ELOVL5 Participates in Embryonic Lipid Determination of Cellular Membranes and Cytoplasmic Droplets
Abstract
1. Introduction
2. Results
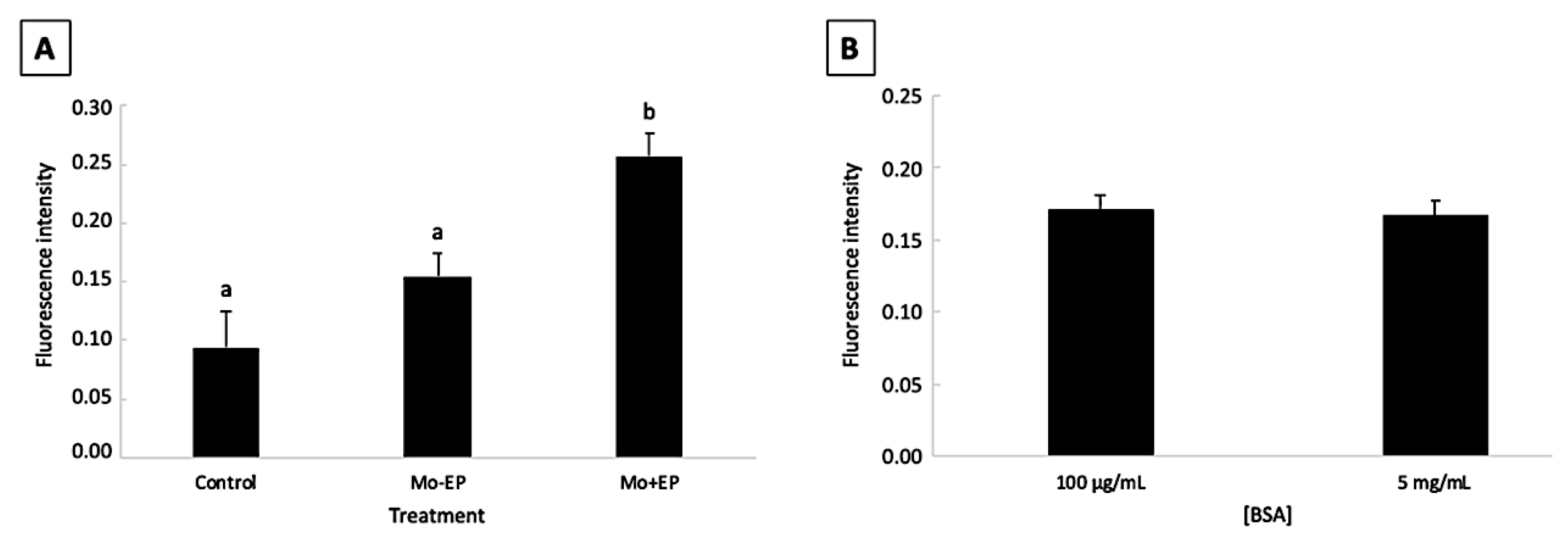
2.1. Pilot Study: Effect of Morpholino, Endo-Porter, and Bovine Serum Albumin Concentration on Bovine Embryo Development and Mo Delivery into the Embryonic Cell
2.1.1. Embryo Development
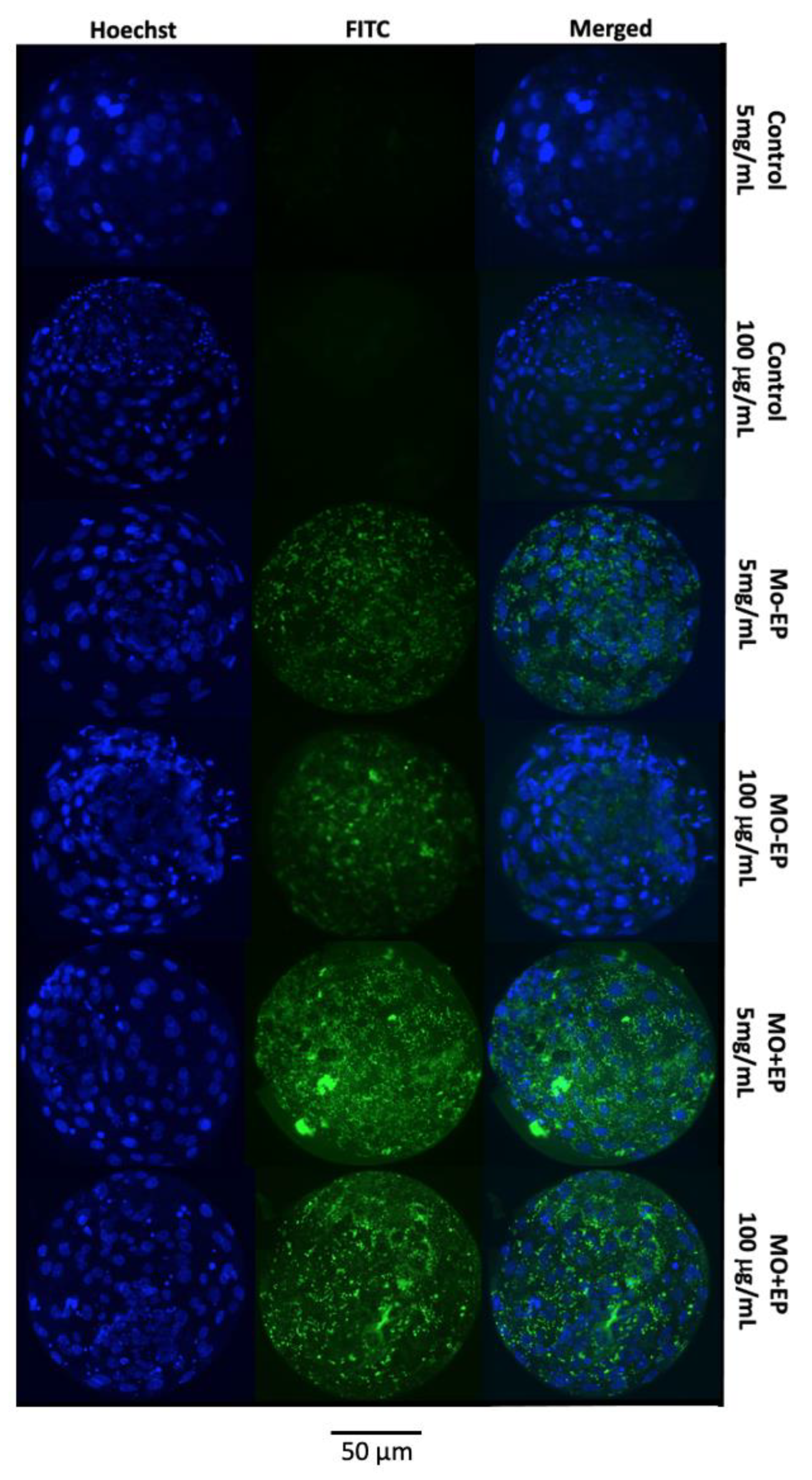
2.1.2. Mo Delivery into the Embryonic Cells
2.2. Main Experiment: Involvement of ELOVL5 in Embryonic Development and Lipid Determination
2.2.1. Embryonic Production and Total Number of Cells in the Blastocysts
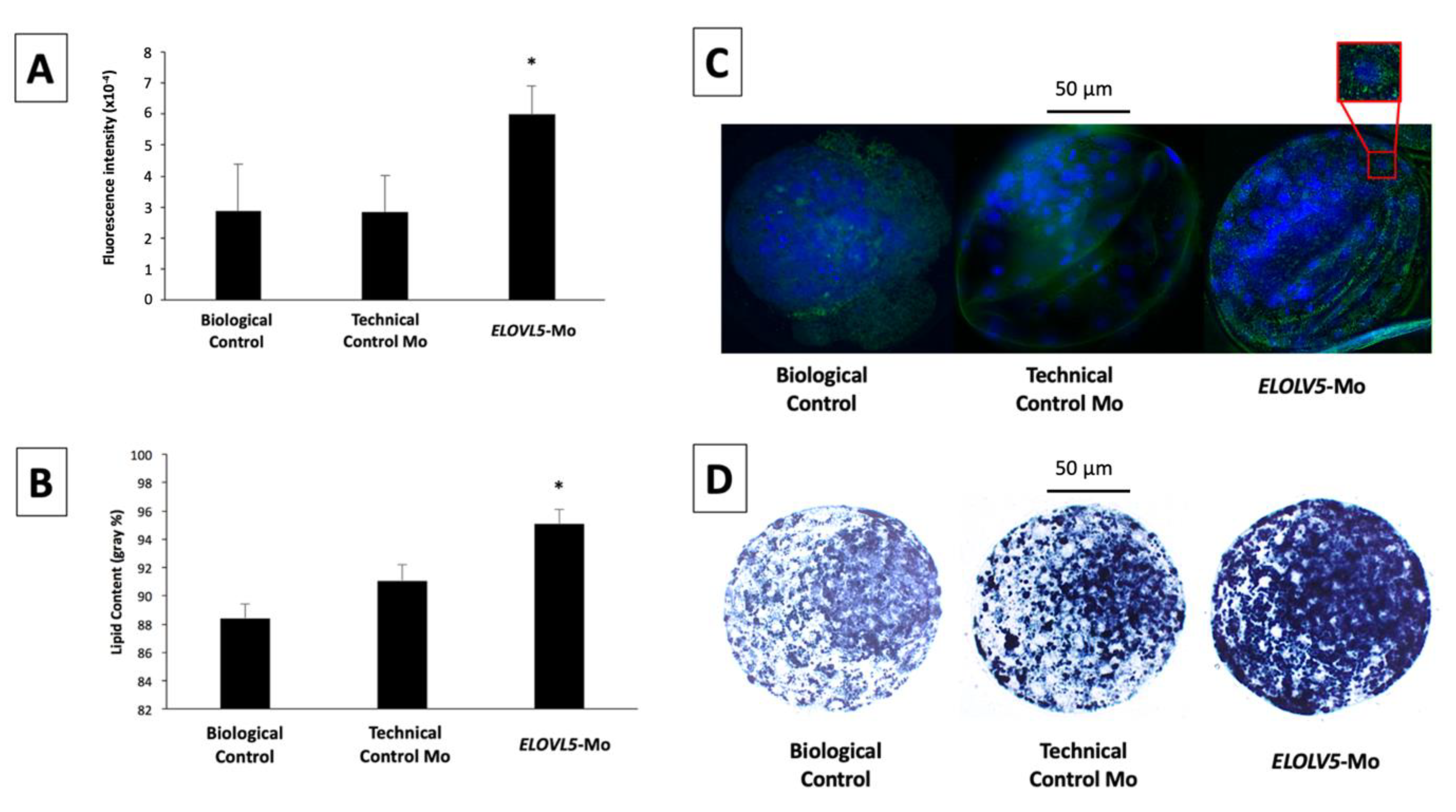
2.2.2. Immunolocalization of ELOVL5 Protein in In Vitro-Produced Blastocysts
2.2.3. Cytoplasmic Lipid Droplet Deposition
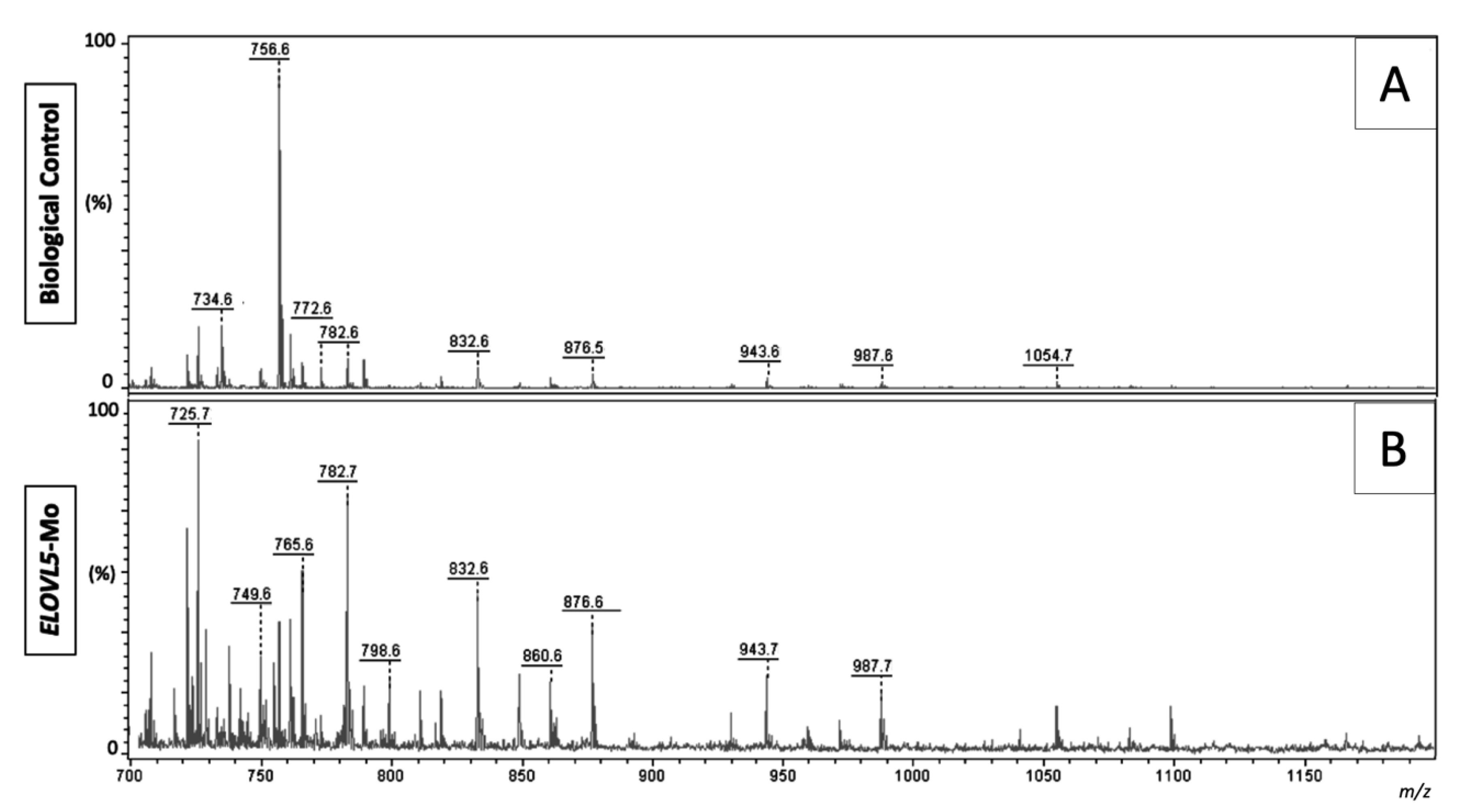
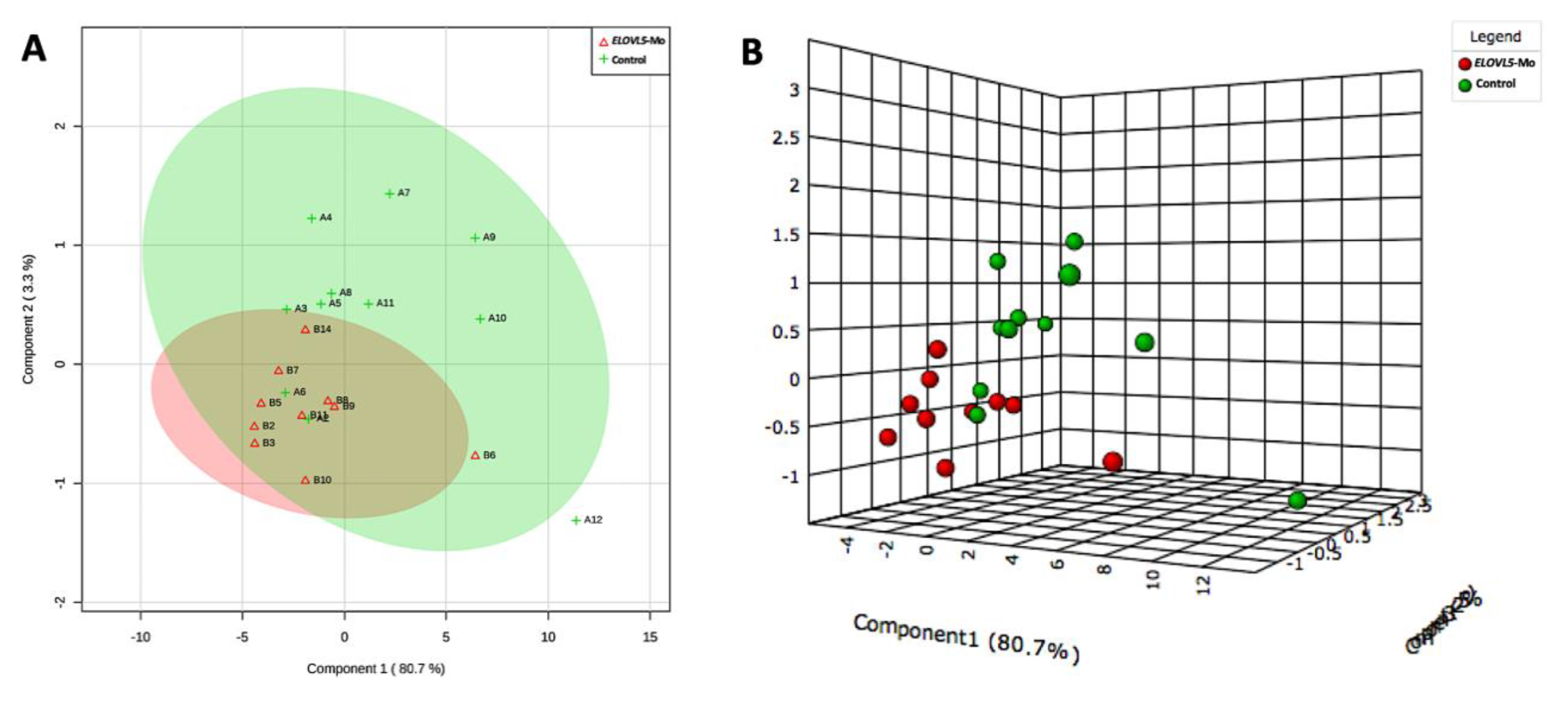
2.2.4. Lipid Fingerprint Analysis by Matrix-Assisted Laser Desorption and Ionization Mass Spectrometry
3. Discussion
4. Material and Methods
4.1. Reagents Used
4.2. Experimental Design
4.3. Pilot Study
4.4. In Vitro Production of Bovine Embryos
4.5. In Vitro Fertilization
4.6. In Vitro Culture
4.7. Immunolocalization of ELOVL5 Protein
4.8. Staining of Lipid Droplets by Sudan Black
4.9. Lipid Fingerprint Analysis by Matrix-Assisted Laser Desorption and Ionization Mass Spectrometry
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Sanches, B.V.; Marinho, L.S.R.; Filho, B.D.O.; Pontes, J.H.F.; Basso, A.C.; Meirinhos, M.L.G.; Silva-Santos, K.C.; Ferreira, C.R.; Seneda, M.M. Cryosurvival and pregnancy rates after exposure of IVF-derived Bosindicus embryos to forskolin before vitrification. Theriogenology 2013, 80, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dey, S.K. Lipid signaling in embryo implantation. Prostaglandins Other Lipid Mediat. 2005, 77, 84–102. [Google Scholar] [CrossRef]
- Hu, K.; Yu, Y. Metabolite availability as a window to view the early embryo microenvironment in vivo. Mol. Reprod. Dev. 2017, 84, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Sturmey, R.G.; Reis, A.; Leese, H.J.; McEvoy, T.G. Role of fatty acids in energy provision during oocyte maturation and early embryo development. Reprod. Domest. Anim. 2009, 44, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, E.M.; Leese, H.J. A potential role for triglyceride as an energy source during bovine oocyte maturation and early embryo development. Mol. Reprod. Dev. 2006, 73, 1195–1201. [Google Scholar] [CrossRef]
- Pavani, K.C.; Rocha, A.; Oliveira, E.; da Silva, F.M.; Sousa, M. Novel ultrastructural findings in bovine oocytes matured in vitro. Theriogenology 2020, 143. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Yamashita, S.; Satoh, T.; Hoshi, H. Accumulation of cytoplasmic lipid droplets in bovine embryos and cryotolerance of embryos developed in different culture systems using serum-free or serum-containing media. Mol. Reprod. Dev. 2002, 61, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, A.; Suzuki, K.; Mori, T.; Ueda, J.; Watanabe, T. Effects of delipidation and oxygen concentration on in vitro development of porcine embryos. J. Reprod. Dev. 2004, 50, 287–295. [Google Scholar] [CrossRef][Green Version]
- Rizos, D.; Ward, F.; Duffy, P.; Boland, M.P.; Lonergan, P. Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: Implications for blastocyst yield and blastocyst quality. Mol. Reprod. Dev. 2002, 61, 234–248. [Google Scholar] [CrossRef]
- Sudano, M.J.; Paschoal, D.M.; da Silva Rascado, T.; Magalhães, L.C.; Crocomo, L.F.; de Lima-Neto, J.F.; da Cruz Landim-Alvarenga, F. Lipid content and apoptosis of in vitro-produced bovine embryos as determinants of susceptibility to vitrification. Theriogenology 2011, 75, 1211–1220. [Google Scholar] [CrossRef]
- Sudano, M.J.; Caixeta, E.S.; Paschoal, D.M.; Martins, A., Jr.; Machado, R.; Buratini, J.; Landim-Alvarenga, F.D.C. Cryotolerance and global gene-expression patterns of Bos taurus indicus and Bos taurus taurus in vitro- and in vivo-produced blastocysts. Reprod. Fertil. Dev. 2014, 26, 1129–1141. [Google Scholar] [CrossRef] [PubMed]
- Paschoal, D.M.; Sudano, M.J.; Schwarz, K.R.L.; Maziero, R.R.D.; Guastali, M.D.; Crocomo, L.F.; Magalhães, L.C.O.; Martins, A.; Leal, C.L.V.; Landim-Alvarenga, F.D.C. Cell apoptosis and lipid content of in vitro–produced, vitrified bovine embryos treated with forskolin. Theriogenology 2017, 87, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Sudano, M.J.; Paschoal, D.M.; Maziero, R.R.D.; Rascado, T.S.; Guastali, M.D.; Crocomo, L.F.; Magalhaes, L.C.O.; Monteiro, B.A.; Martins, A., Jr.; Machado, R.; et al. Improving postcryopreservation survival capacity: An embryo-focused approach. Anim. Reprod. 2013, 10, 160–167. [Google Scholar]
- Murillo, A.; Muñoz, M.; Martín-González, D.; Carrocera, S.; Martínez-Nistal, A.; Gómez, E. Low serum concentration in bovine embryo culture enhances early blastocyst rates on Day-6 with quality traits in the expanded blastocyst stage similar to BSA-cultured embryos. Reprod. Biol. 2017, 17, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Sudano, M.J.; Paschoal, D.M.; da Silva Rascado, T.; Crocomo, L.F.; Magalhães, L.C.O.; Junior, A.M.; Machado, R.; da Cruz Landim-Alvarenga, F. Crucial surviving aspects for vitrified in vitro-produced bovine embryos. Zygote 2014, 22, 124–131. [Google Scholar] [CrossRef]
- Guillou, H.; Zadravec, D.; Martin, P.G.P.; Jacobsson, A. The key roles of elongases and desaturases in mammalian fatty acid metabolism: Insights from transgenic mice. Prog. Lipid Res. 2010, 49, 186–199. [Google Scholar] [CrossRef]
- Jakobsson, A.; Westerberg, R.; Jacobsson, A. Fatty acid elongases in mammals: Their regulation and roles in metabolism. Prog. Lipid Res. 2006, 45, 237–249. [Google Scholar] [CrossRef]
- Sudano, M.J.; Rascado, T.D.; Tata, A.; Belaz, K.R.; Santos, V.G.; Valente, R.S.; Mesquita, F.S.; Ferreira, C.R.; Araújo, J.P.; Eberlin, M.N.; et al. Lipidome signatures in early bovine embryo development. Theriogenology 2016, 86, 472–484. [Google Scholar] [CrossRef]
- Bond, L.M.; Miyazaki, M.; O’Neill, L.M.; Ding, F.; Ntambi, J.M. Chapter 6 Fatty acid desaturation and elongation in mammals. In Lipoproteins and Membranes, 6th ed.; Ridgway, N., McLeod, R., Eds.; Elsevier: Boston, MA, USA, 2016; pp. 185–208. ISBN 978-0-444-63438-2. [Google Scholar]
- Moon, Y.A.; Hammer, R.E.; Horton, J.D. Deletion of ELOVL5 leads to fatty liver through activation of SREBP-1c in mice. J. Lipid Res. 2009, 50, 412–423. [Google Scholar] [CrossRef]
- Sata, R.; Tsujii, H.; Abe, H.; Yamashita, S.; Hoshi, H. Fatty acid composition of bovine embryos cultured in serum-free and serum-containing medium during early embryonic development. J. Reprod. Dev. 1999, 45, 97–103. [Google Scholar] [CrossRef][Green Version]
- McEvoy, T.G.; Coull, G.D.; Broadbent, P.J.; Hutchinson, J.S.; Speake, B.K. Fatty acid composition of lipids in immature cattle, pig and sheep oocytes with intact zona pellucida. J. Reprod. Fertil. 2000, 118, 163–170. [Google Scholar] [CrossRef]
- Schiller, J.; Süß, R.; Arnhold, J.; Fuchs, B.; Leßig, J.; Müller, M.; Petković, M.; Spalteholz, H.; Zschörnig, O.; Arnold, K. Matrix-assisted laser desorption and ionization time-of-flight (MALDI-TOF) mass spectrometry in lipid and phospholipid research. Prog. Lipid. Res. 2004, 43, 449–488. [Google Scholar] [CrossRef]
- Milne, S.; Ivanova, P.; Forrester, J.; Alex Brown, H. Lipidomics: An analysis of cellular lipids by ESI-MS. Methods 2006, 39, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.R.; Saraiva, S.A.; Catharino, R.R.; Garcia, J.S.; Gozzo, F.C.; Sanvido, G.B.; Santos, L.F.A.; Lo Turco, E.G.; Pontes, J.H.F.; Basso, A.C.; et al. Single embryo and oocyte lipid fingerprinting by mass spectrometry. J. Lipid Res. 2010, 51, 1218–1227. [Google Scholar] [CrossRef]
- Belaz, K.R.A.; Tata, A.; Franca, M.R.; Santos da Silva, M.I.; Vendramini, P.H.; Fernandes, A.M.A.P.; D’Alexandri, F.L.; Eberlin, M.N.; Binelli, M. Phospholipid Profile and Distribution in the Receptive Oviduct and Uterus During Early Diestrus in Cattle. Biol. Reprod. 2016, 95, 127. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Yamashita, S.; Itoh, T.; Satoh, T.; Hoshi, H. Ultrastructure of bovine embryos developed from in vitro–matured and –fertilized oocytes: Comparative morphological evaluation of embryos cultured either in serum-free medium or in serum-supplemented medium. Mol. Reprod. Dev. 1999, 53, 325–335. [Google Scholar] [CrossRef]
- Sudano, M.J.; Santos, V.G.; Tata, A.; Ferreira, C.R.; Paschoal, D.M.; Machado, R.; Buratini, J.; Eberlin, M.N.; Landim-Alvarenga, F.D.C. Phosphatidylcholine and sphingomyelin profiles vary in Bos taurus indicus and Bos taurus taurus in vitro- and in vivo-produced Blastocysts. Biol. Reprod. 2012, 87, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Farin, C.E.; Farin, P.W.; Piedrahita, J.A. Development of fetuses from in vitro-produced and cloned bovine embryos. J. Anim. Sci. 2004, 82, E53–E62. [Google Scholar] [PubMed]
- Kim, J.Y.; Kinoshita, M.; Ohnishi, M.; Fukui, Y. Lipid and fatty acid analysis of fresh and frozen-thawed immature and in vitro matured bovine oocytes. Reproduction 2001, 122, 131–138. [Google Scholar] [CrossRef]
- Santos, J.A.R.; Vicente, C.P. Biomembranas. In A célula, 3rd ed.; Carvalho, F.H., Recco-Pimentel, M.S., Eds.; Manole: Barueri, Brazil, 2013; pp. 95–115. [Google Scholar]
- Graham, J.K. Principles of cooled semen. In Equine Reproduction, 2nd ed.; Mckinnon, A.O., Squires, E.L., Vaala, W.E., Varner, D.D., Eds.; Wiley-Blackwell: Oxford, UK, 2011; pp. 1308–1315. [Google Scholar]
- Hudziak, R.M.; Barofsky, E.; Barofsky, D.F.; Weller, D.L.; Ben Huang, S.B.; Weller, D.D. Resistance of morpholino phosphorodiamidate oligomers to enzymatic degradation. Antisense Nucleic Acid Drug Dev. 1996, 6, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Youngblood, D.S.; Hatlevig, S.A.; Hassinger, J.N.; Iversen, P.L.; Moulton, H.M. Stability of cell-penetrating peptide−morpholino oligomer conjugates in human serum and in cells. Bioconjug. Chem. 2007, 18, 50–60. [Google Scholar] [CrossRef]
- Inoue, Y.; Kurihara, R.; Tsuchida, A.; Hasegawa, M.; Nagashima, T.; Mori, T.; Niidome, T.; Katayama, Y.; Okitsu, O. Efficient delivery of siRNA using dendritic poly(l-lysine) for loss-of-function analysis. J. Control. Release 2008, 126, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Wells, D.J. Gene doping: The hype and the reality. Br. J. Pharmacol. 2008, 154, 623–631. [Google Scholar] [CrossRef]
- Bill, B.R.; Petzold, A.M.; Clark, K.J.; Schimmenti, L.A.; Ekker, S.C. A primer for morpholino use in zebrafish. Zebrafish 2009, 6, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Burton, E.A. Zebrafish. In Movement Disorders: Genetics and Models, 2nd ed.; Academic Press-Elsevier: London, UK, 2015; pp. 117–138. [Google Scholar]
- Eisen, J.S.; Smith, J.C. Controlling morpholino experiments: Don’t stop making antisense. Development 2008, 135, 1735–1743. [Google Scholar] [CrossRef]
- Frei, R.E.; Schultz, G.A.; Church, R.B. Qualitative and quantitative changes in protein synthesis occur at the 8–16-cell stage of embryogenesis in the cow. J. Reprod. Fertil. 1989, 86, 637–641. [Google Scholar] [CrossRef]
- Moulton, J.D. Using morpholinos to control gene expression. Curr. Protoc. Nucleic Acid Chem. 2017, 2017. [Google Scholar] [CrossRef]
- Shahzad, Q.; Pu, L.; Ahmed Wadood, A.; Waqas, M.; Xie, L.; Shekhar Pareek, C.; Xu, H.; Liang, X.; Lu, Y. Proteomics analysis reveals that warburg effect along with modification in lipid metabolism improves in vitro embryo development under low oxygen. Int. J. Mol. Sci. 2020, 21, 1996. [Google Scholar] [CrossRef]
- Zhao, B.; Bie, J.; Wang, J.; Marqueen, S.A.; Ghosh, S. Identification of a novel intracellular cholesteryl ester hydrolase (carboxylesterase 3) in human macrophages: Compensatory increase in its expression after carboxylesterase 1 silencing. Am. J. Physiol. Cell Physiol. 2012, 303, C427–C435. [Google Scholar] [CrossRef]
- Sun, S.; Ren, T.; Li, X.; Cao, X.; Gao, J. Polyunsaturated fatty acids synthesized by freshwater fish: A new insight to the roles of elovl2 and elovl5 in vivo. Biochem. Biophys. Res. Commun. 2020, 532, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Stringfellow, D.A.; Seidel, S.M. Manual da Sociedade Internacional de Transferência De Embriões: Um Guia de Procedimento E Informações Gerais Para Uso Em Tecnologia de Transferência de Embriões Enfatizando Procedimentos Sanitários, 3rd ed.; Sociedade Brasileira de Tecnologia de Embriões: Uberlândia, Brazil, 1996; Volume 1, p. 180. [Google Scholar]
- Hoelker, M.; Held, E.; Salilew-Wondim, D.; Schellander, K.; Tesfaye, D. Molecular signatures of bovine embryo developmental competence. Reprod. Fertil. Dev. 2013, 26, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, G.; De Camilli, P. Phosphoinositides in cell regulation and membrane dynamics. Nature 2006, 443, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Burnum, K.E.; Cornett, D.S.; Puolitaival, S.M.; Milne, S.B.; Myers, D.S.; Tranguch, S.; Brown, H.A.; Dey, S.K.; Caprioli, R.M. Spatial and temporal alterations of phospholipids determined by mass spectrometry during mouse embryo implantation. J. Lipid Res. 2009, 50, 2290–2298. [Google Scholar] [CrossRef] [PubMed]
- IInternational guiding principles for biomedical research involving animals, 1985 The Development of Science-Based Guidelines for Laboratory Animal Care—NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK25438/ (accessed on 3 November 2020).
- Parrish, J.J.; Krogenaes, A.; Susko-Parrish, J.L. Effect of bovine sperm separation by either swim-up or Percoll method on success of in vitro fertilization and early embryonic development. Theriogenology 1995, 44, 859–869. [Google Scholar] [CrossRef]
- Holm, P.; Booth, P.J.; Schmidt, M.H.; Greve, T.; Callesen, H. High bovine blastocyst development in a static in vitro production system using SOFaa medium supplemented with sodium citrate and myo-inositol with or without serum-proteins. Theriogenology 1999, 52, 683–700. [Google Scholar] [CrossRef]
- Valente, R.S.; Almeida, T.G.; Alves, M.F.; Camargo, J.; Basso, A.C.; Belaz, K.R.A.; Eberlin, M.N.; Landim-Alvarenga, F.D.C.; Fontes, P.K.; Nogueira, M.F.G.; et al. Modulation of long-chain acyl-CoA synthetase on the development, lipid deposit and cryosurvival of in vitro produced bovine embryos. PLoS ONE 2019, 14, e0220731. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Mandal, R.; Sinelnikov, I.V.; Broadhurst, D.; Wishart, D.S. MetaboAnalyst 2.0 -A comprehensive server for metabolomic data analysis. Nucleic Acids Res. 2012, 40, W127–W133. [Google Scholar] [CrossRef]
| Treatment | Oocytes | Cleavage (%) | Blastocysts/Oocytes (%) * | Blastocysts/Cleaved (%) * |
|---|---|---|---|---|
| Morpholino | ||||
| Control | 81 | 77.7 ± 5.0 | 21.0 ± 4.3 | 27.0 ± 6.8 |
| Mo − EP | 117 | 75.2 ± 4.2 | 21.4 ± 3.3 | 28.4 ± 5.2 |
| Mo + EP | 147 | 76.2 ± 3.7 | 30.6 ± 2.9 | 40.2 ± 4.6 |
| [BSA] | ||||
| 100 μg/mL | 208 | 73.6 ± 3.2 | 25.5 ± 2.8 | 34.6 ± 4.4 |
| 5 mg/mL | 137 | 80.3 ± 3.8 | 24.8 ± 3.0 | 30.9 ± 4.7 |
| Treatment | Oocytes | Cleavage (%) | Blastocysts/Oocytes (%) | Blastocysts/Cleaved (%) | Cells (n) |
|---|---|---|---|---|---|
| Biological Control | 603 | 59.4 ± 3.5 | 16.9 ± 2.8 | 31.3 ± 4.2 | 99.6 ± 7.7 |
| Technical Control Mo | 511 | 63.6 ± 4.1 | 17.2 ± 3.3 | 28.1 ± 4.9 | 100.2 ± 6.2 |
| ELOVL5-Mo | 784 | 65.4 ± 2.2 | 23.6 ± 1.8 | 36.1 ± 2.1 | 86.8 ± 5.6 |
| m/z | Lipid Ion (Carbons: Unsaturation) | Log2 (Fold Change) | p-Value |
|---|---|---|---|
| 706.5 | [PC (30:0) + H]+ and/or [PE (33:0) + H]+ | −13.9 | 0.02 |
| 722.5 | [PC (30:3) + Na]+ and/or [PE (33:3) + Na]+ and/or [PE (35:6) + H]+ | −13.1 | 0.02 |
| 738.6 | [PCp (32:1) + Na]+ and/or [PCp (34:4) + H]+ | −11.3 | 0.03 |
| 766.6 | [PEp (37:1) + Na]+ and/or [PCp (36:4) + H]+ and/or [PCp (34:1) + Na]+ | −11.5 | 0.03 |
| 818.6 | PCp [(40:6) + H]+ and/or PCp [(38:3) + Na]+ and/or [PC (39:7) + H]+ and/or [PC (37:4) + Na]+ and/or [PE (42:7) + H]+ and/or [PE (40:4) + Na]+ | −11.0 | 0.09 |
| 832.6 | [PC (40:7) + H]+ and/or [PC (38:4) + Na]+ and/or [PE (43:7) + H]+ and/or [PE (41:4) + Na]+ | −11.9 | 0.04 |
| 877.7 | [TAG (52:4) + Na]+ and/or [TAG (52:7) + H]+ | −10.1 | 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanzarini, F.; Pereira, F.A.; Camargo, J.d.; Oliveira, A.M.; Belaz, K.R.A.; Melendez-Perez, J.J.; Eberlin, M.N.; Brum, M.C.S.; Mesquita, F.S.; Sudano, M.J. ELOVL5 Participates in Embryonic Lipid Determination of Cellular Membranes and Cytoplasmic Droplets. Int. J. Mol. Sci. 2021, 22, 1311. https://doi.org/10.3390/ijms22031311
Lanzarini F, Pereira FA, Camargo Jd, Oliveira AM, Belaz KRA, Melendez-Perez JJ, Eberlin MN, Brum MCS, Mesquita FS, Sudano MJ. ELOVL5 Participates in Embryonic Lipid Determination of Cellular Membranes and Cytoplasmic Droplets. International Journal of Molecular Sciences. 2021; 22(3):1311. https://doi.org/10.3390/ijms22031311
Chicago/Turabian StyleLanzarini, Franciele, Fernanda Alves Pereira, Janine de Camargo, Andressa Minozzo Oliveira, Katia Roberta Anacleto Belaz, Jose Javier Melendez-Perez, Marcos Nogueira Eberlin, Mário Celso Sperotto Brum, Fernando Silveira Mesquita, and Mateus José Sudano. 2021. "ELOVL5 Participates in Embryonic Lipid Determination of Cellular Membranes and Cytoplasmic Droplets" International Journal of Molecular Sciences 22, no. 3: 1311. https://doi.org/10.3390/ijms22031311
APA StyleLanzarini, F., Pereira, F. A., Camargo, J. d., Oliveira, A. M., Belaz, K. R. A., Melendez-Perez, J. J., Eberlin, M. N., Brum, M. C. S., Mesquita, F. S., & Sudano, M. J. (2021). ELOVL5 Participates in Embryonic Lipid Determination of Cellular Membranes and Cytoplasmic Droplets. International Journal of Molecular Sciences, 22(3), 1311. https://doi.org/10.3390/ijms22031311





