Genetic Variants of ANGPT1, CD39, FGF2 and MMP9 Linked to Clinical Outcome of Bevacizumab Plus Chemotherapy for Metastatic Colorectal Cancer
Abstract
1. Introduction
2. Results
2.1. Gene Variants
2.2. Gene Variants and Tumour Response
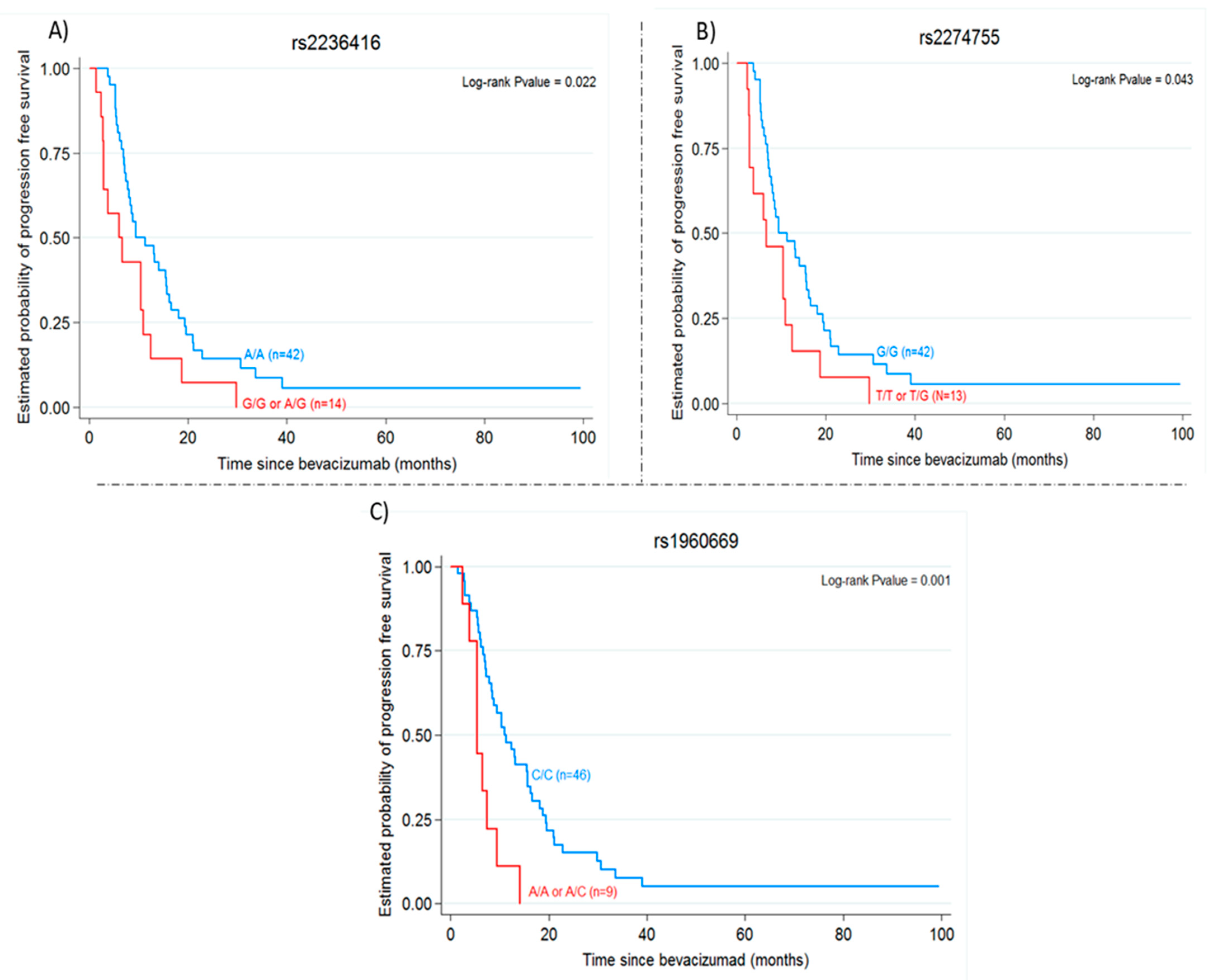
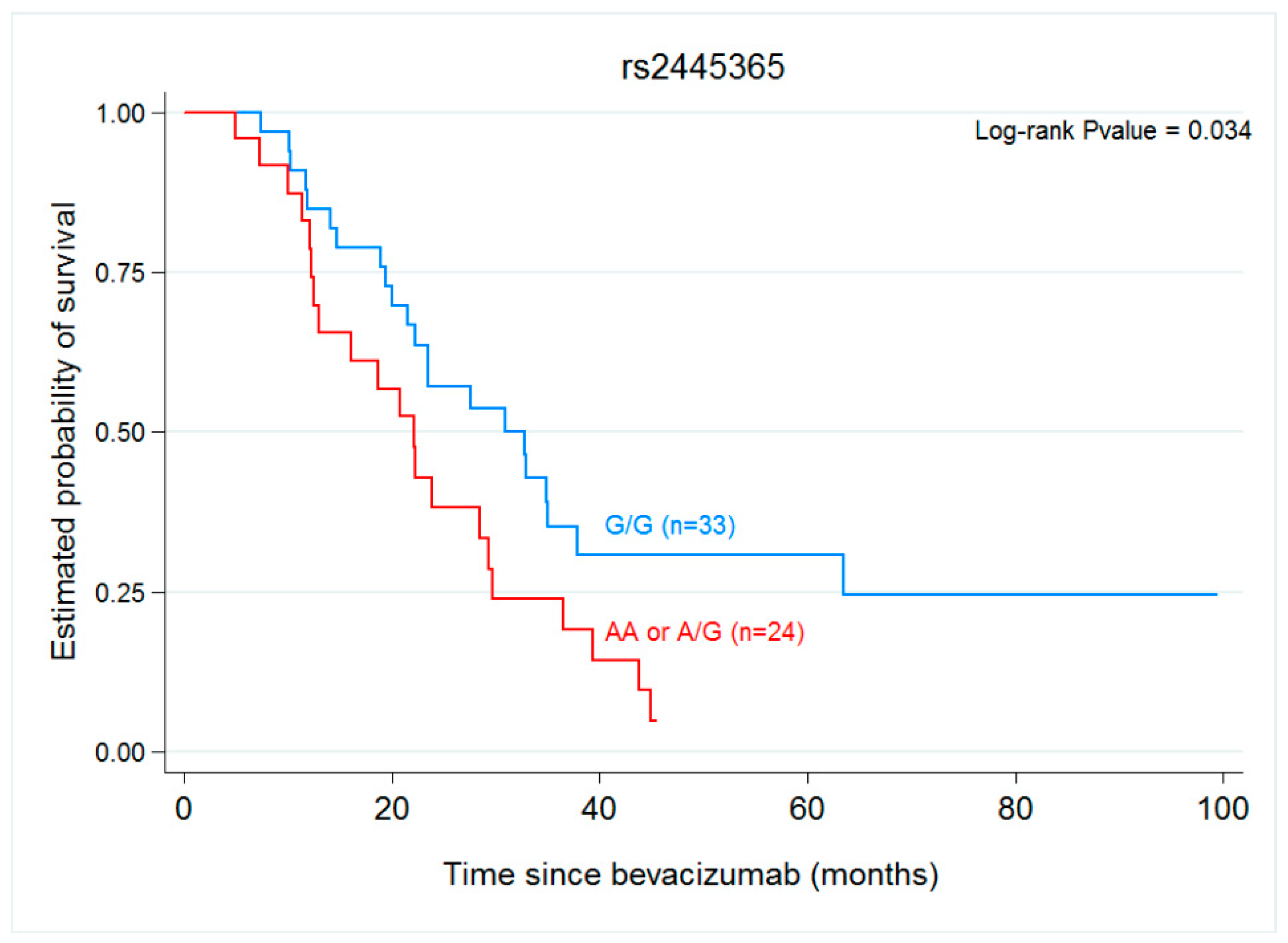
2.3. Gene Variants, Progression-Free Survival and Overall Survival
3. Discussion
4. Materials and Methods
4.1. Design and Patients
4.2. Candidate Polymorphisms
4.3. Tumour DNA Extraction and Genotyping
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A2AR | Adenosine A2a receptor |
| A2BR | Adenosine A2b receptor |
| ANGPT1 | Angiopoietin 1 |
| ANGPT2 | Angiopoietin 2 |
| ANGP-TIE | Angiopoietin receptor TEK |
| BVZ | Bevacizumab |
| CCL5 | C-C motif chemokine ligand 5 |
| CD39 | Ectonucleoside triphosphate diphosphohydrolase 1 (ENTPD1) |
| CRC | Colorectal cancer |
| CT | Chemotherapy |
| EDN1 | Endothelin 1 |
| FGF2 | Fibroblast growth factor 2 |
| IGF1 | Insulin like growth factor 1 |
| mCRC | Metastatic colorectal cancer |
| MKNK1 | MAPK interacting serine/threonine kinase 1 |
| MMP9 | Matrix metallopeptidase 9 |
| NT5E | 5′-Nucleotidase ecto |
| OS | Overall survival |
| pCR | Pathological complete response |
| PFS | Progression-free survival |
| RECIST | Response Evaluation Criteria in Solid Tumors |
| RR | Response rate |
| R | Responders |
| nR | Non-responders |
| SNP | Single nucleotide polymorphism |
| TOP1 | Topoisomerase |
| Tregs | Regulatory T cells |
| VEGFA | Vascular endothelial growth factor A |
References
- Sharma, R. An examination of colorectal cancer burden by socioeconomic status: Evidence from GLOBOCAN 2018. EPMA J. 2020, 11, 95–117. [Google Scholar] [CrossRef]
- Li, H.; Chen, Y.-X.; Wen, J.-G.; Zhou, H.-H. Metastasis-associated in colon cancer 1: A promising biomarker for the metastasis and prognosis of colorectal cancer. Oncol. Lett. 2017, 14, 3899–3908. [Google Scholar] [CrossRef] [PubMed]
- Valle, L.; Vilar, E.; Tavtigian, S.V.; Stoffel, E.M. Genetic predisposition to colorectal cancer: Syndromes, genes, classification of genetic variants and implications for precision medicine. J. Pathol. 2019, 247, 574–588. [Google Scholar] [CrossRef] [PubMed]
- Custodio, A.; Barriuso, J.; De Castro, J.; Martínez-Marín, V.; Moreno, V.; Rodríguez-Salas, N.; Feliu, J. Molecular markers to predict outcome to antiangiogenic therapies in colorectal cancer: Current evidence and future perspectives. Cancer Treat. Rev. 2013, 39, 908–924. [Google Scholar] [CrossRef] [PubMed]
- Eng, L.; Liu, G. VEGF pathway polymorphisms as prognostic and pharmacogenetic factors in cancer: A 2013 update. Pharmacogenomics 2013, 14, 1659–1667. [Google Scholar] [CrossRef]
- Rosen, L.S.; Jacobs, I.A.; Burkes, R.L. Bevacizumab in Colorectal Cancer: Current Role in Treatment and the Potential of Biosimilars. Target. Oncol. 2017, 12, 599–610. [Google Scholar] [CrossRef]
- Welch, S.; Spithoff, K.; Rumble, R.B.; Maroun, J. Bevacizumab combined with chemotherapy for patients with advanced colorectal cancer: A systematic review. Ann. Oncol. 2010, 21, 1152–1162. [Google Scholar] [CrossRef]
- Botrel, T.E.A.; De Clark, L.G.O.; Paladini, L.; Clark, O.A.C. Efficacy and safety of bevacizumab plus chemotherapy compared to chemotherapy alone in previously untreated advanced or metastatic colorectal cancer: A systematic review and meta-analysis. BMC Cancer 2016, 16, 677. [Google Scholar] [CrossRef]
- Tebbutt, N.C.; Wilson, K.; Gebski, V.J.; Cummins, M.M.; Zannino, D.; Van Hazel, G.A.; Robinson, B.; Broad, A.; Ganju, V.; Ackland, S.P.; et al. Capecitabine, bevacizumab, and mitomycin in first-line treatment of metastatic colorectal cancer: Results of the Australasian Gastrointestinal Trials Group Randomized Phase III MAX Study. J. Clin. Oncol. 2010, 28, 3191–3198. [Google Scholar] [CrossRef]
- Ruan, W.-C.; Che, Y.-P.; Ding, L.; Li, H.-F. Efficacy and Toxicity of Addition of Bevacizumab to Chemotherapy in Patients with Metastatic Colorectal Cancer. Comb. Chem. High Throughput Screen. 2018, 21, 718–724. [Google Scholar] [CrossRef]
- Cunningham, D.; Atkin, W.; Lenz, H.-J.; Lynch, H.T.; Minsky, B.; Nordlinger, B.; Starling, N. Colorectal cancer. Lancet 2010, 375, 1030–1047. [Google Scholar] [CrossRef]
- Dirican, A.; Varol, U.; Kucukzeybek, Y.; Alacacioglu, A.; Erten, C.; Somali, I.; Can, A.; Demir, L.; Bayoglu, I.V.; Akyol, M. Treatment of metastatic colorectal cancer with or without bevacizumab: Can the neutrophil/lymphocyte ratio predict the efficiency of bevacizumab. Asian Pac. J. Cancer Prev. 2014, 15, 4781–4786. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shojaei, F.; Ferrara, N. Refractoriness to antivascular endothelial growth factor treatment: Role of myeloid cells. Cancer Res. 2008, 68, 5501–5504. [Google Scholar] [CrossRef] [PubMed]
- Bergers, G.; Hanahan, D. Modes of resistance to anti-angiogenic therapy. Nat. Rev. Cancer 2008, 8, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, S.; Loupakis, F.; Stintzing, S.; Cao, S.; Zhang, W.; Yang, D.; Ning, Y.; Sunakawa, Y.; Stremitzer, S.; Matsusaka, S.; et al. Clinical significance of tlr1 i602s polymorphism for patients with metastatic colorectal cancer treated with folfiri plus bevacizumab. Mol. Cancer Ther. 2016, 15, 1740–1745. [Google Scholar] [CrossRef]
- Di Salvatore, M.; Pietrantonio, F.; Orlandi, A.; Del Re, M.; Berenato, R.; Rossi, E.; Caporale, M.; Guarino, D.; Martinetti, A.; Basso, M.; et al. IL-8 and eNOS polymorphisms predict bevacizumab-based first line treatment outcomes in RAS mutant metastatic colorectal cancer patients. Oncotarget 2017, 8, 16887–16898. [Google Scholar] [CrossRef]
- Sunakawa, Y.; Stintzing, S.; Heinemann, V.; Cremolini, C.; Falcone, A.; Cao, S.; Yang, D.; Zhang, W.; Yamauchi, S.; Ning, Y.; et al. Variations in genes regulating tumor-associated macrophages (TAMs) to predict outcome of bevacizumab (bev)-based treatment in patients with metastatic colorectal cancer (mCRC): Results from TRIBE and FIRE3 trials. J. Clin. Oncol. 2015, 33, 3552. [Google Scholar] [CrossRef]
- Ulivi, P.; Scarpi, E.; Passardi, A.; Marisi, G.; Calistri, D.; Zoli, W.; Del Re, M.; Frassineti, G.L.; Tassinari, D.; Tamberi, S.; et al. eNOS polymorphisms as predictors of efficacy of bevacizumab-based chemotherapy in metastatic colorectal cancer: Data from a randomized clinical trial. J. Transl. Med. 2015, 13, 1–10. [Google Scholar] [CrossRef]
- Matsusaka, S.; Zhang, W.; Cao, S.; Hanna, D.L.; Sunakawa, Y.; Sebio, A.; Ueno, M.; Yang, D.; Ning, Y.; Parekh, A.; et al. TWIST1 Polymorphisms Predict Survival in Patients with Metastatic Colorectal Cancer Receiving First-Line Bevacizumab plus Oxaliplatin-Based Chemotherapy. Mol. Cancer Ther. 2016, 15, 1405–1411. [Google Scholar] [CrossRef]
- Loupakis, F.; Cremolini, C.; Yang, D.; Salvatore, L.; Zhang, W.; Wakatsuki, T.; Bohanes, P.; Schirripa, M.; Benhaim, L.; Lonardi, S.; et al. Prospective Validation of Candidate SNPs of VEGF/VEGFR Pathway in Metastatic Colorectal Cancer Patients Treated with First-Line FOLFIRI Plus Bevacizumab. PLoS ONE 2013, 8, e66774. [Google Scholar] [CrossRef]
- Novillo, A.; Gaibar, M.; Romero-Lorca, A.; Gilsanz, M.F.; Beltrán, L.; Galán, M.; Antón, B.; Malón, D.; Moreno, A.; Fernández-Santander, A. Efficacy of bevacizumab-containing chemotherapy in metastatic colorectal cancer and CXCL5 expression: Six case reports. World J. Gastroenterol. 2020, 26, 1979–1986. [Google Scholar] [CrossRef] [PubMed]
- Auton, A.; Abecasis, G.R.; Altshuler, D.M.; Durbin, R.M.; Bentley, D.R.; Chakravarti, A.; Clark, A.G.; Donnelly, P.; Eichler, E.E.; Flicek, P.; et al. A global reference for human genetic variation. Nature 2015, 526, 68–74. [Google Scholar] [PubMed]
- Raouf, S.; Bertelli, G.; Ograbek, A.; Field, P.; Tran, I. Real-world use of bevacizumab in metastatic colorectal, metastatic breast, advanced ovarian and cervical cancer: A systematic literature review. Future Oncol. 2019, 15, 543–561. [Google Scholar] [CrossRef] [PubMed]
- Makhoul, I.; Todorova, V.K.; Siegel, E.R.; Erickson, S.W.; Dhakal, I.; Raj, V.R.; Lee, J.Y.; Orloff, M.S.; Griffin, R.J.; Henry-Tillman, R.S.; et al. Germline genetic variants in TEK, ANGPT1, ANGPT2, MMP9, FGF2 and VEGFA are associated with pathologic complete response to bevacizumab in breast cancer patients. PLoS ONE 2017, 12, e0168550. [Google Scholar] [CrossRef]
- Aprile, G.; Ferrari, L.; Fontanella, C.; Puglisi, F. Bevacizumab in older patients with advanced colorectal or breast cancer. Crit. Rev. Oncol. Hematol. 2013, 87, 41–54. [Google Scholar] [CrossRef]
- Gerger, A.; El-Khoueiry, A.; Zhang, W.; Yang, D.; Singh, H.; Bohanes, P.; Ning, Y.; Winder, T.; Labonte, M.J.; Wilson, P.M.; et al. Pharmacogenetic angiogenesis profiling for first-line Bevacizumab plus oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Clin. Cancer Res. 2011, 17, 5783–5792. [Google Scholar] [CrossRef]
- Yegutkin, G.G. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim. Biophys. Acta 2008, 1783, 673–694. [Google Scholar] [CrossRef]
- Deaglio, S.; Dwyer, K.M.; Gao, W.; Friedman, D.; Usheva, A.; Erat, A.; Chen, J.-F.; Enjyoji, K.; Linden, J.; Oukka, M.; et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 2007, 204, 1257–1265. [Google Scholar] [CrossRef]
- Häusler, S.F.M.; Montalbán del Barrio, I.; Strohschein, J.; Chandran, P.A.; Engel, J.B.; Hönig, A.; Ossadnik, M.; Horn, E.; Fischer, B.; Krockenberger, M.; et al. Ectonucleotidases CD39 and CD73 on OvCA cells are potent adenosine-generating enzymes responsible for adenosine receptor 2A-dependent suppression of T cell function and NK cell cytotoxicity. Cancer Immunol. Immunother. 2011, 60, 1405–1418. [Google Scholar] [CrossRef]
- Tokunaga, R.; Cao, S.; Naseem, M.; Lo, J.H.; Battaglin, F.; Puccini, A.; Berger, M.D.; Soni, S.; Millstein, J.; Zhang, W.; et al. Prognostic Effect of Adenosine-related Genetic Variants in Metastatic Colorectal Cancer Treated With Bevacizumab-based Chemotherapy. Clin. Colorectal Cancer 2019, 18, e8–e19. [Google Scholar] [CrossRef]
- Liu, J.; Shi, K.; Chen, M.; Xu, L.; Hong, J.; Hu, B.; Yang, X.; Sun, R. Elevated miR-155 expression induces immunosuppression via CD39+ regulatory T-cells in sepsis patient. Int. J. Infect. Dis. 2015, 40, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Cao, Y.; Lei, Z. Selective Depletion of CD4 + CD25 + Foxp3 + Regulatory T Cells by Low-Dose Cyclophosphamide Is Explained by Reduced Intracellular ATP Levels T Cells by Low-Dose Cyclophosphamide Is Explained. Cancer Res. 2010, 70. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Bo, C.; Kang, Y.; Li, H. What else can CD39 tell us? Front. Immunol. 2017, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Deryugina, E.I.; Quigley, J.P. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006, 25, 9–34. [Google Scholar] [CrossRef] [PubMed]
- Egeblad, M.; Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2002, 2, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Beeghly-Fadiel, A.; Lu, W.; Shu, X.-O.; Long, J.; Cai, Q.; Xiang, Y.; Gao, Y.-T.; Zheng, W. MMP9 Polymorphisms and Breast Cancer Risk: A Report from the Shanghai Breast Cancer Genetics Study. Breast Cancer Res. Treat. 2011, 126, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef]
- Huang, H. Matrix Metalloproteinase-9 (MMP-9) as a Cancer Biomarker and MMP-9 Biosensors: Recent Advances. Sensors 2018, 18, 3249. [Google Scholar] [CrossRef]
- Jiménez-Morales, S.; Martínez-Aguilar, N.; Gamboa-Becerra, R.; Jiménez-Ruíz, J.L.; López-Ley, D.; Lou, H.; Saldaña-Alvarez, Y.; Dean, M.; Orozco, L. Polymorphisms in metalloproteinase-9 are associated with the risk for asthma in Mexican pediatric patients. Hum. Immunol. 2013, 74, 998–1002. [Google Scholar] [CrossRef]
- Xu, E.D.; Xiao, Y.F.; Wang, J.J.; Dong, L. Association study between matrix metalloproteinase-9 gene (MMP9) polymorphisms and the risk of Henoch-Schönlein purpura in children. Genet. Mol. Res. 2016, 15, 1–7. [Google Scholar] [CrossRef]
- Johnatty, S.E.; Beesley, J.; Chen, X.; Spurdle, A.B.; DeFazio, A.; Webb, P.M.; Goode, E.L.; Rider, D.N.; Vierkant, R.A.; Anderson, S.; et al. Polymorphisms in the FGF2 gene and risk of serous ovarian cancer: Results from the ovarian cancer association consortium. Twin Res. Hum. Genet. 2009, 12, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.L.; John, E.M.; Stern, M.C.; Herrick, J.; Lundgreen, A.; Giuliano, A.R.; Hines, L.; Baumgartner, K.B.; Torres-Mejia, G.; Wolff, R.K. Associations with growth factor genes (FGF1, FGF2, PDGFB, FGFR2, NRG2, EGF, ERBB2) with breast cancer risk and survival: The Breast Cancer Health Disparities Study. Breast Cancer Res. Treat. 2013, 140, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.; Watanabe, H.; Budson, A.E.; Richie, J.P.; Hayes, D.F.; Folkman, J. Elevated levels of an angiogenic peptide, basic fibroblast growth factor, in the urine of patients with a wide spectrum of cancers. J. Natl. Cancer Inst. 1994, 86, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.E.; Fallon, M.B.; Krowka, M.J.; Brown, R.S.; Trotter, J.F.; Peter, I.; Tighiouart, H.; Knowles, J.A.; Rabinowitz, D.; Benza, R.L.; et al. Genetic risk factors for portopulmonary hypertension in patients with advanced liver disease. Am. J. Respir. Crit. Care Med. 2009, 179, 835–842. [Google Scholar] [CrossRef]
- Thompson, S.D.; Sudman, M.; Ramos, P.S.; Marion, M.C.; Ryan, M.; Tsoras, M.; Weiler, T.; Wagner, M.; Keddache, M.; Haas, J.P.; et al. The susceptibility loci juvenile idiopathic arthritis shares with other autoimmune diseases extend to PTPN2, COG6, and ANGPT1. Arthritis Rheum. 2010, 62, 3265–3276. [Google Scholar] [CrossRef]
- Dai, J.; Wan, S.; Zhou, F.; Myers, R.E.; Guo, X.; Li, B.; Fu, X.; Palazzo, J.P.; Dou, K.; Yang, H.; et al. Genetic polymorphism in a VEGF-independent angiogenesis gene ANGPT1 and overall survival of colorectal cancer patients after surgical resection. PLoS ONE 2012, 7, e34758. [Google Scholar] [CrossRef]
- Huang, H.; Bhat, A.; Woodnutt, G.; Lappe, R. Targeting the ANGPT-TIE2 pathway in malignancy. Nat. Rev. Cancer 2010, 10, 575–585. [Google Scholar] [CrossRef]
- Yu, Q.; Stamenkovic, I. Angiopoietin-2 is implicated in the regulation of tumor angiogenesis. Am. J. Pathol. 2001, 158, 563–570. [Google Scholar] [CrossRef]
- Hawighorst, T.; Skobe, M.; Streit, M.; Hong, Y.-K.; Velasco, P.; Brown, L.F.; Riccardi, L.; Lange-Asschenfeldt, B.; Detmar, M. Activation of the tie2 receptor by angiopoietin-1 enhances tumor vessel maturation and impairs squamous cell carcinoma growth. Am. J. Pathol. 2002, 160, 1381–1392. [Google Scholar] [CrossRef]
- Hayes, A.J.; Huang, W.Q.; Yu, J.; Maisonpierre, P.C.; Liu, A.; Kern, F.G.; Lippman, M.E.; McLeskey, S.W.; Li, L.Y. Expression and function of angiopoietin-1 in breast cancer. Br. J. Cancer 2000, 83, 1154–1160. [Google Scholar] [CrossRef]
- Stoeltzing, O.; Ahmad, S.A.; Liu, W.; McCarty, M.F.; Wey, J.S.; Parikh, A.A.; Fan, F.; Reinmuth, N.; Kawaguchi, M.; Bucana, C.D.; et al. Angiopoietin-1 inhibits vascular permeability, angiogenesis, and growth of hepatic colon cancer tumors. Cancer Res. 2003, 63, 3370–3377. [Google Scholar] [PubMed]
| Characteristic | Number (%) |
|---|---|
| Response to bevacizumab | |
| R (responder) | 30 (52.6%) |
| nR (non responder) | 27 (47.4%) |
| Sex | |
| Female | 16 (28%) |
| Male | 41 (72%) |
| Age (years) | |
| <55 | 13 (22.8%) |
| 55–65 | 20 (35.1%) |
| > 65 | 24 (42.1%) |
| Metastases, | |
| Liver, lung, peritoneum or other | |
| 1 | 16 (27.7%) |
| 2 | 22 (38.6%) |
| >2 | 19 (33.3) |
| Primary tumour site | |
| Right | 17 (30%) |
| Left | 40 (70%) |
| Tumour stage at diagnosis, | |
| I | 0 |
| II | 2 (3.5%) |
| III | 16 (28.1%) |
| IV | 39 (68.4%) |
| KRAS status, | |
| Mutated | 42 (76%) |
| Normal | 13 (24%) |
| Chemotherapy backbone, | |
| FOLFOX | 27 (47%) |
| FOLFORI | 21 (37%) |
| OTHER (Xeloda, 5-Fu, Irotecan) | 9 (16%) |
| BVZ-regimen | |
| 1 | 33 (58.9%) |
| 2 | 15 (26.7%) |
| 3 | 6 (10.7%) |
| 4 | 1 (1.8%) |
| 5 | 1 (1.8%) |
| Surgical resection | |
| Yes | 20 (35%) |
| No | 37 (65%) |
| Gene Ontology | Genetic Variants Type | Genotypes WW WV VV | Treated Patients, n (%) | Alleles (%) W V | 1000 Genomes Allele Frequency European |
|---|---|---|---|---|---|
| Adenosine pathway | A2BR rs2015353 (ADORA2B) | TT | 8 (15.09) | T = 0.39 C = 0.60 | T = 0.46 C = 0.53 |
| T > C | TC | 26 (49.05) | |||
| Coding variant | CC | 19 (35.85) | |||
| ENTPD1 (CD39) rs11188513 | CC | 6 (10.53) | C = 0.31 T = 0.68 | C = 0.36 T = 0.63 | |
| C > T | CT | 24 (42.10) | |||
| Intron variant | TT | 27 (47.37) | |||
| NT5E (CD73) rs2229523 | AA | 3 (5.36) | A = 0.29 G = 0.71 | A = 0.29 G = 0.70 | |
| A > G | AG | 26 (46.43) | |||
| Missense variant | GG | 27 (48.21) | |||
| Direct angiogenesis | ANGPT1 rs2445365 | GG | 33 (57.89) | G = 0.75 A = 0.25 | G = 0.75 A = 0.25 |
| G > A, C | GA | 20 (35.09) | |||
| Intron variant | AA | 4 (7.02) | |||
| ANGPT2 rs10102851 | AA | 53 (92.98) | A = 0.97 G = 0.03 | A = 0.97 G = 0.03 | |
| A > G | AG | 4 (7.02) | |||
| Intron variant | GG | 0 | |||
| ANGPT2 rs1375668 | GG | 6 (10.71) | G = 0.27 A = 0.73 | G = 0.34 A = 0.66 | |
| G > A, C | GA | 18 (32.14) | |||
| Intron variant | AA | 32 (57.14) | |||
| ANGPT2 rs2515462 | AA | 4 (7.02) | A = 0.29 G = 0.71 | A = 0.32 G = 0.68 | |
| A > C, G, T | AG | 25 (43.86) | |||
| Intron variant | GG | 28 (49.12) | |||
| VEGFA rs833061 | CC | 10 (17.54) | C = 0.42 T = 0.58 | C = 0.50 T = 0.50 | |
| C > G, T | CT | 28 (49.12) | |||
| Upstream transcript variant | TT | 19 (33.33) | |||
| VEGFA rs833068 | GG | 17 (29.82) | G = 0.54 A = 0.46 | G = 0.69 A = 0.31 | |
| G > A | GA | 28 (49.12) | |||
| Intron variant | AA | 12 (21.05) | |||
| VEGFA rs833069 | TT | 16 (29.09) | T = 0.54 C = 0.46 | T = 0.69 C = 0.31 | |
| T > C, G | TC | 27 (49.09) | |||
| Intron variant | CC | 12 (21.82) | |||
| VEGFA rs3025039 | CC | 43 (75.44) | C = 0.88 T = 0.12 | C = 0.88 T = 0.12 | |
| C > T | CT | 13 (22.81) | |||
| 3 prime UTR variant | TT | 1 (1.75) | |||
| FGF2 rs1960669 | CC | 46 (82.14) | C = 0.90 A = 0.1 | C = 0.84 A = 0.16 | |
| C > A | CA | 9 (16.07) | |||
| Intron variant | AA | 1 (1.79) | |||
| MMP9 rs2236416 | AA | 43 (75.44) | A = 0.86 G = 0.14 | A = 0.83 G = 0.17 | |
| A > G | AG | 12 (21.05) | |||
| Intron variant | GG | 2 (3.51) | |||
| MMP9 rs2274755 | GG | 43 (76.78) | G = 0.87 T = 0.13 | G = 0.83 T = 0.17 | |
| G > T | GT | 12 (21.43) | |||
| Intron variant | TT | 1 (1.79) | |||
| Cytokine signalling | EDN1 rs5370 | GG | 34 (60.71) | G = 0.79 T = 0.21 | G = 0.78 T = 0.22 |
| G > T | GT | 20 (35.71) | |||
| Missense variant | TT | 2 (3.57) | |||
| CCL5 rs2280789 | AA | 47 (82.46) | A = 0.89 G = 0.11 | A = 0.89 G = 0.11 | |
| A > G, C, T | AG | 7 (12.28) | |||
| Intron variant | GG | 3 (5.26) | |||
| DNA Topological change | TOP1 rs34282819 | CC | 44 (77.19) | C = 0.89 A = 0.11 | C = 0.92 A = 0.08 |
| C > A | CA | 13 (22.81) | |||
| 5 prime transcript variant | AA | 0 | |||
| TOP1 rs6072249 | AA | 18 (31.58) | A = 0.57 G = 0.43 | A = 0.55 G = 0.45 | |
| A > G | AG | 29 (50.88) | |||
| Upstream transcript variant | GG | 10 (17.54) | |||
| Intracellular signal transduction | MKNK1 rs8602 | CC | 29 (53.70) | C = 0.74 A = 0.26 | C = 0.72 A = 0.18 |
| C > A | CA | 22 (40.74) | |||
| Non coding transcript variant | AA | 3 (5.56) | |||
| Growth factor | IGF1 rs6220 | GG | 4 (7.14) | G = 0.23 A = 0.77 | G = 0.27 A = 0.73 |
| G > A | GA | 18 (32.14) | |||
| 3 prime UTR variant | AA | 34 (60.71) |
| Number of Mutated Genes (of 13) Analyzed in This Study | ||||||
|---|---|---|---|---|---|---|
| Tumour Stage | <7 genes | 7 genes | 8 genes | 9 genes | 10 genes | 11 genes |
| Number of patients with mutated genes (% of total patients in each stage) | ||||||
| II | 1 (50) | 1 (50) | ||||
| III | 4 (25) | 5 (31) | 5 (31) | 2 (13) | ||
| IV | 6 (15,5) | 11 (28) | 6 (15,5) | 11 (28) | 3 (8) | 2 (5) |
| Genetic Variant | PFS | OS | ||
|---|---|---|---|---|
| Median (Months) (95% CI) | Hazard Ratio p-Value | Median (Months) (95% CI) | Hazard Ratio p-Value | |
| A2BR rs2015353 | ||||
| TT | 6.20 (2.89–16.56) | 0.57 (0.26–1.24) | 12.52 (4.89-...) | 0.70 (0.31–1.60) |
| TC/CC | 10.39 (7.05–15.64) | 0.149 | 28.39 (21.48–34.89) | 0.397 |
| ENTPD1(CD39) rs11188513 | ||||
| CC | 9.41 (2.43–…) | 1.71 (0.65–4.46) | 43.84 (12.52–…) | 2.32 (0.71–7.54) |
| CT/TT | 8.75 (7.05–13.18) | 0.272 | 23.51 (19.38–30.92) | 0.150 |
| NT5E (CD73) rs229523 | ||||
| AA | 9.48 (6.62–…) | 0.8 (0.25–5.59) | 27.54 (11.84-..) | 0.59 (0.18–1.95) |
| AG/GG | 9.41 (7.05–13.18) | 0.708 | 23.87 (20.72–34.89) | 0.386 |
| ANGPT1 rs2445365 | ||||
| GG | 9.48 (6.62–14.03) | 1.2 (0.689–2.06) | 30.92 (21.48–37.87) | 1.91 (1.04–3.5) |
| GA/AA | 8.75 (5.74–15.7) | 0.519 | 22.07 (12.52–29.28) | 0.034 |
| ANGPT2 rs10102851 | ||||
| AA | 8.75 (6,89–13.05) | 0.97 (0.30–3.15) | - | - |
| AG/GG | 14.03 (12.43–…) | 0.953 | - | - |
| ANGPT2 rs1375668 | ||||
| GG | 10.43 (5.44–…) | 0.66 (0.27–1.58) | 21.48 (12.20–…) | 0.72 (0.24–2.08) |
| GA/AA | 9.41 (7.21–14.03) | 0.343 | 23.87 (20–34.89) | 0.544 |
| ANGPT2 rs2515462 | ||||
| AA | 10.39 (5.74–…) | 0.74 (0.26–2.09) | 21.48 (12.20–…) | 0.82 (0.19–3.51) |
| AG/GG | 8.75 (7.05–13.18) | 0.568 | 27.54 (20–32.89) | 0.787 |
| VEGFA rs833061 | ||||
| CC | 5.61 (2.79–15.64) | 0.71 (0.36–1.43) | 21.48 (4.89–32.89) | 0.77 (0.37–1.61) |
| CT/TT | 9.48 (7.21–13.18) | 0.337 | 23.87 (18.85–35.02) | 0.481 |
| VEGFA rs833068 | ||||
| GG | 9.48 (5.44–15.64) | 1 (0.56–1.80) | 22.2 (14.75–36.52) | 0.93 (0.48–1.78) |
| GA/AA | 9.41 (6.62–13.18) | 0.999 | 23.87 (18.85–34.89) | 0.821 |
| VEGFA rs833069 | ||||
| TT | 8.52 (5.44–15.48) | 0.98 (0.54–1.79) | 28.39 (14.75–36.52) | 1.03 (0.53–2.02) |
| TC/CC | 9.41 (6.62–13.18) | 0.946 | 23.87 (18.62–34.89) | 0.926 |
| VEGFA rs3025039 | ||||
| CC | 9.41 (6.89–13.18) | 1.10 (0.59–2.03) | 23.87 (19.38–32.89) | 0.84 (0.4–1.75) |
| CT/TT | 8.52 (5.74–16.56) | 0.769 | 27.54(11.74–…) | 0.634 |
| FGF2 rs1960669 | ||||
| CC | 10.95 (7.84–15.70) | 3.30 (1.52–7.14) | 27.54 (20–34.89) | 1.45 (0.69–3.05) |
| CA/AA | 5.44 (2.43–9.48) | 0.001 | 22.20 (9.97–32.89) | 0.324 |
| MMP9 rs2236416 | ||||
| AA | 9.48 (7.84–15.64) | 2.04 (1.09–3.80) | 23.51 (19.38–29.74) | 0.91 (0.46–1.81) |
| AG/GG | 6 (2.79–10.95) | 0.022 | 32.74 (12.20–43.84) | 0.786 |
| MMP9 rs2274755 | ||||
| GG | 9.48 (7.84–15.64) | 1.91 (1.01–3.63) | 23.51 (19.38–29.74) | 0.9 (0.44–1.84) |
| GT/TT | 6.62 (2.89–10.95) | 0.043 | 35.02 (12.20–43.84) | 0.780 |
| EDN1 rs5370 | ||||
| GG | 10.43 (7.21–16.26) | 1.29 (0.74–2.26) | 23.51 (18.85–34.89) | 1.02 (0.55–1.88) |
| GT/TT | 7.84 (4.20–14.03) | 0.367 | 27.54 (11.84–35.02) | 0.957 |
| CCL5 rs2280789 | ||||
| AA | 9.48 (7.21–15.48) | 1.44 (0.7–2.98) | 27.54 (21.48–32.89) | 1.17 (0.49–2.79) |
| AG/GG | 7.44 (2.89–12.43) | 0.323 | 16.03 (7.31-...) | 0.723 |
| TOP1 rs34282819 | ||||
| CC | 8.52 (6.89–12.43) | 0.8 (0.42–1.54) | 23.87 (20.72–32.72) | 0.93 (0.46–1.91) |
| CA/AA | 16.26 (4.20–21.08) | 0.501 | 27.54 (9.97–44.49) | 0.853 |
| TOP1 rs6072249 | ||||
| AA | 11.34 (5.44–18.10) | 1.05 (0.58–1.91) | 23.51 (12.10–35.02) | 0.87 (0.45–1.65) |
| AG/GG | 8.75 (7.05–13.05) | 0.862 | 23.87 (19.38–34.89) | 0.662 |
| MKNK1 rs8602 | ||||
| CC | 8.75 (6.89–13.18) | 1.04 (0.59–1.83) | 23.51 (16.03–36.52) | 1.18 (0.63–2.20) |
| CA/AA | 11.34 (7.21–16.56) | 0.904 | 28.39 (21.48–34.89) | 0.614 |
| IGF1 rs6220 | ||||
| GG | 13.05 (12.43–…) | 1.09 (0.38–3.07) | 21.48 (4.89–32.89) | 1.7 (0.41–7.07) |
| GA/AA | 8.52 (6.62–11.34) | 0.874 | 23.87 (18.85–35.02) | 0.457 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaibar, M.; Galán, M.; Romero-Lorca, A.; Antón, B.; Malón, D.; Moreno, A.; Fernández-Santander, A.; Novillo, A. Genetic Variants of ANGPT1, CD39, FGF2 and MMP9 Linked to Clinical Outcome of Bevacizumab Plus Chemotherapy for Metastatic Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 1381. https://doi.org/10.3390/ijms22031381
Gaibar M, Galán M, Romero-Lorca A, Antón B, Malón D, Moreno A, Fernández-Santander A, Novillo A. Genetic Variants of ANGPT1, CD39, FGF2 and MMP9 Linked to Clinical Outcome of Bevacizumab Plus Chemotherapy for Metastatic Colorectal Cancer. International Journal of Molecular Sciences. 2021; 22(3):1381. https://doi.org/10.3390/ijms22031381
Chicago/Turabian StyleGaibar, María, Miguel Galán, Alicia Romero-Lorca, Beatriz Antón, Diego Malón, Amalia Moreno, Ana Fernández-Santander, and Apolonia Novillo. 2021. "Genetic Variants of ANGPT1, CD39, FGF2 and MMP9 Linked to Clinical Outcome of Bevacizumab Plus Chemotherapy for Metastatic Colorectal Cancer" International Journal of Molecular Sciences 22, no. 3: 1381. https://doi.org/10.3390/ijms22031381
APA StyleGaibar, M., Galán, M., Romero-Lorca, A., Antón, B., Malón, D., Moreno, A., Fernández-Santander, A., & Novillo, A. (2021). Genetic Variants of ANGPT1, CD39, FGF2 and MMP9 Linked to Clinical Outcome of Bevacizumab Plus Chemotherapy for Metastatic Colorectal Cancer. International Journal of Molecular Sciences, 22(3), 1381. https://doi.org/10.3390/ijms22031381







