Effect of Increased IL-1β on Expression of HK in Alzheimer’s Disease
Abstract
1. Introduction
2. Results
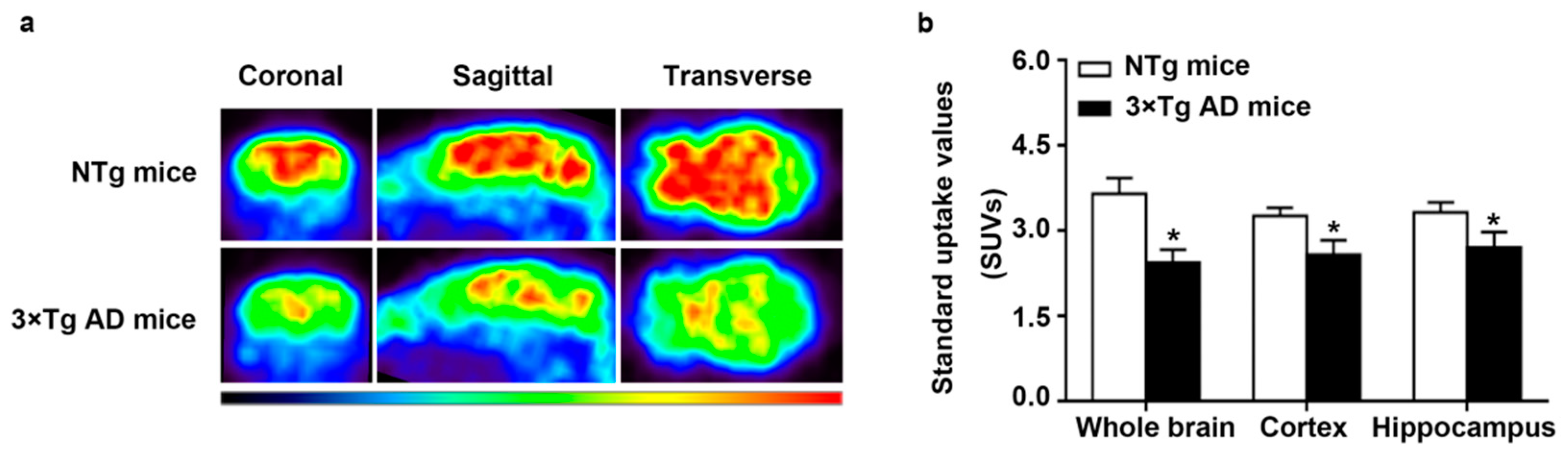
2.1. Decreased Glucose Metabolism in Triple-Transgenic (3 × Tg) AD Mice
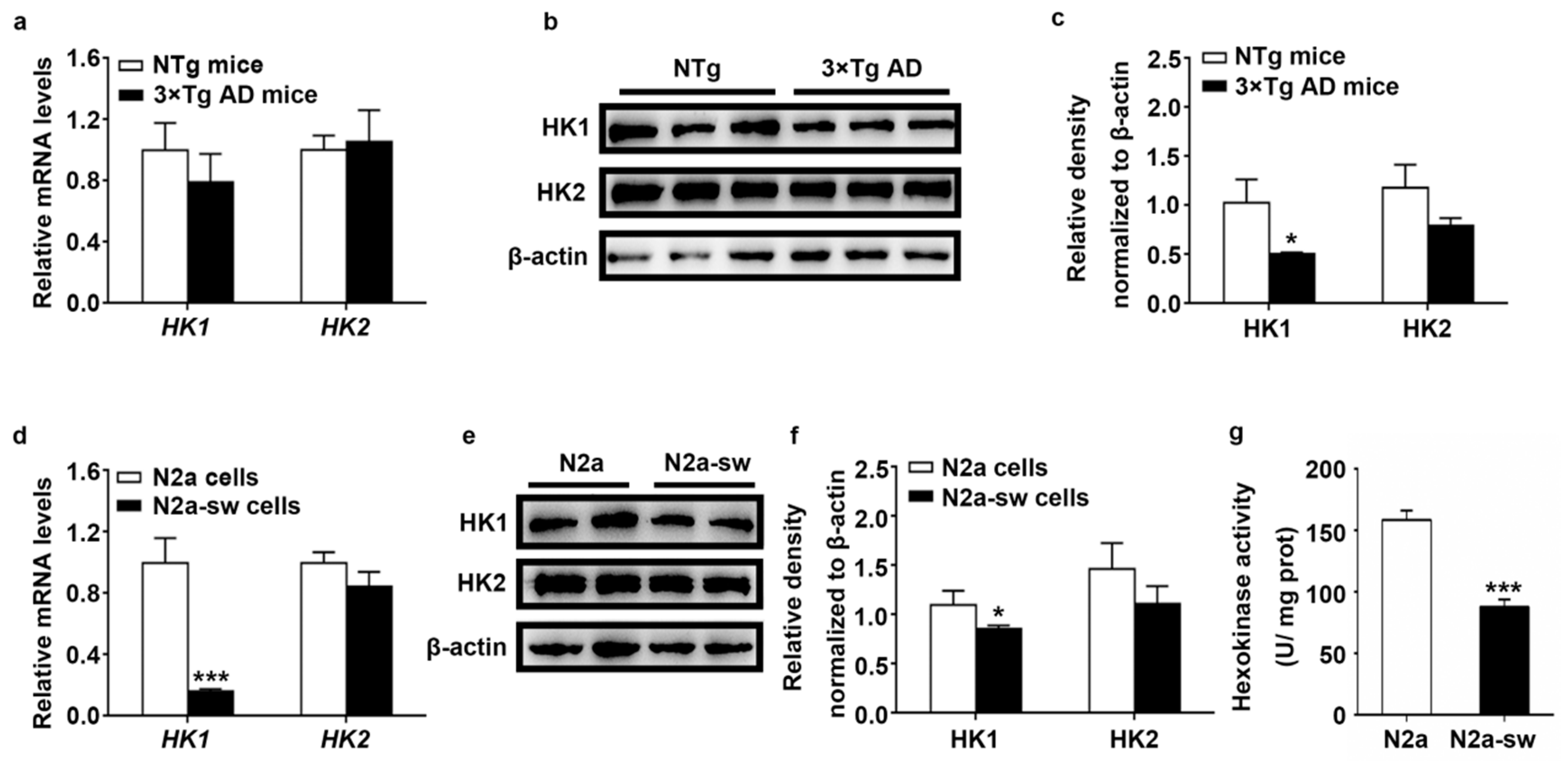
2.2. Decreased Expression of HK In Vivo and In Vitro
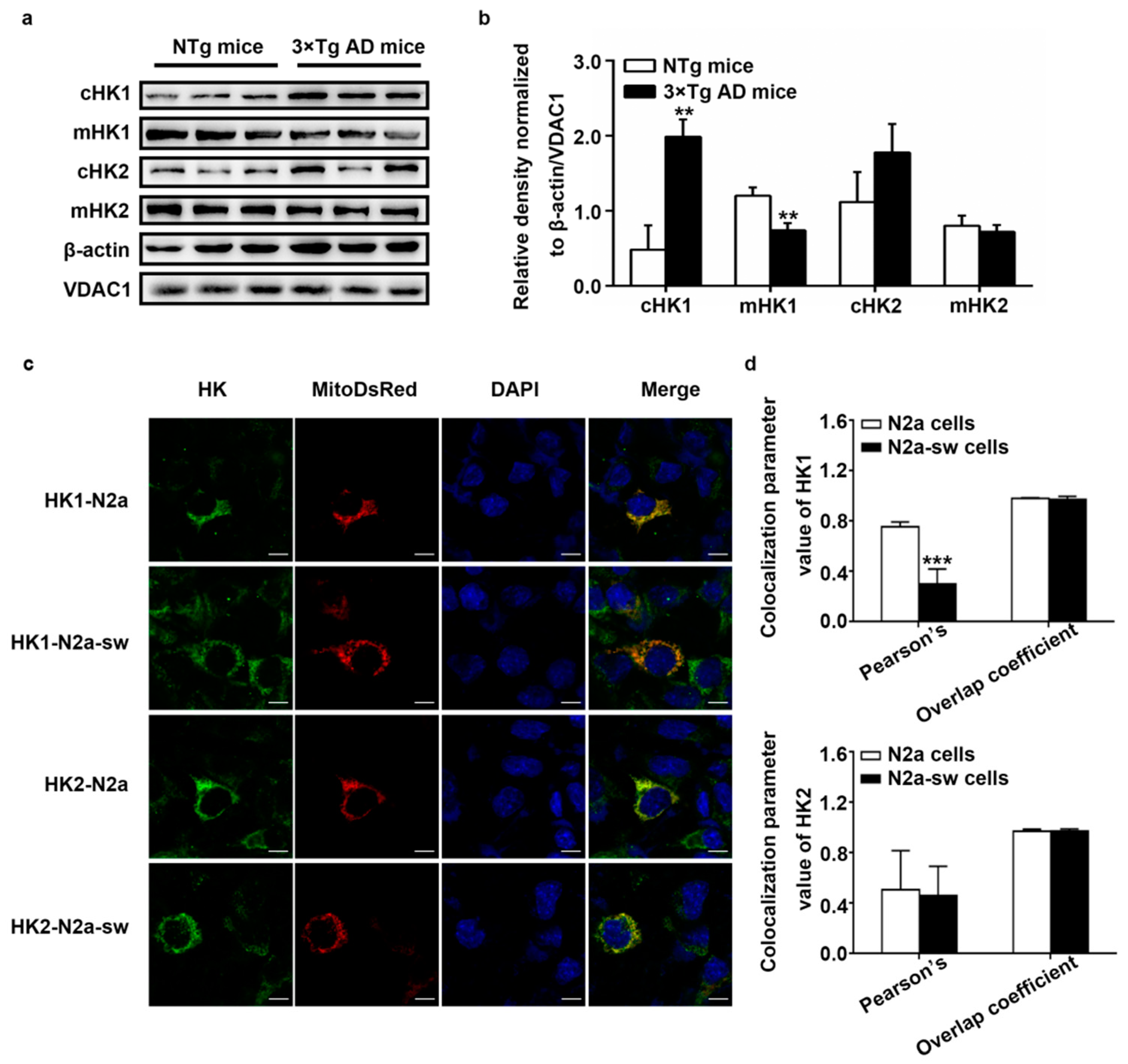
2.3. Dissociation of HK from Mitochondria In Vivo and In Vitro
2.4. Increased Protein Expression of IL-1β In Vivo and In Vitro
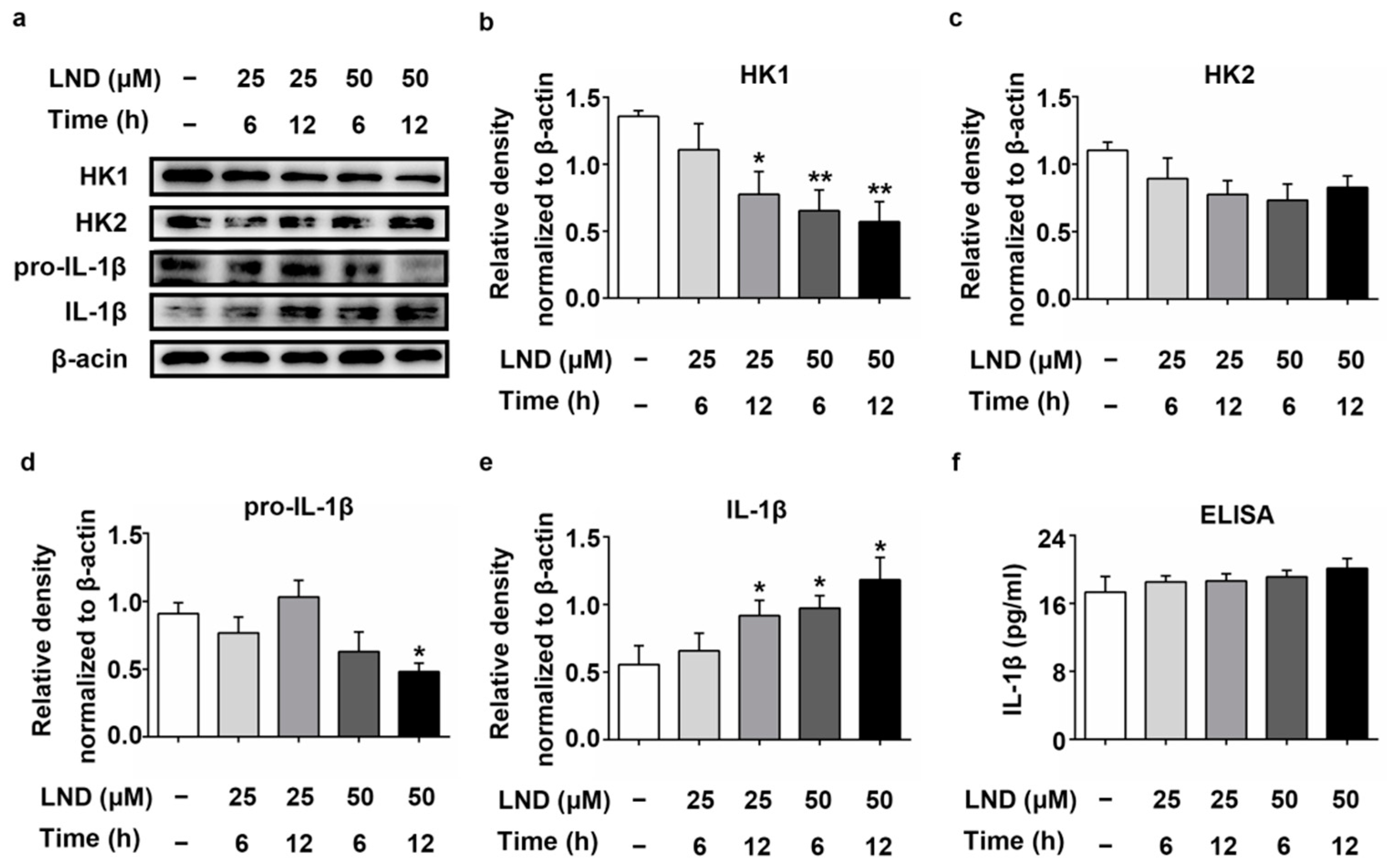
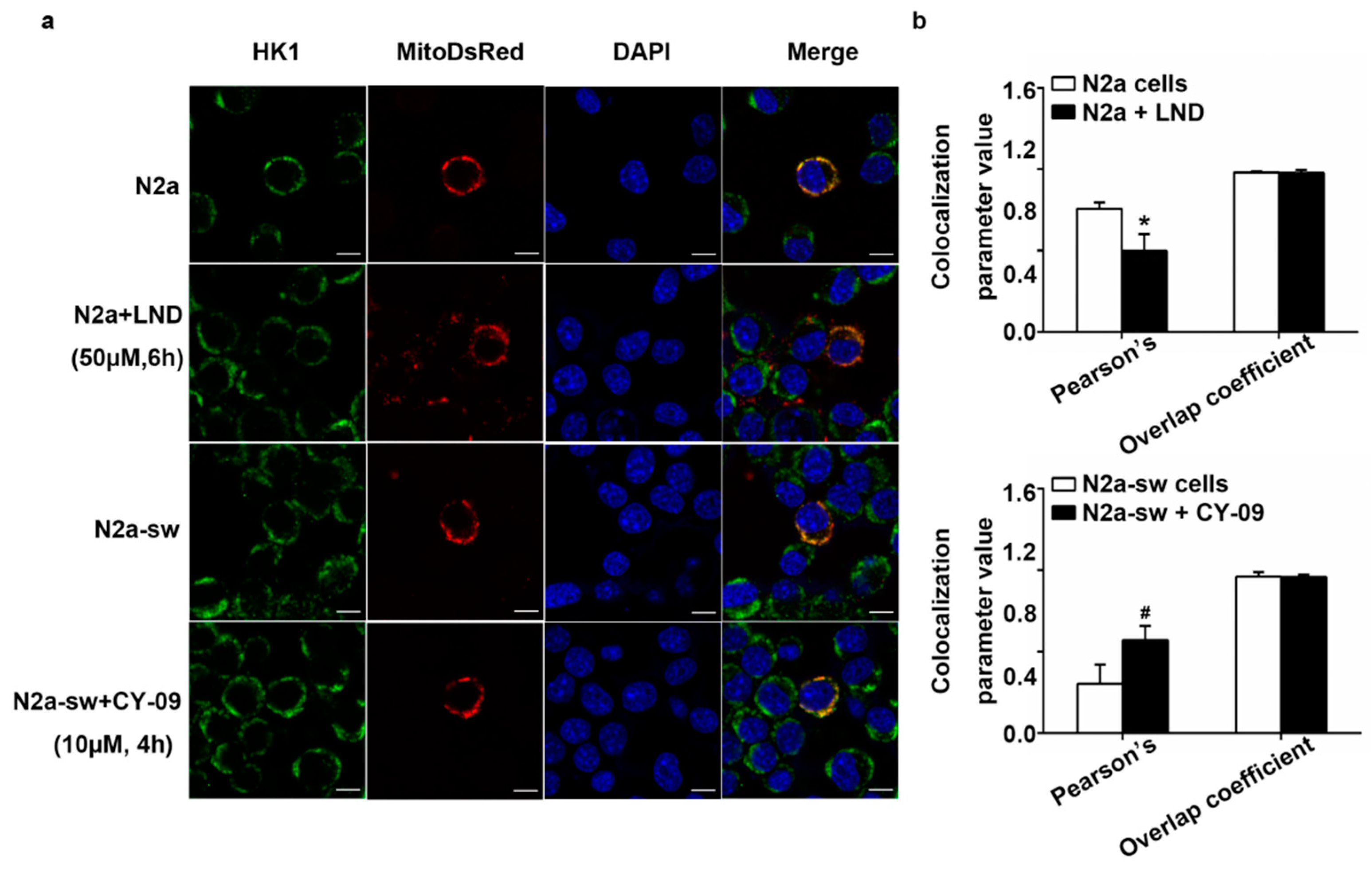
2.5. Decreased HK Causes the Increase in IL-1β in LND-Treated N2a Cells
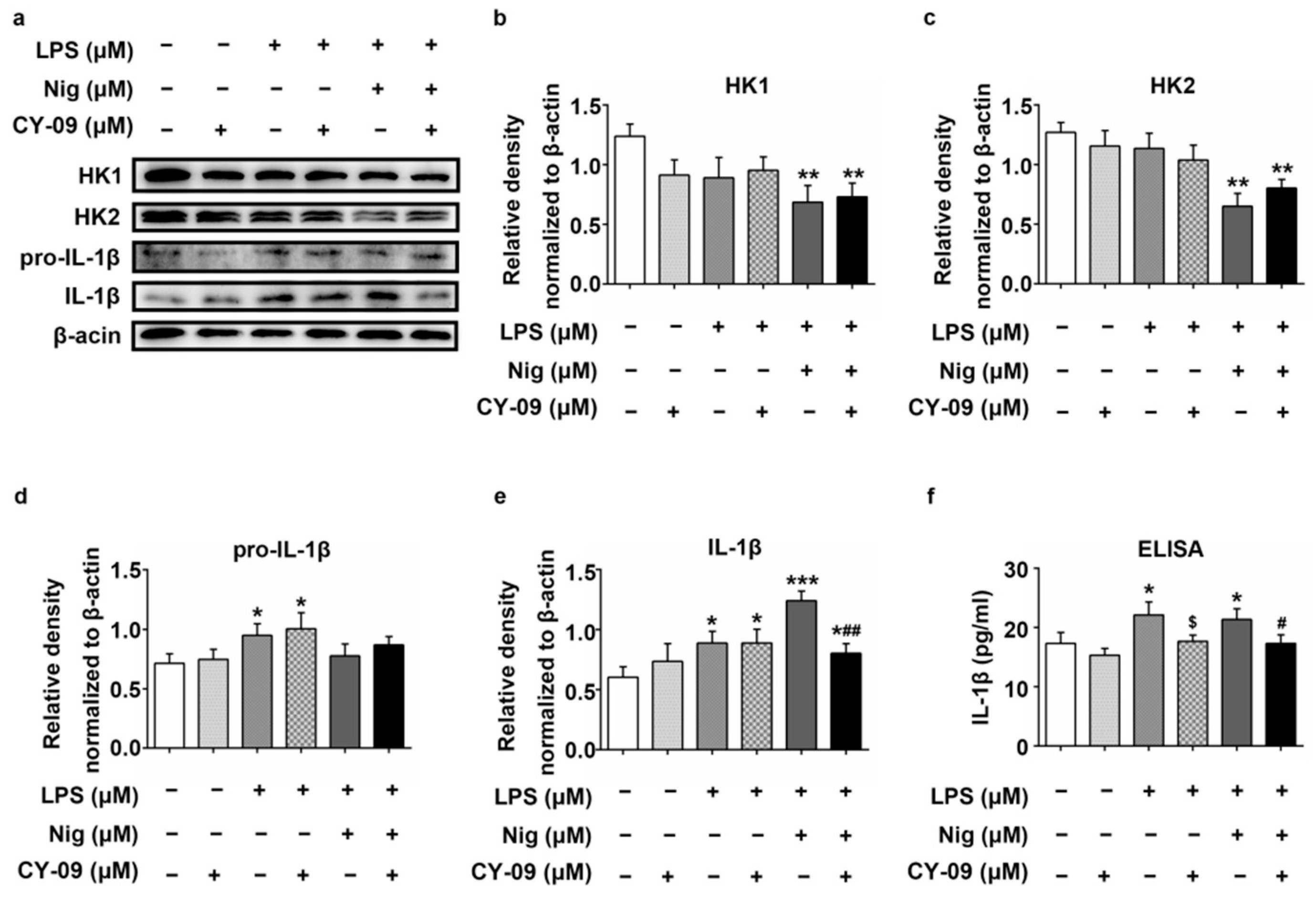
2.6. Increased IL-1β Leads to the Reduction of HK in LPS-, Nig- and CY-09-Treated N2a Cells
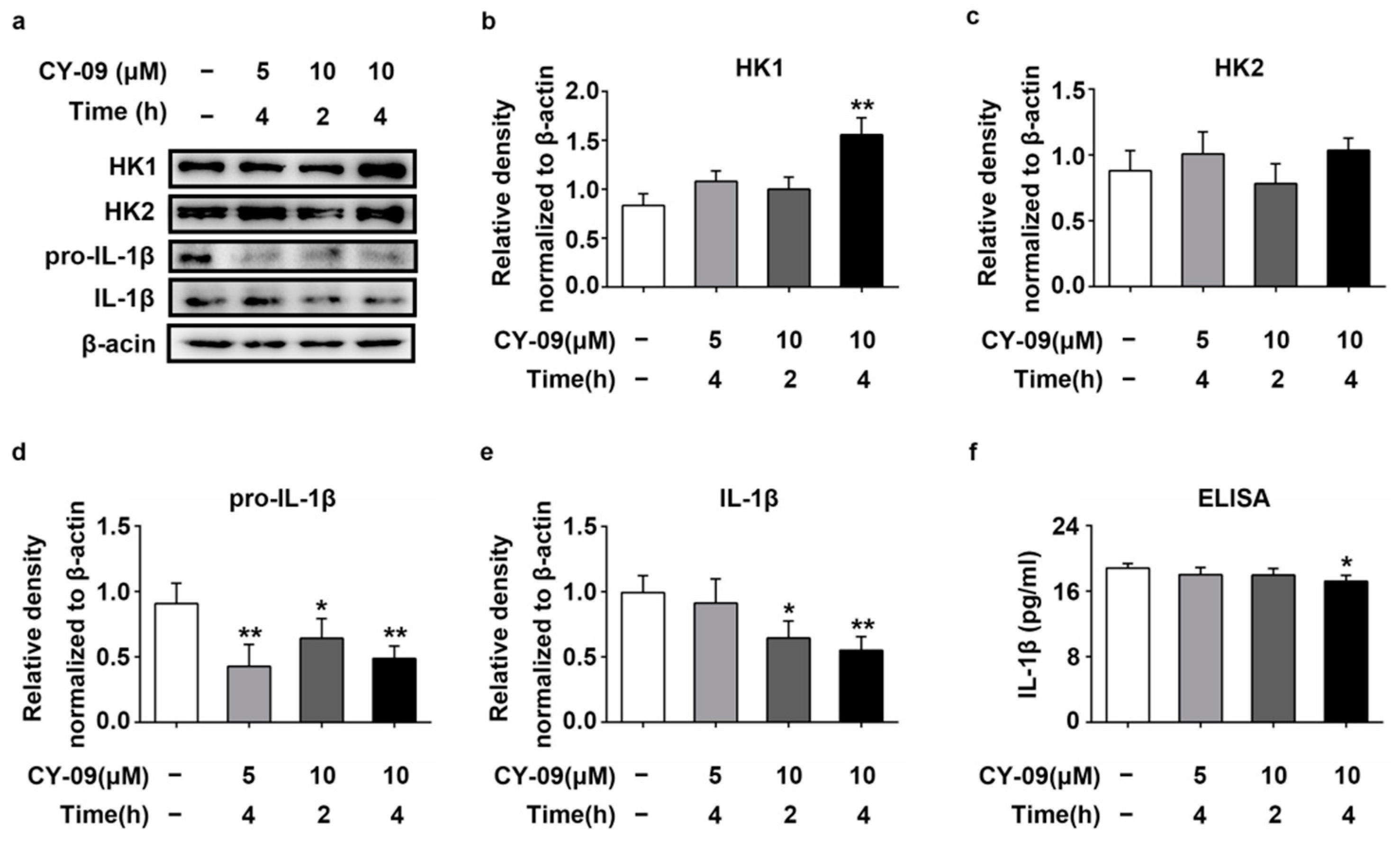
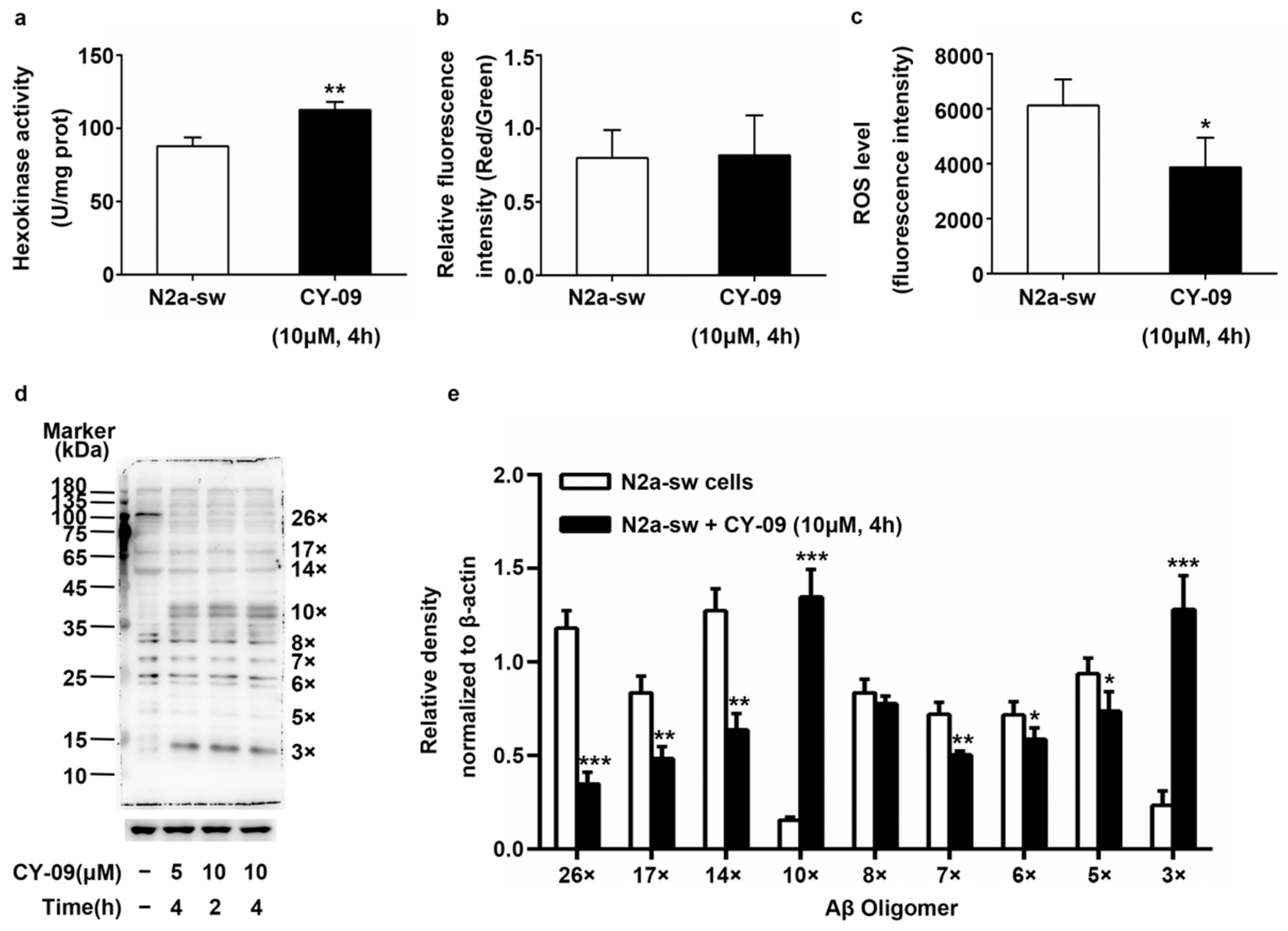
2.7. Increased Expression of HK in CY-09-Treated N2a-sw Cells
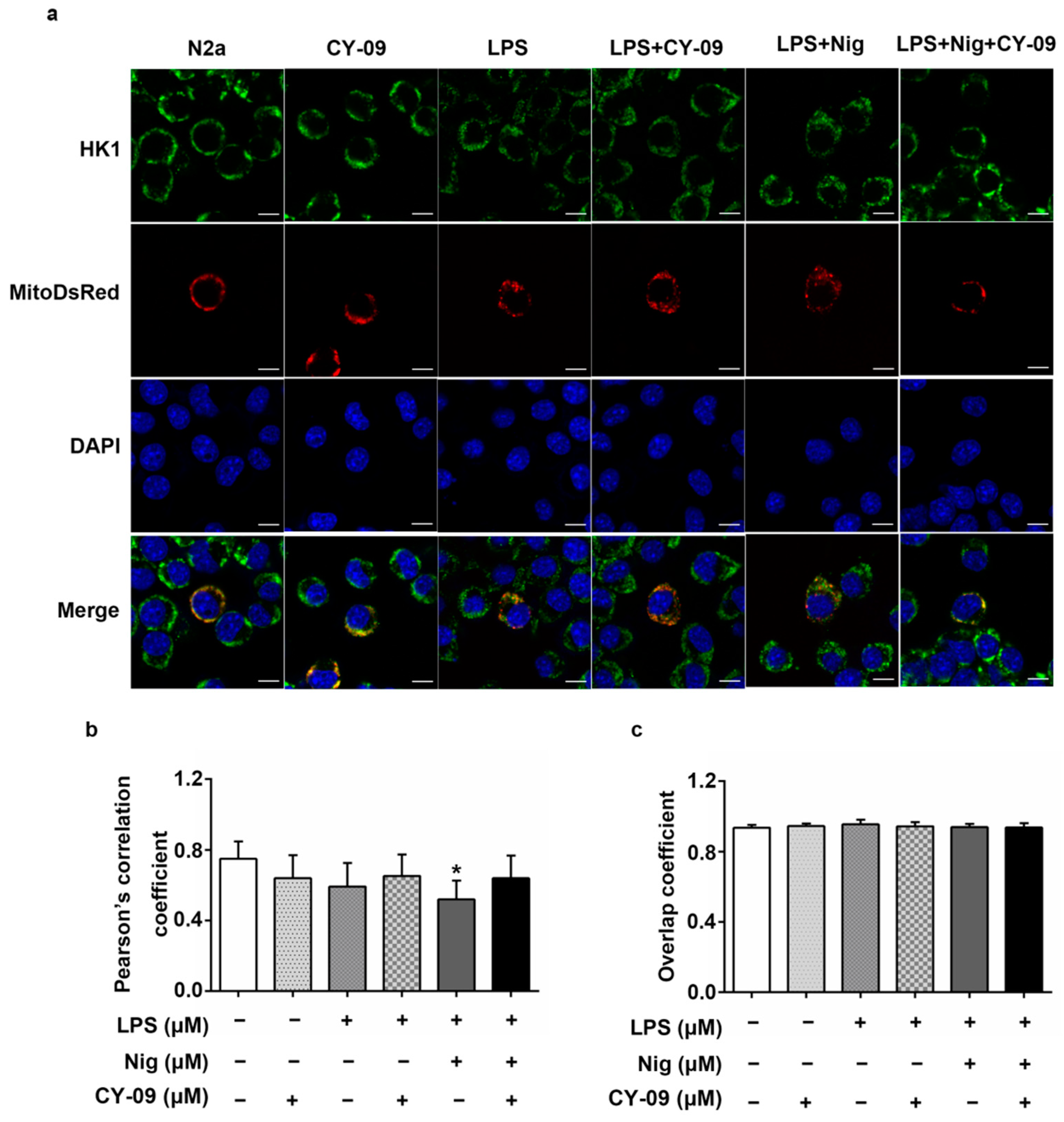
2.8. Decreased IL-1β Can Improve the Abnormal Distribution in Treated Cells
2.9. Decreased ROS Levels and Increased HK Activity in CY-09-Treated N2a-sw Cells
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Positron Emission Tomography (PET) Scanning
4.3. Cell Culture and Treatment
4.4. Quantitative Real-Time PCR (Q-PCR) Assay
4.5. Brain Mitochondrion Isolation
4.6. Western Blotting
4.7. ELISA Assay
4.8. Hexokinase Activity Assay
4.9. Mitochondrial Membrane Potential and ROS Assay
4.10. Transfection Assay
4.11. Immunofluorescence
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| HK | hexokinase |
| NLRP3 | NOD-like receptor protein 3 |
| IL-1β | interleukin-1β |
| 18F-FDG | 18F-flurodeoxyglucose |
| PET | positron emission tomography |
| SUVs | standard uptake values |
| LPS | lipopolysaccharide |
| LND | lonidamine |
| Nig | nigericin |
| Q-PCR | quantitative real-time PCR |
| mHK | mitochondrial hexokinase |
| cHK | cytoplasmic hexokinase |
| ROS | reactive oxygen species |
References
- Calsolaro, V.; Edison, P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimer’s Dement. 2016, 12, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Shao, T.; Yu, P.; Cao, R.; Zhang, M.; Wen, K.; Fan, M.; He, B. Neuronostatin promotes soluble Aβ1-42 oligomers–induced spatial learning and memory impairments in mice. Behav. Brain Res. 2019, 364, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Inui, Y.; Nakamura, A.; Ito, K. Brain fluorodeoxyglucose (FDG) PET in dementia. Ageing Res. Rev. 2016, 30, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Lundgaard, I.; Li, B.; Xie, L.; Kang, H.; Sanggaard, S.; Haswell, J.D.R.; Sun, W.; Goldman, S.A.; Blekot, S.; Nielsen, M.; et al. Direct neuronal glucose uptake heralds activity-dependent increases in cerebral metabolism. Nat. Commun. 2015, 6, 6807. [Google Scholar] [CrossRef]
- Moon, J.-S.; Hisata, S.; Park, M.-A.; DeNicola, G.M.; Ryter, S.W.; Nakahira, K.; Choi, A.M.K. mTORC1-Induced HK1-Dependent Glycolysis Regulates NLRP3 Inflammasome Activation. Cell Rep. 2015, 12, 102–115. [Google Scholar] [CrossRef]
- Wolf, A.J.; Reyes, C.N.; Liang, W.; Becker, C.; Shimada, K.; Wheeler, M.L.; Cho, H.C.; Popescu, N.I.; Coggeshall, K.M.; Arditi, M.; et al. Hexokinase Is an Innate Immune Receptor for the Detection of Bacterial Peptidoglycan. Cell 2016, 166, 624–636. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Qiao, J.; Liu, S.; Wang, S.; Zhao, D.; Bai, X.; Liu, M. Ginsenoside Rh2 inhibits HeLa cell energy metabolism and induces apoptosis by upregulating voltage‑dependent anion channel 1. Int. J. Mol. Med. 2020, 46, 1695–1706. [Google Scholar] [CrossRef]
- Harris, R.A.; Tindale, L.; Cumming, R.C. Age-dependent metabolic dysregulation in cancer and Alzheimer’s disease. Biogerontology 2014, 15, 559–577. [Google Scholar] [CrossRef]
- Regenold, W.T.; Pratt, M.; Nekkalapu, S.; Shapiro, P.; Kristian, T.; Fiskum, G. Mitochondrial detachment of hexokinase 1 in mood and psychotic disorders: Implications for brain energy metabolism and neurotrophic signaling. J. Psychiatr. Res. 2012, 46, 95–104. [Google Scholar] [CrossRef]
- Robey, R.B.; Hay, N. Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene 2006, 25, 4683–4696. [Google Scholar] [CrossRef]
- Da-Silva, W.S.; Gómez-Puyou, A.; de Gómez-Puyou, M.T.; Moreno-Sanchez, R.; De Felice, F.G.; de Meis, L.; Oliveira, M.F.; Galina, A. Mitochondrial bound hexokinase activity as a preventive antioxidant defense: Steady-state ADP formation as a regulatory mechanism of membrane potential and reactive oxygen species generation in mitochondria. J. Biol. Chem. 2004, 279, 39846–39855. [Google Scholar] [CrossRef] [PubMed]
- Schindler, A.; Foley, E. Hexokinase 1 blocks apoptotic signals at the mitochondria. Cell. Signal. 2013, 25, 2685–2692. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.M.; O’Neill, L.A.J. Metabolic regulation of NLRP3. Immunol. Rev. 2018, 281, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Daniels, M.J.D.; Rivers-Auty, J.; Schilling, T.; Spencer, N.G.; Watremez, W.; Fasolino, V.; Booth, S.J.; White, C.S.; Baldwin, A.G.; Freeman, S.; et al. Fenamate NSAIDs inhibit the NLRP3 inflammasome and protect against Alzheimer’s disease in rodent models. Nat. Commun. 2016, 7, 12504. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Kummer, M.P.; Stutz, A.; Delekate, A.; Schwartz, S.; Vieira-Saecker, A.; Griep, A.; Axt, D.; Remus, A.; Tzeng, T.-C.; et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 2013, 493, 674–678. [Google Scholar] [CrossRef]
- Saraiva, L.M.; Da Silva, G.S.S.; Galina, A.; Da-Silva, W.S.; Klein, W.L.; Ferreira, S.T.; De Felice, F.G. Amyloid-β Triggers the Release of Neuronal Hexokinase 1 from Mitochondria. PLoS ONE 2010, 5, e15230. [Google Scholar] [CrossRef]
- Song, L.; Pei, L.; Yao, S.; Wu, Y.; Shang, Y. NLRP3 Inflammasome in Neurological Diseases, from Functions to Therapies. Front. Cell. Neurosci. 2017, 11, 63. [Google Scholar] [CrossRef]
- Smilansky, A.; Dangoor, L.; Nakdimon, I.; Ben-Hail, D.; Mizrachi, D.; Shoshan-Barmatz, V. The Voltage-dependent Anion Channel 1 Mediates Amyloid β Toxicity and Represents a Potential Target for Alzheimer Disease Therapy. J. Biol. Chem. 2015, 290, 30670–30683. [Google Scholar] [CrossRef]
- Bi, D.; Li, X.; Li, T.; Li, X.; Lin, Z.; Yao, L.; Li, H.; Xu, H.; Hu, Z.; Zhang, Z.; et al. Characterization and Neuroprotection Potential of Seleno-Polymannuronate. Front. Pharmacol. 2020, 11, 21. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Wu, Q.-Y.; Chen, C.; Zheng, R.; Chen, Y.; Liu, Q.; Ni, J.-Z.; Song, G.-L. Selenomethionine Attenuates the Amyloid-β Level by Both Inhibiting Amyloid-β Production and Modulating Autophagy in Neuron-2a/AβPPswe Cells. J. Alzheimer’s Dis. 2017, 59, 591–602. [Google Scholar] [CrossRef]
- Bi, D.; Yao, L.; Lin, Z.; Chi, L.; Li, H.; Xu, H.; Du, X.; Liu, Q.; Hu, Z.; Lu, J.; et al. Unsaturated mannuronate oligosaccharide ameliorates β-amyloid pathology through autophagy in Alzheimer’s disease cell models. Carbohydr. Polym. 2021, 251, 117124. [Google Scholar] [CrossRef] [PubMed]
- Turkseven, S.; Zorzano, A. Determination of mitochondrial fragmentation and autophagosome formation in C2C12 skeletal muscle cells. Turk. J. Med Sci. 2013, 43, 775–781. [Google Scholar] [CrossRef]
- Qin, N.; Xu, G.; Gao, Y.; Wang, Z.L.; Fu, S.B.; Liu, H.M.; Xiao, X.H.; Zhao, H.P.; Bai, Z.F. Regulation and mechanism of corylin on inflammasomes. Chin. Pharmacol. Bull. 2019, 35, 364–370. [Google Scholar] [CrossRef]
- Hassel, B.; Elsais, A.; Frøland, A.S.; Taubøll, E.; Gjerstad, L.; Quan, Y.; Dingledine, R.; Rise, F. Uptake and metabolism of fructose by rat neocortical cells in vivo and by isolated nerve terminals in vitro. J. Neurochem. 2015, 133, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, M.F.; Oliveira, P.G.; Garcia-Carbonell, R.; Croft, A.P.; Smith, J.M.; Serrano, R.L.; Sanchez-Lopez, E.; Liu, X.; Kisseleva, T.; Hay, N.; et al. Hexokinase 2 as a novel selective metabolic target for rheumatoid arthritis. Ann. Rheum. Dis. 2018, 77, 1636–1643. [Google Scholar] [CrossRef]
- Takkinen, J.S.; López-Picón, F.R.; Al Majidi, R.; Eskola, O.; Krzyczmonik, A.; Keller, T.; Löyttyniemi, E.; Solin, O.; Rinne, J.O.; Haaparanta-Solin, M. Brain energy metabolism and neuroinflammation in ageing APP/PS1-21 mice using longitudinal 18F-FDG and 18F-DPA-714 PET imaging. Br. J. Pharmacol. 2016, 37, 2870–2882. [Google Scholar] [CrossRef]
- Chen, Y.; Han, S.; Huang, X.; Ni, J.; He, X. The Protective Effect of Icariin on Mitochondrial Transport and Distribution in Primary Hippocampal Neurons from 3× Tg-AD Mice. Int. J. Mol. Sci. 2016, 17, 163. [Google Scholar] [CrossRef]
- Jiang, H.; He, H.; Chen, Y.; Huang, W.; Cheng, J.; Ye, J.; Wang, A.; Tao, J.; Wang, C.; Liu, Q.; et al. Identification of a selec-tive and direct NLRP3 inhibitor to treat inflammatory disorders. J. Exp. Med. 2017, 214, 3219–3238. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.; He, Z.; Jacob, C.; Hu, X.; Liang, X.; Xiao, W.; Wan, L.; Xiao, P.; D’Ascenzo, N.; Ni, J.; et al. Effect of Increased IL-1β on Expression of HK in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 1306. https://doi.org/10.3390/ijms22031306
Han S, He Z, Jacob C, Hu X, Liang X, Xiao W, Wan L, Xiao P, D’Ascenzo N, Ni J, et al. Effect of Increased IL-1β on Expression of HK in Alzheimer’s Disease. International Journal of Molecular Sciences. 2021; 22(3):1306. https://doi.org/10.3390/ijms22031306
Chicago/Turabian StyleHan, Shuangxue, Zhijun He, Cornelius Jacob, Xia Hu, Xiao Liang, Wenchang Xiao, Lu Wan, Peng Xiao, Nicola D’Ascenzo, Jiazuan Ni, and et al. 2021. "Effect of Increased IL-1β on Expression of HK in Alzheimer’s Disease" International Journal of Molecular Sciences 22, no. 3: 1306. https://doi.org/10.3390/ijms22031306
APA StyleHan, S., He, Z., Jacob, C., Hu, X., Liang, X., Xiao, W., Wan, L., Xiao, P., D’Ascenzo, N., Ni, J., Liu, Q., & Xie, Q. (2021). Effect of Increased IL-1β on Expression of HK in Alzheimer’s Disease. International Journal of Molecular Sciences, 22(3), 1306. https://doi.org/10.3390/ijms22031306





