Diazotroph Paenibacillus triticisoli BJ-18 Drives the Variation in Bacterial, Diazotrophic and Fungal Communities in the Rhizosphere and Root/Shoot Endosphere of Maize
Abstract
1. Introduction
2. Results
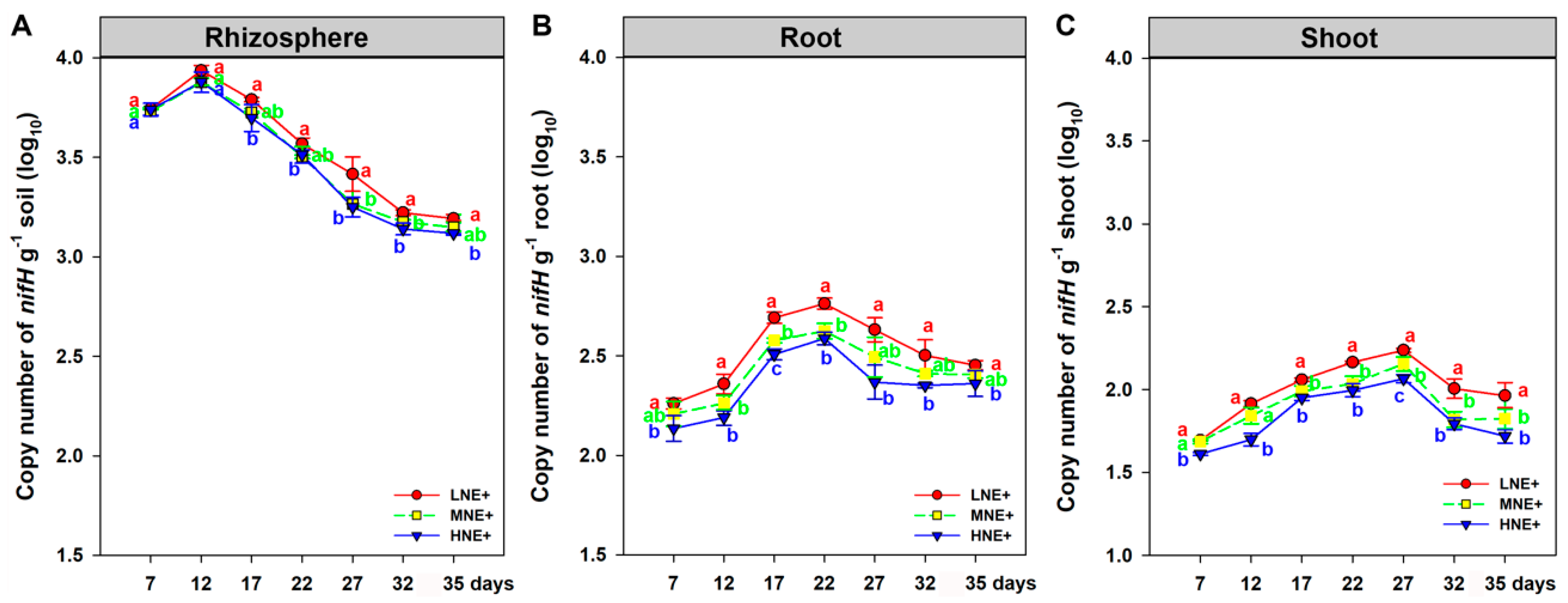
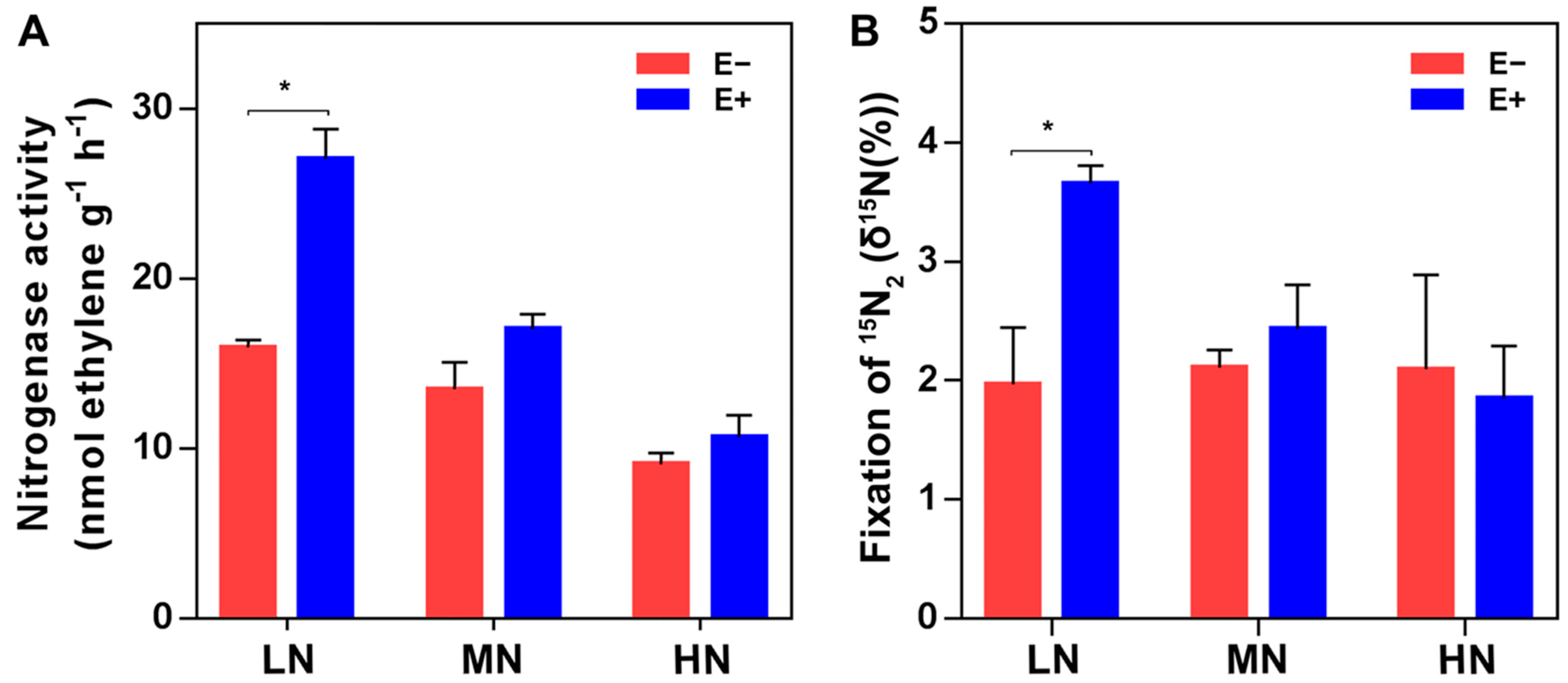
2.1. Population Dynamics of P. triticisoli BJ-18 and Copy Numbers of 16S rRNA, nifH Gene and ITS
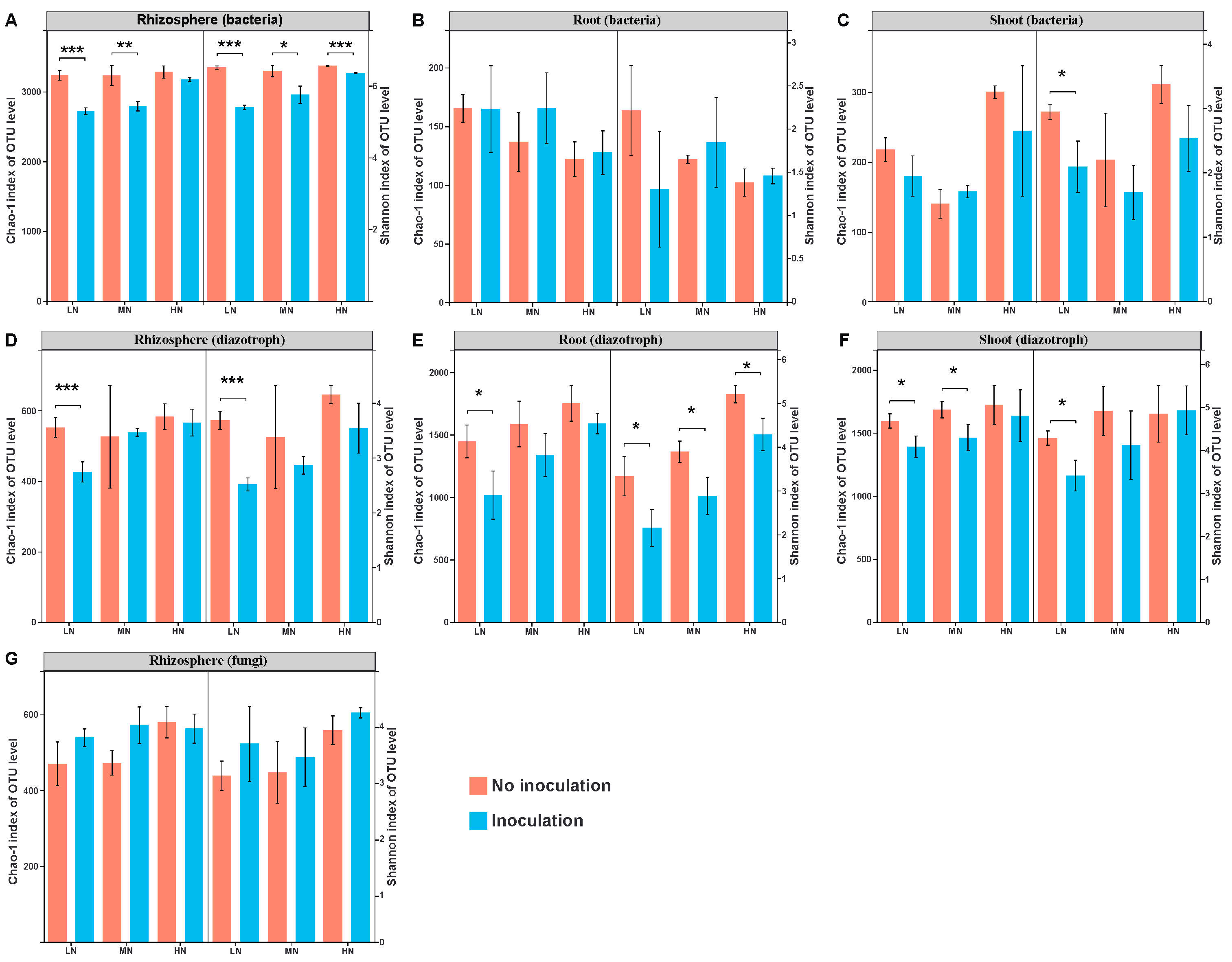
2.2. Microbial Diversity
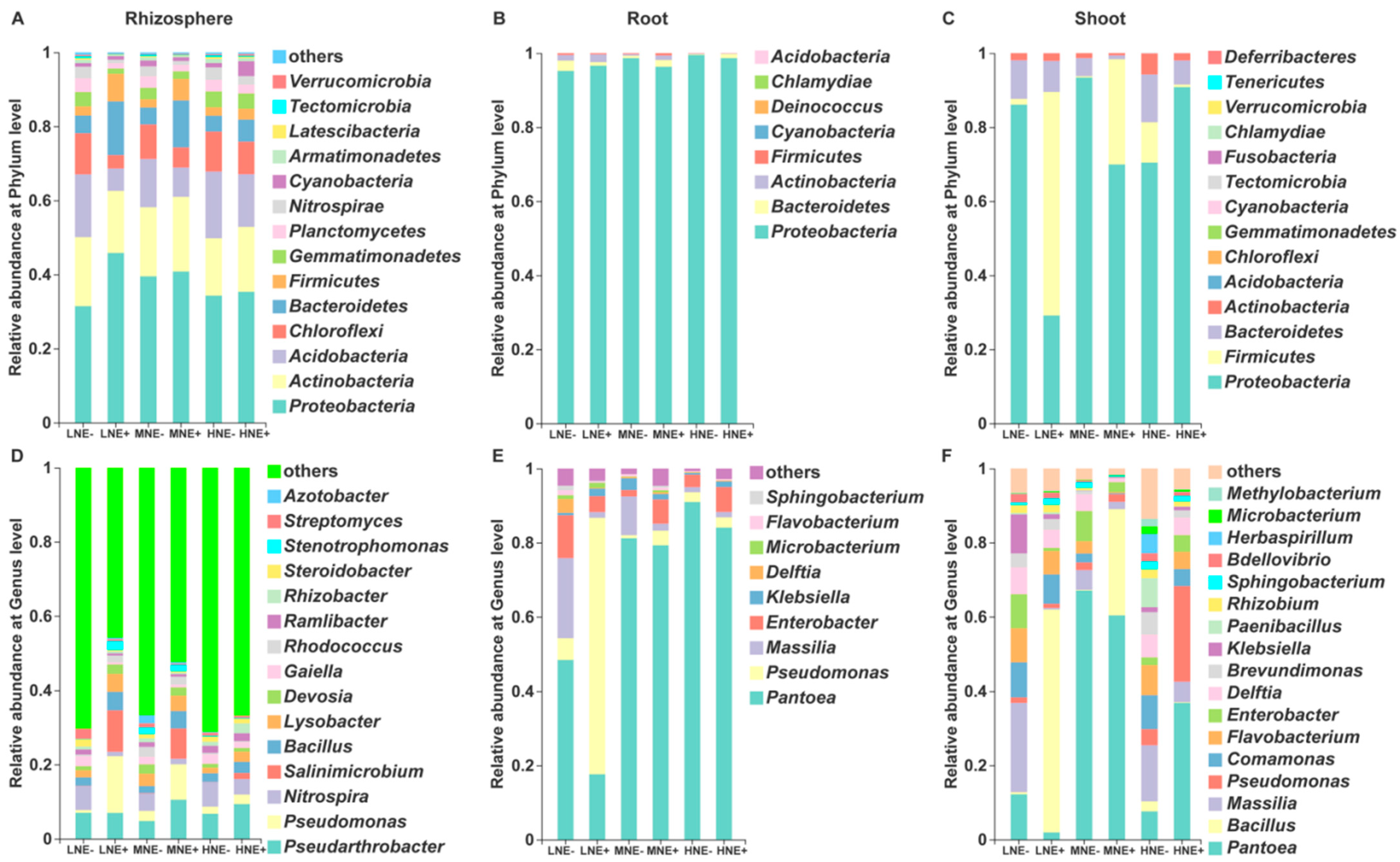
2.3. Bacterial Communities in the Rhizosphere and Root/Shoot Endosphere
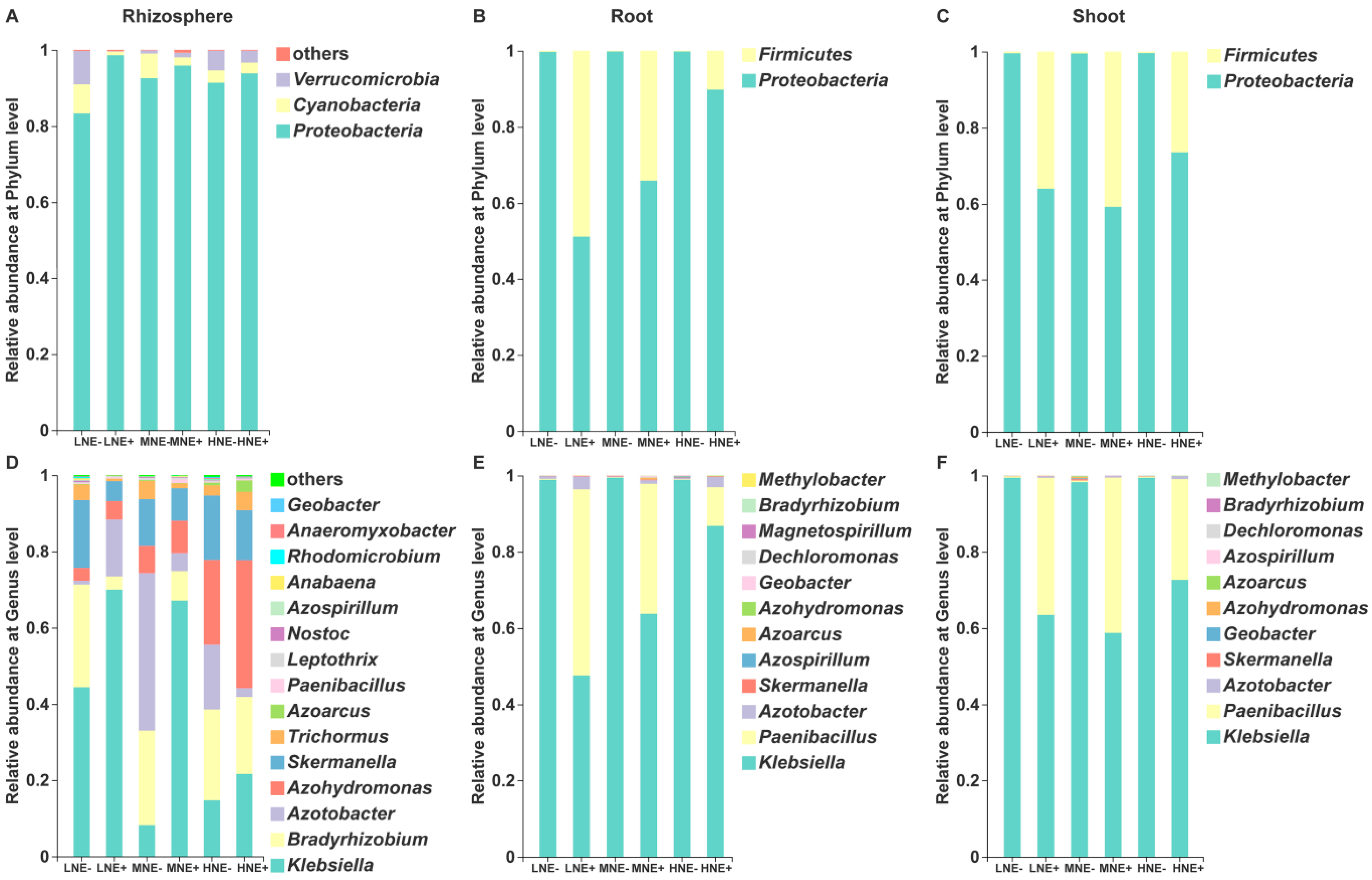
2.4. Diazotrophic Communities in the Rhizosphere and Root/Shoot Endosphere
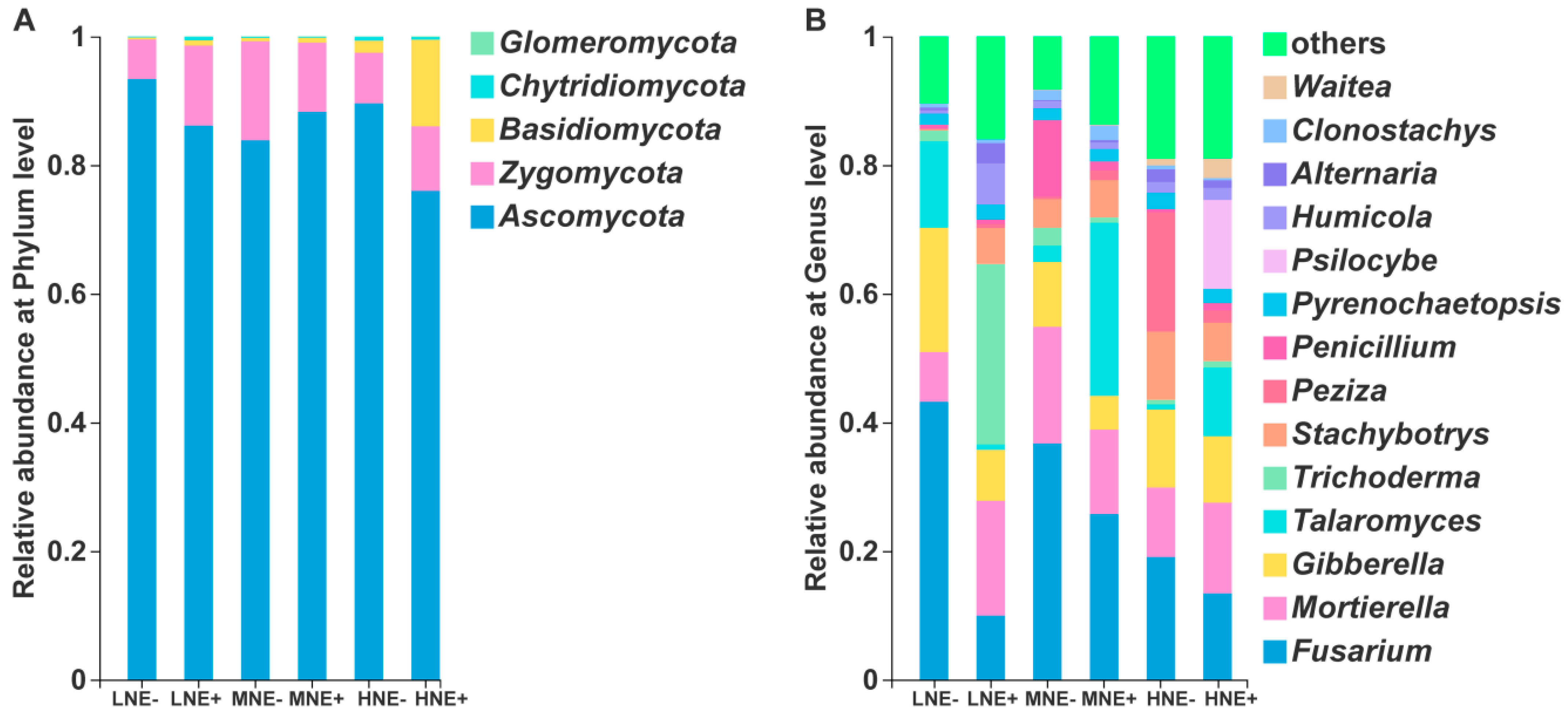
2.5. Fungal Community in the Maize Rhizosphere
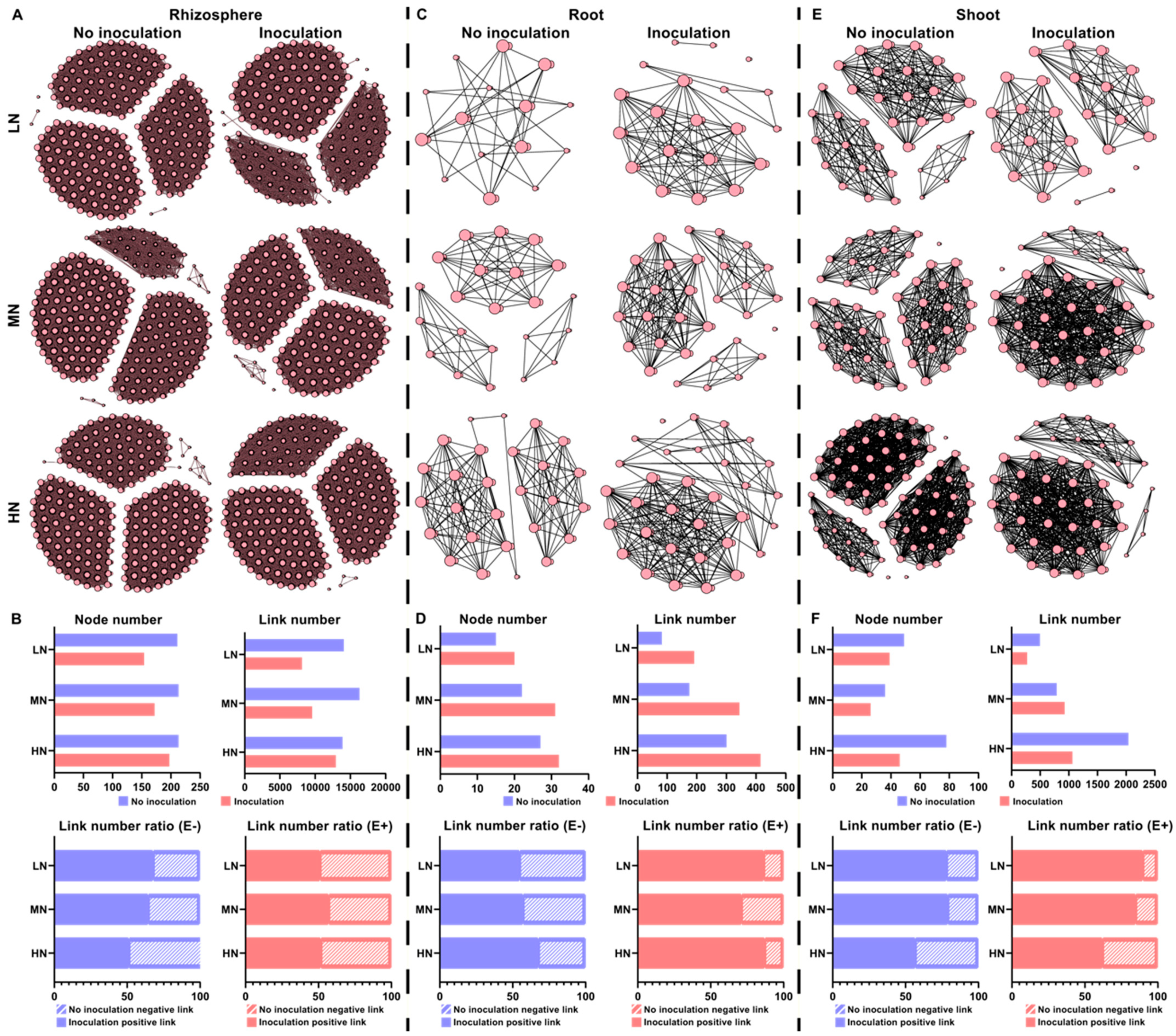
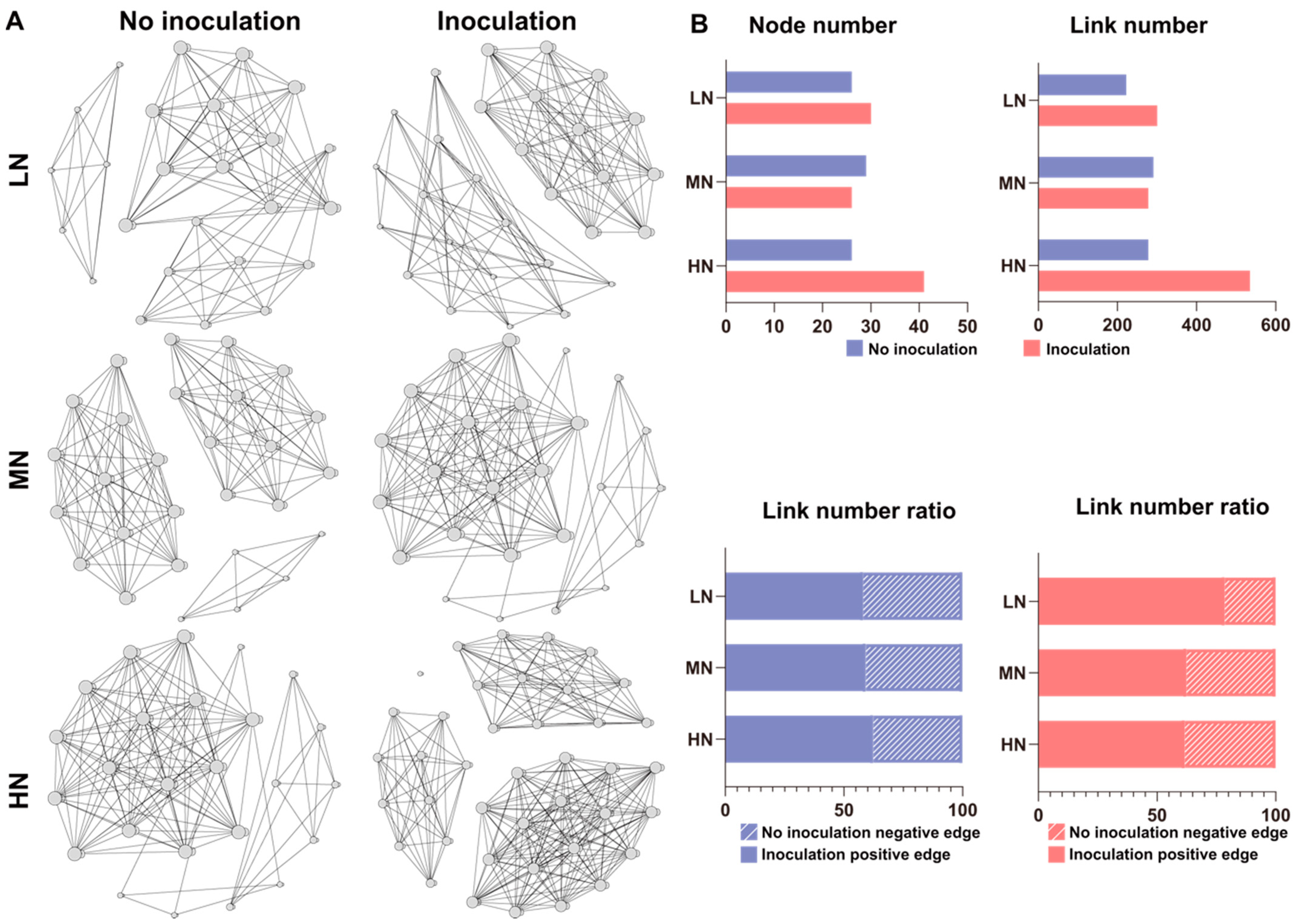
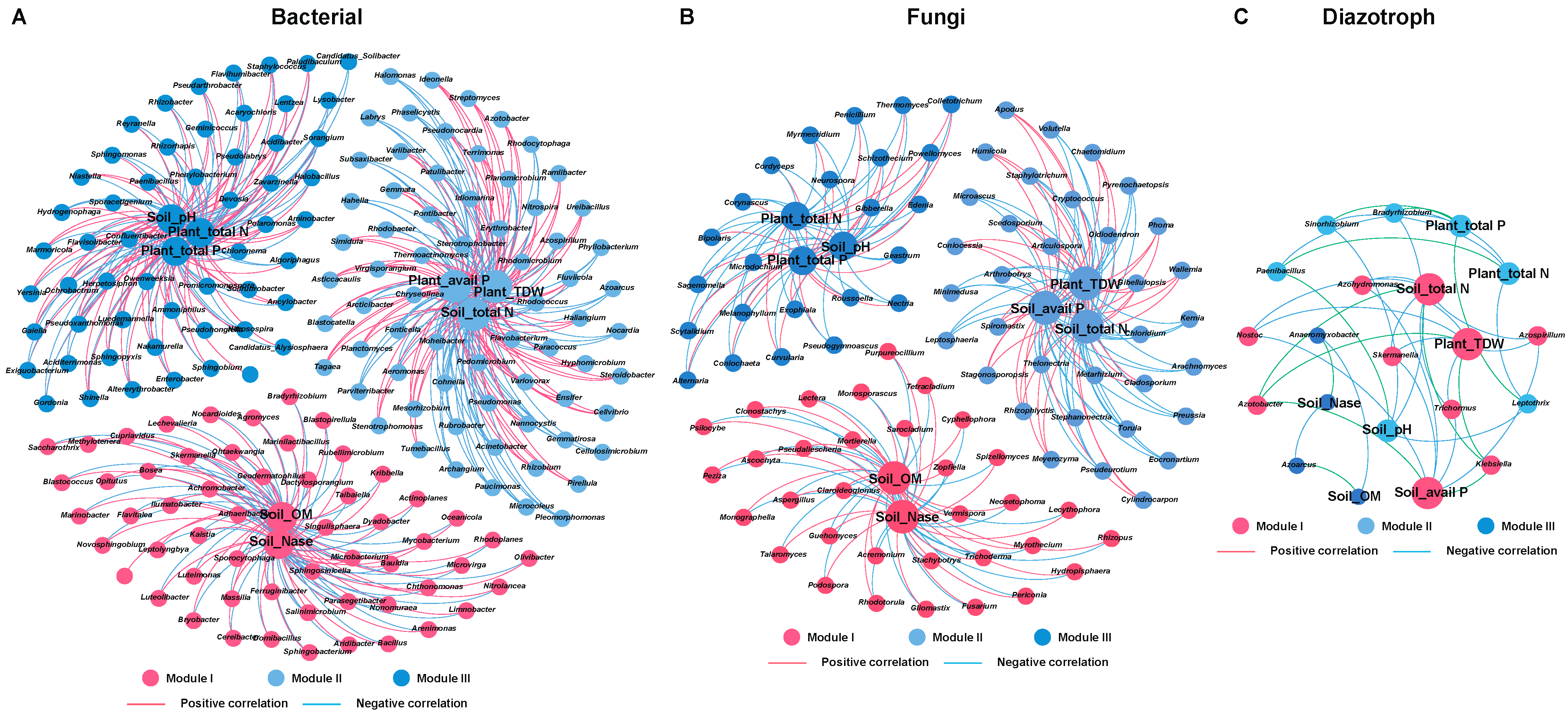
2.6. Microbial Interaction Networks
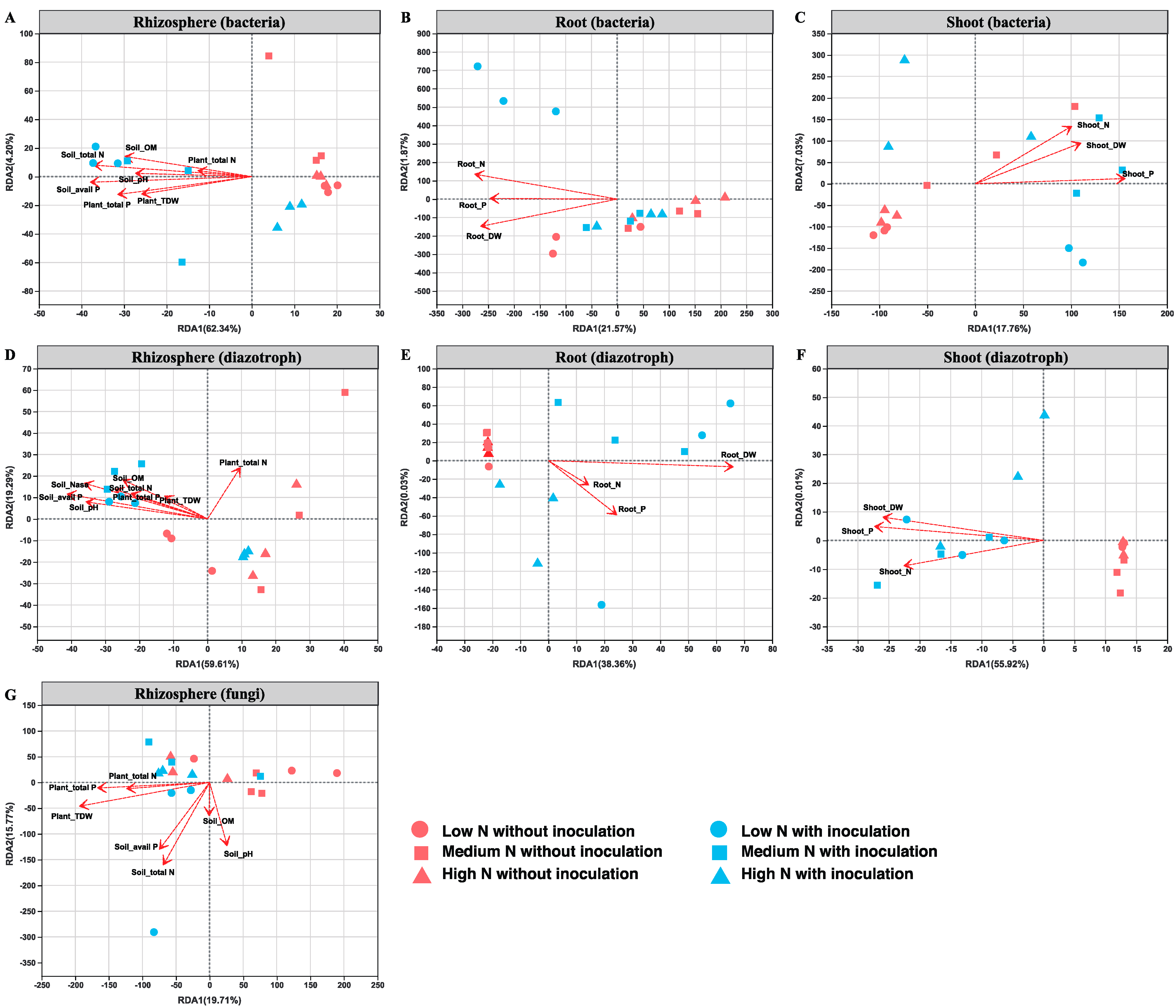
2.7. Relationships between Microbial Community and Environmental Variables in the Rhizosphere and Root/Shoot Endosphere of Maize
3. Discussion
4. Materials and Methods
4.1. Plant Plantation and Inoculation with P. triticisoli BJ-18 Cells
4.2. Sample Collection
4.3. Soil and Plant Physicochemical Property
4.4. Nitrogenase Activity Assay
4.5. 15N2 Incorporation Assay
4.6. Extraction of DNA
4.7. Quantitative-PCR (qPCR) Analysis
4.8. Amplification and Sequencing of 16S rRNA, nifH Gene and ITS Region
4.9. Bioinformatic Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef] [PubMed]
- Bakker, P.; Pieterse, C.M.J.; de Jonge, R.; Berendsen, R.L. The soil-borne legacy. Cell 2018, 172, 1178–1180. [Google Scholar] [CrossRef] [PubMed]
- Dobbelaere, S.; Vanderleyden, J.; Okon, Y. Plant growth-promoting effects of diazotrophs in the rhizosphere. Crit. Rev. Plant Sci. 2003, 22, 107–149. [Google Scholar] [CrossRef]
- Dennis, P.G.; Miller, A.J.; Hirsch, P.R. Are root exudates more important than other sources of rhizodeposits in structuring rhizosphere bacterial communities? FEMS Microbiol. Ecol. 2010, 72, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Maheshwari, D.K. Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246. [Google Scholar] [CrossRef] [PubMed]
- Rosenblueth, M.; Martinez-Romero, E. Bacterial endophytes and their interactions with hosts. Mol. Plant Microbe Interact. 2006, 19, 827–837. [Google Scholar] [CrossRef]
- Hallmann, J.; Quadt, A.; Mahaffee, W.; Kloepper, J. Endophytic bacteria in agricultural crops. Can. J. Microbiol. 1997, 43, 895–914. [Google Scholar] [CrossRef]
- Liu, H.W.; Carvalhais, L.C.; Crawford, M.; Singh, E.; Dennis, P.G.; Pieterse, C.M.J.; Schenk, P.M. Inner plant values: Diversity, colonization and benefits from endophytic bacteria. Front. Microbiol. 2017, 8, 2552. [Google Scholar] [CrossRef]
- Hardoim, P.R.; van Overbeek, L.S.; van Elsas, J.D. Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol. 2008, 16, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Lucy, M.; Reed, E.; Glick, B.R. Applications of free living plant growth-promoting rhizobacteria. Antonie Van Leeuwenhoek 2004, 86, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, M.R.; Yesmin, L.; Vessey, J.K.; Rai, M. Plant-growth-promoting rhizobacteria as biofertilizers and biopesticides. In Handbook of Microbial Biofertilizers; Rai, M.K., Ed.; Food Products Press: Binghamton, NY, USA, 2005; pp. 137–181. [Google Scholar]
- Chi, F.; Shen, S.H.; Cheng, H.P.; Jing, Y.X.; Yanni, Y.G.; Dazzo, F.B. Ascending migration of endophytic rhizobia, from roots to leaves, inside rice plants and assessment of benefits to rice growth physiology. Appl. Environ. Microbiol. 2005, 71, 7271–7278. [Google Scholar] [CrossRef]
- Hao, T.; Chen, S. Colonization of wheat, maize and cucumber by Paenibacillus polymyxa WLY78. PLoS ONE 2017, 12, e0169980. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Zhang, H.; Wang, M.; Chen, S. Diazotrophic Paenibacillus beijingensis BJ-18 provides nitrogen for plant and promotes plant growth, nitrogen uptake and metabolism. Front. Microbiol. 2019, 10, 1119. [Google Scholar] [CrossRef]
- Bodenhausen, N.; Horton, M.W.; Bergelson, J. Bacterial communities associated with the leaves and the roots of Arabidopsis thaliana. PLoS ONE 2013, 8, e56329. [Google Scholar] [CrossRef]
- Bai, Y.; Muller, D.B.; Srinivas, G.; Garrido-Oter, R.; Potthoff, E.; Rott, M.; Dombrowski, N.; Munch, P.C.; Spaepen, S.; Remus-Emsermann, M.; et al. Functional overlap of the Arabidopsis leaf and root microbiota. Nature 2015, 528, 364–370. [Google Scholar] [CrossRef]
- Bulgari, D.; Casati, P.; Quaglino, F.; Bianco, P.A. Endophytic bacterial community of grapevine leaves influenced by sampling date and phytoplasma infection process. BMC Microbiol. 2014, 14, 198. [Google Scholar]
- Liu, H.; Carvalhais, L.C.; Schenk, P.M.; Dennis, P.G. Effects of jasmonic acid signalling on the wheat microbiome differ between body sites. Sci. Rep. 2017, 7, 41766. [Google Scholar] [CrossRef] [PubMed]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; van Themaat, E.V.L.; Schulze-Lefert, P. Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef] [PubMed]
- Gans, J.; Wolinsky, M.; Dunbar, J. Computational improvements reveal great bacterial diversity and high metal toxicity in soil. Science 2005, 309, 1387–1390. [Google Scholar] [CrossRef]
- Berg, G.; Smalla, K. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol. Ecol. 2009, 68, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Peiffer, J.A.; Spor, A.; Koren, O.; Jin, Z.; Tringe, S.G.; Dangl, J.L.; Buckler, E.S.; Ley, R.E. Diversity and heritability of the maize rhizosphere microbiome under field conditions. Proc. Natl. Acad. Sci. USA 2013, 110, 6548–6553. [Google Scholar] [CrossRef]
- Edwards, J.; Johnson, C.; Santos-Medellin, C.; Lurie, E.; Podishetty, N.K.; Bhatnagar, S.; Eisen, J.A.; Sundaresan, V. Structure, variation, and assembly of the root-associated microbiomes of rice. Proc. Natl. Acad. Sci. USA 2015, 112, 911–920. [Google Scholar] [CrossRef]
- Veach, A.M.; Morris, R.; Yip, D.Z.; Yang, Z.K.; Engle, N.L.; Cregger, M.A.; Tschaplinski, T.J.; Schadt, C.W. Rhizosphere microbiomes diverge among Populus trichocarpa plant-host genotypes and chemotypes, but it depends on soil origin. Microbiome 2019, 7, 76. [Google Scholar] [CrossRef]
- Lynch, J.M.; Whipps, J.M. Substrate flow in the rhizosphere. Plant Soil 1990, 129, 1–10. [Google Scholar] [CrossRef]
- Bever, J.D.; Platt, T.G.; Morton, E.R. Microbial population and community dynamics on plant roots and their feedbacks on plant communities. Annu. Rev. Microbiol. 2012, 66, 265–283. [Google Scholar]
- Miransari, M. Soil microbes and the availability of soil nutrients. Acta Physiol. Plant 2013, 35, 3075–3084. [Google Scholar] [CrossRef]
- Badri, D.V.; Chaparro, J.M.; Zhang, R.F.; Shen, Q.R.; Vivanco, J.M. Application of natural blends of phytochemicals derived from the root exudates of arabidopsis to the soil reveal that phenolic-related compounds predominantly modulate the soil microbiome. J. Biol. Chem. 2013, 288, 4502–4512. [Google Scholar] [CrossRef]
- Dakora, F.D.; Phillips, D.A. Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil 2002, 245, 35–47. [Google Scholar] [CrossRef]
- Kamilova, F.; Validov, S.; Azarova, T.; Mulders, I.; Lugtenberg, B. Enrichment for enhanced competitive plant root tip colonizers selects for a new class of biocontrol bacteria. Environ. Microbiol. 2005, 7, 1809–1817. [Google Scholar] [CrossRef] [PubMed]
- Kamilova, F.; Kravchenko, L.V.; Shaposhnikov, A.I.; Azarova, T.; Makarova, N.; Lugtenberg, B. Organic acids, sugars, and L-tryptophane in exudates of vegetables growing on stonewool and their effects on activities of rhizosphere bacteria. Mol. Plant Microbe Interact. 2006, 19, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Mathesius, U. The role of flavonoids in root-rhizosphere signalling: Opportunities and challenges for improving plant-microbe interactions. J. Exp. Bot. 2012, 63, 3429–3444. [Google Scholar] [CrossRef] [PubMed]
- Kudjordjie, E.N.; Sapkota, R.; Steffensen, S.K.; Fomsgaard, I.S.; Nicolaisen, M. Maize synthesized benzoxazinoids affect the host associated microbiome. Microbiome 2019, 7, 59. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, N.; Guo, X.Y.; Zhang, Y.; Ye, B.P. Comparative analysis of bacterial community structure in the rhizosphere of maize by high-throughput pyrosequencing. PLoS ONE 2017, 12, e0178425. [Google Scholar] [CrossRef]
- Xiong, W.; Guo, S.; Jousset, A.; Zhao, Q.Y.; Wu, H.S.; Li, R.; Kowalchuk, G.A.; Shen, Q.R. Bio-fertilizer application induces soil suppressiveness against Fusarium wilt disease by reshaping the soil microbiome. Soil Biol. Biochem. 2017, 114, 238–247. [Google Scholar] [CrossRef]
- Fu, L.; Penton, C.R.; Ruan, Y.; Shen, Z.; Xue, C.; Li, R.; Shen, Q. Inducing the rhizosphere microbiome by biofertilizer application to suppress banana Fusarium wilt disease. Soil Biol. Biochem. 2017, 104, 39–48. [Google Scholar] [CrossRef]
- Ke, X.; Feng, S.; Wang, J.; Lu, W.; Zhang, W.; Chen, M.; Lin, M. Effect of inoculation with nitrogen–fixing bacterium Pseudomonas stutzeri A1501 on maize plant growth and the microbiome indigenous to the rhizosphere. Syst. Appl. Microbiol. 2019, 42, 248–260. [Google Scholar] [CrossRef]
- Gupta, V.; Roper, M.M.; Roget, D.K. Potential for non-symbiotic N2-fixation in different agroecological zones of southern Australia. Aust. J. Soil Res. 2006, 44, 343–354. [Google Scholar] [CrossRef]
- Hurek, T.; Handley, L.L.; Reinhold-Hurek, B.; Piche, Y. Azoarcus grass endophytes contribute fixed nitrogen to the plant in an unculturable state. Mol. Plant Microbe Interact. 2002, 15, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Reiter, B.; Bürgmann, H.; Burg, K.; Sessitsch, A. Endophytic nifH gene diversity in African sweetpotato. Can. J. Microbiol. 2003, 49, 549–555. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carvalho, T.L.G.; Balsemao-Pires, E.; Saraiva, R.M.; Ferreira, P.C.G.; Hemerly, A.S. Nitrogen signalling in plant interactions with associative and endophytic diazotrophic bacteria. J. Exp. Bot. 2014, 65, 5631–5642. [Google Scholar] [CrossRef] [PubMed]
- Mus, F.; Crook, M.B.; Garcia, K.; Costas, A.G.; Geddes, B.A.; Kouri, E.D.; Paramasivan, P.; Ryu, M.H.; Oldroyd, G.E.D.; Poole, P.S.; et al. Symbiotic nitrogen fixation and the challenges to its extension to nonlegumes. Appl. Environ. Microbiol. 2016, 82, 3698–3710. [Google Scholar] [CrossRef] [PubMed]
- Barraquio, W.; Revilla, L.; Ladha, J. Isolation of endophytic diazotrophic bacteria from wetland rice. Plant. Soil 1997, 194, 15–24. [Google Scholar] [CrossRef]
- Ladha, J.K.; Barraquio, W.L.; Watanabe, I. Isolation and identification of nitrogen-fixing Enterobacter cloacae and Klebsiella planticola associated with rice plants. Can. J. Microbiol. 1983, 29, 1301–1308. [Google Scholar] [CrossRef]
- Reinhold-Hurek, B.; Hurek, T. Life in grasses: Diazotrophic endophytes. Trends Microbiol. 1998, 6, 202. [Google Scholar] [CrossRef]
- Reinhold-Hurek, B.; Hurek, T. Living inside plants: Bacterial endophytes. Curr. Opin. Plant Biol. 2011, 14, 435–443. [Google Scholar] [CrossRef]
- Boddey, R.M.; de Oliveira, O.C.; Urquiaga, S.; Reis, V.M.; de Olivares, F.L.; Baldani, V.L.D.; Döbereiner, J. Biological nitrogen fixation associated with sugar cane and rice: Contributions and prospects for improvement. Plant Soil 1995, 174, 195–209. [Google Scholar] [CrossRef]
- Van Deynze, A.; Zamora, P.; Delaux, P.M.; Heitmann, C.; Jayaraman, D.; Rajasekar, S.; Graham, D.; Maeda, J.; Gibson, D.; Schwartz, K.D.; et al. Nitrogen fixation in a landrace of maize is supported by a mucilage-associated diazotrophic microbiota. PLoS Biol. 2018, 16, e2006352. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Li, J.; Li, Q.X.; Chen, S.F. Paenibacillus beijingensis sp. nov., a nitrogen–fixing species isolated from wheat rhizosphere soil. Antonie Van Leeuwenhoek 2013, 105, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Shi, H.; Du, Z.; Wang, T.; Liu, X.; Chen, S. Comparative genomic and functional analysis reveal conservation of plant growth promoting traits in Paenibacillus polymyxa and its closely related species. Sci. Rep. 2016, 6, 21329. [Google Scholar] [CrossRef] [PubMed]
- Yunzhi, Z.; Jinwei, R.; Wenzhao, W.; Baosong, C.; Erwei, L.; Chen, S. Siderophore and indolic acid production by Paenibacillus triticisoli BJ-18 and their plant growth-promoting and antimicrobe abilities. PeerJ 2020, 8, e9403. [Google Scholar]
- Bulgarelli, D.; Rott, M.; Schlaeppi, K.; van Themaat, E.V.L.; Ahmadinejad, N.; Assenza, F.; Rauf, P.; Huettel, B.; Reinhardt, R.; Schmelzer, E.; et al. Revealing structure and assembly cues for Arabidopsis root–inhabiting bacterial microbiota. Nature 2012, 488, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Ramirez, L.E.; Caballero-Mellado, J.; Sepulveda, J.; Martinez-Romero, E. Colonization of sugarcane by Acetobacter diazotrophicus is inhibited by high N-fertilization. FEMS Microbiol. Ecol. 1999, 29, 117–128. [Google Scholar] [CrossRef]
- Johnston-Monje, D.; Lundberg, D.S.; Lazarovits, G.; Reis, V.M.; Raizada, M.N. Bacterial populations in juvenile maize rhizospheres originate from both seed and soil. Plant Soil 2016, 405, 337–355. [Google Scholar] [CrossRef]
- Walters, W.A.; Jin, Z.; Youngblut, N.; Wallace, J.G.; Sutter, J.; Zhang, W.; Gonzalez-Pena, A.; Peiffer, J.; Koren, O.; Shi, Q.J.; et al. Large-scale replicated field study of maize rhizosphere identifies heritable microbes. Proc. Natl. Acad. Sci. USA 2018, 115, 7368–7373. [Google Scholar] [CrossRef]
- Miao, L.; Fuzhen, Y.; Wang, Q.; Hai, Y.; Yang, L. Bacterial diversity of three beet soils based on microorganism isolation and culture. J. Anhui Agri. Sci. 2019, 47, 157–161. [Google Scholar]
- Jing, M.; Sun, X.; Li, S.; Hao, L. Draft genome sequence of Paenarthrobacter nicotinovorans Hce-1. Genome Announc. 2017, 5, e00727-17. [Google Scholar]
- Verma, S.K.; Kingsley, K.; Irizarry, I.; Bergen, M.; Kharwar, R.N.; White, J.F. Seed-vectored endophytic bacteria modulate development of rice seedlings. J. App. Microbiol. 2017, 122, 1680–1691. [Google Scholar] [CrossRef] [PubMed]
- Gerna, D.; Roach, T.; Mitter, B.; Stoggl, W.M.; Kranner, I. Hydrogen peroxide metabolism in inter–kingdom interaction between bacteria and wheat seeds and seedlings. Mol. Plant Microbe Interact. 2019, 33, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Elmore, M.T.; White, J.F.; Kingsley, K.L.; Diehl, K.H.; Verma, S.K. Pantoea spp. Associated with smooth crabgrass (Digitaria ischaemum) seed inhibit competitor plant species. Microorganisms 2019, 7, 143. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Jeong, J.C.; Lee, J.S.; Park, J.M.; Yang, J.W.; Lee, M.H.; Choi, S.H.; Kim, C.Y.; Kim, D.H.; Kim, S.W.; et al. Potential of Pantoea dispersa as an effective biocontrol agent for black rot in sweet potato. Sci. Rep. 2019, 9, 16354. [Google Scholar] [CrossRef]
- Luziatelli, F.; Ficca, A.G.; Melini, F.; Ruzzi, M. Genome sequence of the plant growth-promoting rhizobacterium Pantoea agglomerans C1. Microbiol. Resour. Announc. 2019, 8, e00828-19. [Google Scholar] [CrossRef]
- Doblas-Ibanez, P.; Deng, K.; Vasquez, M.F.; Giese, L.; Cobine, P.A.; Kolkman, J.M.; King, H.; Jamann, T.M.; Balint-Kurti, P.; De, L.; et al. Dominant, heritable resistance to stewart’s wilt in maize is associated with an enhanced vascular defense response to infection with Pantoea stewartii. Mol. Plant Microbe Interact. 2019, 32, 1581–1597. [Google Scholar] [CrossRef]
- Yassin, A.F.; Chen, W.M.; Hupfer, H.; Siering, C.; Kroppenstedt, R.M.; Arun, A.B.; Lai, W.A.; Shen, F.T.; Rekha, P.D.; Young, C.C. Lysobacter defluvii sp. nov., isolated from municipal solid waste. Int. J. Syst. Evol. Microbiol. 2007, 57, 1131–1136. [Google Scholar] [CrossRef]
- Yuen, G.Y.; Zhang, Z. Control of brown patch disease using the bacterium Stenotrophomonas maltophilia strain C3 and culture fluid. Int. Turfgrass Soc. Res. J. 2001, 9, 742–747. [Google Scholar]
- Ash, C.; Priest, F.G.; Collins, M.D. Molecular identification of rRNA group 3 bacilli (Ash, Farrow, Wallbanks and Collins) using a PCR probe test. Antonie Van Leeuwenhoek 1993, 64, 253–260. [Google Scholar] [CrossRef]
- Ma, Y.; Xia, Z.; Liu, X.; Chen, S. Paenibacillus sabinae sp. nov., a nitrogen-fixing species isolated from the rhizosphere soils of shrubs. Int. J. Syst. Evol. Microbiol. 2007, 57, 6–11. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, J.; Chen, S. Paenibacillus zanthoxyli sp. nov., a novel nitrogen-fixing species isolated from the rhizosphere of Zanthoxylum simulans. Int. J. Syst. Evol. Microbiol. 2007, 57, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.C.; Chen, S.F. Paenibacillus forsythiae sp. nov., a nitrogen-fixing species isolated from rhizosphere soil of Forsythia mira. Int. J. Syst. Evol. Microbiol. 2008, 58, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.Y.; Ma, Y.C.; Zhou, Y.G.; Gao, F.; Liu, H.C.; Chen, S.F. Paenibacillus sonchi sp. nov., a nitrogen-fixing species isolated from the rhizosphere of Sonchus oleraceus. Int. J. Syst. Evol. Microbiol. 2009, 59, 2656–2661. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Lv, J.; Chen, S.F. Paenibacillus sophorae sp. nov., a nitrogen-fixing species isolated from the rhizosphere of Sophora japonica. Int. J. Syst. Evol. Microbiol. 2011, 61, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Zhou, Y.G.; Liu, H.C.; Chen, S.F. Paenibacillus jilunlii sp. nov., a nitrogen-fixing species isolated from the rhizosphere of Begonia semperflorens. Int. J. Syst. Evol. Microbiol. 2011, 61, 1350–1355. [Google Scholar] [CrossRef]
- Xie, J.B.; Zhang, L.H.; Zhou, Y.G.; Liu, H.C.; Chen, S.F. Paenibacillus taohuashanense sp. nov., a nitrogen–fixing species isolated from rhizosphere soil of the root of Caragana kansuensis Pojark. Antonie Van Leeuwenhoek 2012, 102, 735–741. [Google Scholar] [CrossRef]
- von der Weid, I.; Duarte, G.F.; van Elsas, J.D.; Seldin, L. Paenibacillus brasilensis sp. nov., a novel nitrogen-fixing species isolated from the maize rhizosphere in Brazil. Int. J. Syst. Evol. Microbiol. 2002, 52, 2147–2153. [Google Scholar]
- Yang, Y.D.; Feng, X.M.; Hu, Y.; Ren, C.Z.; Zeng, Z. Effects of legume-oat intercropping on abundance and community structure of soil N2-fixing bacteria. Chin. J. Appl. Ecol. 2017, 28, 957–965. [Google Scholar]
- Zeffa, D.M.; Perini, L.J.; Silva, M.B.; de Sousa, N.V.; Scapim, C.A.; Oliveira, A.L.M.; Júnior, A.T.D.; Azeredo, G.L.S. Azospirillum brasilense promotes increases in growth and nitrogen use efficiency of maize genotypes. PLoS ONE 2019, 14, e0215332. [Google Scholar] [CrossRef]
- Romero-Perdomo, F.; Abril, J.; Camelo, M.; Moreno-Galván, A.; Pastrana, I.; Rojas-Tapias, D.; Bonilla, R. Azotobacter chroococcum as a potentially useful bacterial biofertilizer for cotton (Gossypium hirsutum): Effect in reducing N fertilization Azotobacter chroococcum como biofertilizante bacteriano potencialmente útil para el algodón (Gossypium hirsutum L.): Efecto en la reducción de la fertilización nitrogenada. Rev. Argent. Microbiol. 2017, 49, 377–383. [Google Scholar]
- de Alencar, M.J.I.; de Matos, F.G.; de Freitas, M.K.; da Conceicao, J.E.; Rouws, L.F.M. Occurrence of diverse Bradyrhizobium spp. in roots and rhizospheres of two commercial Brazilian sugarcane cultivars. Braz. J. Microbiol. 2019, 50, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Gaworzewska, E.T.; Carlile, M.J. Positive chemotaxis of Rhizobium leguminosarum and other bacteria towards root exudates from legumes and other plants. J. Gen. Appl. Microbiol. 1982, 128, 1179–1188. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Zhang, P.; Trivedi, P.; Riera, N.; Wang, Y.; Liu, X.; Fan, G.; Tang, J.; Coletta-Filho, H.D.; et al. The structure and function of the global citrus rhizosphere microbiome. Nat. Commun. 2018, 9, 4894. [Google Scholar] [CrossRef]
- Sarkar, A.; Marszalkowska, M.; Schaefer, M.; Pees, T.; Klingenberg, H.; Macht, F.; Reinhold-Hurek, B. Global expression analysis of the response to microaerobiosis reveals an important cue for endophytic establishment of Azoarcus sp. BH72. Environ. Microbiol. 2017, 19, 198–217. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.N.; Gomaa, A.B.; Yanni, Y.G.; El-Saadany, A.E.Y.; Stedtfeld, T.M.; Stedtfeld, R.D.; Gantner, S.; Chai, B.; Cole, J.; Hashsham, S.A.; et al. Alterations in the endophyte-enriched root-associated microbiome of rice receiving growth–promoting treatments of urea fertilizer and rhizobium biofertilizer. Microb. Ecol. 2020, 79, 367–382. [Google Scholar] [CrossRef]
- Shen, Z.Z.; Ruan, Y.Z.; Chao, X.; Zhang, J.; Li, R.; Shen, Q.R. Rhizosphere microbial community manipulated by 2 years of consecutive biofertilizer application associated with banana Fusarium wilt disease suppression. Biol. Fert. Soils 2015, 51, 553–562. [Google Scholar] [CrossRef]
- Mohammadi, A.; Shams-Ghahfarokhi, M.; Nazarian-Firouzabadi, F.; Kachuei, R.; Gholami-Shabani, M.; Razzaghi-Abyaneh, M. Giberella fujikuroi species complex isolated from maize and wheat in Iran: Distribution, molecular identification and fumonisin B1 in vitro biosynthesis. J. Sci. Food Agric. 2016, 96, 1333–1340. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Y.; Ma, C.; Zhang, D.; Wang, C.; Yang, Q. Transcriptome analysis of maize resistance to Fusarium graminearum. BMC Genom. 2016, 17, 477. [Google Scholar]
- Thangavelu, R.; Palaniswami, A.; Velazhahan, R. Mass production of Trichoderma harzianum for managing fusarium wilt of banana. Agr. Ecosyst. Environ. 2004, 103, 259–263. [Google Scholar] [CrossRef]
- Sezonov, G.; Joseleau-Petit, D.; d’Ari, R. Escherichia coli physiology in Luria-Bertani broth. J. Bacteriol. 2007, 189, 8746–8749. [Google Scholar] [CrossRef]
- Earl, H.J. A precise gravimetric method for simulating drought stress in pot experiments. Crop. Sci. 2003, 43, 1868–1873. [Google Scholar] [CrossRef]
- Evdokia, S.; Stavros, C.; Georgia, G.; Sofie, T.; Nele, W.; Jaco, V.; Nicolas, K. Exploitation of endophytic bacteria to enhance the phytoremediation potential of the wetland helophyte Juncus acutus. Front. Microbiol. 2016, 7, 1016. [Google Scholar]
- Baker, W.; Thompson, T. Determination of total nitrogen in plant samples by Kjeldahl. Plant Anal. Ref. Proced. South. Reg. United States South. Coop. Ser. Bull. 1992, 368, 13–16. [Google Scholar]
- Hanson, W.C. The photometric determination of phosphorus in fertilizers using the phosphovanado-molybdate complex. J. Sci. Food Agric. 1950, 1, 172–173. [Google Scholar] [CrossRef]
- Hedley, M.J.; Stewart, J.W.B. Method to measure microbial phosphate in soils. Soil Biol. Biochem. 1982, 14, 377–385. [Google Scholar] [CrossRef]
- Strickland, T.C.; Sollins, P. Improved method for separating light– and heavy–fraction organic material from soil. Soil Sci. Soc. Am. J. 1987, 51, 1390–1393. [Google Scholar] [CrossRef]
- Li, X.Y.; Deng, Y.; Li, Q.; Lu, C.Y.; Wang, J.J.; Zhang, H.W.; Zhu, J.G.; Zhou, J.H.; He, Z.L. Shifts of functional gene representation in wheat rhizosphere microbial communities under elevated ozone. ISME J. 2013, 7, 660–671. [Google Scholar] [CrossRef]
- Perakis, S.S.; Pett-Ridge, J.C.; Catricala, C.E. Nutrient feedbacks to soil heterotrophic nitrogen fixation in forests. Biogeochemistry 2017, 134, 41–55. [Google Scholar] [CrossRef]
- Jean, M.E.; Phalyvong, K.; Forest-Drolet, J.; Bellenger, J.P. Molybdenum and phosphorus limitation of asymbiotic nitrogen fixation in forests of eastern Canada: Influence of vegetative cover and seasonal variability. Soil Biol. Biochem. 2013, 67, 140–146. [Google Scholar] [CrossRef]
- Fierer, N.; Jackson, J.A.; Vilgalys, R.; Jackson, R.B. Assessment of soil microbial community structure by use of taxon-specific quantitative PCR assays. Appl. Environ. Microbiol. 2005, 71, 4117–4120. [Google Scholar] [CrossRef]
- Teng, Y.; Zhang, M.; Yang, G.; Wang, J.; Christie, P.; Luo, Y. Successive chlorothalonil applications inhibit soil nitrification and discrepantly affect abundances of functional genes in soil nitrogen cycling. Environ. Sci. Pollut. Res. 2017, 24, 3562–3571. [Google Scholar] [CrossRef] [PubMed]
- Lauber, C.L.; Ramirez, K.S.; Aanderud, Z.; Lennon, J.; Fierer, N. Temporal variability in soil microbial communities across land-use types. ISME J. 2013, 7, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
- Yusoff, M.Z.M.; Hu, A.Y.; Feng, C.J.; Maeda, T.; Shirai, Y.; Hassan, M.A.; Yu, C.P. Influence of pretreated activated sludge for electricity generation in microbial fuel cell application. Bioresour. Technol. 2013, 145, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, D.S.; Lebeis, S.L.; Paredes, S.H.; Yourstone, S.; Gehring, J.; Malfatti, S.; Tremblay, J.; Engelbrektson, A.; Kunin, V.; del Rio, T.G.; et al. Defining the core Arabidopsis thaliana root microbiome. Nature 2012, 488, 86. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Garrido-Oter, R.; Muench, P.C.; Weiman, A.; Droege, J.; Pan, Y.; McHardy, A.C.; Schulze-Lefert, P. Structure and function of the bacterial root microbiota in wild and domesticated barley. Cell Host Microbe 2015, 17, 392–403. [Google Scholar] [CrossRef]
- Roesch, C.; Mergel, A.; Bothe, H. Biodiversity of denitrifying and dinitrogen–fixing bacteria in an acid forest soil. Appl. Environ. Microbiol. 2002, 68, 3818–3829. [Google Scholar]
- Adams, R.I.; Miletto, M.; Taylor, J.W.; Bruns, T.D. Dispersal in microbes: Fungi in indoor air are dominated by outdoor air and show dispersal limitation at short distances. ISME J. 2013, 7, 1262–1273. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Yousuf, B.; Sanadhya, P.; Keshri, J.; Jha, B. Comparative molecular analysis of chemolithoautotrophic bacterial diversity and community structure from coastal saline soils, Gujarat, India. BMC Microbiol. 2012, 12, 150. [Google Scholar] [CrossRef]
- Banerjee, S.; Schlaeppi, K.; Van der Heijden, M. Keystone taxa as drivers of microbiome structure and functioning. Nat. Rev. Microbiol. 2018, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Csardi, G.; Nepusz, T. The igraph software package for complex network research. InterJournal Complex. Syst. 2005, 1695, 1–9. [Google Scholar]
- Newman, M.E.J. Modularity and community structure in networks. Proc. Natl. Acad. Sci. USA 2006, 103, 8577. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Li, Q.; Chen, S. Diazotroph Paenibacillus triticisoli BJ-18 Drives the Variation in Bacterial, Diazotrophic and Fungal Communities in the Rhizosphere and Root/Shoot Endosphere of Maize. Int. J. Mol. Sci. 2021, 22, 1460. https://doi.org/10.3390/ijms22031460
Li Y, Li Q, Chen S. Diazotroph Paenibacillus triticisoli BJ-18 Drives the Variation in Bacterial, Diazotrophic and Fungal Communities in the Rhizosphere and Root/Shoot Endosphere of Maize. International Journal of Molecular Sciences. 2021; 22(3):1460. https://doi.org/10.3390/ijms22031460
Chicago/Turabian StyleLi, Yongbin, Qin Li, and Sanfeng Chen. 2021. "Diazotroph Paenibacillus triticisoli BJ-18 Drives the Variation in Bacterial, Diazotrophic and Fungal Communities in the Rhizosphere and Root/Shoot Endosphere of Maize" International Journal of Molecular Sciences 22, no. 3: 1460. https://doi.org/10.3390/ijms22031460
APA StyleLi, Y., Li, Q., & Chen, S. (2021). Diazotroph Paenibacillus triticisoli BJ-18 Drives the Variation in Bacterial, Diazotrophic and Fungal Communities in the Rhizosphere and Root/Shoot Endosphere of Maize. International Journal of Molecular Sciences, 22(3), 1460. https://doi.org/10.3390/ijms22031460





