Biomaterials 2015, 60, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Tulloch, N.L.; Muskheli, V.; Razumova, M.V.; Korte, F.S.; Regnier, M.; Hauch, K.D.; Pabon, L.; Reinecke, H.; Murry, C.E. Growth of Engineered Human Myocardium with Mechanical Loading and Vascular Coculture. Circ. Res. 2011, 109, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, S.; Shibamiya, A.; Mewe, M.; Eder, A.; Stöhr, A.; Hirt, M.N.; Rau, T.; Zimmermann, W.-H.; Conradi, L.; Eschenhagen, T.; et al. Human Engineered Heart Tissue as a Versatile Tool in Basic Research and Preclinical Toxicology. PLoS ONE 2011, 6, e26397. [Google Scholar] [CrossRef]
- Thavandiran, N.; Dubois, N.; Mikryukov, A.; Massé, S.; Beca, B.; Simmons, C.A.; Deshpande, V.S.; McGarry, J.P.; Chen, C.S.; Nanthakumar, K.; et al. Design and Formulation of Functional Pluripotent Stem Cell-Derived Cardiac Microtissues. Proc. Natl. Acad. Sci. USA 2013, 110, E4698–E4707. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Q.; Gablaski, B.; Zhang, X.; Lucchesi, P.; Zhao, Y. A Microdevice for Studying Intercellular Electromechanical Transduction in Adult Cardiac Myocytes. Lab Chip 2013, 13, 3090–3097. [Google Scholar] [CrossRef][Green Version]
- Nunes, S.S.; Miklas, J.W.; Liu, J.; Aschar-Sobbi, R.; Xiao, Y.; Zhang, B.; Jiang, J.; Massé, S.; Gagliardi, M.; Hsieh, A.; et al. Biowire: A Platform for Maturation of Human Pluripotent Stem Cell–Derived Cardiomyocytes. Nat. Methods 2013, 10, 781–787. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, B.; Liu, H.; Miklas, J.W.; Gagliardi, M.; Pahnke, A.; Thavandiran, N.; Sun, Y.; Simmons, C.; Keller, G.; et al. Microfabricated Perfusable Cardiac Biowire: A Platform that Mimics Native Cardiac Bundle. Lab Chip 2014, 14, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, I.C.; Karakikes, I.; Serrao, G.W.; Backeris, P.; Lee, J.-J.; Xie, C.; Senyei, G.; Gordon, R.E.; Li, R.A.; Akar, F.G.; et al. Advancing Functional Engineered Cardiac Tissues Toward a Preclinical Model of Human Myocardium. FASEB J. 2014, 28, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Koo, S.; Finnegan, M.A.; Loskill, P.; Huebsch, N.; Marks, N.C.; Conklin, B.R.; Grigoropoulos, C.P.; Healy, K.E. Three-Dimensional Filamentous Human Diseased Cardiac Tissue Model. Biomaterials 2014, 35, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Wang, J.; Loskill, P.; Huebsch, N.; Koo, S.; Svedlund, F.L.; Marks, N.C.; Hua, E.W.; Grigoropoulos, C.P.; Conklin, B.R.; et al. Self-Organizing Human Cardiac Microchambers Mediated by Geometric Confinement. Nat. Commun. 2015, 6, 7413. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, F.; Breckwoldt, K.; Pecha, S.; Kelly, A.; Geertz, B.; Starbatty, J.; Yorgan, T.; Cheng, K.-H.; Lessmann, K.; Stolen, T.; et al. Cardiac Repair in Guinea Pigs with Human Engineered Heart Tissue from Induced Pluripotent Stem Cells. Sci. Transl. Med. 2016, 8, 363ra148. [Google Scholar] [CrossRef]
- Mannhardt, I.; Breckwoldt, K.; Letuffe-Brenière, D.; Schaaf, S.; Schulz, H.; Neuber, C.; Benzin, A.; Werner, T.; Eder, A.; Schulze, T.; et al. Human Engineered Heart Tissue: Analysis of Contractile Force. Stem Cell Rep. 2016, 7, 29–42. [Google Scholar] [CrossRef]
- Ruan, J.-L.; Tulloch, N.L.; Razumova, M.V.; Saiget, M.; Muskheli, V.; Pabon, L.; Reinecke, H.; Regnier, M.; Murry, C.E. Mechanical Stress Conditioning and Electrical Stimulation Promote Contractility and Force Maturation of Induced Pluripotent Stem Cell-Derived Human Cardiac Tissue. Circulation 2016, 134, 1557–1567. [Google Scholar] [CrossRef]
- Jackman, C.P.; Carlson, A.L.; Bursac, N. Dynamic Culture Yields Engineered Myocardium with Near-Adult Functional Output. Biomaterials 2016, 111, 66–79. [Google Scholar] [CrossRef]
- Lemoine, M.D.; Mannhardt, I.; Breckwoldt, K.; Prondzynski, M.; Flenner, F.; Ulmer, B.; Hirt, M.N.; Neuber, C.; Horváth, A.; Kloth, B.; et al. Human iPSC-Derived Cardiomyocytes Cultured in 3D Engineered Heart Tissue Show Physiological Upstroke Velocity and Sodium Current Density. Sci. Rep. 2017, 7, 5464. [Google Scholar] [CrossRef]
- Nakane, T.; Masumoto, H.; Tinney, J.P.; Yuan, F.; Kowalski, W.J.; Ye, F.; LeBlanc, A.J.; Sakata, R.; Yamashita, J.K.; Keller, B.B. Impact of Cell Composition and Geometry on Human Induced Pluripotent Stem Cells-Derived Engineered Cardiac Tissue. Sci. Rep. 2017, 7, 45641. [Google Scholar] [CrossRef]
- Tiburcy, M.; Hudson, J.E.; Balfanz, P.; Schlick, S.; Meyer, T.; Chang Liao, M.-L.; Levent, E.; Raad, F.; Zeidler, S.; Wingender, E.; et al. Defined Engineered Human Myocardium with Advanced Maturation for Applications in Heart Failure Modeling and Repair. Circulation 2017, 135, 1832–1847. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, M.; Ahadian, S.; Davenport Huyer, L.; Lo Rito, M.; Civitarese, R.A.; Vanderlaan, R.D.; Wu, J.; Reis, L.A.; Momen, A.; Akbari, S.; et al. Flexible Shape-Memory Scaffold for Minimally Invasive Delivery of Functional Tissues. Nat. Mater. 2017, 16, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Gregorich, Z.R.; Zhu, W.; Mattapally, S.; Oduk, Y.; Lou, X.; Kannappan, R.; Borovjagin, A.V.; Walcott, G.P.; Pollard, A.E.; et al. Large Cardiac Muscle Patches Engineered from Human Induced-Pluripotent Stem Cell-Derived Cardiac Cells Improve Recovery from Myocardial Infarction in Swine. Circulation 2018, 137, 1712–1730. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Rafatian, N.; Feric, N.T.; Cox, B.J.; Aschar-Sobbi, R.; Wang, E.Y.; Aggarwal, P.; Zhang, B.; Conant, G.; Ronaldson-Bouchard, K.; et al. A Platform for Generation of Chamber-Specific Cardiac Tissues and Disease Modeling. Cell 2019, 176, 913–927.e18. [Google Scholar] [CrossRef] [PubMed]
- Ronaldson-Bouchard, K.; Ma, S.P.; Yeager, K.; Chen, T.; Song, L.; Sirabella, D.; Morikawa, K.; Teles, D.; Yazawa, M.; Vunjak-Novakovic, G. Author Correction: Advanced Maturation of Human Cardiac Tissue Grown from Pluripotent Stem Cells. Nature 2019, 572, E16–E17. [Google Scholar] [CrossRef] [PubMed]
- Noor, N.; Shapira, A.; Edri, R.; Gal, I.; Wertheim, L.; Dvir, T. 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Adv. Sci. 2019, 6, 1900344. [Google Scholar] [CrossRef]
- Shimizu, T.; Yamato, M.; Isoi, Y.; Akutsu, T.; Setomaru, T.; Abe, K.; Kikuchi, A.; Umezu, M.; Okano, T. Fabrication of Pulsatile Cardiac Tissue Grafts Using a Novel 3-Dimensional Cell Sheet Manipulation Technique and Temperature-Responsive Cell Culture Surfaces. Circ. Res. 2002, 90, e40. [Google Scholar] [CrossRef]
- Sekine, H.; Shimizu, T.; Sakaguchi, K.; Dobashi, I.; Wada, M.; Yamato, M.; Kobayashi, E.; Umezu, M.; Okano, T. In Vitro Fabrication of Functional Three-Dimensional Tissues with Perfusable Blood Vessels. Nat. Commun. 2013, 4, 1399. [Google Scholar] [CrossRef]
- Kawatou, M.; Masumoto, H.; Fukushima, H.; Morinaga, G.; Sakata, R.; Ashihara, T.; Yamashita, J.K. Modelling Torsade de Pointes Arrhythmias In Vitro in 3D Human iPS Cell-Engineered Heart Tissue. Nat. Commun. 2017, 8, 1078. [Google Scholar] [CrossRef]
- Zimmermann, W.H.; Fink, C.; Kralisch, D.; Remmers, U.; Weil, J.; Eschenhagen, T. Three-Dimensional Engineered Heart Tissue from Neonatal Rat Cardiac Myocytes. Biotechnol. Bioeng. 2000, 68, 106–114. [Google Scholar] [CrossRef]
- Akins, R.E.; Boyce, R.A.; Madonna, M.L.; Schroedl, N.A.; Gonda, S.R.; McLaughlin, T.A.; Hartzell, C.R. Cardiac Organogenesis In Vitro: Reestablishment of Three-Dimensional Tissue Architecture by Dissociated Neonatal Rat Ventricular Cells. Tissue Eng. 1999, 5, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Carrier, R.L.; Papadaki, M.; Rupnick, M.; Schoen, F.J.; Bursac, N.; Langer, R.; Freed, L.E.; Vunjak-Novakovic, G. Cardiac Tissue Engineering: Cell Seeding, Cultivation Parameters, and Tissue Construct Characterization. Biotechnol. Bioeng. 1999, 64, 580–589. [Google Scholar] [CrossRef]
- Yang, L.; Soonpaa, M.H.; Adler, E.D.; Roepke, T.K.; Kattman, S.J.; Kennedy, M.; Henckaerts, E.; Bonham, K.; Abbott, G.W.; Linden, R.M.; et al. Human Cardiovascular Progenitor Cells Develop from A KDR+ Embryonic-Stem-Cell-Derived Population. Nature 2008, 453, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Hsiao, C.; Wilson, G.; Zhu, K.; Hazeltine, L.B.; Azarin, S.M.; Raval, K.K.; Zhang, J.; Kamp, T.J.; Palecek, S.P. Robust Cardiomyocyte Differentiation from Human Pluripotent Stem Cells Via Temporal Modulation of Canonical Wnt Signaling. Proc. Natl. Acad. Sci. USA 2012, 109, E1848–E1857. [Google Scholar] [CrossRef] [PubMed]
- Burridge, P.W.; Holmström, A.; Wu, J.C. Chemically Defined Culture and Cardiomyocyte Differentiation of Human Pluripotent Stem Cells. Curr. Protoc. Hum. Genet. 2015, 87, 21.3.1–21.3.15. [Google Scholar] [CrossRef] [PubMed]
- Louch, W.E.; Sheehan, K.A.; Wolska, B.M. Methods in Cardiomyocyte Isolation, Culture, and Gene Transfer. J. Mol. Cell. Cardiol. 2011, 51, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Wolska, B.M.; Solaro, R.J. Method for Isolation of Adult Mouse Cardiac Myocytes for Studies of Contraction and microfluorimetry. Am. J. Physiol. 1996, 271, H1250–H1255. [Google Scholar] [CrossRef]
- Zimmermann, W.-H.; Zimmermann, W.; Schneiderbanger, K.; Schubert, P.; Didié, M.; Münzel, F.; Heubach, J.F.; Kostin, S.; Neuhuber, W.L.; Eschenhagen, T. Tissue Engineering of a Differentiated Cardiac Muscle Construct. Circ. Res. 2002, 90, 223–230. [Google Scholar] [CrossRef]
- Bursac, N.; Papadaki, M.; Cohen, R.J.; Schoen, F.J.; Eisenberg, S.R.; Carrier, R.; Vunjak-Novakovic, G.; Freed, L.E. Cardiac Muscle Tissue Engineering: Toward an In Vitro Model for Electrophysiological Studies. Am. J. Physiol. Heart Circul. Physiol. 1999, 277, H433–H444. [Google Scholar] [CrossRef]
- Papadaki, M.; Bursac, N.; Langer, R.; Merok, J.; Vunjak-Novakovic, G.; Freed, L.E. Tissue Engineering of Functional Cardiac Muscle: Molecular, Structural, and Electrophysiological Studies. Am. J. Physiol. Heart Circul. Physiol. 2001, 280, H168–H178. [Google Scholar] [CrossRef]
- Bursac, N.; Papadaki, M.; White, J.A.; Eisenberg, S.R.; Vunjak-Novakovic, G.; Freed, L.E. Cultivation in Rotating Bioreactors Promotes Maintenance of Cardiac Myocyte Electrophysiology and Molecular Properties. Tissue Eng. 2003, 9, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Radisic, M.; Park, H.; Chen, F.; Salazar-Lazzaro, J.E.; Wang, Y.; Dennis, R.; Langer, R.; Freed, L.E.; Vunjak-Novakovic, G. Biomimetic Approach to Cardiac Tissue Engineering: Oxygen Carriers and Channeled Scaffolds. Tissue Eng. 2006, 12, 2077–2091. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Zhang, J.; Azarin, S.M.; Zhu, K.; Hazeltine, L.B.; Bao, X.; Hsiao, C.; Kamp, T.J.; Palecek, S.P. Directed Cardiomyocyte Differentiation from Human Pluripotent Stem Cells by Modulating Wnt/β-catenin Signaling Under Fully Defined Conditions. Nat. Protoc. 2013, 8, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Burridge, P.W.; Thompson, S.; Millrod, M.A.; Weinberg, S.; Yuan, X.; Peters, A.; Mahairaki, V.; Koliatsos, V.E.; Tung, L.; Zambidis, E.T. A Universal System for Highly Efficient Cardiac Differentiation of Human Induced Pluripotent Stem Cells that Eliminates Interline Variability. PLoS ONE 2011, 6, e18293. [Google Scholar] [CrossRef]
- Van Laake, L.W.; Passier, R.; Monshouwer-Kloots, J.; Verkleij, A.J.; Lips, D.J.; Freund, C.; den Ouden, K.; Ward-van Oostwaard, D.; Korving, J.; Tertoolen, L.G.; et al. Human Embryonic Stem Cell-Derived Cardiomyocytes Survive and Mature in the Mouse Heart and Transiently Improve Function after Myocardial Infarction. Stem Cell Res. 2007, 1, 9–24. [Google Scholar] [CrossRef]
- Laflamme, M.A.; Chen, K.Y.; Naumova, A.V.; Muskheli, V.; Fugate, J.A.; Dupras, S.K.; Reinecke, H.; Xu, C.; Hassanipour, M.; Police, S.; et al. Cardiomyocytes Derived from Human Embryonic Stem Cells in Pro-Survival Factors Enhance Function of Infarcted Rat Hearts. Nat. Biotechnol. 2007, 25, 1015–1024. [Google Scholar] [CrossRef]
- Caspi, O.; Huber, I.; Kehat, I.; Habib, M.; Arbel, G.; Gepstein, A.; Yankelson, L.; Aronson, D.; Beyar, R.; Gepstein, L. Transplantation of Human Embryonic Stem Cell-Derived Cardiomyocytes Improves Myocardial Performance in Infarcted Rat Hearts. J. Am. Coll. Cardiol. 2007, 50, 1884–1893. [Google Scholar] [CrossRef]
- Carpenter, L.; Carr, C.; Yang, C.T.; Stuckey, D.J.; Clarke, K.; Watt, S.M. Efficient Differentiation of Human Induced Pluripotent Stem Cells Generates Cardiac Cells that Provide Protection Following Myocardial Infarction in the Rat. Stem Cells Dev. 2012, 21, 977–986. [Google Scholar] [CrossRef]
- Shiba, Y.; Fernandes, S.; Zhu, W.-Z.; Filice, D.; Muskheli, V.; Kim, J.; Palpant, N.J.; Gantz, J.; Moyes, K.W.; Reinecke, H.; et al. Human ES-Cell-Derived Cardiomyocytes Electrically Couple and Suppress Arrhythmias in Injured Hearts. Nature 2012, 489, 322–325. [Google Scholar] [CrossRef]
- Templin, C.; Zweigerdt, R.; Schwanke, K.; Olmer, R.; Ghadri, J.-R.; Emmert, M.Y.; Müller, E.; Küest, S.M.; Cohrs, S.; Schibli, R.; et al. Transplantation and Tracking of Human-Induced Pluripotent Stem Cells in a Pig Model of Myocardial Infarction: Assessment of Cell Survival, Engraftment, and Distribution by Hybrid Single Photon Emission Computed Tomography/Computed Tomography of Sodium Iodide Symporter Transgene Expression. Circulation 2012, 126, 430–439. [Google Scholar]
- Chong, J.J.H.; Murry, C.E. Cardiac Regeneration Using Pluripotent Stem Cells--Progression to Large Animal Models. Stem Cell Res. 2014, 13, 654–665. [Google Scholar] [CrossRef] [PubMed]
- Shiba, Y.; Gomibuchi, T.; Seto, T.; Wada, Y.; Ichimura, H.; Tanaka, Y.; Ogasawara, T.; Okada, K.; Shiba, N.; Sakamoto, K.; et al. Allogeneic transplantation of iPS Cell-Derived Cardiomyocytes Regenerates Primate Hearts. Nature 2016, 538, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, M.; Miyagawa, S.; Miki, K.; Saito, A.; Fukushima, S.; Higuchi, T.; Kawamura, T.; Kuratani, T.; Daimon, T.; Shimizu, T.; et al. Feasibility, Safety, and Therapeutic Efficacy of Human Induced Pluripotent Stem Cell-Derived Cardiomyocyte Sheets in a porcine Ischemic Cardiomyopathy Model. Circulation 2012, 126, S29–S37. [Google Scholar] [CrossRef]
- Xiong, Q.; Ye, L.; Zhang, P.; Lepley, M.; Tian, J.; Li, J.; Zhang, L.; Swingen, C.; Vaughan, J.T.; Kaufman, D.S.; et al. Functional Consequences of human Induced Pluripotent Stem Cell Therapy: Myocardial ATP turnover Rate in the In Vivo Swine Heart with Postinfarction Remodeling. Circulation 2013, 127, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Chang, Y.-H.; Xiong, Q.; Zhang, P.; Zhang, L.; Somasundaram, P.; Lepley, M.; Swingen, C.; Su, L.; Wendel, J.S.; et al. Cardiac Repair in a Porcine Model of Acute Myocardial Infarction with Human Induced Pluripotent Stem Cell-Derived Cardiovascular Cells. Cell Stem Cell 2014, 15, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Menasché, P.; Vanneaux, V.; Fabreguettes, J.-R.; Bel, A.; Tosca, L.; Garcia, S.; Bellamy, V.; Farouz, Y.; Pouly, J.; Damour, O.; et al. Towards Clinical Use of Human Embryonic Stem Cell-Derived Cardiac Progenitors: A Translational Experience. Eur. Heart J. 2015, 36, 743–750. [Google Scholar] [PubMed]
- Menasché, P.; Vanneaux, V.; Hagège, A.; Bel, A.; Cholley, B.; Parouchev, A.; Cacciapuoti, I.; Al-Daccak, R.; Benhamouda, N.; Blons, H.; et al. Transplantation of Human Embryonic Stem Cell-Derived Cardiovascular Progenitors for Severe Ischemic Left Ventricular Dysfunction. J. Am. Coll. Cardiol. 2018, 71, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Ankrum, J.A.; Ong, J.F.; Karp, J.M. Mesenchymal Stem Cells: Immune Evasive, Not Immune privileged. Nat. Biotechnol. 2014, 32, 252–260. [Google Scholar] [CrossRef]
- Golpanian, S.; Wolf, A.; Hatzistergos, K.E.; Hare, J.M. Rebuilding the Damaged Heart: Mesenchymal Stem Cells, Cell-Based Therapy, and Engineered Heart Tissue. Physiol. Rev. 2016, 96, 1127–1168. [Google Scholar] [CrossRef]
- Williams, A.R.; Hare, J.M. Mesenchymal Stem Cells: Biology, Pathophysiology, Translational Findings, and Therapeutic Implications for Cardiac Disease. Circ. Res. 2011, 109, 923–940. [Google Scholar] [CrossRef]
- Valina, C.; Pinkernell, K.; Song, Y.-H.; Bai, X.; Sadat, S.; Campeau, R.J.; Le Jemtel, T.H.; Alt, E. Intracoronary Administration of Autologous Adipose Tissue-Derived Stem Cells Improves Left Ventricular Function, Perfusion, and Remodelling after Acute Myocardial Infarction. Eur. Heart J. 2007, 28, 2667–2677. [Google Scholar] [CrossRef] [PubMed]
- Schuleri, K.H.; Feigenbaum, G.S.; Centola, M.; Weiss, E.S.; Zimmet, J.M.; Turney, J.; Kellner, J.; Zviman, M.M.; Hatzistergos, K.E.; Detrick, B.; et al. Autologous Mesenchymal Stem Cells Produce Reverse Remodelling in Chronic Ischaemic Cardiomyopathy. Eur. Heart J. 2009, 30, 2722–2732. [Google Scholar] [CrossRef] [PubMed]
- Mazo, M.; Hernández, S.; Gavira, J.J.; Abizanda, G.; Araña, M.; López-Martínez, T.; Moreno, C.; Merino, J.; Martino-Rodríguez, A.; Uixeira, A.; et al. Treatment of Reperfused Ischemia with Adipose-Derived Stem Cells in a Preclinical Swine Model of Myocardial Infarction. Cell Transplant. 2012, 21, 2723–2733. [Google Scholar] [CrossRef] [PubMed]
- Hatzistergos, K.E.; Quevedo, H.; Oskouei, B.N.; Hu, Q.; Feigenbaum, G.S.; Margitich, I.S.; Mazhari, R.; Boyle, A.J.; Zambrano, J.P.; Rodriguez, J.E.; et al. Bone Marrow Mesenchymal Stem Cells Stimulate Cardiac Stem Cell Proliferation and Differentiation. Circ. Res. 2010, 107, 913–922. [Google Scholar] [CrossRef]
- Williams, A.R.; Suncion, V.Y.; McCall, F.; Guerra, D.; Mather, J.; Zambrano, J.P.; Heldman, A.W.; Hare, J.M. Durable Scar Size Reduction due to Allogeneic Mesenchymal Stem Cell Therapy Regulates Whole-Chamber Remodeling. J. Am. Heart Assoc. 2013, 2, e000140. [Google Scholar] [CrossRef]
- Liu, J.; Hu, Q.; Wang, Z.; Xu, C.; Wang, X.; Gong, G.; Mansoor, A.; Lee, J.; Hou, M.; Zeng, L.; et al. Autologous Stem Cell Transplantation for Myocardial Repair. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H501–H511. [Google Scholar] [CrossRef]
- Godier-Furnemont, A.F.G.; Martens, T.P.; Koeckert, M.S.; Wan, L.; Parks, J.; Arai, K.; Zhang, G.; Hudson, B.; Homma, S.; Vunjak-Novakovic, G. Composite Scaffold Provides a Cell Delivery Platform for Cardiovascular Repair. Proc. Natl. Acad. Sci. USA 2011, 108, 7974–7979. [Google Scholar] [CrossRef]
- Gilsbach, R.; Preissl, S.; Grüning, B.A.; Schnick, T.; Burger, L.; Benes, V.; Würch, A.; Bönisch, U.; Günther, S.; Backofen, R.; et al. Dynamic DNA Methylation Orchestrates Cardiomyocyte Development, Maturation and Disease. Nat. Commun. 2014, 5, 5288. [Google Scholar] [CrossRef]
- Liau, B.; Christoforou, N.; Leong, K.W.; Bursac, N. Pluripotent Stem Cell-Derived Cardiac Tissue Patch with Advanced Structure and Function. Biomaterials 2011, 32, 9180–9187. [Google Scholar] [CrossRef]
- Zhang, D.; Shadrin, I.Y.; Lam, J.; Xian, H.-Q.; Snodgrass, H.R.; Bursac, N. Tissue-engineered Cardiac Patch for Advanced Functional Maturation of Human ESC-Derived Cardiomyocytes. Biomaterials 2013, 34, 5813–5820. [Google Scholar] [CrossRef]
- Liau, B.; Jackman, C.P.; Li, Y.; Bursac, N. Developmental Stage-Dependent Effects of Cardiac Fibroblasts on Function of Stem Cell-Derived Engineered Cardiac Tissues. Sci. Rep. 2017, 7, 42290. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Liu, H.; Klein, M.; Ostrominski, J.; Hong, S.G.; Yada, R.C.; Chen, G.; Navarengom, K.; Schwartzbeck, R.; San, H.; et al. Efficient Differentiation of Cardiomyocytes and Generation of Calcium-Sensor Reporter Lines from Nonhuman Primate iPSCs. Sci. Rep. 2018, 8, 5907. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, W.-H.; Melnychenko, I.; Wasmeier, G.; Didié, M.; Naito, H.; Nixdorff, U.; Hess, A.; Budinsky, L.; Brune, K.; Michaelis, B.; et al. Engineered Heart Tissue Grafts Improve Systolic and Diastolic Function in Infarcted Rat Hearts. Nat. Med. 2006, 12, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, E.; Bellin, M.; Sala, L.; van Meer, B.J.; Tertoolen, L.G.J.; Orlova, V.V.; Mummery, C.L. Three-Dimensional Cardiac Microtissues Composed of Cardiomyocytes and Endothelial Cells Co-Differentiated from Human Pluripotent Stem Cells. Development 2017, 144, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Jackman, C.P.; Shadrin, I.Y.; Carlson, A.L.; Bursac, N. Human Cardiac Tissue Engineering: From Pluripotent Stem Cells to Heart Repair. Curr. Opin. Chem. Eng. 2015, 7, 57–64. [Google Scholar] [CrossRef]
- Yamato, M.; Okano, T. Cell sheet engineering. Mater. Today 2004, 7, 42–47. [Google Scholar] [CrossRef]
- Yang, J.; Yamato, M.; Kohno, C.; Nishimoto, A.; Sekine, H.; Fukai, F.; Okano, T. Cell Sheet Engineering: Recreating Tissues without Biodegradable Scaffolds. Biomaterials 2005, 26, 6415–6422. [Google Scholar] [CrossRef]
- Shimizu, T.; Yamato, M.; Kikuchi, A.; Okano, T. Cell Sheet Engineering for Myocardial Tissue Reconstruction. Biomaterials 2003, 24, 2309–2316. [Google Scholar] [CrossRef]
- Sekine, H.; Shimizu, T.; Dobashi, I.; Matsuura, K.; Hagiwara, N.; Takahashi, M.; Kobayashi, E.; Yamato, M.; Okano, T. Cardiac Cell Sheet Transplantation Improves Damaged Heart Function via Superior Cell Survival in Comparison with Dissociated Cell Injection. Tissue Eng. Part A 2011, 17, 2973–2980. [Google Scholar] [CrossRef]
- Sawa, Y.; Yoshikawa, Y.; Toda, K.; Fukushima, S.; Yamazaki, K.; Ono, M.; Sakata, Y.; Hagiwara, N.; Kinugawa, K.; Miyagawa, S. Safety and Efficacy of Autologous Skeletal Myoblast Sheets (TCD-51073) for the Treatment of Severe Chronic Heart Failure Due to Ischemic Heart Disease. Circ. J. 2015, 79, 991–999. [Google Scholar] [CrossRef]
- Yui, Y. Concerns on a New Therapy for Severe Heart Failure Using Cell Sheets with Skeletal Muscle or Myocardial Cells from iPS Cells in Japan. NPJ Regen. Med. 2018, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Pomeroy, J.E.; Helfer, A.; Bursac, N. Biomaterializing the Promise of Cardiac Tissue Engineering. Biotechnol. Adv. 2019. [Google Scholar] [CrossRef] [PubMed]
- Radisic, M.; Park, H.; Martens, T.P.; Salazar-Lazaro, J.E.; Geng, W.; Wang, Y.; Langer, R.; Freed, L.E.; Vunjak-Novakovic, G. Pre-treatment of Synthetic Elastomeric Scaffolds by Cardiac Fibroblasts Improves Engineered Heart Tissue. J. Biomed. Mater. Res. A 2008, 86, 713–724. [Google Scholar] [CrossRef]
- Zandonella, C. Tissue Engineering: The Beat Goes On. Nature 2003, 421, 884–886. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N.; Hassan, M.I.; Lim, M.M. Scaffolding Biomaterials. In Composite Synthetic Scaffolds for Tissue Engineering and Regenerative Medicine; Sultana, N., Hassan, M.I., Lim, M.M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–11. ISBN 9783319097558. [Google Scholar]
- Badrossamay, M.R.; McIlwee, H.A.; Goss, J.A.; Parker, K.K. Nanofiber Assembly by Rotary Jet-Spinning. Nano Lett. 2010, 10, 2257–2261. [Google Scholar] [CrossRef]
- Kenar, H.; Kose, G.T.; Toner, M.; Kaplan, D.L.; Hasirci, V. A 3D Aligned Microfibrous Myocardial Tissue Construct Cultured Under Transient Perfusion. Biomaterials 2011, 32, 5320–5329. [Google Scholar] [CrossRef]
- Bursac, N.; Loo, Y.; Leong, K.; Tung, L. Novel Anisotropic Engineered Cardiac Tissues: Studies of Electrical Propagation. Biochem. Biophys. Res. Commun. 2007, 361, 847–853. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & Scaffolds for Tissue Engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Gaetani, R.; Doevendans, P.A.; Metz, C.H.G.; Alblas, J.; Messina, E.; Giacomello, A.; Sluijter, J.P.G. Cardiac Tissue Engineering Using Tissue Printing Technology and Human Cardiac Progenitor Cells. Biomaterials 2012, 33, 1782–1790. [Google Scholar] [CrossRef]
- Liberski, A.; Latif, N.; Raynaud, C.; Bollensdorff, C.; Yacoub, M. Alginate for Cardiac Regeneration: From Seaweed to Clinical Trials. Glob. Cardiol. Sci. Pract. 2016, 2016, e201604. [Google Scholar] [CrossRef]
- Fleischer, S.; Shapira, A.; Regev, O.; Nseir, N.; Zussman, E.; Dvir, T. Albumin Fiber Scaffolds for Engineering Functional Cardiac Tissues. Biotechnol. Bioeng. 2014, 111, 1246–1257. [Google Scholar] [CrossRef] [PubMed]
- Bian, W.; Jackman, C.P.; Bursac, N. Controlling the Structural and Functional Anisotropy of Engineered Cardiac Tissues. Biofabrication 2014, 6, 024109. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, L.; Kikuiri, T.; Akiyama, K.; Chen, C.; Xu, X.; Yang, R.; Chen, W.; Wang, S.; Shi, S. Mesenchymal Stem Cell-Based Tissue Regeneration is Governed by Recipient T Lymphocytes via IFN-γ and TNF-α. Nat. Med. 2011, 17, 1594–1601. [Google Scholar] [CrossRef]
- Radisic, M.; Euloth, M.; Yang, L.; Langer, R.; Freed, L.E.; Vunjak-Novakovic, G. High-Density Seeding of Myocyte Cells for Cardiac Tissue Engineering. Biotechnol. Bioeng. 2003, 82, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Alkhawaji, A.; Ding, Y.; Mei, J. Decellularized Scaffolds in Regenerative Medicine. Oncotarget 2016, 7, 58671–58683. [Google Scholar] [CrossRef]
- Hodgson, M.J.; Knutson, C.C.; Momtahan, N.; Cook, A.D. Extracellular Matrix from Whole Porcine Heart Decellularization for Cardiac Tissue Engineering. Methods Mol. Biol. 2017, 95–102. [Google Scholar] [CrossRef]
- Fontana, G.; Gershlak, J.; Adamski, M.; Lee, J.-S.; Matsumoto, S.; Le, H.D.; Binder, B.; Wirth, J.; Gaudette, G.; Murphy, W.L. Biofunctionalized Plants as Diverse Biomaterials for Human Cell Culture. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef]
- Modulevsky, D.J.; Lefebvre, C.; Haase, K.; Al-Rekabi, Z.; Pelling, A.E. Apple Derived Cellulose Scaffolds for 3D Mammalian Cell Culture. PLoS ONE 2014, 9, e97835. [Google Scholar] [CrossRef]
- Modulevsky, D.J.; Cuerrier, C.M.; Pelling, A.E. Biocompatibility of Subcutaneously Implanted Plant-Derived Cellulose Biomaterials. PLoS ONE 2016, 11, e0157894. [Google Scholar] [CrossRef]
- Gershlak, J.R.; Hernandez, S.; Fontana, G.; Perreault, L.R.; Hansen, K.J.; Larson, S.A.; Binder, B.Y.K.; Dolivo, D.M.; Yang, T.; Dominko, T.; et al. Crossing Kingdoms: Using Decellularized Plants as Perfusable Tissue Engineering Scaffolds. Biomaterials 2017, 125, 13–22. [Google Scholar] [CrossRef]
- Entcheva, E.; Bien, H.; Yin, L.; Chung, C.-Y.; Farrell, M.; Kostov, Y. Functional Cardiac Cell Constructs on Cellulose-Based Scaffolding. Biomaterials 2004, 25, 5753–5762. [Google Scholar] [CrossRef] [PubMed]
- Wippermann, J.; Schumann, D.; Klemm, D.; Albes, J.M.; Wittwer, T.; Strauch, J.; Wahlers, T. Preliminary Results of Small Arterial Substitutes Performed with a New Cylindrical Biomaterial Composed of Bacterial Cellulose. Thorac. Cardiovasc. Surg. 2008, 56, 592–596. [Google Scholar] [CrossRef]
- Oberwallner, B.; Brodarac, A.; Anić, P.; Šarić, T.; Wassilew, K.; Neef, K.; Choi, Y.-H.; Stamm, C. Human Cardiac Extracellular Matrix Supports Myocardial Lineage Commitment of Pluripotent Stem Cells. Eur. J. Cardiothorac. Surg. 2015, 47, 416–425, discussion 425. [Google Scholar] [CrossRef] [PubMed]
- Kc, P.; Hong, Y.; Zhang, G. Cardiac Tissue-Derived Extracellular Matrix Scaffolds for Myocardial Repair: Advantages and Challenges. Regen Biomater 2019, 6, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Oberwallner, B.; Brodarac, A.; Choi, Y.-H.; Saric, T.; Anić, P.; Morawietz, L.; Stamm, C. Preparation of Cardiac Extracellular Matrix Scaffolds by Decellularization of Human Myocardium. J. Biomed. Mater. Res. A 2014, 102, 3263–3272. [Google Scholar] [CrossRef] [PubMed]
- Garreta, E.; de Oñate, L.; Fernández-Santos, M.E.; Oria, R.; Tarantino, C.; Climent, A.M.; Marco, A.; Samitier, M.; Martínez, E.; Valls-Margarit, M.; et al. Myocardial Commitment From Human Pluripotent Stem Cells: Rapid Production Of Human Heart Grafts. Biomaterials 2016, 98, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, P.L.; Fernández-Santos, M.E.; Costanza, S.; Climent, A.M.; Moscoso, I.; Gonzalez-Nicolas, M.A.; Sanz-Ruiz, R.; Rodríguez, H.; Kren, S.M.; Garrido, G.; et al. Acellular Human Heart Matrix: A Critical Step Toward Whole Heart Grafts. Biomaterials 2015, 61, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Guyette, J.P.; Charest, J.M.; Mills, R.W.; Jank, B.J.; Moser, P.T.; Gilpin, S.E.; Gershlak, J.R.; Okamoto, T.; Gonzalez, G.; Milan, D.J.; et al. Bioengineering Human Myocardium on Native Extracellular Matrix. Circ. Res. 2016, 118, 56–72. [Google Scholar] [CrossRef]
- Pedron, S.; Van Lierop, S.; Horstman, P.; Penterman, R.; Broer, D.J.; Peeters, E. Stimuli Responsive Delivery Vehicles for Cardiac Microtissue Transplantation. Adv. Funct. Mater. 2011, 21, 1624–1630. [Google Scholar] [CrossRef]
- Birla, R.K.; Borschel, G.H.; Dennis, R.G.; Brown, D.L. Myocardial Engineering in Vivo: Formation and Characterization of Contractile, Vascularized Three-Dimensional Cardiac Tissue. Tissue Eng. 2005, 11, 803–813. [Google Scholar] [CrossRef]
- Sepantafar, M.; Maheronnaghsh, R.; Mohammadi, H.; Rajabi-Zeleti, S.; Annabi, N.; Aghdami, N.; Baharvand, H. Stem Cells and Injectable Hydrogels: Synergistic Therapeutics in Myocardial Repair. Biotechnol. Adv. 2016, 34, 362–379. [Google Scholar] [CrossRef] [PubMed]
- Kopeček, J.; Yang, J. Smart Self-Assembled Hybrid Hydrogel Biomaterials. Angew. Chem. Int. Ed. 2012, 51, 7396–7417. [Google Scholar] [CrossRef] [PubMed]
- Camci-Unal, G.; Annabi, N.; Dokmeci, M.R.; Liao, R.; Khademhosseini, A. Hydrogels for Cardiac Tissue Engineering. NPG Asia Mater. 2014, 6, e99. [Google Scholar] [CrossRef]
- Black, L.D., 3rd; Meyers, J.D.; Weinbaum, J.S.; Shvelidze, Y.A.; Tranquillo, R.T. Cell-Induced Alignment Augments Twitch Force in Fibrin Gel-Based Engineered Myocardium via Gap Junction Modification. Tissue Eng. Part A 2009, 15, 3099–3108. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.J.; Laflamme, M.A.; Gaudette, G.R. Development of a Contractile Cardiac Fiber from Pluripotent Stem Cell Derived Cardiomyocytes. Front. Cardiovasc. Med. 2018, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Naito, H.; Melnychenko, I.; Didié, M.; Schneiderbanger, K.; Schubert, P.; Rosenkranz, S.; Eschenhagen, T.; Zimmermann, W.-H. Optimizing Engineered Heart Tissue for Therapeutic Applications as Surrogate Heart Muscle. Circulation 2006, 114, I72–I78. [Google Scholar] [CrossRef]
- Lau, H.K.; Kiick, K.L. Opportunities for Multicomponent Hybrid Hydrogels in Biomedical Applications. Biomacromolecules 2015, 16, 28–42. [Google Scholar] [CrossRef]
- Fennema, E.; Rivron, N.; Rouwkema, J.; van Blitterswijk, C.; de Boer, J. Spheroid Culture as a Tool for Creating 3D Complex Tissues. Trends Biotechnol. 2013, 31, 108–115. [Google Scholar] [CrossRef]
- Beauchamp, P.; Moritz, W.; Kelm, J.M.; Ullrich, N.D.; Agarkova, I.; Anson, B.D.; Suter, T.M.; Zuppinger, C. Development and Characterization of a Scaffold-Free 3D Spheroid Model of Induced Pluripotent Stem Cell-Derived Human Cardiomyocytes. Tissue Eng. Part C Methods 2015, 21, 852–861. [Google Scholar] [CrossRef]
- Nguyen, D.C.; Hookway, T.A.; Wu, Q.; Jha, R.; Preininger, M.K.; Chen, X.; Easley, C.A.; Spearman, P.; Deshpande, S.R.; Maher, K.; et al. Microscale Generation of Cardiospheres Promotes Robust Enrichment of Cardiomyocytes Derived from Human Pluripotent Stem Cells. Stem Cell Rep. 2014, 3, 260–268. [Google Scholar] [CrossRef]
- Kofron, C.M.; Kim, T.Y.; King, M.E.; Xie, A.; Feng, F.; Park, E.; Qu, Z.; Choi, B.; Mende, U. Gq-Activated Fibroblasts Induce Cardiomyocyte Action Potential Prolongation and Automaticity in a Three-Dimensional Microtissue Environment. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H810–H827. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Bejoy, J.; Xia, J.; Griffin, K.; Guan, J.; Li, Y. Cell Population Balance of Cardiovascular Spheroids Derived from Human Induced Pluripotent Stem Cells. Sci. Rep. 2019, 9, 1295. [Google Scholar] [CrossRef] [PubMed]
- Polonchuk, L.; Chabria, M.; Badi, L.; Hoflack, J.-C.; Figtree, G.; Davies, M.J.; Gentile, C. Cardiac Spheroids as Promising In Vitro Models to Study the Human Heart Microenvironment. Sci. Rep. 2017, 7, 7005. [Google Scholar] [CrossRef] [PubMed]
- Oltolina, F.; Zamperone, A.; Colangelo, D.; Gregoletto, L.; Reano, S.; Pietronave, S.; Merlin, S.; Talmon, M.; Novelli, E.; Diena, M.; et al. Human Cardiac Progenitor Spheroids Exhibit Enhanced Engraftment Potential. PLoS ONE 2015, 10, e0137999. [Google Scholar] [CrossRef]
- Mattapally, S.; Zhu, W.; Fast, V.G.; Gao, L.; Worley, C.; Kannappan, R.; Borovjagin, A.V.; Zhang, J. Spheroids of Cardiomyocytes Derived from Human-Induced Pluripotent Stem Cells Improve Recovery from Myocardial Injury in Mice. Am. J. Physiol. Heart Circul. Physiol. 2018, 315, H327–H339. [Google Scholar] [CrossRef]
- Noguchi, R.; Nakayama, K.; Itoh, M.; Kamohara, K.; Furukawa, K.; Oyama, J.-I.; Node, K.; Morita, S. Development of a Three-Dimensional Pre-Vascularized Scaffold-Free Contractile Cardiac Patch for Treating Heart Disease. J. Heart Lung Transplant. 2016, 35, 137–145. [Google Scholar] [CrossRef]
- Ong, C.S.; Fukunishi, T.; Zhang, H.; Huang, C.Y.; Nashed, A.; Blazeski, A.; DiSilvestre, D.; Vricella, L.; Conte, J.; Tung, L.; et al. Biomaterial-Free Three-Dimensional Bioprinting of Cardiac Tissue using Human Induced Pluripotent Stem Cell Derived Cardiomyocytes. Sci. Rep. 2017, 7, 4566. [Google Scholar] [CrossRef]
- Kim, T.Y.; Kofron, C.M.; King, M.E.; Markes, A.R.; Okundaye, A.O.; Qu, Z.; Mende, U.; Choi, B.-R. Directed Fusion of Cardiac Spheroids into Larger Heterocellular Microtissues Enables Investigation of Cardiac Action Potential Propagation via Cardiac Fibroblasts. PLoS ONE 2018, 13, e0196714. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a Dish: Modeling Development and Disease Using Organoid Technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef]
- Evans, M. Discovering Pluripotency: 30 Years of Mouse Embryonic Stem Cells. Nat. Rev. Mol. Cell Biol. 2011, 12, 680–686. [Google Scholar] [CrossRef]
- Knight, E.; Przyborski, S. Advances in 3D Cell Culture Technologies Enabling Tissue-Like Structures to Be Created In Vitro. J. Anat. 2015, 227, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Shkumatov, A.; Baek, K.; Kong, H. Matrix Rigidity-Modulated Cardiovascular Organoid Formation from Embryoid Bodies. PLoS ONE 2014, 9, e94764. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.J.; Parker, B.L.; Quaife-Ryan, G.A.; Voges, H.K.; Needham, E.J.; Bornot, A.; Ding, M.; Andersson, H.; Polla, M.; Elliott, D.A.; et al. Drug Screening in Human PSC-Cardiac Organoids Identifies Pro-Proliferative Compounds Acting via the Mevalonate Pathway. Cell Stem Cell 2019, 24, 895–907.e6. [Google Scholar] [CrossRef] [PubMed]
- Hoang, P.; Wang, J.; Conklin, B.R.; Healy, K.E.; Ma, Z. Generation of Spatial-Patterned Early-Developing Cardiac Organoids Using Human Pluripotent Stem Cells. Nat. Protoc. 2018, 13, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Varzideh, F.; Pahlavan, S.; Ansari, H.; Halvaei, M.; Kostin, S.; Feiz, M.-S.; Latifi, H.; Aghdami, N.; Braun, T.; Baharvand, H. Human Cardiomyocytes Undergo Enhanced Maturation in Embryonic Stem Cell-Derived Organoid Transplants. Biomaterials 2019, 192, 537–550. [Google Scholar] [CrossRef]
- Richards, D.J.; Li, Y.; Kerr, C.M.; Yao, J.; Beeson, G.C.; Coyle, R.C.; Chen, X.; Jia, J.; Damon, B.; Wilson, R.; et al. Human Cardiac Organoids for the Modelling of Myocardial Infarction and Drug Cardiotoxicity. Nat. Biomed. Eng. 2020, 4, 446–462. [Google Scholar] [CrossRef]
- Richards, D.J.; Coyle, R.C.; Tan, Y.; Jia, J.; Wong, K.; Toomer, K.; Menick, D.R.; Mei, Y. Inspiration from Heart Development: Biomimetic Development of Functional Human Cardiac Organoids. Biomaterials 2017, 142, 112–123. [Google Scholar] [CrossRef]
- Lee, J.; Sutani, A.; Kaneko, R.; Takeuchi, J.; Sasano, T.; Kohda, T.; Ihara, K.; Takahashi, K.; Yamazoe, M.; Morio, T.; et al. In Vitro Generation of Functional Murine Heart Organoids via FGF4 and Extracellular Matrix. Nat. Commun. 2020, 11, 4283. [Google Scholar] [CrossRef]
- Huh, D.; Torisawa, Y.-S.; Hamilton, G.A.; Kim, H.J.; Ingber, D.E. Microengineered Physiological Biomimicry: Organs-on-Chips. Lab Chip 2012, 12, 2156–2164. [Google Scholar] [CrossRef]
- Ewart, L.; Dehne, E.-M.; Fabre, K.; Gibbs, S.; Hickman, J.; Hornberg, E.; Ingelman-Sundberg, M.; Jang, K.-J.; Jones, D.R.; Lauschke, V.M.; et al. Application of Microphysiological Systems to Enhance Safety Assessment in Drug Discovery. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 65–82. [Google Scholar] [CrossRef]
- Jastrzebska, E.; Tomecka, E.; Jesion, I. Heart-on-a-Chip Based on Stem Cell Biology. Biosens. Bioelectron. 2016, 75, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Ribas, J.; Sadeghi, H.; Manbachi, A.; Leijten, J.; Brinegar, K.; Zhang, Y.S.; Ferreira, L.; Khademhosseini, A. Cardiovascular Organ-on-a-Chip Platforms for Drug Discovery and Development. Appl. In Vitro Toxicol. 2016, 2, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Kujala, V.J.; Pasqualini, F.S.; Goss, J.A.; Nawroth, J.C.; Parker, K.K. Laminar Ventricular Myocardium on a Microelectrode Array-Based Chip. J. Mater. Chem. B Mater. Biol. Med. 2016, 4, 3534–3543. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chan, H.N.; Michael, S.A.; Shen, Y.; Chen, Y.; Tian, Q.; Huang, L.; Wu, H. A Microfluidic Circulatory System Integrated with Capillary-Assisted Pressure Sensors. Lab Chip 2017, 17, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Ghiaseddin, A.; Pouri, H.; Soleimani, M.; Vasheghani-Farahani, E.; Ahmadi Tafti, H.; Hashemi-Najafabadi, S. Cell Laden Hydrogel Construct on-a-Chip for Mimicry of Cardiac Tissue in-Vitro Study. Biochem. Biophys. Res. Commun. 2017, 484, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Simmons, C.S.; Petzold, B.C.; Pruitt, B.L. Microsystems for Biomimetic Stimulation of Cardiac Cells. Lab Chip 2012, 12, 3235–3248. [Google Scholar] [CrossRef] [PubMed]
- Kobuszewska, A.; Tomecka, E.; Zukowski, K.; Jastrzebska, E.; Chudy, M.; Dybko, A.; Renaud, P.; Brzozka, Z. Heart-on-a-Chip: An Investigation of the Influence of Static and Perfusion Conditions on Cardiac (H9C2) Cell Proliferation, Morphology, and Alignment. SLAS Technol. 2017, 22, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.-D.; Tinney, J.P.; Ye, F.; Elnakib, A.A.; Yuan, F.; El-Baz, A.; Sethu, P.; Keller, B.B.; Giridharan, G.A. Effects of Physiologic Mechanical Stimulation on Embryonic Chick Cardiomyocytes Using a Microfluidic Cardiac Cell Culture Model. Anal. Chem. 2015, 87, 2107–2113. [Google Scholar] [CrossRef]
- Marsano, A.; Conficconi, C.; Lemme, M.; Occhetta, P.; Gaudiello, E.; Votta, E.; Cerino, G.; Redaelli, A.; Rasponi, M. Beating Heart on a Chip: A Novel Microfluidic Platform to Generate Functional 3D Cardiac Microtissues. Lab Chip 2016, 16, 599–610. [Google Scholar] [CrossRef]
- Grosberg, A.; Alford, P.W.; McCain, M.L.; Parker, K.K. Ensembles of Engineered Cardiac Tissues for Physiological and Pharmacological Study: Heart on a Chip. Lab Chip 2011, 11, 4165–4173. [Google Scholar] [CrossRef]
- Tu, C.; Chao, B.S.; Wu, J.C. Strategies for Improving the Maturity of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Circ. Res. 2018, 123, 512–514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Aleman, J.; Arneri, A.; Bersini, S.; Piraino, F.; Shin, S.R.; Dokmeci, M.R.; Khademhosseini, A. From Cardiac Tissue Engineering to Heart-on-a-Chip: Beating Challenges. Biomed. Mater. 2015, 10, 034006. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Bleasby, K.; Evers, R. Species Differences in Drug Transporters and Implications for Translating Preclinical Findings to Humans. Expert Opin. Drug Metab. Toxicol. 2013, 9, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Hirt, M.N.; Boeddinghaus, J.; Mitchell, A.; Schaaf, S.; Börnchen, C.; Müller, C.; Schulz, H.; Hubner, N.; Stenzig, J.; Stoehr, A.; et al. Functional Improvement and Maturation of Rat and Human Engineered Heart Tissue by Chronic Electrical Stimulation. J. Mol. Cell. Cardiol. 2014, 74, 151–161. [Google Scholar] [CrossRef]
- Zhang, B.; Montgomery, M.; Chamberlain, M.D.; Ogawa, S.; Korolj, A.; Pahnke, A.; Wells, L.A.; Massé, S.; Kim, J.; Reis, L.; et al. Biodegradable Scaffold with Built-In Vasculature for Organ-on-a-Chip Engineering and Direct Surgical Anastomosis. Nat. Mater. 2016, 15, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.; Loskill, P.; Shao, K.; Huebsch, N.; Hong, S.; Marcus, S.G.; Marks, N.; Mandegar, M.; Conklin, B.R.; Lee, L.P.; et al. Human iPSC-Based Cardiac Microphysiological System for Drug Screening Applications. Sci. Rep. 2015, 5, 8883. [Google Scholar] [CrossRef]
- Homan, K.A.; Kolesky, D.B.; Skylar-Scott, M.A.; Herrmann, J.; Obuobi, H.; Moisan, A.; Lewis, J.A. Bioprinting of 3D Convoluted Renal Proximal Tubules on Perfusable Chips. Sci. Rep. 2016, 6, 34845. [Google Scholar] [CrossRef]
- Kolesky, D.B.; Homan, K.A.; Skylar-Scott, M.A.; Lewis, J.A. Three-Dimensional Bioprinting of Thick Vascularized Tissues. Proc. Natl. Acad. Sci. USA 2016, 113, 3179–3184. [Google Scholar] [CrossRef]
- Dhariwala, B.; Hunt, E.; Boland, T. Rapid Prototyping of Tissue-Engineering Constructs, Using Photopolymerizable Hydrogels and Stereolithography. Tissue Eng. 2004, 10, 1316–1322. [Google Scholar] [CrossRef]
- Boland, T.; Xu, T.; Damon, B.; Cui, X. Application of Inkjet Printing to Tissue Engineering. Biotechnol. J. 2006, 1, 910–917. [Google Scholar] [CrossRef]
- Yan, J.; Huang, Y.; Chrisey, D.B. Laser-Assisted Printing of Alginate Long Tubes and Annular Constructs. Biofabrication 2013, 5, 015002. [Google Scholar] [CrossRef] [PubMed]
- Beyer, S.T.; Bsoul, A.; Ahmadi, A.; Walus, K. 3D Alginate Constructs for Tissue Engineering Printed Using a Coaxial Flow Focusing Microfluidic Device. In Proceedings of the 2013 Transducers & Eurosensors XXVII: The 17th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS & EUROSENSORS XXVII), Barcelona, Spain, 6–20 June 2013; pp. 1206–1209. [Google Scholar] [CrossRef]
- Gaetani, R.; Feyen, D.A.M.; Verhage, V.; Slaats, R.; Messina, E.; Christman, K.L.; Giacomello, A.; Doevendans, P.A.F.M.; Sluijter, J.P.G. Epicardial Application of Cardiac Progenitor Cells in a 3D-Printed Gelatin/Hyaluronic Acid Patch Preserves Cardiac Function after Myocardial Infarction. Biomaterials 2015, 61, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Maiullari, F.; Costantini, M.; Milan, M.; Pace, V.; Chirivì, M.; Maiullari, S.; Rainer, A.; Baci, D.; Marei, H.E.-S.; Seliktar, D.; et al. A Multi-Cellular 3D Bioprinting Approach for Vascularized Heart Tissue Engineering Based on HUVECs and iPSC-Derived Cardiomyocytes. Sci. Rep. 2018, 8, 13532. [Google Scholar] [CrossRef]
- Lee, H.; Cho, D.-W. One-Step Fabrication of an Organ-on-a-Chip with Spatial Heterogeneity Using a 3D Bioprinting Technology. Lab Chip 2016, 16, 2618–2625. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A Fascinating Fiber Fabrication Technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Huang, S.; Yang, Y.; Yang, Q.; Zhao, Q.; Ye, X. Engineered Circulatory Scaffolds for Building Cardiac Tissue. J. Thorac. Dis. 2018, 10, S2312–S2328. [Google Scholar] [CrossRef] [PubMed]
- Castilho, M.; Hochleitner, G.; Wilson, W.; van Rietbergen, B.; Dalton, P.D.; Groll, J.; Malda, J.; Ito, K. Mechanical Behavior of a Soft Hydrogel Reinforced with Three-Dimensional Printed Microfibre Scaffolds. Sci. Rep. 2018, 8, 1245. [Google Scholar] [CrossRef]
- Yildirim, Y.; Naito, H.; Didie, M.; Karikkineth, B.C.; Biermann, D.; Eschenhagen, T.; Zimmermann, W. Development of a Biological Ventricular Assist Device: Preliminary Data from a Small Animal Model. Circulation 2007, 116, I16–I23. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, D.E.; Azeloglu, E.U.; Costa, K.D. Engineered Cardiac Organoid Chambers: Toward a Functional Biological Model Ventricle. Tissue Eng. Part A 2008, 14, 215–225. [Google Scholar] [CrossRef]
- Li, R.A.; Keung, W.; Cashman, T.J.; Backeris, P.C.; Johnson, B.V.; Bardot, E.S.; Wong, A.O.T.; Chan, P.K.W.; Chan, C.W.Y.; Costa, K.D. Bioengineering an Electro-Mechanically Functional Miniature Ventricular Heart Chamber from Human Pluripotent Stem Cells. Biomaterials 2018, 163, 116–127. [Google Scholar] [CrossRef]
- MacQueen, L.A.; Sheehy, S.P.; Chantre, C.O.; Zimmerman, J.F.; Pasqualini, F.S.; Liu, X.; Goss, J.A.; Campbell, P.H.; Gonzalez, G.M.; Park, S.-J.; et al. A Tissue-Engineered Scale Model of the Heart Ventricle. Nat. Biomed. Eng. 2018, 2, 930–941. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D Bioprinting of Collagen to Rebuild Components of the Human Heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Kupfer, M.E.; Lin, W.-H.; Ravikumar, V.; Qiu, K.; Wang, L.; Gao, L.; Bhuiyan, D.; Lenz, M.; Ai, J.; Mahutga, R.R.; et al. In Situ Expansion, Differentiation and Electromechanical Coupling of Human Cardiac Muscle in a 3D Bioprinted, Chambered Organoid. Circ. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Garoffolo, G.; Pesce, M. Mechanotransduction in the Cardiovascular System: From Developmental Origins to Homeostasis and Pathology. Cells 2019, 8, 1607. [Google Scholar] [CrossRef]
- Fink, C.; Ergün, S.; Kralisch, D.; Remmers, U.; Weil, J.; Eschenhagen, T. Chronic Stretch of Engineered Heart Tissue Induces Hypertrophy and Functional Improvement. FASEB J. 2000, 14, 669–679. [Google Scholar] [CrossRef]
- Lasher, R.A.; Pahnke, A.Q.; Johnson, J.M.; Sachse, F.B.; Hitchcock, R.W. Electrical Stimulation Directs Engineered Cardiac Tissue to an Age-Matched Native Phenotype. J. Tissue Eng. 2012, 3. [Google Scholar] [CrossRef]
- Ruwhof, C. Mechanical Stress-Induced Cardiac Hypertrophy: Mechanisms and Signal Transduction Pathways. Cardiovasc. Res. 2000, 47, 23–37. [Google Scholar] [CrossRef]
- Severs, N.J. The cardiac Muscle Cell. Bioessays 2000, 22, 188–199. [Google Scholar] [CrossRef]
- Lie-Venema, H.; van den Akker, N.M.S.; Bax, N.A.M.; Winter, E.M.; Maas, S.; Kekarainen, T.; Hoeben, R.C.; DeRuiter, M.C.; Poelmann, R.E.; Gittenberger-de Groot, A.C. Origin, Fate, and Function of Epicardium-Derived Cells (EPDCs) in Normal and Abnormal Cardiac Development. Sci. World J. 2007, 7, 1777–1798. [Google Scholar] [CrossRef]
- Goette, A.; Lendeckel, U. Electrophysiological Effects of Angiotensin II. Part I: Signal Transduction and Basic Electrophysiological Mechanisms. Europace 2008, 10, 238–241. [Google Scholar] [CrossRef]
- Zhang, P.; Su, J.; Mende, U. Cross Talk between Cardiac Myocytes and Fibroblasts: From Multiscale Investigative Approaches to Mechanisms and Functional Consequences. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H1385–H1396. [Google Scholar] [CrossRef] [PubMed]
- Brutsaert, D.L. Cardiac Endothelial-Myocardial Signaling: Its Role in Cardiac Growth, Contractile Performance, and Rhythmicity. Physiol. Rev. 2003, 83, 59–115. [Google Scholar] [CrossRef] [PubMed]
- Iyer, R.K.; Odedra, D.; Chiu, L.L.Y.; Vunjak-Novakovic, G.; Radisic, M. Vascular Endothelial Growth Factor Secretion by Nonmyocytes Modulates Connexin-43 Levels in Cardiac Organoids. Tissue Eng. Part A 2012, 18, 1771–1783. [Google Scholar] [CrossRef] [PubMed]
- Garzoni, L.R.; Rossi, M.I.D.; de Barros, A.P.D.N.; Guarani, V.; Keramidas, M.; Balottin, L.B.L.; Adesse, D.; Takiya, C.M.; Manso, P.P.; Otazú, I.B.; et al. Dissecting Coronary Angiogenesis: 3D Co-Culture of Cardiomyocytes with Endothelial or Mesenchymal Cells. Exp. Cell Res. 2009, 315, 3406–3418. [Google Scholar] [CrossRef] [PubMed]
- Liau, B.; Zhang, D.; Bursac, N. Functional Cardiac Tissue Engineering. Regen. Med. 2012, 7, 187–206. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Cho, H.C.; Akar, F.G.; Tsang, S.-Y.; Jones, S.P.; Marbán, E.; Tomaselli, G.F.; Li, R.A. Functional Integration of Electrically Active Cardiac Derivatives from Genetically Engineered Human Embryonic Stem Cells with Quiescent Recipient Ventricular Cardiomyocytes: Insights into the Development of Cell-Based Pacemakers. Circulation 2005, 111, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Ponard, J.G.C.; Kondratyev, A.A.; Kucera, J.P. Mechanisms of Intrinsic Beating Variability in Cardiac Cell Cultures and Model Pacemaker Networks. Biophys. J. 2007, 92, 3734–3752. [Google Scholar] [CrossRef]
- Radisic, M.; Fast, V.G.; Sharifov, O.F.; Iyer, R.K.; Park, H.; Vunjak-Novakovic, G. Optical Mapping of Impulse Propagation in Engineered Cardiac Tissue. Tissue Eng. Part A 2009, 15, 851–860. [Google Scholar]
- Schaffer, P.; Ahammer, H.; Müller, W.; Koidl, B.; Windisch, H. Di-4-ANEPPS Causes Photodynamic Damage to Isolated Cardiomyocytes. Pflug. Arch. 1994, 426, 548–551. [Google Scholar] [CrossRef]
- Hamill, O.P.; Marty, A.; Neher, E.; Sakmann, B.; Sigworth, F.J. Improved Patch-Clamp Techniques for high-Resolution Current Recording from Cells and Cell-Free Membrane Patches. Pflug. Arch. 1981, 391, 85–100. [Google Scholar] [CrossRef]
- Del Corsso, C.; Srinivas, M.; Urban-Maldonado, M.; Moreno, A.P.; Fort, A.G.; Fishman, G.I.; Spray, D.C. Transfection of Mammalian Cells with Connexins and Measurement of Voltage Sensitivity of Their Gap Junctions. Nat. Protoc. 2006, 1, 1799–1809. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.T. Advanced Micropipette Techniques for Cell Physiology; Wiley: Hoboken, NJ, USA, 1986; ISBN 9780471909521. [Google Scholar]
- Kehat, I.; Gepstein, A.; Spira, A.; Itskovitz-Eldor, J.; Gepstein, L. High-Resolution Electrophysiological Assessment of Human Embryonic Stem Cell-Derived Cardiomyocytes. Circ. Res. 2002, 91, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Reppel, M.; Pillekamp, F.; Brockmeier, K.; Matzkies, M.; Bekcioglu, A.; Lipke, T.; Nguemo, F.; Bonnemeier, H.; Hescheler, J. The Electrocardiogram of Human Embryonic Stem Cell-Derived Cardiomyocytes. J. Electrocardiol. 2005, 38, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Tung, L.; Zhang, Y. Optical Imaging of Arrhythmias in Tissue Culture. J. Electrocardiol. 2006, 39, S2–S6. [Google Scholar] [CrossRef]
- Efimov, I.R.; Nikolski, V.P.; Salama, G. Optical Imaging of the Heart. Circ. Res. 2004, 95, 21–33. [Google Scholar] [CrossRef]
- Lieu, D.K.; Turnbull, I.C.; Costa, K.D.; Li, R.A. Engineered Human Pluripotent Stem Cell-Derived Cardiac Cells and Tissues for Electrophysiological Studies. Drug Discov. Today Dis. Models 2012, 9, e209–e217. [Google Scholar] [CrossRef][Green Version]
- Van Der Velden, J.; Klein, L.J.; Zaremba, R.; Boontje, N.M.; Huybregts, M.A.; Stooker, W.; Eijsman, L.; de Jong, J.W.; Visser, C.A.; Visser, F.C.; et al. Effects of Calcium, Inorganic Phosphate, and pH on Isometric Force in Single Skinned Cardiomyocytes from Donor and Failing Human Hearts. Circulation 2001, 104, 1140–1146. [Google Scholar] [CrossRef]
- Karakikes, I.; Ameen, M.; Termglinchan, V.; Wu, J.C. Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes: Insights into Molecular, Cellular, and Functional Phenotypes. Circ. Res. 2015, 117, 80–88. [Google Scholar] [CrossRef]
- Hunter, P.J.; Pullan, A.J.; Smaill, B.H. Modeling Total Heart Function. Annu. Rev. Biomed. Eng. 2003, 5, 147–177. [Google Scholar] [CrossRef]
| CTE Construct | Cell Source | Culture Conditions | Functional Analyses | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type | Area (mm2) | Thickness (mm) | Species | Cell Type | Stimulation | Perfusion | Time (d) | CM Maturation | Tissue Maturation | |
| Scaffold | 70 | 2 | Rat | Neonatal CM | No | Yes | 10 | Protein expression | No functional analyses | [21] |
| Scaffold | 95 | 1.5 | Rat | Neonatal CM | No | Yes | 7 | Protein expression | Contractile activity | [22] |
| Scaffold | 48 | 1.5 | Rat | Neonatal CM | Electrical stimulation | No | 8 | Protein expression, sarcomere structure | Contractile activity | [23] |
| Scaffold | 95 | 1.5 | Rat | Neonatal CM | No | Yes | 14 | No functional analyses | Contractile activity | [24] |
| Scaffold | 48 | 1.5 | Rat | Neonatal CM | Electrical stimulation | No | 8 | Protein expression, sarcomere structure | Contractile activity | [25] |
| Scaffold | 20 | 2 | Rat | Neonatal CM | Electrical stimulation | Yes | 4 | Protein expression | No functional analyses | [26] |
| Scaffold | 50 | 1 | Rat | Neonatal CM | Electrical stimulation | Yes | 8 | No functional analyses | Contractile activity | [27] |
| Scaffold | 20 | 2 | Rat | Neonatal CM | Mechanical stimulation | Yes | 4 | Protein expression | No functional analyses | [28] |
| Scaffold | 80 | 5 | Rat | Neonatal CM | No | No | 7 | No functional analyses | Calcium imaging | [29] |
| Scaffold | n/a | n/a | Human | ESC-CM | Mechanical stimulation | No | 14 | Protein expression | Force of contraction | [30] |
| Scaffold | 56 | 3.5 | Human | ESC-CM | Mechanical | No | 5 | Gene/Protein expression | Calcium imaging | [31] |
| Scaffold | 80 | 1 | Rat/human | Neonatal CM/iPSC-CM | Electrical stimulation | Yes | 14 | Gene/protein expression, sarcomere structure | Force of contraction, contractile activity, whole construct electrical activity | [32] |
| Hydrogel | n/a | n/a | Rat | Neonatal CM | Mechanical stimulation | No | 14 | Protein expression, sarcomere structure, electrical signal propagation | Force of contraction | [33] |
| Hydrogel | n/a | 0.9 | Rat | Neonatal CM | Mechanical stimulation | Yes | 14 | Gene/protein expression | Force of contraction | [34] |
| Hydrogel | n/a | n/a | Rat | Neonatal CM | Electro-mechanical stimulation | No | 13 | Protein expression | Force of contraction, calcium imaging | [35] |
| Hydrogel | 60 | n/a | Human | ESC-CM/iPSC-CM+HUVEC+MSC | Mechanical stimulation | No | 4 | Gene expression, sarcomere structure | Force of contraction | [36] |
| Hydrogel | 0.4 | n/a | Human | ESC-CM | No | No | 14 | Gene/protein expression, patch clamp | Force of contraction | [37] |
| Hydrogel | n/a | 0.1 | Rat/human | Neonatal CM/ESC-CM | Electro-mechanical stimulation | No | 7 | Gene/protein expression | Contractile activity, optical mapping | [38] |
| Hydrogel | 49 | n/a | Human | ESC-CM | No | No | 14 | Gene/protein expression, sarcomere structure | Force of contraction, optical mapping | [39] |
| Hydrogel | 3 | 0.3 | Human | iPSC-CM | Electrical stimulation | No | 14 | Protein expression, sarcomere structure, patch clamp | Contractile activity, optical mapping, calcium imaging | [40] |
| Hydrogel | 15 | n/a | Rat/human | Neonatal CM/ESC-CM | Electrical stimulation | Yes | 14 | Protein expression | Force of contraction, contractile activity | [41] |
| Hydrogel | 5 | n/a | Human | ESC-CM | No | No | 24 | Gene/protein expression, sarcomere structure | Force of contraction, optical mapping | [42] |
| Hydrogel | n/a | n/a | Human | iPSC-CM | No | No | 7 | Protein expression, electrical signal propagation | No functional analyses | [43] |
| Hydrogel | 0.125 | n/a | Human | iPSC-CM | No | No | 15 | Gene/protein expression | No functional analyses | [44] |
| Hydrogel | 27 | 0.2 | Human | iPSC-CM+iPSC-EC/HUVEC | No | No | 15 | Gene/Protein expression | Optical mapping | [45] |
| Hydrogel | 4 | n/a | Human | iPSC-CM | No | No | 40 | Protein expression, sarcomere structure | Force of contraction | [46] |
| Hydrogel | 20 | 0.3 | Human | iPSC-CM | Electro-mechanical stimulation | No | 14 | Protein expression, sarcomere structure | Force of contraction, calcium imaging | [47] |
| Hydrogel | 14 | 0.2 | Rat/human | Neonatal CM/iPSC-CM | No | No | 14 | Gene/protein expression | Force of contraction, optical mapping | [48] |
| Hydrogel | 200 | n/a | Human | iPSC-CM | No | No | 60 | Protein expression, electrical signal propagation | No functional analyses | [49] |
| Hydrogel | 900 | n/a | Human | iPSC-CM | No | No | 28 | Protein expression | Force of contraction, contractile activity | [50] |
| Hydrogel | 1190 | 0.5 | Human | ESC-CM/iPSC-CM | Mechanical stimulation | No | 45 | Gene expression, sarcomere structure, patch clamp | Force of contraction | [51] |
| Hydrogel | 1296 | 0.1 | Human | iPSC-CM | No | No | 21 | Gene/protein expression, sarcomere structure | Force of contraction, optical mapping | [11] |
| Hydrogel | 100 | 0.1 | Rat/human | Neonatal CM/ESC-CM | Electrical stimulation | No | 7 | No functional analyses | Force of contraction, contractile activity | [52] |
| Hydrogel | 800 | n/a | Human | iPSC-CM+iPSC-EC+iPS-SMC | No | No | 7 | Protein expression | Force of contraction, optical mapping | [53] |
| Hydrogel | 5 | 0.3 | Human | ESC-CM/iPSC-CM+hcFB | Electrical stimulation | No | 42 | Gene/Protein expression, sarcomere structure, patch clamp | Force of contraction, contractile activity, calcium imaging | [54] |
| Hydrogel | 11 | n/a | Human | iPSC-CM | Electrical stimulation | No | 30 | Gene/protein expression, sarcomere structure, patch clamp | Force of contraction, calcium imaging | [55] |
| Hydrogel | 14 | 2 | Human/Pig/Rat | iPSC-CM+iPSC-EC/Neonatal CM+HUVEC+FB | No | No | 7 | Gene/Protein expression | Optical mapping, calcium imaging | [56] |
| Cell sheets | 116 | 0.045 | Rat | Neonatal CM | No | No | 4 | Protein expression, sarcomere structure, electrical signal propagation | Force of contraction | [57] |
| Cell sheets | 960 | 0.1 | Rat/human | Neonatal CM+EC | No | Yes | 10 | Protein expression | No functional analyses | [58] |
| Cell sheets | 70 | 0.1 | Human | iPSC-CM+MSC | No | No | 4 | Protein expression, electrical signal propagation | Contractile activity | [59] |
| Approach | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Patch-clamp |
|
| [217] |
| Multielectrode arrays |
|
| [59,108,217,218,219] |
| Optical mapping |
|
| [29,35,39,48,55,118,220,221] |
| Force transducers |
|
| [36,39,42,47,48,50,57,217] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tadevosyan, K.; Iglesias-García, O.; Mazo, M.M.; Prósper, F.; Raya, A. Engineering and Assessing Cardiac Tissue Complexity. Int. J. Mol. Sci. 2021, 22, 1479. https://doi.org/10.3390/ijms22031479
Tadevosyan K, Iglesias-García O, Mazo MM, Prósper F, Raya A. Engineering and Assessing Cardiac Tissue Complexity. International Journal of Molecular Sciences. 2021; 22(3):1479. https://doi.org/10.3390/ijms22031479
Chicago/Turabian StyleTadevosyan, Karine, Olalla Iglesias-García, Manuel M. Mazo, Felipe Prósper, and Angel Raya. 2021. "Engineering and Assessing Cardiac Tissue Complexity" International Journal of Molecular Sciences 22, no. 3: 1479. https://doi.org/10.3390/ijms22031479
APA StyleTadevosyan, K., Iglesias-García, O., Mazo, M. M., Prósper, F., & Raya, A. (2021). Engineering and Assessing Cardiac Tissue Complexity. International Journal of Molecular Sciences, 22(3), 1479. https://doi.org/10.3390/ijms22031479







