The Progress of Stem Cell Technology for Skeletal Regeneration
Abstract
1. Introduction
2. Skeletal Development in Embryos
2.1. Paraxial Mesoderm
2.1.1. Development of the Paraxial Mesoderm in Embryo
2.1.2. Recapitulating Development of the Paraxial Mesoderm in a Dish
2.1.3. Chondrocyte Differentiation through the Paraxial Mesoderm in a Dish
2.1.4. Osteoblast Differentiation through the Paraxial Mesoderm in a Dish
2.2. Lateral Plate Mesoderm
2.2.1. Development of the Lateral Plate Mesoderm in Embryo
2.2.2. Recapitulating Development of the Lateral Plate Mesoderm in a Dish
2.2.3. Chondrocyte and Osteoblast Differentiation through the Lateral Plate Mesoderm in a Dish
2.3. Neural Crest
2.3.1. Development of the Neural Crest in Embryo
2.3.2. Recapitulating Development of the Neural Crest and Its Derivatives in a Dish
3. MSCs
4. SSCs
5. CAR Cells
6. Summary and Future Perspectives
Funding
Conflicts of Interest
References
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Brinker, M.R.; Trivedi, A.; O’Connor, D.P. Debilitating Effects of Femoral Nonunion on Health-Related Quality of Life. J. Orthop. Trauma 2017, 31, e37–e42. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Hu, X.; Cheng, J.; Zhang, X.; Zhao, F.; Shi, W.; Ren, B.; Yu, H.; Yang, P.; Li, Z.; et al. A small molecule promotes cartilage extracellular matrix generation and inhibits osteoarthritis development. Nat. Commun. 2019, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.S.; Lee, M.A.; Reddi, A.H. Nonunions and the Potential of Stem Cells in Fracture-Healing. JBJS 2008, 90 (Suppl. 1), 92–98. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Yamanaka, Y.; Uemura, M.; Osawa, M.; Saito, M.K.; Nagahashi, A.; Nishio, M.; Guo, L.; Ikegawa, S.; Sakurai, S.; et al. Recapitulating the human segmentation clock with pluripotent stem cells. Nat. Cell Biol. 2020, 580, 124–129. [Google Scholar] [CrossRef]
- Kidwai, F.K.; Mui, B.W.H.; Arora, D.; Iqbal, K.; Hockaday, M.; Diaz, L.F.D.C.; Cherman, N.; Martin, D.; Myneni, V.D.; Ahmad, M.; et al. Lineage-specific differentiation of osteogenic progenitors from pluripotent stem cells reveals the FGF1-RUNX2 association in neural crest-derived osteoprogenitors. Stem Cells 2020, 38, 1107–1123. [Google Scholar] [CrossRef]
- Kim, E.; Wu, F.; Wu, X.; Choo, H.J. Generation of craniofacial myogenic progenitor cells from human induced pluripotent stem cells for skeletal muscle tissue regeneration. Biomaterials 2020, 248, 119995. [Google Scholar] [CrossRef]
- Labibzadeh, N.; Emadedin, M.; Fazeli, R.; Mohseni, F.; Hosseini, S.E.; Moghadasali, R.; Mardpour, S.; Azimian, V.; Liastani, M.G.; Bafghi, A.M.; et al. Mesenchymal Stromal Cells Implantation in Combination with Platelet Lysate Product Is Safe for Reconstruction of Human Long Bone Nonunion. Cell J. 2016, 18, 302–309. [Google Scholar]
- Moll, G.; Ankrum, J.A.; Kamhieh-Milz, J.; Bieback, K.; Ringdén, O.; Volk, H.-D.; Geissler, S.; Reinke, P. Intravascular Mesenchymal Stromal/Stem Cell Therapy Product Diversification: Time for New Clinical Guidelines. Trends Mol. Med. 2019, 25, 149–163. [Google Scholar] [CrossRef]
- Bloor, A.J.C.; Patel, A.; Griffin, J.E.; Gilleece, M.H.; Radia, R.; Yeung, D.T.; Drier, D.; Larson, L.S.; Uenishi, G.I.; Hei, D.; et al. Production, safety and efficacy of iPSC-derived mesenchymal stromal cells in acute steroid-resistant graft versus host disease: A phase I, multicenter, open-label, dose-escalation study. Nat. Med. 2020, 26, 1720–1725. [Google Scholar] [CrossRef]
- Chan, C.K.F.; Gulati, G.S.; Sinha, R.; Tompkins, J.V.; Lopez, M.; Carter, A.C.; Ransom, R.C.; Reinisch, A.; Wearda, T.; Murphy, M.; et al. Identification of the Human Skeletal Stem Cell. Cell 2018, 175, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Omatsu, Y.; Sugiyama, T.; Kohara, H.; Kondoh, G.; Fujii, N.; Kohno, K.; Nagasawa, T. The Essential Functions of Adipo-osteogenic Progenitors as the Hematopoietic Stem and Progenitor Cell Niche. Immunity 2010, 33, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; De Crombrugghe, B. Transcriptional mechanisms in osteoblast differentiation and bone formation. Trends Genet. 2003, 19, 458–466. [Google Scholar] [CrossRef]
- Loh, K.M.; Chen, A.; Koh, P.W.; Deng, T.Z.; Sinha, R.; Tsai, J.M.; Barkal, A.A.; Shen, K.Y.; Jain, R.; Morganti, R.M.; et al. Mapping the Pairwise Choices Leading from Pluripotency to Human Bone, Heart, and Other Mesoderm Cell Types. Cell 2016, 166, 451–467. [Google Scholar] [CrossRef]
- Nakajima, T.; Shibata, M.; Nishio, M.; Nagata, S.; Alev, C.; Sakurai, H.; Toguchida, J.; Ikeya, M. Modeling human somite development and fibrodysplasia ossificans progressiva with induced pluripotent stem cells. Development 2018, 145, dev165431. [Google Scholar] [CrossRef]
- De Bree, K.; De Bakker, B.S.; Oostra, R.-J. The development of the human notochord. PLoS ONE 2018, 13, e0205752. [Google Scholar] [CrossRef]
- James, R.G.; Schultheiss, T.M. Patterning of the Avian Intermediate Mesoderm by Lateral Plate and Axial Tissues. Dev. Biol. 2003, 253, 109–124. [Google Scholar] [CrossRef]
- Christ, B.; Huang, R.; Scaal, M. Formation and differentiation of the avian sclerotome. Anat. Embryol. 2004, 208, 333–350. [Google Scholar] [CrossRef]
- Nguyen, P.D.; Hollway, G.E.; Sonntag, C.; Miles, L.B.; Hall, T.E.; Berger, S.; Fernandez, K.J.; Gurevich, D.B.; Cole, N.J.; Alaei, S.; et al. Haematopoietic stem cell induction by somite-derived endothelial cells controlled by meox1. Nature 2014, 512, 314–318. [Google Scholar] [CrossRef]
- Ben-Yair, R.; Kalcheim, C. Lineage analysis of the avian dermomyotome sheet reveals the existence of single cells with both dermal and muscle progenitor fates. Development 2005, 132, 689–701. [Google Scholar] [CrossRef]
- Takada, S.; Stark, K.L.; Shea, M.J.; Vassileva, G.; McMahon, J.A.; McMahon, A.P. Wnt-3a regulates somite and tailbud formation in the mouse embryo. Genes Dev. 1994, 8, 174–189. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.; Fujiwara, W.; Gonzalez, K.; Jan, M.; Liebscher, S.; Van Handel, B.; Schenke-Layland, K.; Pyle, A.D. In Vivo Human Somitogenesis Guides Somite Development from hPSCs. Cell Rep. 2017, 18, 1573–1585. [Google Scholar] [CrossRef] [PubMed]
- Tonegawa, A.; Takahashi, Y. Somitogenesis Controlled by Noggin. Dev. Biol. 1998, 202, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Tani, S.; Chung, U.-I.; Ohba, S.; Hojo, H. Understanding paraxial mesoderm development and sclerotome specification for skeletal repair. Exp. Mol. Med. 2020, 52, 1–12. [Google Scholar] [CrossRef]
- Niwa, Y.; Masamizu, Y.; Liu, T.; Nakayama, R.; Deng, C.-X.; Kageyama, R. The Initiation and Propagation of Hes7 Oscillation Are Cooperatively Regulated by Fgf and Notch Signaling in the Somite Segmentation Clock. Dev. Cell 2007, 13, 298–304. [Google Scholar] [CrossRef]
- Cairns, D.M.; Sato, M.E.; Lee, P.G.; Lassar, A.B.; Zeng, L. A gradient of Shh establishes mutually repressing somitic cell fates induced by Nkx3.2 and Pax3. Dev. Biol. 2008, 323, 152–165. [Google Scholar] [CrossRef]
- Henrique, D.; Abranches, E.; Verrier, L.; Storey, K. Neuromesodermal progenitors and the making of the spinal cord. Development 2015, 142, 2864–2875. [Google Scholar] [CrossRef]
- Zhao, J.; Li, S.; Trilok, S.; Tanaka, M.; Jokubaitis-Jameson, V.; Wang, B.; Niwa, H.; Nakayama, N. Small molecule-directed specification of sclerotome-like chondroprogenitors and induction of a somitic chondrogenesis program from embryonic stem cells. Development 2014, 141, 3848–3858. [Google Scholar] [CrossRef]
- Chu, L.-F.; Mamott, D.; Ni, Z.; Bacher, R.; Liu, C.; Swanson, S.; Kendziorski, C.; Stewart, R.; Thomson, J.A. An In Vitro Human Segmentation Clock Model Derived from Embryonic Stem Cells. Cell Rep. 2019, 28, 2247–2255. [Google Scholar] [CrossRef]
- Robertson, E.J. Dose-dependent Nodal/Smad signals pattern the early mouse embryo. Semin. Cell Dev. Biol. 2014, 32, 73–79. [Google Scholar] [CrossRef]
- Chal, J.; Oginuma, M.; Al Tanoury, Z.; Gobert, B.; Sumara, O.; Hick, A.; Bousson, F.; Zidouni, Y.; Mursch, C.; Moncuquet, P.; et al. Differentiation of pluripotent stem cells to muscle fiber to model Duchenne muscular dystrophy. Nat. Biotechnol. 2015, 33, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Oldershaw, R.A.; Baxter, M.A.; Lowe, E.T.; Bates, N.; Grady, L.M.; Soncin, F.; Brison, D.R.; Hardingham, T.E.; Kimber, S.J. Directed differentiation of human embryonic stem cells toward chondrocytes. Nat. Biotechnol. 2010, 28, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Morioka, M.; Yahara, Y.; Okada, M.; Kobayashi, T.; Kuriyama, S.; Matsuda, S.; Tsumaki, N. Generation of Scaffoldless Hyaline Cartilaginous Tissue from Human iPSCs. Stem Cell Rep. 2015, 4, 404–418. [Google Scholar] [CrossRef]
- Kawata, M.; Mori, D.; Kanke, K.; Hojo, H.; Ohba, S.; Chung, U.-I.; Yano, F.; Masaki, H.; Otsu, M.; Nakauchi, H.; et al. Simple and Robust Differentiation of Human Pluripotent Stem Cells toward Chondrocytes by Two Small-Molecule Compounds. Stem Cell Rep. 2019, 13, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Morioka, M.; Kishi, H.; Kimura, T.; Yahara, Y.; Okada, M.; Fujita, K.; Sawai, H.; Ikegawa, S.; Tsumaki, N. Statin treatment rescues FGFR3 skeletal dysplasia phenotypes. Nature 2014, 513, 507–511. [Google Scholar] [CrossRef]
- Craft, A.M.; Rockel, J.S.; Nartiss, Y.; Kandel, R.A.; Alman, B.A.; Keller, G.M. Generation of articular chondrocytes from human pluripotent stem cells. Nat. Biotechnol. 2015, 33, 638–645. [Google Scholar] [CrossRef]
- Chijimatsu, R.; Ikeya, M.; Yasui, Y.; Ikeda, Y.; Ebina, K.; Moriguchi, Y.; Shimomura, K.; Hart, D.A.; Yoshikawa, H.; Nakamura, N. Characterization of Mesenchymal Stem Cell-Like Cells Derived From Human iPSCs via Neural Crest Development and Their Application for Osteochondral Repair. Stem Cells Int. 2017, 2017, 1–18. [Google Scholar] [CrossRef]
- Wu, L.; Bluguermann, C.; Kyupelyan, L.; Latour, B.; Gonzalez, S.; Shah, S.; Galic, Z.; Ge, S.; Zhu, Y.; Petrigliano, F.A.; et al. Human Developmental Chondrogenesis as a Basis for Engineering Chondrocytes from Pluripotent Stem Cells. Stem Cell Rep. 2013, 1, 575–589. [Google Scholar] [CrossRef]
- Rim, Y.A.; Nam, Y.; Park, N.; Lee, J.; Park, S.-H.; Ju, J.H. Repair potential of nonsurgically delivered induced pluripotent stem cell-derived chondrocytes in a rat osteochondral defect model. J. Tissue Eng. Regen. Med. 2018, 12, 1843–1855. [Google Scholar] [CrossRef]
- Caron, M.M.J.; Emans, P.; Coolsen, M.; Voss, L.; Surtel, D.; Cremers, A.; Van Rhijn, L.; Welting, T. Redifferentiation of dedifferentiated human articular chondrocytes: Comparison of 2D and 3D cultures. Osteoarthr. Cartil. 2012, 20, 1170–1178. [Google Scholar] [CrossRef]
- Kanke, K.; Masaki, H.; Saito, T.; Komiyama, Y.; Hojo, H.; Nakauchi, H.; Lichtler, A.C.; Takato, T.; Chung, U.-I.; Ohba, S. Stepwise Differentiation of Pluripotent Stem Cells into Osteoblasts Using Four Small Molecules under Serum-free and Feeder-free Conditions. Stem Cell Rep. 2014, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Zujur, D.; Kanke, K.; Onodera, S.; Tani, S.; Lai, J.; Azuma, T.; Xin, X.; Lichtler, A.C.; Rowe, D.W.; Saito, T.; et al. Stepwise strategy for generating osteoblasts from human pluripotent stem cells under fully defined xeno-free conditions with small-molecule inducers. Regen. Ther. 2020, 14, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Ochiai-Shino, H.; Kato, H.; Sawada, T.; Onodera, S.; Saito, A.; Takato, T.; Shibahara, T.; Muramatsu, T.; Azuma, T. A Novel Strategy for Enrichment and Isolation of Osteoprogenitor Cells from Induced Pluripotent Stem Cells Based on Surface Marker Combination. PLoS ONE 2014, 9, e99534. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Niyibizi, C. Cells derived from murine induced pluripotent stem cells (iPSC) by treatment with members of TGF-beta family give rise to osteoblasts differentiation and form bone in vivo. BMC Cell Biol. 2012, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Kishida, T.; Sato, Y.; Nishioka, K.; Ejima, A.; Fujiwara, H.; Kubo, T.; Yamamoto, T.; Kanamura, N.; Mazda, O. Direct conversion of human fibroblasts into functional osteoblasts by defined factors. Proc. Natl. Acad. Sci. USA 2015, 112, 6152–6157. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Kishida, T.; Nakai, K.; Sato, Y.; Kotani, S.-I.; Nishizawa, Y.; Yamamoto, T.; Kanamura, N.; Mazda, O. Direct phenotypic conversion of human fibroblasts into functional osteoblasts triggered by a blockade of the transforming growth factor-β signal. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Kawai, S.; Yoshitomi, H.; Sunaga, J.; Alev, C.; Nagata, S.; Nishio, M.; Hada, M.; Koyama, Y.; Uemura, M.; Sekiguchi, K.; et al. In vitro bone-like nodules generated from patient-derived iPSCs recapitulate pathological bone phenotypes. Nat. Biomed. Eng. 2019, 3, 558–570. [Google Scholar] [CrossRef]
- Zujur, D.; Kanke, K.; Lichtler, A.; Hojo, H.; Chung, U.-I.; Ohba, S. Three-dimensional system enabling the maintenance and directed differentiation of pluripotent stem cells under defined conditions. Sci. Adv. 2017, 3, e1602875. [Google Scholar] [CrossRef]
- Jeon, O.H.; Panicker, L.M.; Lu, Q.; Chae, J.J.; Feldman, R.A.; Elisseeff, J.H. Human iPSC-derived osteoblasts and osteoclasts together promote bone regeneration in 3D biomaterials. Sci. Rep. 2016, 6, 26761. [Google Scholar] [CrossRef]
- Funayama, N.; Sato, Y.; Matsumoto, K.; Ogura, T.; Takahashi, Y. Coelom formation: Binary decision of the lateral plate mes-oderm is controlled by the ectoderm. Development 1999, 126, 4129–4138. [Google Scholar]
- Niederreither, K.; Subbarayan, V.; Dollé, P.; Chambon, P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat. Genet. 1999, 21, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, S.; Wilde, S.M.; Wood, S.; Logan, M.P.O. RA Acts in a Coherent Feed-Forward Mechanism with Tbx5 to Control Limb Bud Induction and Initiation. Cell Rep. 2015, 12, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Kengaku, M. Distinct WNT Pathways Regulating AER Formation and Dorsoventral Polarity in the Chick Limb Bud. Science 1998, 280, 1274–1277. [Google Scholar] [CrossRef] [PubMed]
- Galceran, J.; Fariñas, I.; Depew, M.J.; Clevers, H.; Grosschedl, R. Wnt3a–/–-like phenotype and limb deficiency in Lef1–/–Tcf1–/– mice. Genes Dev. 1999, 13, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Arango, N.A.; Lovell-Badge, R.; Behringer, R.R. Targeted Mutagenesis of the Endogenous Mouse Mis Gene Promoter. Cell 1999, 99, 409–419. [Google Scholar] [CrossRef]
- Laufer, E.; Nelson, C.E.; Johnson, R.L.; Morgan, B.A.; Tabin, C. Sonic hedgehog and Fgf-4 act through a signaling cascade and feedback loop to integrate growth and patterning of the developing limb bud. Cell 1994, 79, 993–1003. [Google Scholar] [CrossRef]
- Verheyden, J.M.; Sun, X. An Fgf/Gremlin inhibitory feedback loop triggers termination of limb bud outgrowth. Nature 2008, 454, 638–641. [Google Scholar] [CrossRef]
- Grotewold, L.; Rüther, U. Bmp, Fgf and Wnt signalling in programmed cell death and chondrogenesis during vertebrate limb development: The role of Dickkopf-1. Int. J. Dev. Biol. 2002, 46, 943–947. [Google Scholar]
- Mori, S.; Sakakura, E.; Tsunekawa, Y.; Hagiwara, M.; Suzuki, T.; Eiraku, M. Self-organized formation of developing appendages from murine pluripotent stem cells. Nat. Commun. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Tan, J.Y.; Sriram, G.; Rufaihah, A.J.; Neoh, K.G.; Cao, T. Efficient Derivation of Lateral Plate and Paraxial Mesoderm Subtypes from Human Embryonic Stem Cells Through GSKi-Mediated Differentiation. Stem Cells Dev. 2013, 22, 1893–1906. [Google Scholar] [CrossRef]
- Sakurai, H.; Era, T.; Jakt, L.M.; Okada, M.; Nakai, S.; Nishikawa, S.; Nishikawa, S.-I. In Vitro Modeling of Paraxial and Lateral Mesoderm Differentiation Reveals Early Reversibility. Stem Cells 2006, 24, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.S.; Charney, R.M.; García-Castro, M.I. Specification and formation of the neural crest: Perspectives on lineage segregation. Genesis 2019, 57, e23276. [Google Scholar] [CrossRef] [PubMed]
- Simões-Costa, M.; Bronner, M.E. Establishing neural crest identity: A gene regulatory recipe. Development 2015, 142, 242–257. [Google Scholar] [CrossRef] [PubMed]
- Khudyakov, J.; Bronner, M.E. Comprehensive spatiotemporal analysis of early chick neural crest network genes. Dev. Dyn. 2009, 238, 716–723. [Google Scholar] [CrossRef]
- Sauka-Spengler, T.; Bronner-Fraser, M. A gene regulatory network orchestrates neural crest formation. Nat. Rev. Mol. Cell Biol. 2008, 9, 557–568. [Google Scholar] [CrossRef]
- Le Douarin, N.M.; Creuzet, S.; Couly, G.; Dupin, E. Neural crest cell plasticity and its limits. Development 2004, 131, 4637–4650. [Google Scholar] [CrossRef]
- Schussler, O.; Gharibeh, L.; Mootoosamy, P.; Murith, N.; Tien, V.; Rougemont, A.-L.; Sologashvili, T.; Suuronen, E.; Lecarpentier, Y.; Ruel, M. Cardiac Neural Crest Cells: Their Rhombomeric Specification, Migration, and Association with Heart and Great Vessel Anomalies. Cell. Mol. Neurobiol. 2020, 1–27. [Google Scholar] [CrossRef]
- Harris, M.L.; Erickson, C.A. Lineage specification in neural crest cell pathfinding. Dev. Dyn. 2006, 236, 1–19. [Google Scholar] [CrossRef]
- Gendron-Maguire, M.; Mallo, M.; Zhang, M.; Gridley, T. Hoxa-2 mutant mice exhibit homeotic transformation of skeletal elements derived from cranial neural crest. Cell 1993, 75, 1317–1331. [Google Scholar] [CrossRef]
- Couly, G.; Grapin-Botton, A.; Coltey, P.; Ruhin, B.; Le Douarin, N.M. Determination of the identity of the derivatives of the cephalic neural crest: Incompatibility between Hox gene expression and lower jaw development. Development 1998, 125, 3445. [Google Scholar]
- Fukuta, M.; Nakai, Y.; Kirino, K.; Nakagawa, M.; Sekiguchi, K.; Nagata, S.; Matsumoto, Y.; Yamamoto, T.; Umeda, K.; Heike, T.; et al. Derivation of Mesenchymal Stromal Cells from Pluripotent Stem Cells through a Neural Crest Lineage using Small Molecule Compounds with Defined Media. PLoS ONE 2014, 9, e112291. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.W.; Murdoch, B.; Salem, A.F.; Prasad, M.S.; Gomez, G.A.; García-Castro, M.I. WNT/β-catenin signaling mediates human neural crest induction via a pre-neural border intermediate. Development 2016, 143, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Menendez, L.; Kulik, M.J.; Page, A.T.; Park, S.S.; Lauderdale, J.D.; Cunningham, M.L.; Dalton, S. Directed differentiation of human pluripotent cells to neural crest stem cells. Nat. Protoc. 2013, 8, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Wymeersch, F.J.; Huang, Y.; Blin, G.; Cambray, N.; Wilkie, R.; Wong, F.C.K.; Wilson, V. Position-dependent plasticity of distinct progenitor types in the primitive streak. eLife 2016, 5, e10042. [Google Scholar] [CrossRef]
- Jamal, M.; Lewandowski, S.L.; Lawton, M.L.; Huang, G.T.-J.; Ikonomou, L. Derivation and characterization of putative craniofacial mesenchymal progenitor cells from human induced pluripotent stem cells. Stem Cell Res. 2018, 33, 100–109. [Google Scholar] [CrossRef]
- Zhu, Q.; Lu, Q.; Gao, R.; Cao, T. Prospect of Human Pluripotent Stem Cell-Derived Neural Crest Stem Cells in Clinical Application. Stem Cells Int. 2016, 2016, 1–11. [Google Scholar] [CrossRef]
- Taïhi, I.; Nassif, A.; Isaac, J.; Fournier, B.P.; Ferré, F. Head to Knee: Cranial Neural Crest-Derived Cells as Promising Candidates for Human Cartilage Repair. Stem Cells Int. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Frieedenstein, A.J.; Petrakova, K.V.; Kurolesova, A.L.; Frolova, G.P. Hetrotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoetic tissue. Transplantacion 1968, 6, 230. [Google Scholar]
- Squillaro, T.; Peluso, G.; Galderisi, U. Clinical Trials with Mesenchymal Stem Cells: An Update. Cell Transpl. 2016, 25, 829–848. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Dezawa, M.; Ishikawa, H.; Itokazu, Y.; Yoshihara, T.; Hoshino, M.; Takeda, S.; Ide, C.; Nabeshima, Y.-I. Bone Marrow Stromal Cells Generate Muscle Cells and Repair Muscle Degeneration. Science 2005, 309, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Urrutia, D.N.; Caviedes, P.; Mardones, R.; Minguell, J.J.; Vega-Letter, A.M.; Jofre, C. Comparative study of the neural differentiation capacity of mesenchymal stromal cells from different tissue sources: An approach for their use in neural regeneration therapies. PLoS ONE 2019, 14, e0213032. [Google Scholar] [CrossRef] [PubMed]
- Parekkadan, B.; Van Poll, D.; Suganuma, K.; Carter, E.A.; Berthiaume, F.; Tilles, A.W.; Yarmush, M.L. Mesenchymal Stem Cell-Derived Molecules Reverse Fulminant Hepatic Failure. PLoS ONE 2007, 2, e941. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.H.; Pulin, A.A.; Seo, M.J.; Kota, D.J.; Ylostalo, J.; Larson, B.L.; Semprun-Prieto, L.; Delafontaine, P.; Prockop, D.J. Intravenous hMSCs Improve Myocardial Infarction in Mice because Cells Embolized in Lung Are Activated to Secrete the Anti-inflammatory Protein TSG-6. Cell Stem Cell 2009, 5, 54–63. [Google Scholar] [CrossRef]
- Morelli, A.E.; Larregina, A.T. Concise Review: Mechanisms Behind Apoptotic Cell-Based Therapies Against Transplant Rejection and Graft versus Host Disease. Stem Cells 2016, 34, 1142–1150. [Google Scholar] [CrossRef]
- Cheung, T.S.; Bertolino, G.M.; Giacomini, C.; Bornhäuser, M.; Dazzi, F.; Galleu, A. Mesenchymal Stromal Cells for Graft Versus Host Disease: Mechanism-Based Biomarkers. Front. Immunol. 2020, 11, 1338. [Google Scholar] [CrossRef]
- Le Blanc, K.; Tammik, C.; Rosendahl, K.; Zetterberg, E.; Ringdén, O. HLA expression and immunologic propertiesof differen-tiated and undifferentiated mesenchymal stem cells. Exp. Hematol. 2003, 31, 890–896. [Google Scholar] [CrossRef]
- Di Nicola, M.; Carlo-Stella, C.; Magni, M.; Milanesi, M.; Longoni, P.D.; Matteucci, P.; Grisanti, S.; Gianni, A.M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002, 99, 3838–3843. [Google Scholar] [CrossRef]
- Saeed, H.; Ahsan, M.; Saleem, Z.; Iqtedar, M.; Islam, M.; Danish, Z.; Khan, A.M. Mesenchymal stem cells (MSCs) as skeletal therapeutics–an update. J. Biomed. Sci. 2016, 23, 1–15. [Google Scholar] [CrossRef]
- Horwitz, E.M.; Prockop, D.J.; Gordon, P.L.; Koo, W.W.K.; Fitzpatrick, L.A.; Neel, M.D.; McCarville, M.E.; Orchard, P.J.; Pyeritz, R.E.; Brenner, M.K. Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood 2001, 97, 1227–1231. [Google Scholar] [CrossRef]
- Vega, A.; Martín-Ferrero, M.A.; Del Canto, F.; Alberca, M.; García, V.; Munar, A.; Orozco, L.; Soler, R.; Fuertes, J.J.; Huguet, M.; et al. Treatment of Knee Osteoarthritis With Allogeneic Bone Marrow Mesenchymal Stem Cells. A Randomized Controlled Trial. Transplantation 2015, 99, 1681–1690. [Google Scholar] [CrossRef] [PubMed]
- Morata-Tarifa, C.; Macías-Sánchez, M.D.M.; Gutiérrez-Pizarraya, A.; Sanchez-Pernaute, R. Mesenchymal stromal cells for the prophylaxis and treatment of graft-versus-host disease—A meta-analysis. Stem Cell Res. Ther. 2020, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Kim, H.-Y.; Kim, H.-W.; Chae, G.-N.; Oh, H.-T.; Park, J.-Y.; Shim, H.; Seo, M.; Shin, E.-Y.; Kim, E.-G.; et al. Increased caveolin-1, a cause for the declined adipogenic potential of senescent human mesenchymal stem cells. Mech. Ageing Dev. 2005, 126, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Pelekanos, R.A.; Ellis, R.L.; Horne, R.; Wolvetang, E.J.; Fisk, N.M. Small Molecule Mesengenic Induction of Human Induced Pluripotent Stem Cells to Generate Mesenchymal Stem/Stromal Cells. Stem Cells Transl. Med. 2012, 1, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Barberi, T.; Willis, L.M.; Socci, N.D.; Studer, L. Derivation of Multipotent Mesenchymal Precursors from Human Embryonic Stem Cells. PLoS Med. 2005, 2, e161. [Google Scholar] [CrossRef]
- Zhou, B.O.; Yue, R.; Murphy, M.M.; Peyer, J.G.; Morrison, S.J. Leptin-Receptor-Expressing Mesenchymal Stromal Cells Represent the Main Source of Bone Formed by Adult Bone Marrow. Cell Stem Cell 2014, 15, 154–168. [Google Scholar] [CrossRef]
- Matsushita, Y.; Nagata, M.; Kozloff, K.M.; Welch, J.D.; Mizuhashi, K.; Tokavanich, N.; Hallett, S.A.; Link, D.C.; Nagasawa, T.; Ono, W.; et al. A Wnt-mediated transformation of the bone marrow stromal cell identity orchestrates skeletal regeneration. Nat. Commun. 2020, 11, 1–17. [Google Scholar] [CrossRef]
- Chan, C.K.F.; Seo, E.Y.; Chen, J.Y.; Taylor, W.; McArdle, A.; Sinha, R.; Tevlin, R.; Seita, J.; Vincent-Tompkins, J.; Wearda, T.; et al. Identification and Specification of the Mouse Skeletal Stem Cell. Cell 2015, 160, 285–298. [Google Scholar] [CrossRef]
- Marecic, O.; Tevlin, R.; McArdle, A.; Seo, E.Y.; Wearda, T.; Duldulao, C.; Walmsley, G.G.; Nguyen, A.; Weissman, I.L.; Chan, C.K.F.; et al. Identification and characterization of an injury-induced skeletal progenitor. Proc. Natl. Acad. Sci. USA 2015, 112, 9920–9925. [Google Scholar] [CrossRef]
- Murphy, M.P.; Koepke, L.S.; Lopez, M.T.; Tong, X.; Ambrosi, T.H.; Gulati, G.S.; Marecic, O.; Wang, Y.; Ransom, R.C.; Hoover, M.Y.; et al. Articular cartilage regeneration by activated skeletal stem cells. Nat. Med. 2020, 26, 1583–1592. [Google Scholar] [CrossRef]
- Méndez-Ferrer, S.; Michurina, T.V.; Ferraro, F.; Mazloom, A.R.; MacArthur, B.D.; Lira, S.A.; Scadden, D.T.; Ma’Ayan, A.; Enikolopov, G.N.; Frenette, P.S. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nat. Cell Biol. 2010, 466, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Worthley, D.L.; Churchill, M.; Compton, J.T.; Tailor, Y.; Rao, M.; Si, Y.; Levin, D.; Schwartz, M.G.; Uygur, A.; Hayakawa, Y.; et al. Gremlin 1 Identifies a Skeletal Stem Cell with Bone, Cartilage, and Reticular Stromal Potential. Cell 2015, 160, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Mizuhashi, K.; Ono, W.; Matsushita, Y.; Sakagami, N.; Takahashi, A.; Saunders, T.L.; Nagasawa, T.; Kronenberg, H.M.; Ono, N. Resting zone of the growth plate houses a unique class of skeletal stem cells. Nat. Cell Biol. 2018, 563, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Mizuhashi, K.; Nagata, M.; Matsushita, Y.; Ono, W.; Ono, N. Growth Plate Borderline Chondrocytes Behave as Transient Mesenchymal Precursor Cells. J. Bone Miner. Res. 2019, 34, 1387–1392. [Google Scholar] [CrossRef]
- Usami, Y.; Gunawardena, A.T.; Francois, N.B.; Otsuru, S.; Takano, H.; Hirose, K.; Matsuoka, M.; Suzuki, A.; Huang, J.; Qin, L.; et al. Possible Contribution of Wnt-Responsive Chondroprogenitors to the Postnatal Murine Growth Plate. J. Bone Miner. Res. 2019, 34, 964–974. [Google Scholar] [CrossRef]
- Debnath, S.; Yallowitz, A.R.; McCormick, J.; Lalani, S.; Zhang, T.; Xu, R.; Li, N.; Liu, Y.; Yang, Y.S.; Eiseman, M.; et al. Discovery of a periosteal stem cell mediating intramembranous bone formation. Nat. Cell Biol. 2018, 562, 133–139. [Google Scholar] [CrossRef]
- Ehninger, A.; Trumpp, A. The bone marrow stem cell niche grows up: Mesenchymal stem cells and macrophages move in. J. Exp. Med. 2011, 208, 421–428. [Google Scholar] [CrossRef]
- Greenbaum, A.; Hsu, Y.-M.S.; Day, R.B.; Schuettpelz, L.G.; Christopher, M.J.; Borgerding, J.N.; Nagasawa, T.; Link, D.C. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 2013, 495, 227–230. [Google Scholar] [CrossRef]
- Sugiyama, T.; Kohara, H.; Noda, M.; Nagasawa, T. Maintenance of the Hematopoietic Stem Cell Pool by CXCL12-CXCR4 Chemokine Signaling in Bone Marrow Stromal Cell Niches. Immunity 2006, 25, 977–988. [Google Scholar] [CrossRef]
- Kozhemyakina, E.; Zhang, M.; Ionescu, A.; Ayturk, U.M.; Ono, N.; Kobayashi, A.; Kronenberg, H.M.; Warman, M.L.; Lassar, A.B. Identification of aPrg4-Expressing Articular Cartilage Progenitor Cell Population in Mice. Arthritis Rheumatol. 2015, 67, 1261–1273. [Google Scholar] [CrossRef]
- Li, L.; Newton, P.T.; Bouderlique, T.; Sejnohova, M.; Zikmund, T.; Kozhemyakina, E.; Xie, M.; Krivanek, J.; Kaiser, J.; Qian, H.; et al. Superficial cells are self-renewing chondrocyte progenitors, which form the articular cartilage in juvenile mice. FASEB J. 2017, 31, 1067–1084. [Google Scholar] [CrossRef] [PubMed]
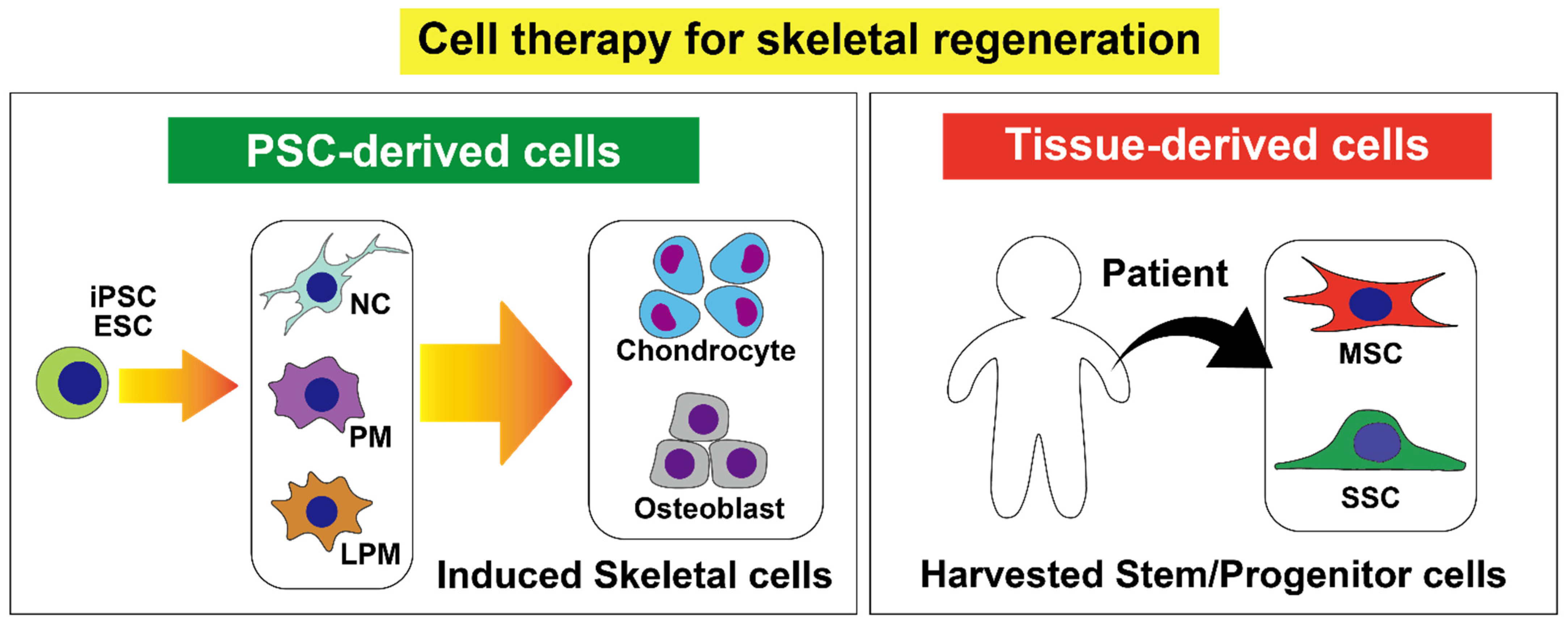
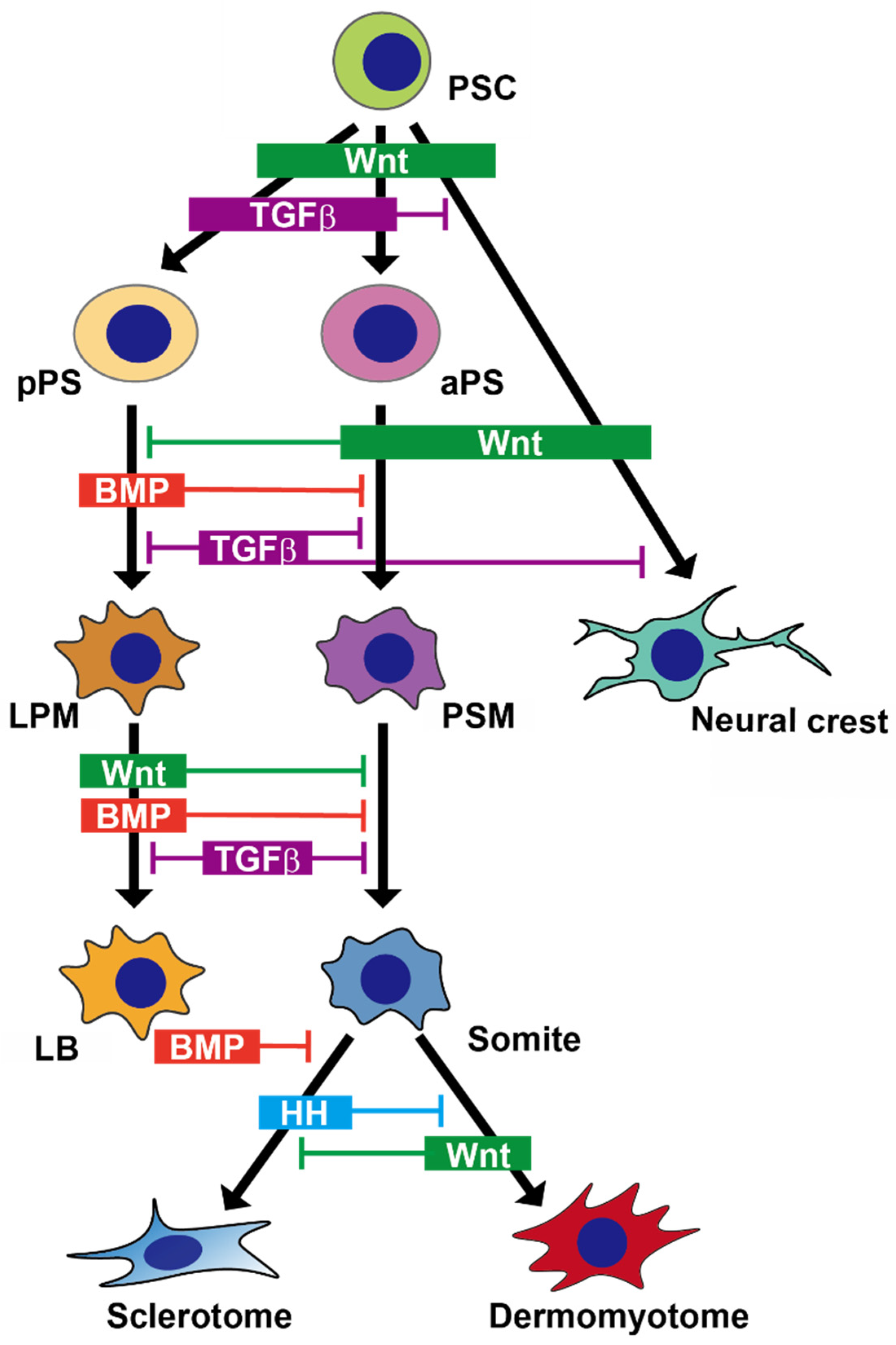
| Cell Type | Condition | Method | Cell Source | Phase |
|---|---|---|---|---|
| iPS (1) | Knee articular cartilage damage | Implantation of iPSC-derived Cartilage | Alogenic (iPSCs) | N/A |
| MSC (2) | Knee articular cartilage damage | Arthroscopy, Microfracture | Autologus (synovium) | N/A |
| MSC (3) | Knee osteoarthritis | Intra-articular injection | Autologus (bone marrow) | 1 |
| MSC (4) | Knee osteoarthritis | Transplantation with high tibial osteotomy | Alogenic (umbilical cord blood) | 2 |
| MSC (5) | Knee osteoarthritis | Intra-articular injection | Autologus (adipose, bone marrow) | 3 |
| MSC (6) | Knee osteoarthritis | Intra-articular injection | Autologus (adipose) | 4 |
| MSC (7) | Osteoporotic Spinal fracture | Intravenous Infusion | Autologus (bone marrow) | 1 |
| MSC (8) | Nonunion of Fracture | Injection at the fracture site | Autologus (adipose) | 1, 2 |
| MSC (9) | Nonunion of Fracture | Implantation with biomaterial | Autologus (bone marrow) | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tani, S.; Okada, H.; Chung, U.-i.; Ohba, S.; Hojo, H. The Progress of Stem Cell Technology for Skeletal Regeneration. Int. J. Mol. Sci. 2021, 22, 1404. https://doi.org/10.3390/ijms22031404
Tani S, Okada H, Chung U-i, Ohba S, Hojo H. The Progress of Stem Cell Technology for Skeletal Regeneration. International Journal of Molecular Sciences. 2021; 22(3):1404. https://doi.org/10.3390/ijms22031404
Chicago/Turabian StyleTani, Shoichiro, Hiroyuki Okada, Ung-il Chung, Shinsuke Ohba, and Hironori Hojo. 2021. "The Progress of Stem Cell Technology for Skeletal Regeneration" International Journal of Molecular Sciences 22, no. 3: 1404. https://doi.org/10.3390/ijms22031404
APA StyleTani, S., Okada, H., Chung, U.-i., Ohba, S., & Hojo, H. (2021). The Progress of Stem Cell Technology for Skeletal Regeneration. International Journal of Molecular Sciences, 22(3), 1404. https://doi.org/10.3390/ijms22031404






