A Closer Look at the Cellular and Molecular Components of the Deep/Muscular Fasciae
Abstract
1. Introduction
2. Classification of the Fasciae
- -
- Superficial fascia, which is found directly under the skin and superficial adipose layer. It can show stratification both grossly and microscopically. It is conventionally described as being made up of membranous layers with loosely packed interwoven collagen and elastic fibers more superficial than other types and containing more elastic fibers [19];
- -
- Deep/muscular fasciae: depending on their orientations, composition architectures, and anatomical locations, the two main types of deep/muscular fasciae are the aponeurotic fasciae and the epimysial fasciae [13,20]. The former refers to all the “well-defined fibrous sheaths that cover and keep in place a group of muscles or serve for the insertion of a broad muscle”, as in the case of the deep fasciae of the limbs, the thoracolumbar fascia and the rectus sheath of the abdomen [21]. The latter refers to the connective tissue sheath surrounding skeletal muscle and, in some cases, directly connected to the periosteum of the bones as in the case of the deep fascia of the trunk and the epimysium of the limb muscles;
- -
- Visceral fasciae, which are all the fasciae closely connected to individual organs and giving shape to them, support the parenchyma as well as all the fibrous sheets forming the compartments for the organs and connect them to the musculoskeletal system [22];
- -
- Neural fasciae, which are all the meningeal layers and the connective tissues that envelop the peripheral nerves.
3. Thickness of the Deep/Muscular Fasciae
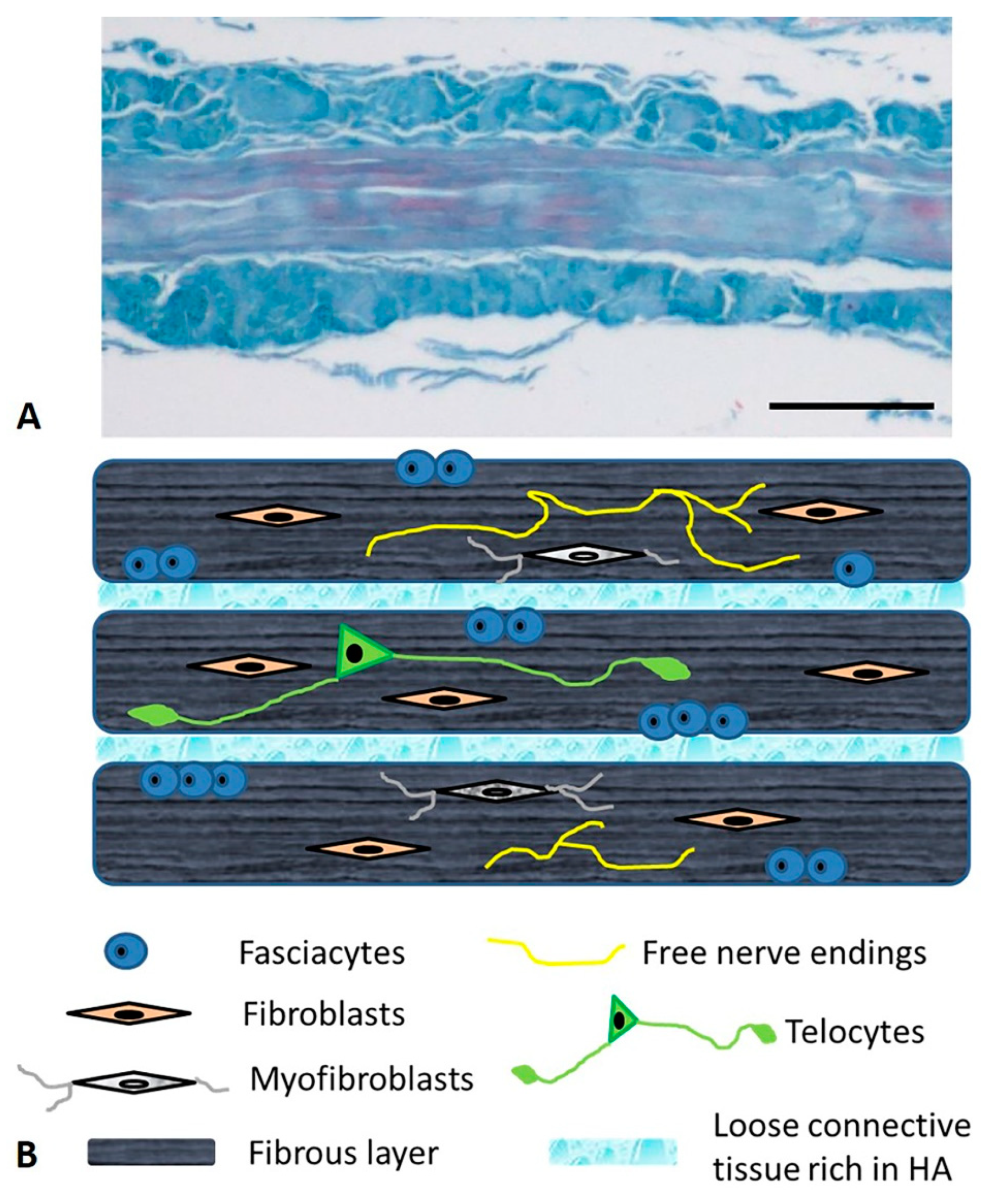
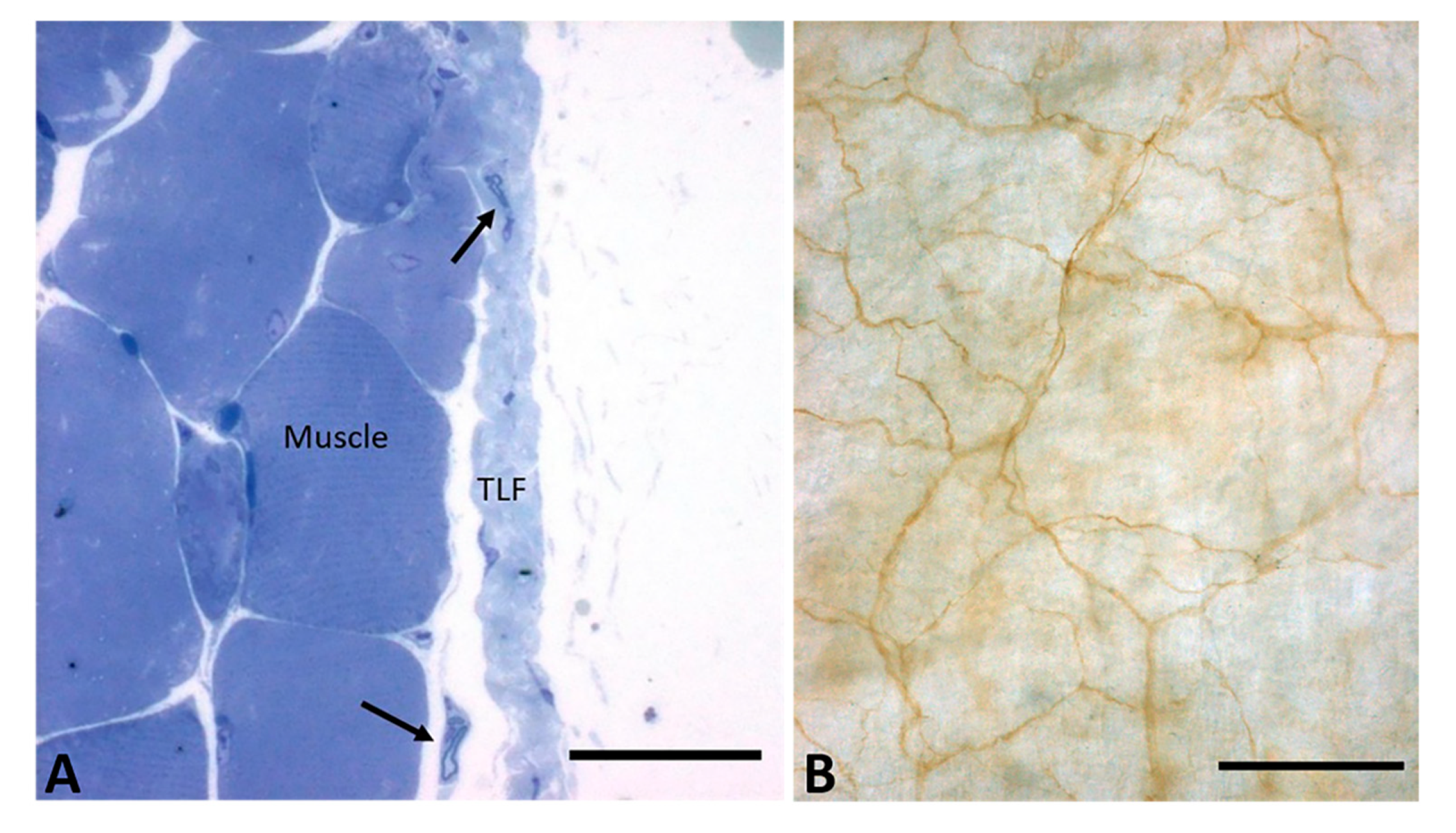
4. Microscopic Anatomy of the Deep/Muscular Fasciae
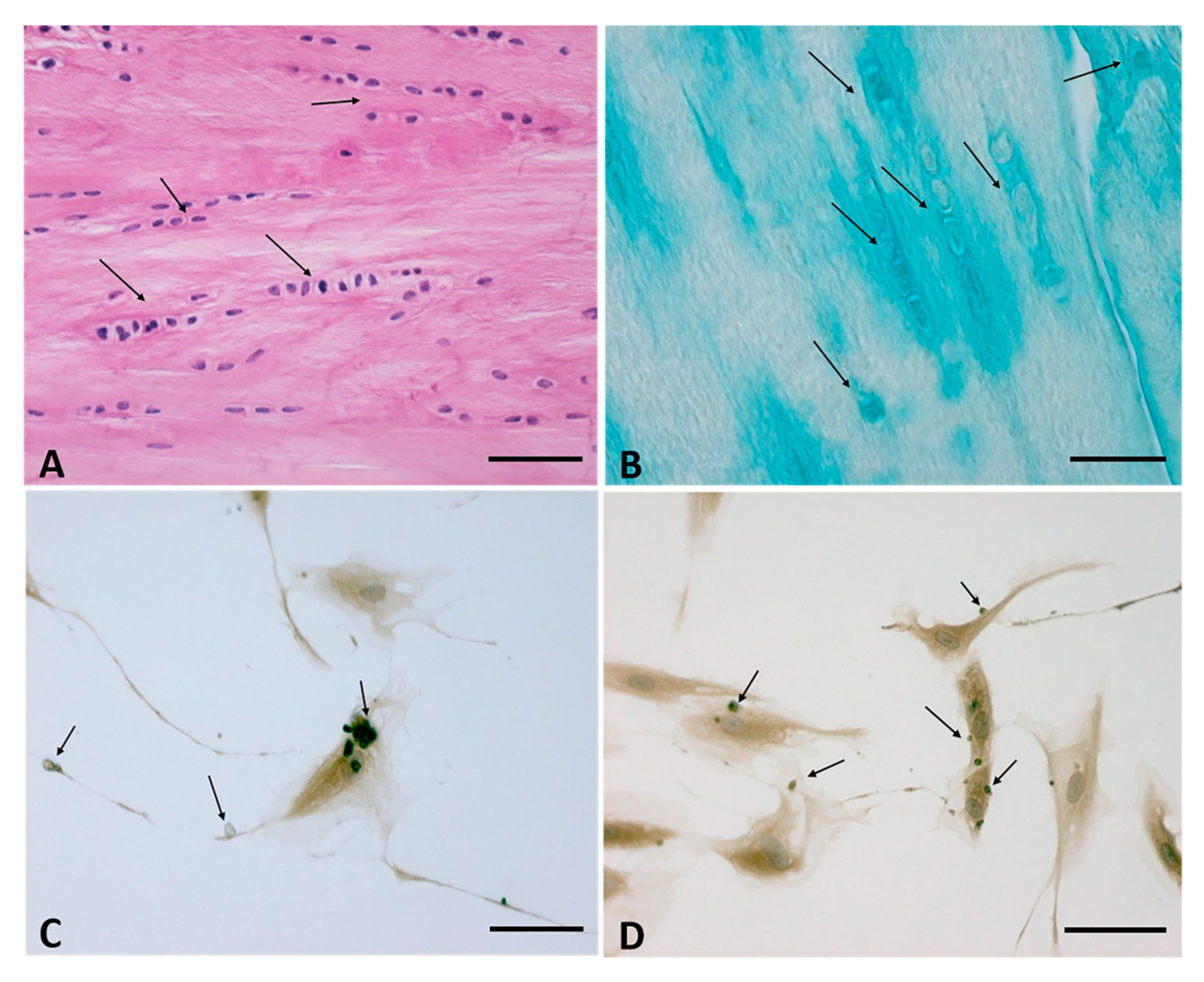
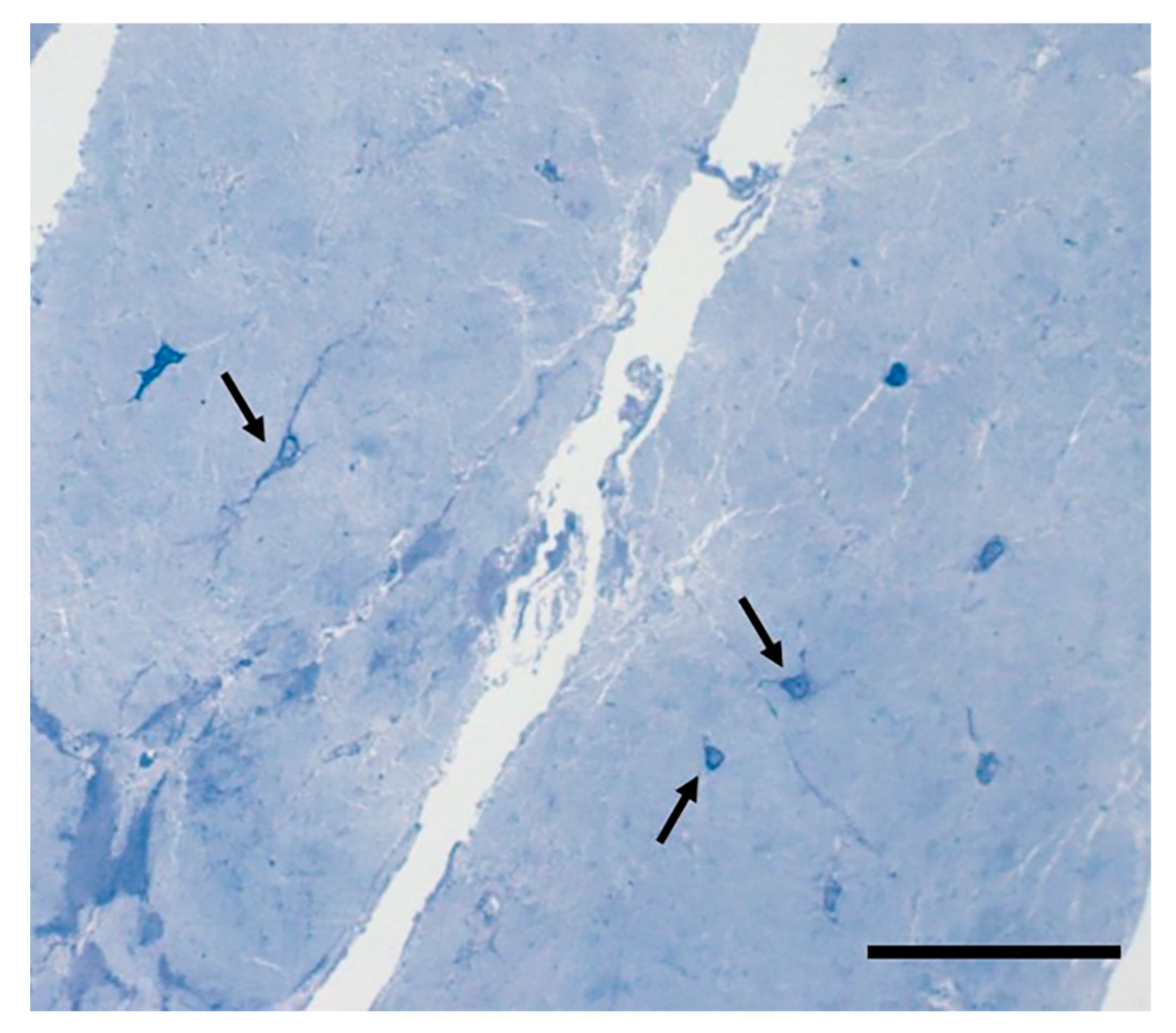
5. The Cells of the Fascial Tissue
6. The Nervous Fibers of the Fascia
7. The Extracellular Matrix: The Fibrous Component
8. The Extracellular Matrix: The Aqueous Matrix
9. Perspectives
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pirri, C.; Todros, S.; Fede, C.; Pianigiani, S.; Fan, C.; Foti, C.; Stecco, C.; Pavan, P. Inter-rater reliability and variability of ultrasound measurements of abdominal muscles and fasciae thickness. Clin. Anat. 2019, 32, 948–960. [Google Scholar] [CrossRef] [PubMed]
- Pirri, C.; Stecco, C.; Fede, C.; Macchi, V.; Özçakar, L. Ultrasound Imaging of the Fascial Layers: You See (Only) What You Know. J. Ultrasound Med. 2020, 39, 827–828. [Google Scholar] [CrossRef] [PubMed]
- Pirri, C.; Stecco, A.; Fede, C.; De Caro, R.; Stecco, C.; Özçakar, L. Ultrasound imaging of a scar on the knee: Sonopalpation for fascia and subcutaneous tissues. Eur. J. Transl. Myol. 2020, 30, 8909. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Fede, C.; Pirri, C.; Guidolin, D.; Biz, C.; Macchi, V.; De Caro, R.; Stecco, C. Quantitative Evaluation of the Echo Intensity of Paraneural Area and Myofascial Structure around Median Nerve in Carpal Tunnel Syndrome. Diagnostics 2020, 10, 914. [Google Scholar] [CrossRef] [PubMed]
- Almazán-Polo, J.; López-López, D.; Romero-Morales, C.; Rodríguez-Sanz, D.; Becerro-de-Bengoa-Vallejo, R.; Losa-Iglesias, M.E.; Bravo-Aguilar, M.; Calvo-Lobo, C. Quantitative Ultrasound Imaging Differences in Multifidus and Thoracolumbar Fasciae between Athletes with and without Chronic Lumbopelvic Pain: A Case-Control Study. J. Clin. Med. 2020, 9, 2647. [Google Scholar] [CrossRef]
- Menon, R.G.; Oswald, S.F.; Raghavan, P.; Regatte, R.R.; Stecco, A. T1ρ-Mapping for Musculoskeletal Pain Diagnosis: Case Series of Variation of Water Bound Glycosaminoglycans Quantification before and after Fascial Manipulation® in Subjects with Elbow Pain. Int. J. Environ. Res. Public Health 2020, 17, 708. [Google Scholar] [CrossRef]
- Geneser, F. Textbook of Histology; Munksgaard: Copenhagen, Denmark, 1986. [Google Scholar]
- Gray, H.; Standring, S.; Ellis, H.; Berkovitz, B.K.B. Gray’s Anatomy: The Anatomical Basis of Clinical Practice, 39th ed.; Elsevier Churchill Livingstone: New York, NY, USA, 2005. [Google Scholar]
- Bogduk, N.; Macintosh, J.E. The applied anatomy of the thoracolumbar fascia. Spine 1984, 9, 164–170. [Google Scholar] [CrossRef]
- Martini, F.H.; Timmons, M.J.; Tallitsch, R.B. Anatomia Umana, 2nd ed.; EdiSES: Naples, Italy, 2004. [Google Scholar]
- Fawcett, D.W. Bloom & Fawcett: A Textbook of Histology, 12th ed.; McGraw-Hill: Milano, Italy, 1996. [Google Scholar]
- Gerlach, U.J.; Lierse, W. Functional construction of the superficial and deep fascia system of the lower limb in man. Acta Anat. 1990, 139, 11–25. [Google Scholar] [CrossRef]
- Stecco, C. Functional Atlas of the Human Fascial System, 1st ed.; Elsevier: Edinburgh, UK, 2015; p. 4. [Google Scholar]
- Mense, S. Innervation of the thoracolumbar fascia. Eur. J. Transl. Myol. 2019, 29, 8297. [Google Scholar] [CrossRef]
- Fede, C.; Porzionato, A.; Petrelli, L.; Fan, C.; Pirri, C.; Biz, C.; De Caro, R.; Stecco, C. Fascia and soft tissues innervation in the human hip and their possible role in post-surgical pain. J. Orthop. Res. 2020, 38, 1646–1654. [Google Scholar] [CrossRef]
- Guimberteau, J.C.; Armstrong, C.; Findley, T.W.; Kapandji, M.D.; Adalbert, I. Architecture of Human Living Fascia: The Extracellular Matrix and Cells Revealed Through Endoscopy; Handspring Publishing: Pencaitland, East Lothian, Scotland, 2015. [Google Scholar]
- Levin, S.M.; Martin, D.C. Biotensegrity: The mechanics of fascia. In Fascia and the Tensional Network of the Human Body. The Science and Clinical Applications in Manual and Movement Therapy; Chapter: 3.5; Elsevier: Edinburgh, UK, 2012; pp. 137–142. [Google Scholar]
- Adstrum, S.; Hedley, G.; Schleip, R.; Stecco, C.; Yucesoy, C.A. Defining the fascial system. J. Bodyw. Mov. Ther. 2017, 21, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Gatt, A.; Agarwal, S.; Zito, P.M. Anatomy, Fascia Layers. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Stecco, A.; Stern, R.; Fantoni, I.; De Caro, R.; Stecco, C. Fascial Disorders: Implications for Treatment. PM&R 2016, 8, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Stedman’s Medical Dictionary, 26th ed.; Williams & Wilkins: Baltimore, MD, USA, 1995; p. 628.
- Stecco, C.; Sfriso, M.M.; Porzionato, A.; Rambaldo, A.; Albertin, G.; Macchi, V.; De Caro, R. Microscopic anatomy of the visceral fasciae. J. Anat. 2017, 231, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Pirri, C.; Fede, C.; Petrelli, L.; Guidolin, D.; Fan, C.; De Caro, R.; Stecco, C. An anatomical comparison of the fasciae of the thigh: A macroscopic, microscopic and ultrasound imaging study. J. Anat. 2020. [Google Scholar] [CrossRef]
- Wilke, J.; Macchi, V.; De Caro, R.; Stecco, C. Fascia thickness, aging and flexibility: Is there an association? J. Anat. 2019, 234, 43–49. [Google Scholar] [CrossRef]
- Fan, C.; Guidolin, D.; Ragazzo, S.; Fede, C.; Pirri, C.; Gaudreault, N.; Porzionato, A.; Macchi, V.; De Caro, R.; Stecco, C. Effects of Cesarean Section and Vaginal Delivery on Abdominal Muscles and Fasciae. Medicina 2020, 56, 260. [Google Scholar] [CrossRef]
- Stecco, A.; Macchi, V.; Masiero, S.; Porzionato, A.; Tiengo, C.; Stecco, C.; Delmas, V.; De Caro, R. Pectoral and femoral fasciae: Common aspects and regional specializations. Surg. Radiol. Anat. 2009, 31, 35–42. [Google Scholar] [CrossRef]
- Benetazzo, L.; Bizzego, A.; De Caro, R.; Frigo, G.; Guidolin, D.; Stecco, C. 3D reconstruction of the crural and thoracolumbar fasciae. Surg. Radiol. Anat. 2011, 33, 855–862. [Google Scholar] [CrossRef]
- Tesarz, J.; Hoheisel, U.; Wiedenhöfer, B.; Mense, S. Sensory innervation of the thoracolumbar fascia in rats and humans. Neuroscience 2011, 194, 302–308. [Google Scholar] [CrossRef]
- Purslow, P.P. The structure and functional significance of variations in the connective tissue within muscle. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 133, 947–966. [Google Scholar] [CrossRef]
- Purslow, P.P. The Structure and Role of Intramuscular Connective Tissue in Muscle Function. Front. Physiol. 2020, 11, 495. [Google Scholar] [CrossRef] [PubMed]
- Kumka, M.; Bonar, J. Fascia: A morphological description and classification system based on a literature review. J. Can. Chiropr. Assoc. 2012, 56, 179–191. [Google Scholar] [PubMed]
- Langevin, H.M.; Cornbrooks, C.J.; Taatjes, D.J. Fibroblasts form a body-wide cellular network. Histochem. Cell Biol. 2004, 122, 7–15. [Google Scholar]
- Benjamin, M. The fascia of the limbs and back—A review. J. Anat. 2009, 214, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Stecco, C.; Fede, C.; Macchi, V.; Petrelli, L.; Biz, C.; Stern, R.; De Caro, R. The fasciacytes: A new cell devoted to fascial gliding regulation. Clin. Anat. 2018, 31, 667–676. [Google Scholar] [CrossRef]
- Bartok, B.; Firestein, G.S. Fibroblast-like synoviocytes: Key effector cells in rheumatoid arthritis. Immunol. Rev. 2010, 233, 233–255. [Google Scholar] [CrossRef]
- Iwanaga, T.; Shikichi, M.; Kitamura, H.; Yanase, H.; Nozawa-Inoue, K. Morphology and functional roles of synoviocytes in the joint. Arch. Histol. Cytol. 2000, 63, 17–31. [Google Scholar] [CrossRef]
- Sakamoto, T.; Ishibashi, T. Hyalocytes: Essential cells of the vitreous cavity in vitreoretinal pathophysiology? Retina 2011, 31, 222–228. [Google Scholar] [CrossRef]
- Holness, C.L.; Simmons, D.L. Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood 1993, 81, 1607–1613. [Google Scholar] [CrossRef]
- Klein, D.M.; Katzman, B.M.; Mesa, J.A.; Lipton, J.F.; Caligiuri, D.A. Histology of the extensor retinaculum of the wrist and the ankle. J. Hand Surg. Am. 1999, 24, 799–802. [Google Scholar] [CrossRef]
- Schleip, R.; Gabbiani, G.; Wilke, J.; Naylor, I.; Hinz, B.; Zorn, A.; Jäger, H.; Breul, R.; Schreiner, S.; Klingler, W. Fascia Is Able to Actively Contract and May Thereby Influence Musculoskeletal Dynamics: A Histochemical and Mechanographic Investigation. Front. Physiol. 2019, 10, 336. [Google Scholar] [CrossRef] [PubMed]
- Follonier, L.; Schaub, S.; Meister, J.J.; Hinz, B. Myofibroblast communication is controlled by intercellular mechanical coupling. J. Cell Sci. 2008, 121, 3305–3316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.Y.; Kargel, J.S. The Basic Science of Dupuytren Disease. Hand Clin. 2018, 34, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Schleip, R.; Klingler, W. Active contractile properties of fascia. Clin. Anat. 2019, 32, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Dawidowicz, J.; Szotek, S.; Matysiak, N.; Mielańczyk, Ł.; Maksymowicz, K. Electron microscopy of human fascia lata: Focus on telocytes. J. Cell Mol. Med. 2015, 19, 2500–2506. [Google Scholar] [CrossRef] [PubMed]
- Dawidowicz, J.; Matysiak, N.; Szotek, S.; Maksymowicz, K. Telocytes of Fascial Structures. Adv. Exp. Med. Biol. 2016, 913, 403–424. [Google Scholar]
- Blottner, D.; Huang, Y.; Trautmann, G.; Sun, L. The fascia: Continuum linking bone and myofascial bag for global and local body movement control on Earth and in Space. A scoping review. Reach 2019, 14–15, 100030. [Google Scholar] [CrossRef]
- Chaitow, L. Telocytes: Connective tissue repair and communication cells. J. Bodyw. Mov. Ther. 2017, 21, 231–233. [Google Scholar] [CrossRef]
- Stecco, C.; Macchi, V.; Barbieri, A.; Tiengo, C.; Porzionato, A.; De Caro, R. Hand fasciae innervation: The palmar aponeurosis. Clin. Anat. 2018, 31, 677–683. [Google Scholar] [CrossRef]
- Stecco, C.; Corradin, M.; Macchi, V.; Morra, A.; Porzionato, A.; Biz, C.; De Caro, R. Plantar fascia anatomy and its relationship with Achilles tendon and paratenon. J. Anat. 2013, 223, 665–676. [Google Scholar] [CrossRef]
- Stecco, C.; Porzionato, A.; Lancerotto, L.; Stecco, A.; Macchi, V.; Day, J.A.; De Caro, R. Histological study of the deep fasciae of the limbs. J. Bodyw. Mov. Ther. 2008, 12, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Stecco, C.; Macchi, V.; Porzionato, A.; Morra, A.; Parenti, A.; Stecco, A.; Delmas, V.; De Caro, R. The ankle retinacula: Morphological evidence of the proprioceptive role of the fascial system. Cells Tissues Organs 2010, 192, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Hoheisel, U.; Rosner, J.; Mense, S. Innervation changes induced by inflammation of the rat thoracolumbar fascia. Neuroscience 2015, 300, 351–359. [Google Scholar] [CrossRef]
- Taguchi, T.; Yasui, M.; Kubo, A.; Abe, M.; Kiyama, H.; Yamanaka, A.; Mizumura, K. Nociception originating from the crural fascia in rats. Pain 2013, 154, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Schilder, A.; Hoheisel, U.; Magerl, W.; Benrath, J.; Klein, S.; Treede, R.D. Sensory findings after stimulation of the thoracolumbar fascia with hypertonic saline suggest its contribution to low back pain. Pain 2014, 155, 222–231. [Google Scholar] [CrossRef]
- Mense, S.; Hoheisel, U. Evidence for the existence of nociceptors in rat thoracolumbar fascia. J. Bodyw. Mov. Ther. 2016, 20, 623–628. [Google Scholar] [CrossRef]
- Fede, C.; Albertin, G.; Petrelli, L.; Sfriso, M.M.; Biz, C.; De Caro, R.; Stecco, C. Expression of the endocannabinoid receptors in human fascial tissue. Eur. J. Histochem. 2016, 60, 2643. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, G.J.; Nakamura, A.; Lee, P.R.; Kim, Y.; Won, C.H.; Furue, H.; Oh, S.B. Involvement of cannabinoid type 1 receptor in fasting-induced analgesia. Mol. Pain. 2020, 16, 1744806920969476. [Google Scholar] [CrossRef]
- Nagarkatti, P.; Pandey, R.; Rieder, S.A.; Hegde, V.L.; Nagarkatti, M. Cannabinoids as novel anti-inflammatory drugs. Future Med. Chem. 2009, 1, 1333–1349. [Google Scholar] [CrossRef]
- Di Marzo, V. Targeting the endocannabinoid system: To enhance or reduce? Nat. Rev. Drug Discov. 2008, 7, 438–455. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef] [PubMed]
- Turrina, A.; Martínez-González, M.A.; Stecco, C. The muscular force transmission system: Role of the intramuscular connective tissue. J. Bodyw. Mov. Ther. 2013, 17, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Ushiki, T. Collagen fibers, reticular fibers and elastic fibers. A comprehensive understanding from a morphological viewpoint. Arch. Histol. Cytol. 2002, 65, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Willard, F.H.; Vleeming, A.; Schuenke, M.D.; Danneels, L.; Schleip, R. The thoracolumbar fascia: Anatomy, function and clinical considerations. J. Anat. 2012, 221, 507–536. [Google Scholar] [CrossRef] [PubMed]
- Giordani, F.; Bernini, A.; Müller-Ehrenberg, H.; Stecco, C.; Masiero, S. A global approach for plantar fasciitis with extracorporeal shockwaves treatment. Eur. J. Transl. Myol. 2019, 29, 8372. [Google Scholar] [CrossRef] [PubMed]
- Frairia, R.; Berta, L. Biological effects of extracorporeal shock waves on fibroblasts. A review. Muscles Ligaments Tendons J. 2011, 1, 138–147. [Google Scholar] [PubMed]
- Pavan, P.; Monti, E.; Bondí, M.; Fan, C.; Stecco, C.; Narici, M.; Reggiani, C.; Marcucci, L. Alterations of Extracellular Matrix Mechanical Properties Contribute to Age-Related Functional Impairment of Human Skeletal Muscles. Int. J. Mol. Sci. 2020, 21, 3992. [Google Scholar] [CrossRef]
- Slimani, L.; Micol, D.; Amat, J.; Delcros, G.; Meunier, B.; Taillandier, D.; Polge, C.; Béchet, D.; Dardevet, D.; Picard, B.; et al. The worsening of tibialis anterior muscle atrophy during recovery post-immobilization correlates with enhanced connective tissue area, proteolysis, and apoptosis. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1335–E1347. [Google Scholar] [CrossRef]
- Fede, C.; Pirri, C.; Fan, C.; Albertin, G.; Porzionato, A.; Macchi, V.; De Caro, R.; Stecco, C. Sensitivity of the fasciae to sex hormone levels: Modulation of collagen-I, collagen-III and fibrillin production. PLoS ONE. 2019, 14, e0223195. [Google Scholar] [CrossRef]
- Rollman, G.B.; Lautenbacher, S. Sex differences in musculoskeletal pain. Clin. J. Pain 2001, 17, 20–24. [Google Scholar] [CrossRef]
- Vita, M.; Sedlackova, Z.; Herman, M.; Furst, T.; Smekal, D.; Cech, Z. Influence of female hormones on fascia elasticity: An elastography study. Clin. Anat. 2019, 32, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Petrofsky, J.; Lee, H. Greater Reduction of Balance as a Result of Increased Plantar Fascia Elasticity at Ovulation during the Menstrual Cycle. Tohoku J. Exp. Med. 2015, 237, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Stecco, C.; Stern, R.; Porzionato, A.; Macchi, V.; Masiero, S.; Stecco, A.; De Caro, R. Hyaluronan within fascia in the etiology of myofascial pain. Surg. Radiol. Anat. 2011, 33, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Fede, C.; Angelini, A.; Stern, R.; Macchi, V.; Porzionato, A.; Ruggieri, P.; De Caro, R.; Stecco, C. Quantification of hyaluronan in human fasciae: Variations with function and anatomical site. J. Anat. 2018, 233, 552–556. [Google Scholar] [CrossRef]
- Viola, M.; Vigetti, D.; Karousou, E.; D’Angelo, M.L.; Caon, I.; Moretto, P.; De Luca, G.; Passi, A. Biology and biotechnology of hyaluronan. Glycoconj. J. 2015, 32, 93–103. [Google Scholar] [CrossRef]
- Fede, C.; Pirri, C.; Petrelli, L.; Guidolin, D.; Fan, C.; De Caro, R.; Stecco, C. Sensitivity of the Fasciae to the Endocannabinoid System: Production of Hyaluronan-Rich Vesicles and Potential Peripheral Effects of Cannabinoids in Fascial Tissue. Int. J. Mol. Sci. 2020, 21, 2936. [Google Scholar] [CrossRef]
- Cowman, M.K.; Lee, H.G.; Schwertfeger, K.L.; McCarthy, J.B.; Turley, E.A. The Content and Size of Hyaluronan in Biological Fluids and Tissues. Front. Immunol. 2015, 6, 261. [Google Scholar] [CrossRef]
- Tammi, M.I.; Day, A.J.; Turley, E.A. Hyaluronan and homeostasis: A balancing act. J. Biol. Chem. 2002, 277, 4581–4584. [Google Scholar] [CrossRef]
- Joy, R.A.; Vikkath, N.; Ariyannur, P.S. Metabolism and mechanisms of action of hyaluronan in human biology. Drug Metab. Pers. Ther. 2018, 33, 15–32. [Google Scholar] [CrossRef]
- Cowman, M.K.; Schmidt, T.A.; Raghavan, P.; Stecco, A. Viscoelastic properties of hyaluronan in physiological conditions. F1000Res 2015, 4, 622. [Google Scholar] [CrossRef]
- Forgacs, G.; Newman, S.A.; Hinner, B.; Maier, C.W.; Sackmann, E. Assembly of collagen matrices as a phase transition revealed by structural and rheologic studies. Biophys. J. 2003, 84, 1272–1280. [Google Scholar] [CrossRef]
- Gatej, I.; Popa, M.; Rinaudo, M. Role of the pH on hyaluronan behavior in aqueous solution. Biomacromolecules 2005, 6, 61–67. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fede, C.; Pirri, C.; Fan, C.; Petrelli, L.; Guidolin, D.; De Caro, R.; Stecco, C. A Closer Look at the Cellular and Molecular Components of the Deep/Muscular Fasciae. Int. J. Mol. Sci. 2021, 22, 1411. https://doi.org/10.3390/ijms22031411
Fede C, Pirri C, Fan C, Petrelli L, Guidolin D, De Caro R, Stecco C. A Closer Look at the Cellular and Molecular Components of the Deep/Muscular Fasciae. International Journal of Molecular Sciences. 2021; 22(3):1411. https://doi.org/10.3390/ijms22031411
Chicago/Turabian StyleFede, Caterina, Carmelo Pirri, Chenglei Fan, Lucia Petrelli, Diego Guidolin, Raffaele De Caro, and Carla Stecco. 2021. "A Closer Look at the Cellular and Molecular Components of the Deep/Muscular Fasciae" International Journal of Molecular Sciences 22, no. 3: 1411. https://doi.org/10.3390/ijms22031411
APA StyleFede, C., Pirri, C., Fan, C., Petrelli, L., Guidolin, D., De Caro, R., & Stecco, C. (2021). A Closer Look at the Cellular and Molecular Components of the Deep/Muscular Fasciae. International Journal of Molecular Sciences, 22(3), 1411. https://doi.org/10.3390/ijms22031411









