Combination of Entecavir or Tenofovir with Pegylated Interferon-α for Long-Term Reduction in Hepatitis B Surface Antigen Levels: Simultaneous, Sequential, or Add-on Combination Therapy
Abstract
1. Introduction
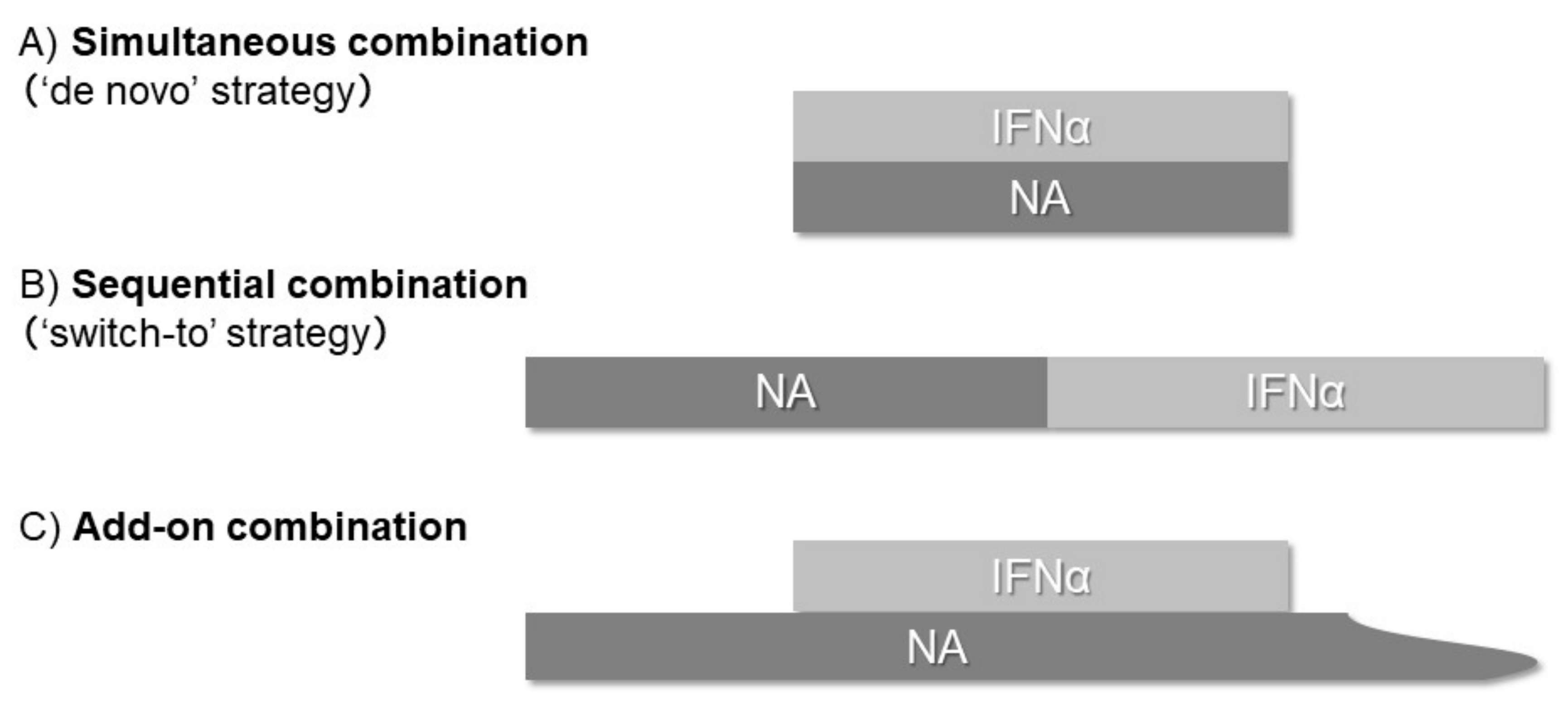
2. Simultaneous (“De Novo”) Combination Strategy
3. Sequential (“Switch-to”) Combination Strategy
4. Add-on Combination Strategy
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALT | Alanine aminotransferase |
| cccDNA | Covalently closed circular DNA |
| ETV | Entecavir |
| HBeAg | Hepatitis B e-antigen |
| HBsAg | Hepatitis B surface antigen |
| HBV | Hepatitis B virus |
| HCC | Hepatocellular carcinoma |
| NA | Nucleoside/nucleotide analogue |
| peg-IFNα | Pegylated interferon-α |
| TAF | Tenofovir alafenamide |
| TDF | Tenofovir disoproxil fumarate |
References
- European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef] [PubMed]
- Terrault, N.A.; Lok, A.; McMahon, B.J.; Chang, K.M.; Hwang, J.P.; Jonas, M.M.; Brown, R.S., Jr.; Bzowej, N.H.; Wong, J.B. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018, 67, 1560–1599. [Google Scholar] [CrossRef] [PubMed]
- Sarin, S.K.; Kumar, M.; Lau, G.K.; Abbas, Z.; Chan, H.L.; Chen, C.J.; Chen, D.S.; Chen, H.L.; Chen, P.J.; Chien, R.N.; et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: A 2015 update. Hepatol. Int. 2016, 10, 1–98. [Google Scholar] [CrossRef] [PubMed]
- Drafting Committee for Hepatitis Management Guidelines, the Japan Society of Hepatology. Japan Society of Hepatology Guidelines for the Management of Hepatitis B Virus Infection: 2019 update. Hepatol. Res. 2020, 50, 892–923. [Google Scholar] [CrossRef] [PubMed]
- Mouzannar, K.; Liang, T.J. Hepatitis B virus—Recent therapeutic advances and challenges to cure. J. Hepatol. 2020, 73, 694–695. [Google Scholar] [CrossRef] [PubMed]
- Thimme, R.; Dandri, M. Dissecting the divergent effects of interferon-alpha on immune cells: Time to rethink combination therapy in chronic hepatitis B. J. Hepatol. 2013, 58, 205–209. [Google Scholar] [CrossRef]
- Murata, K.; Asano, M.; Matsumoto, A.; Sugiyama, M.; Nishida, N.; Tanaka, E.; Inoue, T.; Sakamoto, M.; Enomoto, N.; Shirasaki, T.; et al. Induction of IFN-λ3 as an additional effect of nucleotide, not nucleoside, analogues: A new potential target for HBV infection. Gut 2018, 67, 362–371. [Google Scholar] [CrossRef]
- Koike, K.; Suyama, K.; Ito, H.; Itoh, H.; Sugiura, W. Randomized prospective study showing the non-inferiority of tenofovir to entecavir in treatment-naïve chronic hepatitis B patients. Hepatol. Res. 2018, 48, 59–68. [Google Scholar] [CrossRef]
- Choi, J.; Kim, H.J.; Lee, J.; Cho, S.; Ko, M.J.; Lim, Y.S. Risk of Hepatocellular Carcinoma in Patients Treated with Entecavir vs Tenofovir for Chronic Hepatitis B: A Korean Nationwide Cohort Study. JAMA Oncol. 2019, 5, 30–36. [Google Scholar] [CrossRef]
- Tseng, C.H.; Hsu, Y.C.; Chen, T.H.; Ji, F.; Chen, I.S.; Tsai, Y.N.; Hai, H.; Thuy, L.; Hosaka, T.; Sezaki, H.; et al. Hepatocellular carcinoma incidence with tenofovir versus entecavir in chronic hepatitis B: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 1039–1052. [Google Scholar] [CrossRef]
- Boni, C.; Laccabue, D.; Lampertico, P.; Giuberti, T.; Viganò, M.; Schivazappa, S.; Alfieri, A.; Pesci, M.; Gaeta, G.B.; Brancaccio, G.; et al. Restored function of HBV-specific T cells after long-term effective therapy with nucleos(t)ide analogues. Gastroenterology 2012, 143, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; Liu, X.; Li, X.; Kong, H.; Tian, L.; Chen, Y. T-cell exhaustion in chronic hepatitis B infection: Current knowledge and clinical significance. Cell Death Dis. 2015, 6, e1694. [Google Scholar] [CrossRef] [PubMed]
- Wieland, S.F.; Eustaquio, A.; Whitten-Bauer, C.; Boyd, B.; Chisari, F.V. Interferon prevents formation of replication-competent hepatitis B virus RNA-containing nucleocapsids. Proc. Natl. Acad. Sci. USA 2005, 102, 9913–9917. [Google Scholar] [CrossRef] [PubMed]
- Belloni, L.; Allweiss, L.; Guerrieri, F.; Pediconi, N.; Volz, T.; Pollicino, T.; Petersen, J.; Raimondo, G.; Dandri, M.; Levrero, M. IFN-α inhibits HBV transcription and replication in cell culture and in humanized mice by targeting the epigenetic regulation of the nuclear cccDNA minichromosome. J. Clin. Investig. 2012, 122, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Micco, L.; Peppa, D.; Loggi, E.; Schurich, A.; Jefferson, L.; Cursaro, C.; Panno, A.M.; Bernardi, M.; Brander, C.; Bihl, F.; et al. Differential boosting of innate and adaptive antiviral responses during pegylated-interferon-alpha therapy of chronic hepatitis B. J. Hepatol. 2013, 58, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Marcellin, P.; Lau, G.K.; Bonino, F.; Farci, P.; Hadziyannis, S.; Jin, R.; Lu, Z.M.; Piratvisuth, T.; Germanidis, G.; Yurdaydin, C.; et al. Peginterferon Alfa-2a HBeAg-Negative Chronic Hepatitis B Study Group. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N. Engl. J. Med. 2004, 351, 1206–1217. [Google Scholar] [CrossRef] [PubMed]
- Lau, G.K.; Piratvisuth, T.; Luo, K.X.; Marcellin, P.; Thongsawat, S.; Cooksley, G.; Gane, E.; Fried, M.W.; Chow, W.C.; Paik, S.W.; et al. Peginterferon Alfa-2a HBeAg-Positive Chronic Hepatitis B Study Group. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N. Engl. J. Med. 2005, 352, 2682–2695. [Google Scholar] [CrossRef]
- Enomoto, M.; Tamori, A.; Kohmoto, M.T.; Hayashi, T.; Jomura, H.; Habu, D.; Sakaguchi, H.; Takeda, T.; Kawada, N.; Seki, S.; et al. Lamivudine and IFN-beta sequential therapy in HBe antigen-positive patients with chronic hepatitis B virus genotype C infection. J. Interferon Cytokine Res. 2007, 27, 201–207. [Google Scholar] [CrossRef]
- Enomoto, M.; Nishiguchi, S.; Tamori, A.; Kobayashi, S.; Sakaguchi, H.; Shiomi, S.; Kim, S.R.; Enomoto, H.; Saito, M.; Imanishi, H.; et al. Entecavir and interferon-α sequential therapy in Japanese patients with hepatitis B e antigen-positive chronic hepatitis B. J. Gastroenterol. 2013, 48, 397–404. [Google Scholar] [CrossRef]
- Enomoto, M.; Tamori, A.; Nishiguchi, S.; Kawada, N. Combination therapy with a nucleos(t)ide analogue and interferon for chronic hepatitis B: Simultaneous or sequential. J. Gastroenterol. 2013, 48, 999–1005. [Google Scholar] [CrossRef]
- Chang, T.T.; Gish, R.G.; de Man, R.; Gadano, A.; Sollano, J.; Chao, Y.C.; Lok, A.S.; Han, K.H.; Goodman, Z.; Zhu, J.; et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N. Engl. J. Med. 2006, 354, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.L.; Shouval, D.; Lok, A.S.; Chang, T.T.; Cheinquer, H.; Goodman, Z.; DeHertogh, D.; Wilber, R.; Zink, R.C.; Cross, A.; et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N. Engl. J. Med. 2006, 354, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Marcellin, P.; Heathcote, E.J.; Buti, M.; Gane, E.; de Man, R.A.; Krastev, Z.; Germanidis, G.; Lee, S.S.; Flisiak, R.; Kaita, K.; et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N. Engl. J. Med. 2008, 359, 2442–2455. [Google Scholar] [CrossRef]
- Chan, H.L.; Fung, S.; Seto, W.K.; Chuang, W.L.; Chen, C.Y.; Kim, H.J.; Hui, A.J.; Janssen, H.L.; Chowdhury, A.; Tsang, T.Y.; et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAg-positive chronic hepatitis B virus infection: A randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol. Hepatol. 2016, 1, 185–195. [Google Scholar] [CrossRef]
- Buti, M.; Gane, E.; Seto, W.K.; Chan, H.L.; Chuang, W.L.; Stepanova, T.; Hui, A.J.; Lim, Y.S.; Mehta, R.; Janssen, H.L. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: A randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol. Hepatol. 2016, 1, 196–206. [Google Scholar] [CrossRef]
- Tangkijvanich, P.; Chittmittraprap, S.; Poovorawan, K.; Limothai, U.; Khlaiphuengsin, A.; Chuaypen, N.; Wisedopas, N.; Poovorawan, Y. A randomized clinical trial of peginterferon alpha-2b with or without entecavir in patients with HBeAg-negative chronic hepatitis B: Role of host and viral factors associated with treatment response. J. Viral Hepat. 2016, 23, 427–438. [Google Scholar] [CrossRef]
- Hagiwara, S.; Kudo, M.; Osaki, Y.; Matsuo, H.; Inuzuka, T.; Matsumoto, A.; Tanaka, E.; Sakurai, T.; Ueshima, K.; Inoue, T. Impact of peginterferon alpha-2b and entecavir hydrate combination therapy on persistent viral suppression in patients with chronic hepatitis B. J. Med. Virol. 2013, 85, 987–995. [Google Scholar] [CrossRef]
- Hagiwara, S.; Nishida, N.; Watanabe, T.; Ida, H.; Sakurai, T.; Ueshima, K.; Takita, M.; Komeda, Y.; Nishijima, N.; Osaki, Y.; et al. Sustained antiviral effects and clearance of hepatitis surface antigen after combination therapy with entecavir and pegylated interferon in chronic hepatitis B. Antivir. Ther. 2018, 23, 513–521. [Google Scholar] [CrossRef]
- Marcellin, P.; Ahn, S.H.; Ma, X.; Caruntu, F.A.; Tak, W.Y.; Elkashab, M.; Chuang, W.L.; Lim, S.G.; Tabak, F.; Mehta, R.; et al. Combination of Tenofovir Disoproxil Fumarate and Peginterferon α-2a Increases Loss of Hepatitis B Surface Antigen in Patients with Chronic Hepatitis B. Gastroenterology 2016, 150, 134–144. [Google Scholar] [CrossRef]
- Ahn, S.H.; Marcellin, P.; Ma, X.; Caruntu, F.A.; Tak, W.Y.; Elkhashab, M.; Chuang, W.L.; Tabak, F.; Mehta, R.; Petersen, J.; et al. Hepatitis B Surface Antigen Loss with Tenofovir Disoproxil Fumarate Plus Peginterferon Alfa-2a: Week 120 Analysis. Dig. Dis. Sci. 2018, 63, 3487–3497. [Google Scholar] [CrossRef]
- Zheng, C.; Yan, H.; Zeng, J.; Cai, S.; Wu, X. Comparison of pegylated interferon monotherapy and de novo pegylated interferon plus tenofovir combination therapy in patients with chronic hepatitis B. Infect. Drug Resist. 2019, 12, 845–854. [Google Scholar] [CrossRef] [PubMed]
- de Niet, A.; Jansen, L.; Stelma, F.; Willemse, S.B.; Kuiken, S.D.; Weijer, S.; van Nieuwkerk, C.; Zaaijer, H.L.; Molenkamp, R.; Takkenberg, R.B. Peg-interferon plus nucleotide analogue treatment versus no treatment in patients with chronic hepatitis B with a low viral load: A randomised controlled, open-label trial. Lancet Gastroenterol. Hepatol. 2017, 2, 576–584. [Google Scholar] [CrossRef]
- Ning, Q.; Han, M.; Sun, Y.; Jiang, J.; Tan, D.; Hou, J.; Tang, H.; Sheng, J.; Zhao, M. Switching from entecavir to PegIFN alfa-2a in patients with HBeAg-positive chronic hepatitis B: A randomised open-label trial (OSST trial). J. Hepatol. 2014, 61, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Jiang, J.; Hou, J.; Tan, D.; Sun, Y.; Zhao, M.; Ning, Q. Sustained immune control in HBeAg-positive patients who switched from entecavir therapy to pegylated interferon-α2a: 1 year follow-up of the OSST study. Antivir. Ther. 2016, 21, 337–344. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, R.; Ru, G.Q.; Yu, L.L.; Yao, J.; Wang, H. Pegylated-interferon consolidation treatment versus nucleos(t)ide analogue consolidation treatment in non-cirrhotic hepatitis B patients with hepatitis B e antigen seroconversion: An open-label pilot trial. Hepatol. Int. 2019, 13, 422–430. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, K.; Chen, W.; Liao, J.; Luo, X.; Chen, R. Switching to PegIFNα-2b leads to HBsAg loss in patients with low HBsAg levels and HBV DNA suppressed by NAs. Sci. Rep. 2017, 7, 13383. [Google Scholar] [CrossRef]
- Hu, P.; Shang, J.; Zhang, W.; Gong, G.; Li, Y.; Chen, X.; Jiang, J.; Xie, Q.; Dou, X.; Sun, Y.; et al. HBsAg Loss with Peg-interferon Alfa-2a in Hepatitis B Patients with Partial Response to Nucleos(t)ide Analog: New Switch Study. J. Clin. Transl. Hepatol. 2018, 6, 25–34. [Google Scholar] [CrossRef]
- Xie, Q.; Zhou, H.; Bai, X.; Wu, S.; Chen, J.J.; Sheng, J.; Xie, Y.; Chen, C.; Chan, H.L.; Zhao, M. A randomized, open-label clinical study of combined pegylated interferon Alfa-2a (40KD) and entecavir treatment for hepatitis B “e” antigen-positive chronic hepatitis B. Clin. Infect. Dis. 2014, 59, 1714–1723. [Google Scholar] [CrossRef]
- Tamaki, N.; Kurosaki, M.; Kusakabe, A.; Orito, E.; Joko, K.; Kojima, Y.; Kimura, H.; Uchida, Y.; Hasebe, C.; Asahina, Y.; et al. Hepatitis B surface antigen reduction by switching from long-term nucleoside/nucleotide analogue administration to pegylated interferon. J. Viral Hepat. 2017, 24, 672–678. [Google Scholar] [CrossRef]
- Matsumoto, A.; Nishiguchi, S.; Enomoto, H.; Kang, J.H.; Tanaka, Y.; Shinkai, N.; Kurosaki, M.; Enomoto, M.; Kanda, T.; Yokosuka, O.; et al. Combinational use of hepatitis B viral antigens predicts responses to nucleos(t)ide analogue/peg-interferon sequential therapy. J. Gastroenterol. 2018, 53, 247–257. [Google Scholar] [CrossRef]
- Kimura, T.; Rokuhara, A.; Sakamoto, Y.; Yagi, S.; Tanaka, E.; Kiyosawa, K.; Maki, N. Sensitive enzyme immunoassay for hepatitis B virus core-related antigens and their correlation to virus load. J. Clin. Microbiol. 2002, 40, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Desbiolles, A.; Feldman, S.F.; Ahn, S.H.; Alidjinou, E.K.; Atsukawa, M.; Bocket, L.; Brunetto, M.R.; Buti, M.; Carey, I.; et al. Assay for Hepatitis B Core-related Antigen Identify Patients with High Viral Load: Systematic Review and Meta-analysis of Individual Participant Data. Clin. Gastroenterol. Hepatol. 2020, 46–60. [Google Scholar] [CrossRef]
- Enomoto, M.; Nishiguchi, S.; Tamori, A.; Kozuka, R.; Fujii, H.; Uchida-Kobayashi, S.; Fukunishi, S.; Tsuda, Y.; Higuchi, K.; Saito, M.; et al. Sequential therapy involving an early switch from entecavir to pegylated interferon-α in Japanese patients with chronic hepatitis B. Hepatol. Res. 2018, 48, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, W.P.; Xie, Q.; Sonneveld, M.J.; Zhang, N.; Zhang, Q.; Tabak, F.; Streinu-Cercel, A.; Wang, J.Y.; Idilman, R.; Reesink, H.W.; et al. Adding pegylated interferon to entecavir for hepatitis B e antigen-positive chronic hepatitis B: A multicenter randomized trial (ARES study). Hepatology 2015, 61, 1512–1522. [Google Scholar] [CrossRef]
- Van Campenhout, M.; Brouwer, W.P.; Xie, Q.; Guo, S.; Chi, H.; Qi, X.; Tabak, F.; Streinu-Cercel, A.; Wang, J.Y.; Zhang, N.P.; et al. Long-term follow-up of patients treated with entecavir and peginterferon add-on therapy for HBeAg-positive chronic hepatitis B infection: ARES long-term follow-up. J. Viral. Hepat. 2019, 26, 109–117. [Google Scholar] [CrossRef]
- Chi, H.; Hansen, B.E.; Guo, S.; Zhang, N.P.; Qi, X.; Chen, L.; Guo, Q.; Arends, P.; Wang, J.Y.; Verhey, E.; et al. Pegylated Interferon Alfa-2b Add-on Treatment in Hepatitis B Virus Envelope Antigen-Positive Chronic Hepatitis B Patients Treated with Nucleos(t)ide Analogue: A Randomized, Controlled Trial (PEGON). J. Infect. Dis. 2017, 215, 1085–1093. [Google Scholar] [CrossRef]
- Liem, K.S.; van Campenhout, M.; Xie, Q.; Brouwer, W.P.; Chi, H.; Qi, X.; Chen, L.; Tabak, F.; Hansen, B.E.; Janssen, H. Low hepatitis B surface antigen and HBV DNA levels predict response to the addition of pegylated interferon to entecavir in hepatitis B e antigen positive chronic hepatitis B. Aliment. Pharmacol. Ther. 2019, 49, 448–456. [Google Scholar] [CrossRef]
- Bourlière, M.; Rabiega, P.; Ganne-Carrie, N.; Serfaty, L.; Marcellin, P.; Barthe, Y.; Thabut, D.; Guyader, D.; Hezode, C.; Picon, M.; et al. Effect on HBs antigen clearance of addition of pegylated interferon alfa-2a to nucleos(t)ide analogue therapy versus nucleos(t)ide analogue therapy alone in patients with HBe antigen-negative chronic hepatitis B and sustained undetectable plasma hepatitis B virus DNA: A randomised, controlled, open-label trial. Lancet Gastroenterol. Hepatol. 2017, 2, 177–188. [Google Scholar]
- Li, G.J.; Yu, Y.Q.; Chen, S.L.; Fan, P.; Shao, L.Y.; Chen, J.Z.; Li, C.S.; Yi, B.; Chen, W.C.; Xie, S.Y.; et al. Sequential combination therapy with pegylated interferon leads to loss of hepatitis B surface antigen and hepatitis B e antigen (HBeAg) seroconversion in HBeAg-positive chronic hepatitis B patients receiving long-term entecavir treatment. Antimicrob. Agents Chemother. 2015, 59, 4121–4128. [Google Scholar] [CrossRef]
- Matsumoto, A.; Nishiguchi, S.; Enomoto, H.; Tanaka, Y.; Shinkai, N.; Okuse, C.; Kang, J.H.; Matsui, T.; Miyase, S.; Yatsuhashi, H.; et al. Pilot study of tenofovir disoproxil fumarate and pegylated interferon-alpha 2a add-on therapy in Japanese patients with chronic hepatitis B. J. Gastroenterol. 2020, 55, 977–989. [Google Scholar] [CrossRef]
- Lampertico, P.; Brunetto, M.R.; Craxì, A.; Gaeta, G.B.; Rizzetto, M.; Rozzi, A.; Colombo, M.; HERMES Study Group. Add-on peginterferon alfa-2a to nucleos(t)ide analogue therapy for Caucasian patients with hepatitis B ‘e’ antigen-negative chronic hepatitis B genotype D. J. Viral. Hepat. 2019, 26, 118–125. [Google Scholar] [CrossRef]
- Enomoto, M.; Nishiguchi, S.; Tamori, A.; Kozuka, R.; Hayashi, T.; Kohmoto, M.T.; Jomura, H.; Morikawa, H.; Murakami, Y.; Shiomi, S.; et al. Long-Term Outcome of Sequential Therapy with Lamivudine Followed by Interferon-β in Nucleoside-Naive, Hepatitis B e-Antigen-Positive Patients with Chronic Hepatitis B Virus Genotype C Infection. J. Interferon Cytokine Res. 2015, 35, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Bazinet, M.; Pântea, V.; Placinta, G.; Moscalu, I.; Cebotarescu, V.; Cojuhari, L.; Jimbei, P.; Iarovoi, L.; Smesnoi, V.; Musteata, T.; et al. Safety and Efficacy of 48 Weeks REP 2139 or REP 2165, Tenofovir Disoproxil, and Pegylated Interferon Alfa-2a in Patients With Chronic HBV Infection Naïve to Nucleos(t)ide Therapy. Gastroenterology 2020, 158, 2180–2194. [Google Scholar] [CrossRef] [PubMed]
- Janssen, H.L.A.; Hou, J.; Asselah, T.; Chan, H.L.Y.; Zoulim, F.; Tanaka, Y.; Janczewska, E.; Nahass, R.; Bourgeois, S.; Buti, M.; et al. Efficacy and Safety Results of the Phase 2 JNJ-56136379 JADE Study in Patients with Chronic Hepatitis B: Interim Week 24 Data. J. Hepatol. 2020, 73, S129–S130. [Google Scholar] [CrossRef]
| Author (Year) | HBeAg | n (Genotype, %) | Age * | Male (%) | Regimens | HBV DNA Response (%) | Seroconversion of HBeAg (%) | Decline in HBsAg (log) | HBsAg Seroclearance (%) |
|---|---|---|---|---|---|---|---|---|---|
| Tangkijvanich et al. (2016) [26] | − | 63 (B16/C81) | 40 + 9.8 | 73 | 48-wk ETV + Peg-IFNα | 38.1% < 2000 IU/mL, 6.3% < 10 IU/mL | N.D. | −0.5 | 4.8% |
| Hagiwara et al. (2013) [27] | +/− | 17 (C100) | 47 + 12 | 76 | 48-wk ETV + Peg-IFNα | 71% < 10,000 copies/mL at EOF | 73% at EOF | −0.4 | 5.9% |
| Hagiwara et al. (2018) [28] | +/− | 26 (C100) | 44 + 10 | 69 | 48-wk ETV + Peg-IFNα | 62% < 4.0 log copies/mL at EOF | 60% at EOF | N.D. | 15% |
| Marcellin et al. (2016) [29] | +/− | 186 (A9/B27/C42/D21) | 38 + 17 | 68 | 48-wk TDF + Peg-IFNα | 9.1% < 15 IU/mL at wk 72 | 25% at wk 72 | −1.3 | 9.1% at wk 72 |
| Ahn et al. (2018) [30] | +/− | 186 (A9/B27/C42/D21) | 38 + 17 | 68 | 48-wk TDF + Peg-IFNα | 24.3% < 15 IU/mL at wk 120 | 29.5% at wk 120 | −2.4 | 10.4% at wk 120 |
| Zheng et al. (2019) [31] | +/− | 77 (N.D.) | 30 + 7.3 | 71 | 48-wk TDF + Peg-IFNα | 33.8% < 100 IU/mL at EOT | 34% at EOT | N.D. | 13% |
| de Niet et al. (2017) [32] | − | 45 (A22/B7/C2/D29/E16) | 43 + 12 | 47 | 48-wk TDF + Peg-IFNα | N.D. | N.D. | −0.59 | 4% |
| Author (Year) | HBeAg | n (Genotype, %) | Age * | Male (%) | Regimens | HBV DNA Response (%) | Seroconversion of HBeAg (%) | Decline in HBsAg (log) | HBsAg Seroclearance (%) |
|---|---|---|---|---|---|---|---|---|---|
| Ning et al. (2014) [33] | + | 94 (N.D.) | 33 + 8.3 * | 80 | >12-mo ETV →48-wk Peg-IFNα | 72% < 1000 copies/mL at EOT | 14.9% at EOT | −0.82 | 8.5 |
| Han et al. (2016) [34] | + | 62 (N.D.) | 34 + 8.3 * | 81 | >12-mo ETV →48-wk Peg-IFNα | 51.6% < 1000 copies/mL at EOT | 38.7% at 1 year post-treatment | N.D. | 9.7 |
| Zhou et al. (2019) [35] | + | 24 (N.D.) | 35 + 7 * | 67 | >12-mo NA →48-wk Peg-IFNα | N.D. | 27.2% at EOF | −2.2 | 36 |
| Huang et al. (2017) [36] | +/− | 43 (N.D.) | 32 + 7.8 * | 70 | >2-yr NA →60-wk Peg-IFNα | N.D. | 65.1% at EOF | −1.6 | 32.6 |
| Hu et al. (2018) [37] | + | 153 (N.D.) | 35 + 10 * | 82 | 1–3-yr NA →48-wk Peg-IFNα | 34.6% < 200 IU/mL at EOF | 51% at EOF | −1.09 | 9.8 |
| Hu et al. (2018) [38] | + | 150 (N.D.) | 33 + 8.8 * | 80 | 1–3-yr NA →96-wk Peg-IFNα | 48.7% < 200 IU/mL at EOF | 55% at EOF | −1.30 | 15.3 |
| Xie et al. (2014) [38] | + | 73 (N.D.) | 30 + 8.4 * | 82 | 21-wk ETV →48-wk Peg-IFNα | 37% < 1000 copies/mL at EOF | 26% at EOF | −0.4 at EOF | 1.4 |
| Tamaki et al. (2017) [39] | +/− | 49 (B14/C78) | 50 + 11 * | 69 | >12-mo NA →48-wk Peg-IFNα | 78% < 2.1 log copies/mL at 48 wk | 44% at EOT | −0.81 | 4 |
| Matsumoto et al. (2018) [40] | +/− | 95 (A7/B4/C82) | 45 (27–87) † | 95 | >12-mo NA →48-wk Peg-IFNα | N.D. | N.D. | −0.8 (inresponders) | N.D. |
| Enomoto et al. (2018) [43] | +/− | 24 (B4/C96) | 35 + 7 * | 67 | 36–52-wk ETV →48-wk Peg-IFNα | 29% < 10,000 copies at EOF | 68% at EOF | −0.49 (inresponders) | 8.3 |
| Author (Year) | HBeAg | n (Genotype, %) | Age * | Male (%) | Regimens | HBV DNA Response (%) | Seroconversion of HBeAg (%) | Decline in HBsAg (log) | HBsAg Seroclearance (%) |
|---|---|---|---|---|---|---|---|---|---|
| Brouwer et al. (2015) [44] | + | 85 (A5/B23/C39/D33) | 32 + 10 * | 74 | 24-wk Peg-IFNα on 24-wk ETV | 77% < 200 IU/mL, 57% < 20 IU/mL at EOF | 26% at EOF | −0.8 | 1.2 |
| von Campenhout et al. (2019) [45] | + | 48 (A8/B23/C31/D38) | 33 + 11 * | 73 | 24-wk Peg-IFNα on 24-wk ETV | 69% undetectable | 29% | −1.3 | 2.1 |
| Chi et al. (2017) [46] | + | 39 (B8/C39/D8) | 35 + 9 * | 72 | 48-wk Peg-IFNα on >12-mo ETV/TDF | 77% < 20 IU/mL at EOF | 21% at EOF | −0.35 | 0 |
| Liem et al. (2019) [47] | + | 118 (A3/B19/C38/D25) | 33 + 10 * | 74 | 24–48-wk Peg-IFNα on >24-wk ETV | 85% < 2000 IU/mL, 82% < 2 00 IU/mL at EOF | 24% at EOF | 23% > 0.5 log decline | 0.8 |
| Bourliere et al. (2017) [48] | − | 90 (N.D.) | 47 (41–57) † | 83 | 48-wk Peg-IFNα on >12-mo NA | N.D. | N.D. | −1.03 | 10.0 |
| Li et al. (2015) [49] | + | 81 (N.D.) | 32 (23–54) † | 62 | 48-wk Peg-IFNα on ETV | N.D. | 48% | -0.96 | 4 |
| Matsumoto et al. (2020) [50] | +/− | 32 (A3/B3/C88/D3) | 43 + 8 * | 63 | 48-wk Peg-IFNα on >12-wk TDF | N.D. | 43% at EOT | −0.44 | 0 |
| Lampertico et al. (2018) [51] | − | 70 (D100) | 51 (29–64) † | 81.4 | 48-wk Peg-IFNα on NA | N.D. | N.D. | 10.9% ≥ 1 log decline | 1.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, K.; Enomoto, M.; Tamori, A.; Nishiguchi, S.; Kawada, N. Combination of Entecavir or Tenofovir with Pegylated Interferon-α for Long-Term Reduction in Hepatitis B Surface Antigen Levels: Simultaneous, Sequential, or Add-on Combination Therapy. Int. J. Mol. Sci. 2021, 22, 1456. https://doi.org/10.3390/ijms22031456
Yoshida K, Enomoto M, Tamori A, Nishiguchi S, Kawada N. Combination of Entecavir or Tenofovir with Pegylated Interferon-α for Long-Term Reduction in Hepatitis B Surface Antigen Levels: Simultaneous, Sequential, or Add-on Combination Therapy. International Journal of Molecular Sciences. 2021; 22(3):1456. https://doi.org/10.3390/ijms22031456
Chicago/Turabian StyleYoshida, Kanako, Masaru Enomoto, Akihiro Tamori, Shuhei Nishiguchi, and Norifumi Kawada. 2021. "Combination of Entecavir or Tenofovir with Pegylated Interferon-α for Long-Term Reduction in Hepatitis B Surface Antigen Levels: Simultaneous, Sequential, or Add-on Combination Therapy" International Journal of Molecular Sciences 22, no. 3: 1456. https://doi.org/10.3390/ijms22031456
APA StyleYoshida, K., Enomoto, M., Tamori, A., Nishiguchi, S., & Kawada, N. (2021). Combination of Entecavir or Tenofovir with Pegylated Interferon-α for Long-Term Reduction in Hepatitis B Surface Antigen Levels: Simultaneous, Sequential, or Add-on Combination Therapy. International Journal of Molecular Sciences, 22(3), 1456. https://doi.org/10.3390/ijms22031456






