Natriuretic Peptide Expression and Function in GH3 Somatolactotropes and Feline Somatotrope Pituitary Tumours
Abstract
1. Introduction
2. Results
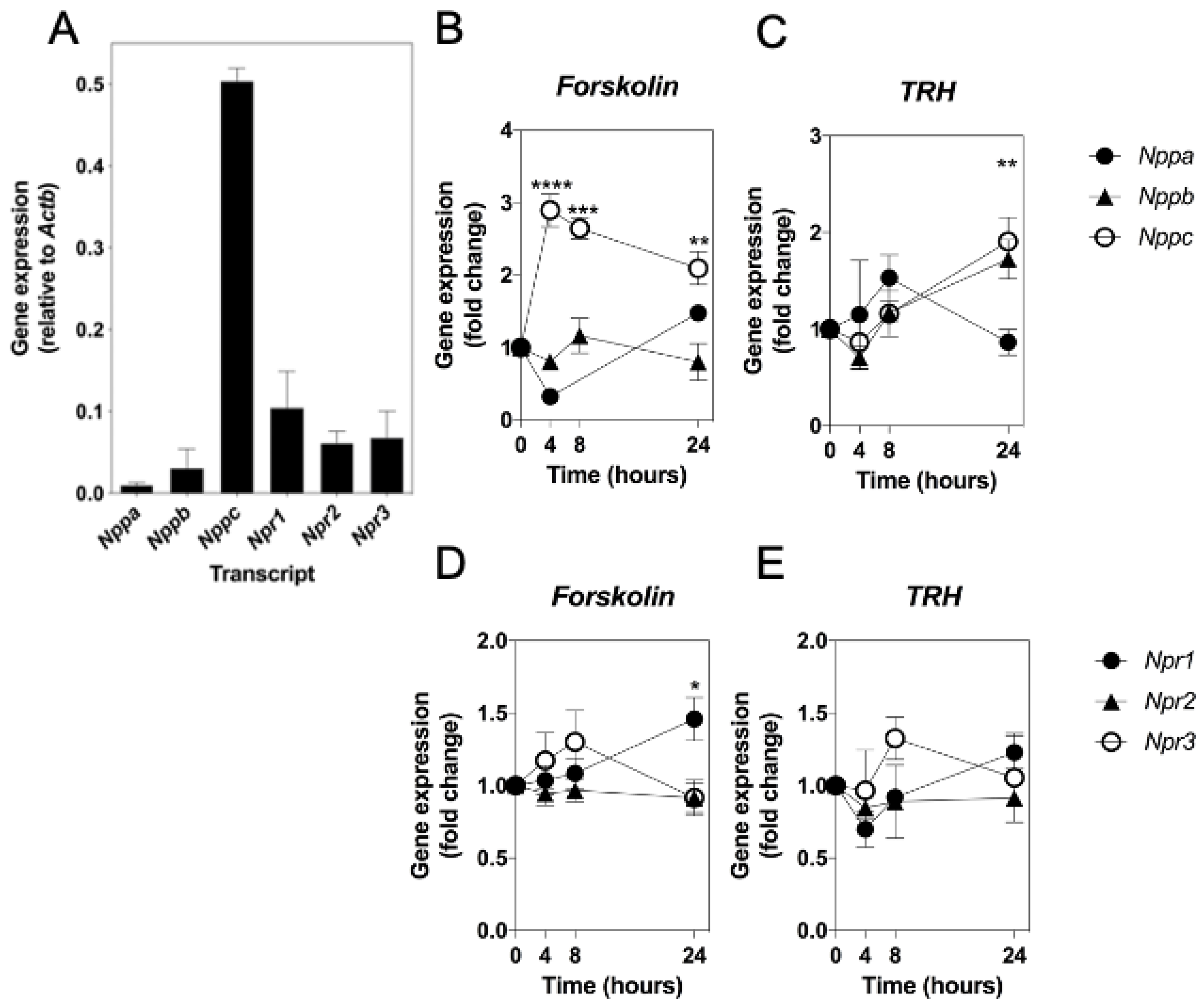
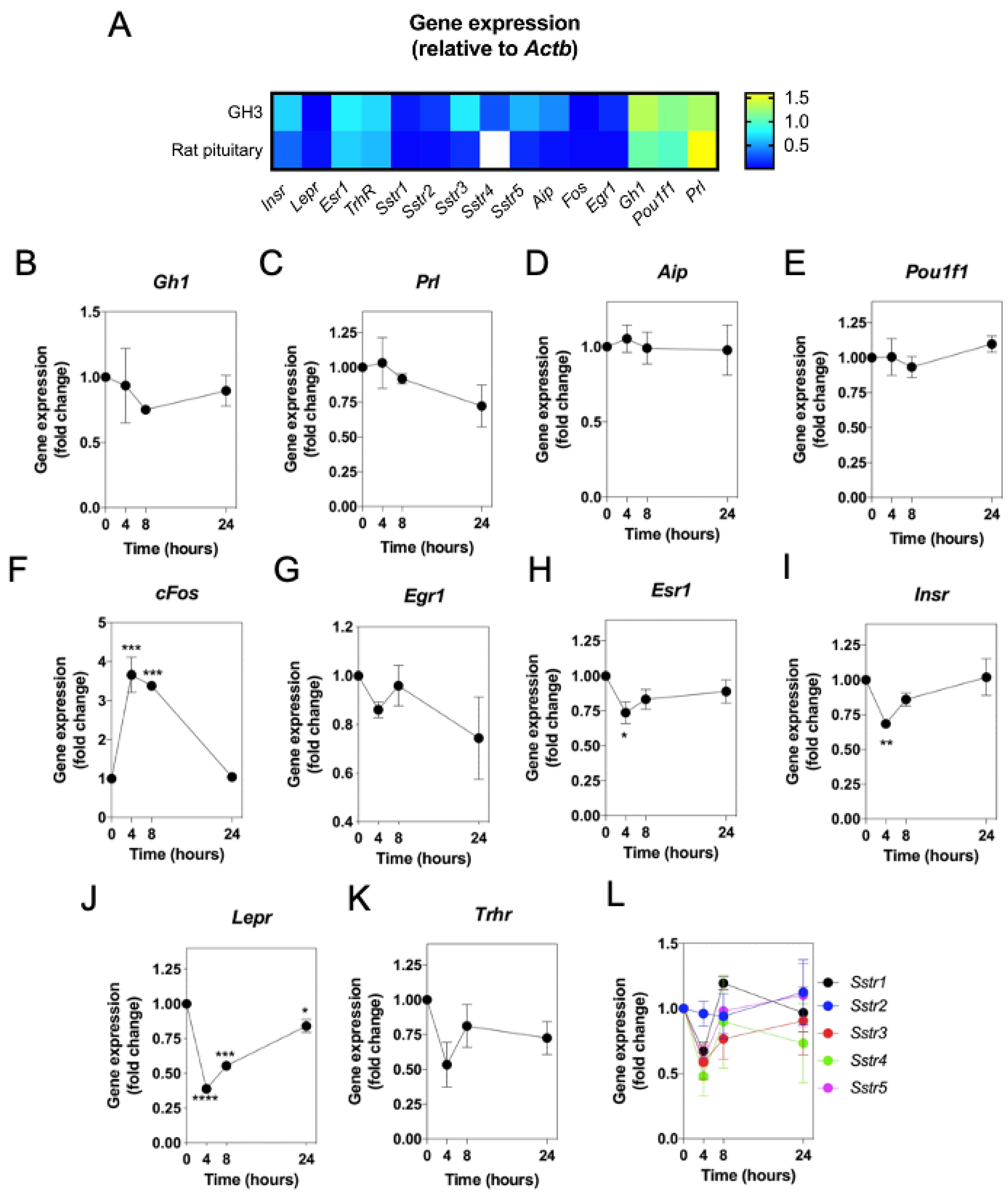
2.1. Molecular Characterization of a Custom Multiplex RT-qPCR Assay for Natriuretic Peptides and Somatotrope Markers in GH3 Somatolactotropes and Primary Rat Pituitary Tissue
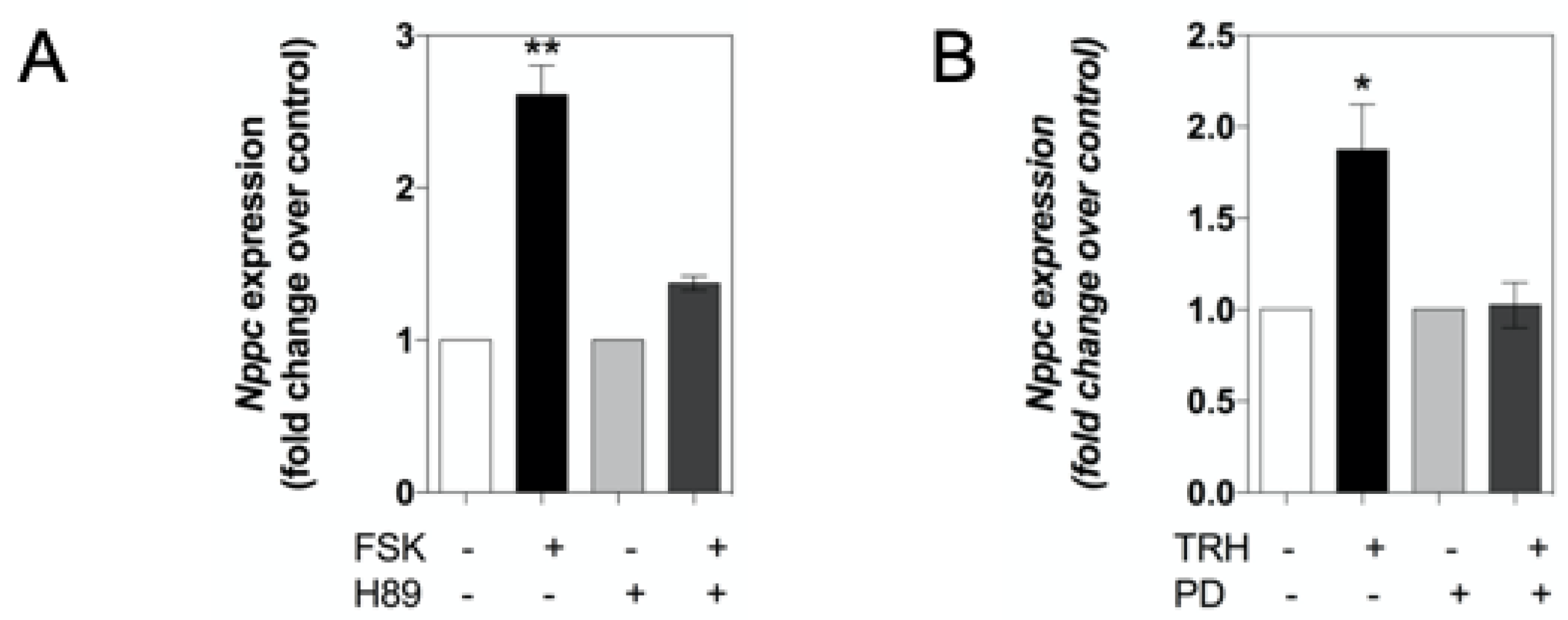
2.2. cAMP and TRH Signalling Pathways Differentially Regulate Expression of Natriuretic Peptides and Their Receptors
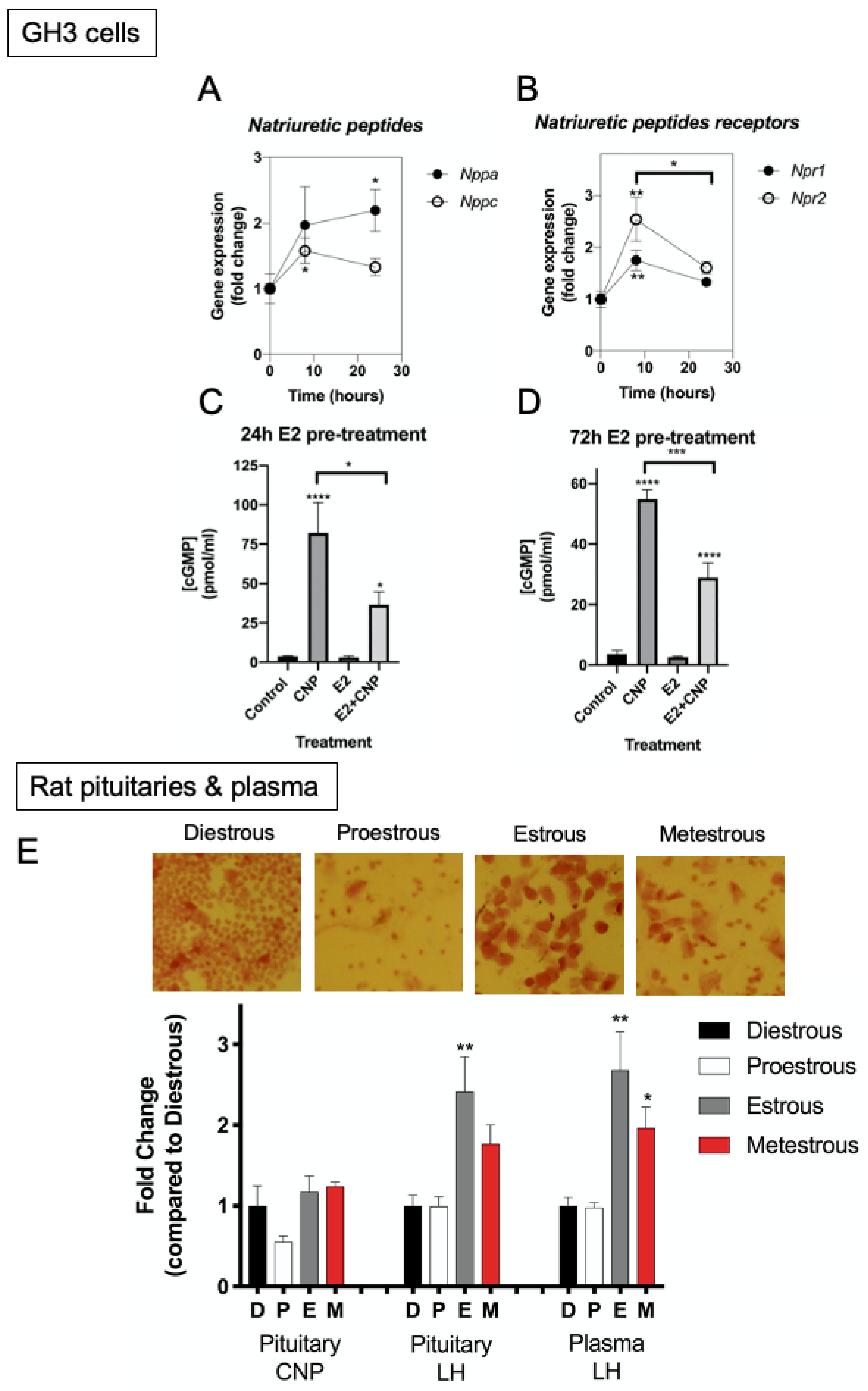
2.3. Identification of Putative CNP Target Genes in GH3 Somatolactotropes
2.4. Interaction of Oestrogen and Natriuretic Peptides in GH3 Somatolactotropes
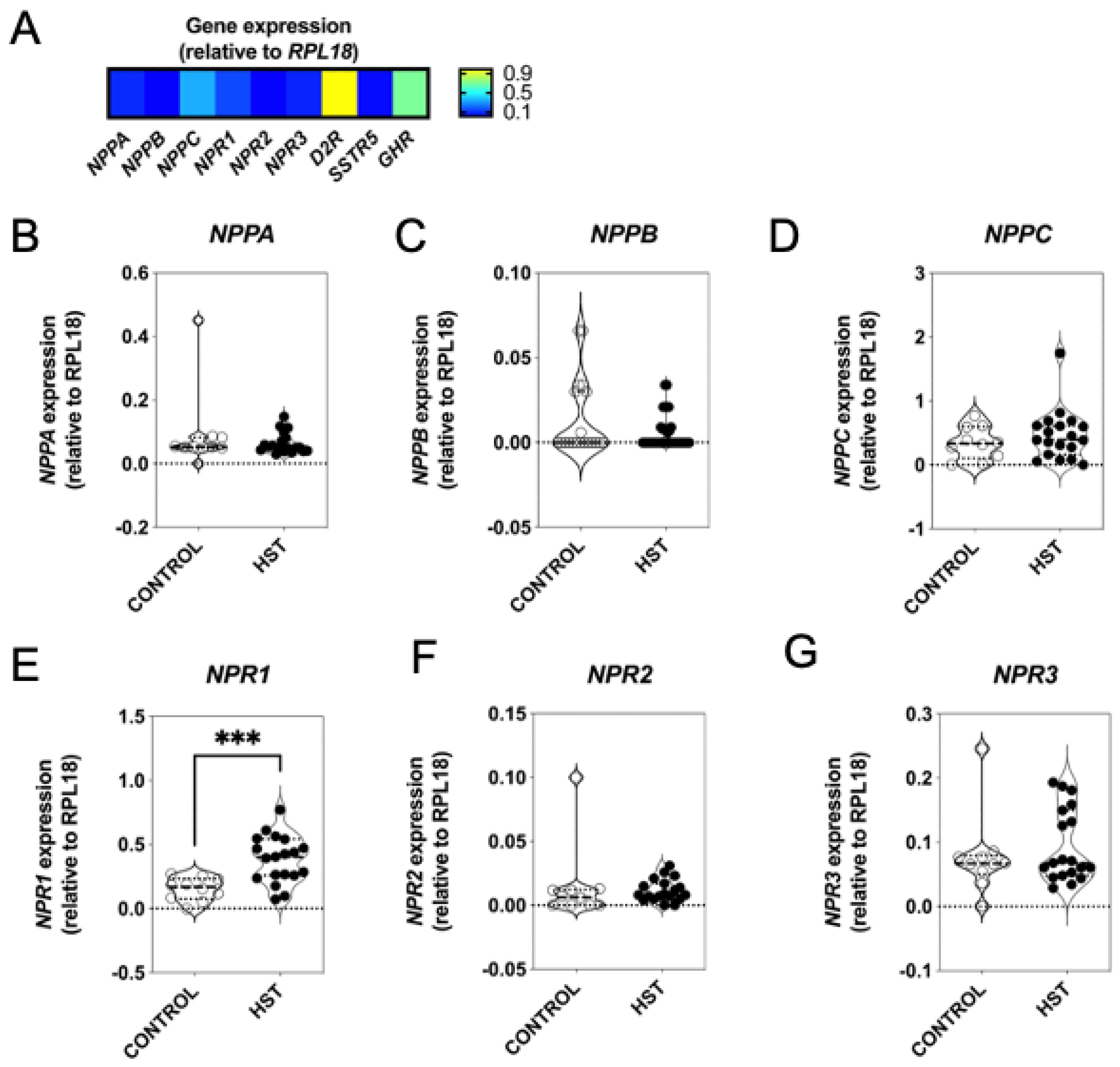
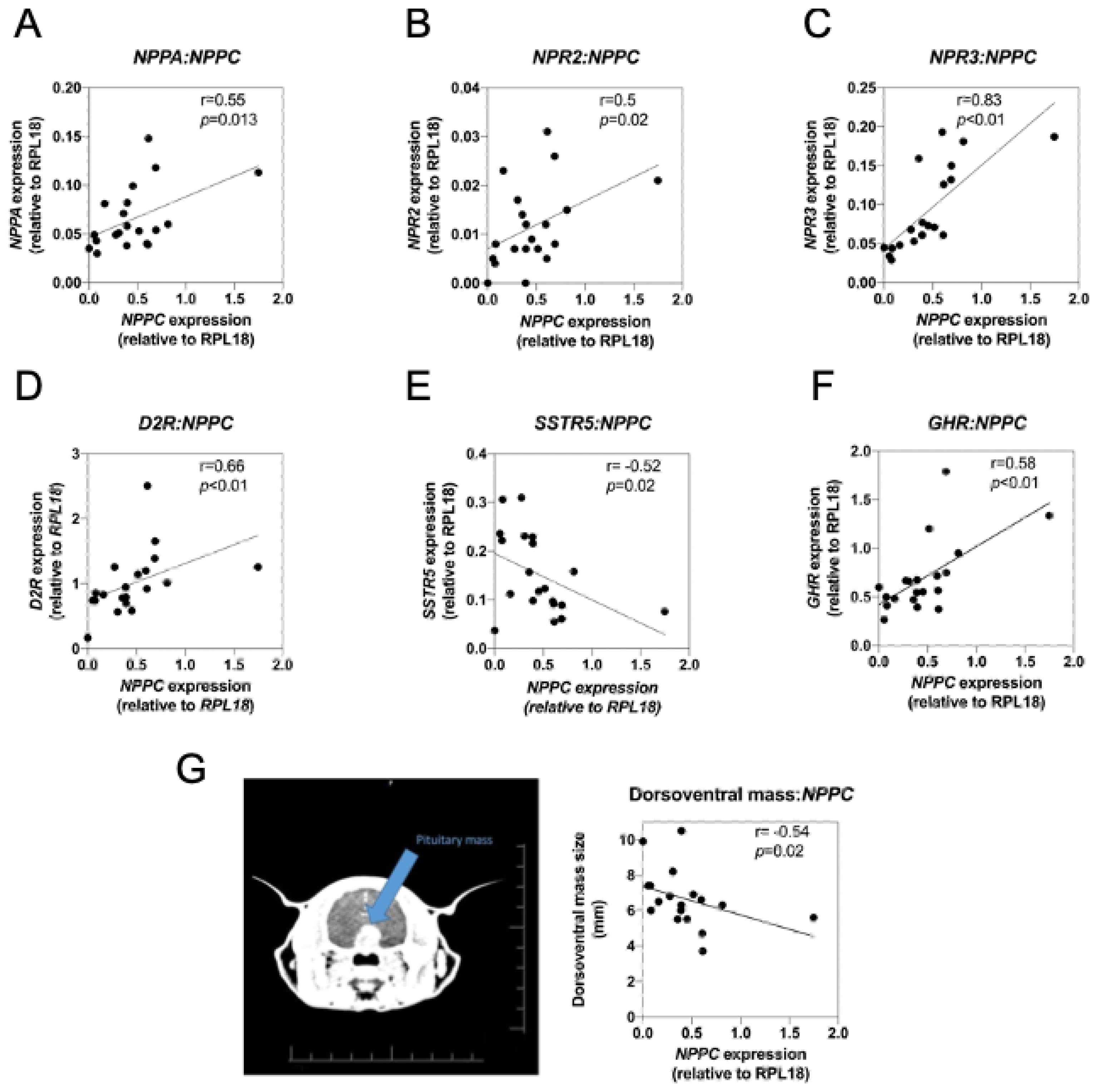
2.5. Natriuretic Peptide System in Feline Somatotrope Tumours
3. Materials and Methods
3.1. Materials
3.2. Cell Culture and cGMP Enzyme Immunoassay
3.3. Patient Data, Ethics, and Hypophysectomy Surgery
3.4. RNA Extraction
3.5. GeXP Multiplex RT-qPCR
3.6. Immunoassays and Vaginal Cytology
3.7. Data Presentation and Statistical Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fowkes, R.C.; McArdle, C.A. C-type Natriuretic Peptide: An Important Neuroendocrine Regulator? Trends Endocrinol. Metab. 2000, 11, 333–338. [Google Scholar] [CrossRef]
- Potter, L.R.; Abbey-Hosch, S.; Dickey, D.M. Natriuretic Peptides, Their Receptors, and Cyclic Guanosine Monophosphate-Dependent Signaling Functions. Endocr. Rev. 2006, 27, 47–72. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, M. Molecular Physiology of Membrane Guanylyl Cyclase Receptors. Physiol. Rev. 2016, 96, 751–804. [Google Scholar] [CrossRef] [PubMed]
- Waschek, J.A. Developmental actions of natriuretic peptides in the brain and skeleton. Cell. Mol. Life Sci. 2004, 61, 2332–2342. [Google Scholar] [CrossRef]
- Chusho, H.; Tamura, N.; Ogawa, Y.; Yasoda, A.; Suda, M.; Miyazawa, T.; Nakamura, K.; Nakao, K.; Kurihara, T.; Komatsu, Y.; et al. Dwarfism and early death in mice lacking C-type natriuretic peptide. Proc. Natl. Acad. Sci. USA 2001, 98, 4016–4021. [Google Scholar] [CrossRef]
- Tamura, N.; Doolittle, L.K.; Hammer, R.E.; Shelton, J.M.; Richardson, J.A.; Garbers, D.L. Critical roles of the guanylyl cyclase B receptor in endochondral ossification and development of female reproductive organs. Proc. Natl. Acad. Sci. USA 2004, 101, 17300–17305. [Google Scholar] [CrossRef]
- Vasques, G.A.; Arnhold, I.J.; Jorge, A.A. Role of the Natriuretic Peptide System in Normal Growth and Growth Disorders. Horm. Res. Paediatr. 2014, 82, 222–229. [Google Scholar] [CrossRef]
- Jee, Y.H.; Baron, J.; Nilsson, O. New developments in the genetic diagnosis of short stature. Curr. Opin. Pediatr. 2018, 30, 541–547. [Google Scholar] [CrossRef]
- Thompson, I.R.; Chand, A.N.; King, P.J.; Ansorge, O.; Karavitaki, N.; Jones, C.A.; Rahmutula, L.; Gardner, D.G.; Zivkovic, V.; Wheeler-Jones, C.P.; et al. Expression of guanylyl cyclase-B (GC-B/NPR2) receptors in normal human fetal pituitaries and human pituitary adenomas implicates a role for C-type natriuretic peptide. Endocr. Relat. Cancer 2012, 19, 497–508. [Google Scholar] [CrossRef]
- Fowkes, R.C.; Forrest-Owen, W.; McArdle, C.A. C-type natriuretic peptide (CNP) effects in anterior pituitary cell lines: Evidence for homologous desensitisation of CNP-stimulated cGMP accumulation in αT3-1 gonadotroph-derived cells. J. Endocrinol. 2000, 166, 195–203. [Google Scholar] [CrossRef]
- Thompson, I.R.; Chand, A.N.; Jonas, K.C.; Burrin, J.M.; Steinhelper, M.E.; Wheeler-Jones, C.P.; McArdle, C.A.; Fowkes, R.C. Molecular characterisation and functional interrogation of a local natriuretic peptide system in rodent pituitaries, αT3-1 and LβT2 gonadotroph cells. J. Endocrinol. 2009, 203, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Thompson, I.R.; Mirczuk, S.M.; Smith, L.; Lessey, A.J.; Simbi, B.; Sunters, A.; Baxter, G.F.; Lipscomb, V.J.; McGonnell, I.M.; Wheeler-Jones, C.P.; et al. Homologous and heterologous desensitization of guanylyl cyclase-B signaling in GH3 somatolactotropes. Cell Tissue Res. 2014, 355, 425–436. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barry, S.; Korbonits, M. Update on the Genetics of Pituitary Tumors. Endocrinol. Metab. Clin. N. Am. 2020, 49, 433–452. [Google Scholar] [CrossRef] [PubMed]
- Niessen, S.; Forcada, Y.; Mantis, P.; Lamb, C.R.; Harrington, N.; Fowkes, R.C.; Korbonits, M.; Smith, K.; Church, D.B. Studying Cat (Felis catus) Diabetes: Beware of the Acromegalic Imposter. PLoS ONE 2015, 10, e0127794. [Google Scholar] [CrossRef] [PubMed]
- Scudder, C.J.; Mirczuk, S.M.; Richardson, K.M.; Crossley, V.J.; Regan, J.T.C.; Gostelow, R.; Forcada, Y.; Hazuchova, K.; Harrington, N.; McGonnell, I.M.; et al. Pituitary Pathology and Gene Expression in Acromegalic Cats. J. Endocr. Soc. 2018, 3, 181–200. [Google Scholar] [CrossRef]
- Lin, X.-W.; Lin, H.-R.; Peter, R.E. The regulatory effects of thyrotropin-releasing hormone on growth hormone secretion from the pituitary of common carp in vitro. Fish Physiol. Biochem. 1993, 11, 71–76. [Google Scholar] [CrossRef]
- Hubina, E.; Nanzer, A.M.; Hanson, M.R.; Ciccarelli, E.; Losa, M.; Gaia, D.; Papotti, M.; Terreni, M.R.; Khalaf, S.; Jordan, S.; et al. Somatostatin analogues stimulate p27 expression and inhibit the MAP kinase pathway in pituitary tumours. Eur. J. Endocrinol. 2006, 155, 371–379. [Google Scholar] [CrossRef]
- Szabó, M.; Stachura, M.E.; Paleologos, N.; Bybee, D.E.; Frohman, L.A. Thyrotropin-Releasing Hormone Stimulates Growth Hormone Release from the Anterior Pituitary of Hypothyroid Rats in Vitro. Endocrinology 1984, 114, 1344–1351. [Google Scholar] [CrossRef]
- Watson, C.S.; Jeng, Y.; Kochukov, M.Y. Nongenomic actions of estradiol compared with estrone and estriol in pituitary tumor cell signaling and proliferation. FASEB J. 2008, 22, 3328–3336. [Google Scholar] [CrossRef]
- Kanasaki, H.; Oride, A.; Mijiddorj, T.; Kyo, S. Role of thyrotropin-releasing hormone in prolactin-producing cell models. Neuropeptides 2015, 54, 73–77. [Google Scholar] [CrossRef]
- Denef, C. Paracrinicity: The story of thirty years of cellular pituitary crosstalk. J. Neuroendocr. 2008, 20, 1–70. [Google Scholar] [CrossRef]
- Hartt, D.; Ogiwara, T.; Ho, A.; Chik, C. Cyclic GMP Stimulates Growth Hormone Release in Rat Anterior Pituitary Cells. Biochem. Biophys. Res. Commun. 1995, 214, 918–926. [Google Scholar] [CrossRef]
- Shimekake, Y.; Ohta, S.; Nagata, K. C-type natriuretic peptide stimulates secretion of growth hormone from rat-pituitary-derived GH3 cells via a cyclic-GMP-mediated pathway. Eur. J. Biochem. 1994, 222, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Wendt, D.; Dvorak-Ewell, M.; Bullens, S.; Lorget, F.; Bell, S.M.; Peng, J.; Castillo, S.; Aoyagi-Scharber, M.; O’Neill, C.A.; Krejci, P.; et al. Neutral Endopeptidase-Resistant C-Type Natriuretic Peptide Variant Represents a New Therapeutic Approach for Treatment of Fibroblast Growth Factor Receptor 3—Related Dwarfism. J. Pharmacol. Exp. Ther. 2015, 353, 132–149. [Google Scholar] [CrossRef] [PubMed]
- Lorget, F.; Kaci, N.; Peng, J.; Benoist-Lasselin, C.; Mugniery, E.; Oppeneer, T.; Wendt, D.J.; Bell, S.M.; Bullens, S.; Bunting, S.; et al. Evaluation of the Therapeutic Potential of a CNP Analog in a Fgfr3 Mouse Model Recapitulating Achondroplasia. Am. J. Hum. Genet. 2012, 91, 1108–1114. [Google Scholar] [CrossRef]
- Breinholt, V.M.; Rasmussen, C.E.; Mygind, P.H.; Kjelgaard-Hansen, M.; Faltinger, F.; Bernhard, A.; Zettler, J.; Hersel, U. TransCon CNP, a Sustained-Release C-Type Natriuretic Peptide Prodrug, a Potentially Safe and Efficacious New Therapeutic Modality for the Treatment of Comorbidities Associated with Fibroblast Growth Factor Receptor 3—Related Skeletal Dysplasias. J. Pharmacol. Exp. Ther. 2019, 370, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Morozumi, N.; Yotsumoto, T.; Yamaki, A.; Yoshikiyo, K.; Yoshida, S.; Nakamura, R.; Jindo, T.; Furuya, M.; Maeda, H.; Minamitake, Y.; et al. ASB20123: A novel C-type natriuretic peptide derivative for treatment of growth failure and dwarfism. PLoS ONE 2019, 14, e0212680. [Google Scholar] [CrossRef]
- Hisado-Oliva, A.; Ruzafa-Martin, A.; Sentchordi, L.; Funari, M.F.A.; Bezanilla-López, C.; Alonso-Bernáldez, M.; Barraza-García, J.; Rodriguez-Zabala, M.; Lerario, A.M.; Benito-Sanz, S.; et al. Mutations in C-natriuretic peptide (NPPC): A novel cause of autosomal dominant short stature. Genet. Med. 2018, 20, 91–97. [Google Scholar] [CrossRef]
- Amano, N.; Mukai, T.; Ito, Y.; Narumi, S.; Tanaka, T.; Yokoya, S.; Ogata, T.; Hasegawa, T. Identification and Functional Characterization of Two Novel NPR2 Mutations in Japanese Patients With Short Stature. J. Clin. Endocrinol. Metab. 2014, 99, E713–E718. [Google Scholar] [CrossRef]
- Tassano, E.; Buttgereit, J.; Bader, M.; Lerone, M.; Divizia, M.T.; Bocciardi, R.; Napoli, F.; Pala, G.; Sloan-Béna, F.; Gimelli, S.; et al. Genotype-Phenotype Correlation of 2q37 Deletions Including NPPC Gene Associated with Skeletal Malformations. PLoS ONE 2013, 8, e66048. [Google Scholar] [CrossRef]
- Robinson, J.W.; Blixt, N.C.; Norton, A.; Mansky, K.C.; Aparicio, C.; Wagner, B.M.; Benton, A.M.; Warren, G.L.; Khosla, S.; Gaddy, D.; et al. Male mice with elevated C-type natriuretic peptide-dependent guanylyl cyclase-B activity have increased osteoblasts, bone mass and bone strength. Bone 2020, 135, 115320. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Furuya, M.; Morozumi, N.; Yoshikiyo, K.; Yotsumoto, T.; Jindo, T.; Nakamura, R.; Murakami, K.; Ueda, Y.; Hanada, T.; et al. Exogenous C-type natriuretic peptide restores normal growth and prevents early growth plate closure in its deficient rats. PLoS ONE 2018, 13, e0204172. [Google Scholar] [CrossRef] [PubMed]
- Caufriez, A. The pubertal spurt: Effects of sex steroids on growth hormone and insulin-like growth factor I. Eur. J. Obstet. Gynecol. Reprod. Biol. 1997, 71, 215–217. [Google Scholar] [CrossRef]
- Lissett, C.A.; Shalet, S.M. The Impact of Dose and Route of Estrogen Administration on the Somatotropic Axis in Normal Women. J. Clin. Endocrinol. Metab. 2003, 88, 4668–4672. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mahmoodzadeh, S.; Pham, T.H.; Kuehne, A.; Fielitz, B.; Dworatzek, E.; Kararigas, G.; Petrov, G.; Davidson, M.M.; Regitz-Zagrosek, V. 17β-Estradiol-induced interaction of ERα with NPPA regulates gene expression in cardiomyocytes. Cardiovasc. Res. 2012, 96, 411–421. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vaillancourt, P.; Omer, S.; Palfree, R.; Varma, D.R.; Mulay, S. Downregulation of adrenal atrial natriuretic peptide receptor mRNAs and proteins by pregnancy in rats. J. Endocrinol. 1997, 155, 523–530. [Google Scholar] [CrossRef]
- Gutkowska, J.; Jankowski, M.; Sairam, M.R.; Fujio, N.; Reis, A.M.; Mukaddam-Daher, S.; Tremblay, J. Hormonal Regulation of Natriuretic Peptide System during Induced Ovarian Follicular Development in the Rat. Biol. Reprod. 1999, 61, 162–170. [Google Scholar] [CrossRef]
- Reis, A.M.; Jankowski, M.; Mukaddam-Daher, S.; Tremblay, J.; Dam, T.V.; Gutkowska, J. Regulation of the natriuretic peptide system in rat uterus during the estrous cycle. J. Endocrinol. 1997, 153, 345–355. [Google Scholar] [CrossRef]
- Tashjian, A.H.; Yasumura, Y.; Levine, L.; Sato, G.H.; Parker, M.L. Establishment of Clonal Strains of Rat Pituitary Tumor Cells That Secrete Growth Hormone. Endocrinology 1968, 82, 342–352. [Google Scholar] [CrossRef]
- Faivre-Bauman, A.; Gourdji, D.; Grouselle, D.; Tixier-Vidal, A. Binding of thyrotropin releasing hormone and prolactin release by synthronized GH3 rat pituitary cell line. Biochem. Biophys. Res. Commun. 1975, 67, 50–57. [Google Scholar] [CrossRef]
- Hagiwara, H.; Inoue, A.; Yamaguchi, A.; Yokose, S.; Furuya, M.; Tanaka, S.; Hirose, S. cGMP produced in response to ANP and CNP regulates proliferation and differentiation of osteoblastic cells. Am. J. Physiol. Cell Physiol. 1996, 270, C1311–C1318. [Google Scholar] [CrossRef] [PubMed]
- Kaneki, H.; Kurokawa, M.; Ide, H. The receptor attributable to C-type natriuretic peptide-induced differentiation of osteoblasts is switched from type B- to type C-natriuretic peptide receptor with aging. J. Cell. Biochem. 2008, 103, 753–764. [Google Scholar] [CrossRef]
- Staines, K.A.; Madi, K.; Mirczuk, S.M.; Parker, S.; Burleigh, A.; Poulet, B.; Hopkinson, M.; Bodey, A.J.; Fowkes, R.C.; Farquharson, C.; et al. Endochondral Growth Defect and Deployment of Transient Chondrocyte Behaviors Underlie Osteoarthritis Onset in a Natural Murine Model. Arthritis Rheumatol. 2016, 68, 880–891. [Google Scholar] [CrossRef] [PubMed]
- Mirczuk, S.M.; Lessey, A.J.; Catterick, A.R.; Perrett, R.M.; Scudder, C.J.; Read, J.E.; Lipscomb, V.J.; Niessen, S.; Childs, A.J.; McArdle, C.A.; et al. Regulation and Function of C-Type Natriuretic Peptide (CNP) in Gonadotrope-Derived Cell Lines. Cells 2019, 8, 1086. [Google Scholar] [CrossRef] [PubMed]
- Cora, M.C.; Kooistra, L.; Travlos, G. Vaginal Cytology of the Laboratory Rat and Mouse: Review and Criteria for the Staging of the Estrous Cycle Using Stained Vaginal Smears. Toxicol. Pathol. 2015, 43, 776–793. [Google Scholar] [CrossRef] [PubMed]
- McArdle, C.A.; Olcese, J.; Schmidt, C.; Poch, A.; Kratzmeier, M.; Middendorff, R. C-type natriuretic peptide (CNP) in the pituitary: Is CNP an autocrine regulator of gonadotropes? Endocrinology 1994, 135, 2794–2801. [Google Scholar] [CrossRef]
- Komatsu, Y.; Nakao, K.; Suga, S.-I.; Ogawa, Y.; Mukoyama, M.; Arai, H.; Shirakami, G.; Hosoda, K.; Nakagawa, O.; Hama, N.; et al. C-Type Natriuretic Peptide (CNP) in Rats and Humans. Endocrinology 1991, 129, 1104–1106. [Google Scholar] [CrossRef]
- Wilson, M.O.; Barrell, G.; Prickett, T.C.; Espiner, E.A. Molecular forms of C-type natriuretic peptide in cerebrospinal fluid and plasma reflect differential processing in brain and pituitary tissues. Peptides 2018, 99, 223–230. [Google Scholar] [CrossRef]
- Wilson, M.O.; McNeill, B.A.; Barrell, G.K.; Prickett, T.C.R.; Espiner, E.A. Dexamethasone increases production of C-type natriuretic peptide in the sheep brain. J. Endocrinol. 2017, 235, 15–25. [Google Scholar] [CrossRef]
- Hwang, I.T.; Mizuno, Y.; Amano, N.; Lee, H.J.; Shim, Y.S.; Nam, H.-K.; Rhie, Y.-J.; Yang, S.; Lee, K.-H.; Hasegawa, T.; et al. Role of NPR2 mutation in idiopathic short stature: Identification of two novel mutations. Mol. Genet. Genom. Med. 2020, 8, e1146. [Google Scholar] [CrossRef]
- Jonas, K.C.; Melrose, T.; Thompson, I.R.; Baxter, G.F.; Lipscomb, V.J.; Niessen, S.J.; Lawson, C.; McArdle, C.A.; Roberson, M.S.; McGonnell, I.M.; et al. Natriuretic peptide activation of extracellular regulated kinase 1/2 (ERK1/2) pathway by particulate guanylyl cyclases in GH3 somatolactotropes. Cell Tissue Res. 2017, 369, 567–578. [Google Scholar] [CrossRef] [PubMed]
- John, S.W.; Krege, J.H.; Oliver, P.M.; Hagaman, J.R.; Hodgin, J.B.; Pang, S.C.; Flynn, T.G.; Smithies, O. Genetic decreases in atrial natriuretic peptide and salt-sensitive hypertension. Science 1995, 267, 679–681. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.J.; Wong, S.K.-F.; Kishimoto, I.; Dubois, S.; Mach, V.; Friesen, J.; Garbers, D.L.; Beuve, A. Salt-resistant hypertension in mice lacking the guanylyl cyclase-A receptor for atrial natriuretic peptide. Nat. Cell Biol. 1995, 378, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Kenny, P.; Scudder, C.; Keyte, S.; Swann, J.; Fowkes, R.; Church, D.; Forcada, Y.; Niessen, S. Treatment of Feline Hypersomatotropism—Efficacy, morbidity and mortality of hypophysectomy. In Proceedings of the ACVIM Forum Research Reports Program, Indianapolis, IN, USA, 3–6 June 2015. [Google Scholar]
- Jankowski, M.; Reis, A.M.; Mukaddam-Daher, S.; Dam, T.-V.; Farookhi, R.; Gutkowska, J. C-Type Natriuretic Peptide and the Guanylyl Cyclase Receptors in the Rat Ovary are Modulated by the Estrous Cycle1. Biol. Reprod. 1997, 56, 59–66. [Google Scholar] [CrossRef]
- De Cesaro, M.; Dos Santos, J.; Ferst, J.; Nóbrega, J.; Rosa, P.; Rovani, M.; Ilha, G.; Bohrer, R.; Ferreira, R.; Gasperin, B.G.; et al. Natriuretic peptide system regulation in granulosa cells during follicle deviation and ovulation in cattle. Reprod. Domest. Anim. 2018, 53, 710–717. [Google Scholar] [CrossRef]
- Ohta, S.; Shimekake, Y.; Nagata, K. Cell-type-specific function of the C-type natriuretic peptide gene promoter in rat anterior pituitary-derived cultured cell lines. Mol. Cell. Biol. 1993, 13, 4077–4086. [Google Scholar] [CrossRef]
- Gonzalez, G.A.; Yamamoto, K.K.; Fischer, W.H.; Karr, D.; Menzel, P.; Biggs, W., III; Vale, W.W.; Montminy, M.R. A cluster of phosphorylation sites on the cyclic AMP-regulated nuclear factor CREB predicted by its sequence. Nature 1989, 337, 749–752. [Google Scholar] [CrossRef]
- Xing, J.; Ginty, D.D.; Greenberg, M.E. Coupling of the RAS-MAPK Pathway to Gene Activation by RSK2, a Growth Factor-Regulated CREB Kinase. Science 1996, 273, 959–963. [Google Scholar] [CrossRef]
- West, A.; Dupré, S.M.; Yu, L.; Paton, I.R.; Miedzinska, K.; McNeilly, A.S.; Davis, J.; Burt, D.W.; Loudon, A.S.I. Npas4 is Activated by Melatonin, and Drives the Clock Gene Cry1 in the Ovine Pars Tuberalis. Mol. Endocrinol. 2013, 27, 979–989. [Google Scholar] [CrossRef]
- Sellitti, D.F.; Puggina, E.F.; Lagranha, C.; Doi, S.Q. cAMP inhibits natriuretic peptide receptor-B activity and increases C-type natriuretic peptide in FRTL-5 rat thyroid cells. J. Endocrinol. 2004, 180, 23–34. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Maurer, R.A. A Role for the Mitogen-Activated Protein Kinase in Mediating the Ability of Thyrotropin-Releasing Hormone to Stimulate the Prolactin Promoter. Mol. Endocrinol. 1999, 13, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- Garner, K.L.; Voliotis, M.; Alobaid, H.M.S.; Perrett, R.M.; Pham, T.; Tsaneva-Atanasova, K.; McArdle, C.A. Information Transfer via Gonadotropin-Releasing Hormone Receptors to ERK and NFAT: Sensing GnRH and Sensing Dynamics. J. Endocr. Soc. 2017, 1, 260–277. [Google Scholar] [CrossRef] [PubMed]
- Caunt, C.J.; Finch, A.R.; Sedgley, K.R.; McArdle, C.A. GnRH receptor signalling to ERK: Kinetics and compartmentalization. Trends Endocrinol. Metab. 2006, 17, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Roh, J. LH-induced Transcriptional Regulation of Klf4 Expression in Granulosa Cells Occurs via the cAMP/PKA Pathway and Requires a Putative Sp1 Binding Site. Int. J. Mol. Sci. 2020, 21, 7385. [Google Scholar] [CrossRef]
- Anjali, G.; Kaur, S.; Lakra, R.; Taneja, J.; Kalsey, G.S.; Nagendra, A.; Shrivastav, T.; Devi, M.G.; Malhotra, N.; Kriplani, A.; et al. FSH stimulates IRS-2 expression in human granulosa cells through cAMP/SP1, an inoperative FSH action in PCOS patients. Cell. Signal. 2015, 27, 2452–2466. [Google Scholar] [CrossRef]
- Liang, F.; Schaufele, F.; Gardner, D.G. Sp1 Dependence of Natriuretic Peptide Receptor A Gene Transcription in Rat Aortic Smooth Muscle Cells. Endocrinology 1999, 140, 1695–1701. [Google Scholar] [CrossRef]
- Thiriet, N.; Esteve, L.; Aunis, D.; Zwiller, J. Immediate early gene induction by natriuretic peptides in PC12 phaeochromocytoma and C6 glioma cells. Neuroreport 1997, 8, 399–402. [Google Scholar] [CrossRef]
- Broderick, K.E.; Zhang, T.; Rangaswami, H.; Zeng, Y.; Zhao, X.; Boss, G.R.; Pilz, R.B. Guanosine 3′,5′-Cyclic Monophosphate (cGMP)/cGMP-Dependent Protein Kinase Induce Interleukin-6 Transcription in Osteoblasts. Mol. Endocrinol. 2007, 21, 1148–1162. [Google Scholar] [CrossRef][Green Version]
- Yan, M.; Jones, M.E.E.; Hernandez, M.; Liu, D.; Simpson, E.R.; Chen, C. Functional Modification of Pituitary Somatotropes in the Aromatase Knockout Mouse and the Effect of Estrogen Replacement. Endocrinology 2004, 145, 604–612. [Google Scholar] [CrossRef]
- Gahete, M.D.; Córdoba-Chacón, J.; Lin, Q.; Brüning, J.C.; Kahn, C.R.; Castaño, J.P.; Christian, H.; Luque, R.M.; Kineman, R.D. Insulin and IGF-I Inhibit GH Synthesis and Release in Vitro and in Vivo by Separate Mechanisms. Endocrinology 2013, 154, 2410–2420. [Google Scholar] [CrossRef]
- Childs, G.V.; Akhter, N.; Haney, A.; Syed, M.; Odle, A.; Cozart, M.; Brodrick, Z.; Gaddy, D.; Suva, L.J.; Akel, N.; et al. The Somatotrope as a Metabolic Sensor: Deletion of Leptin Receptors Causes Obesity. Endocrinology 2011, 152, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Akhter, N.; Odle, A.K.; Allensworth-James, M.L.; Haney, A.C.; Syed, M.M.; Cozart, M.A.; Chua, S.; Kineman, R.; Childs, G.V. Ablation of Leptin Signaling to Somatotropes: Changes in Metabolic Factors that Cause Obesity. Endocrinology 2012, 153, 4705–4715. [Google Scholar] [CrossRef]
- Zaccolo, M.; Movsesian, M.A. cAMP and cGMP Signaling Cross-Talk. Circ. Res. 2007, 100, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Pilz, R.B.; Casteel, D.E. Regulation of Gene Expression by Cyclic GMP. Circ. Res. 2003, 93, 1034–1046. [Google Scholar] [CrossRef]
- Renate, B.P. Role of cyclic GMP in gene regulation. Front. Biosci. 2005, 10, 1239–1268. [Google Scholar] [CrossRef]
- Shaulian, E.; Karin, M. AP-1 as a regulator of cell life and death. Nat. Cell Biol. 2002, 4, E131–E136. [Google Scholar] [CrossRef]
- Legeai-Mallet, L. C-Type Natriuretic Peptide Analog as Therapy for Achondroplasia. Adv. Ther. Pediatr. Endocrinol. Diabetol. 2015, 30, 98–105. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirczuk, S.M.; Scudder, C.J.; Read, J.E.; Crossley, V.J.; Regan, J.T.; Richardson, K.M.; Simbi, B.; McArdle, C.A.; Church, D.B.; Fenn, J.; et al. Natriuretic Peptide Expression and Function in GH3 Somatolactotropes and Feline Somatotrope Pituitary Tumours. Int. J. Mol. Sci. 2021, 22, 1076. https://doi.org/10.3390/ijms22031076
Mirczuk SM, Scudder CJ, Read JE, Crossley VJ, Regan JT, Richardson KM, Simbi B, McArdle CA, Church DB, Fenn J, et al. Natriuretic Peptide Expression and Function in GH3 Somatolactotropes and Feline Somatotrope Pituitary Tumours. International Journal of Molecular Sciences. 2021; 22(3):1076. https://doi.org/10.3390/ijms22031076
Chicago/Turabian StyleMirczuk, Samantha M., Christopher J. Scudder, Jordan E. Read, Victoria J. Crossley, Jacob T. Regan, Karen M. Richardson, Bigboy Simbi, Craig A. McArdle, David B. Church, Joseph Fenn, and et al. 2021. "Natriuretic Peptide Expression and Function in GH3 Somatolactotropes and Feline Somatotrope Pituitary Tumours" International Journal of Molecular Sciences 22, no. 3: 1076. https://doi.org/10.3390/ijms22031076
APA StyleMirczuk, S. M., Scudder, C. J., Read, J. E., Crossley, V. J., Regan, J. T., Richardson, K. M., Simbi, B., McArdle, C. A., Church, D. B., Fenn, J., Kenny, P. J., Volk, H. A., Wheeler-Jones, C. P., Korbonits, M., Niessen, S. J., McGonnell, I. M., & Fowkes, R. C. (2021). Natriuretic Peptide Expression and Function in GH3 Somatolactotropes and Feline Somatotrope Pituitary Tumours. International Journal of Molecular Sciences, 22(3), 1076. https://doi.org/10.3390/ijms22031076





