CdSe/ZnS Core-Shell-Type Quantum Dot Nanoparticles Disrupt the Cellular Homeostasis in Cellular Blood–Brain Barrier Models
Abstract
1. Introduction
2. Results
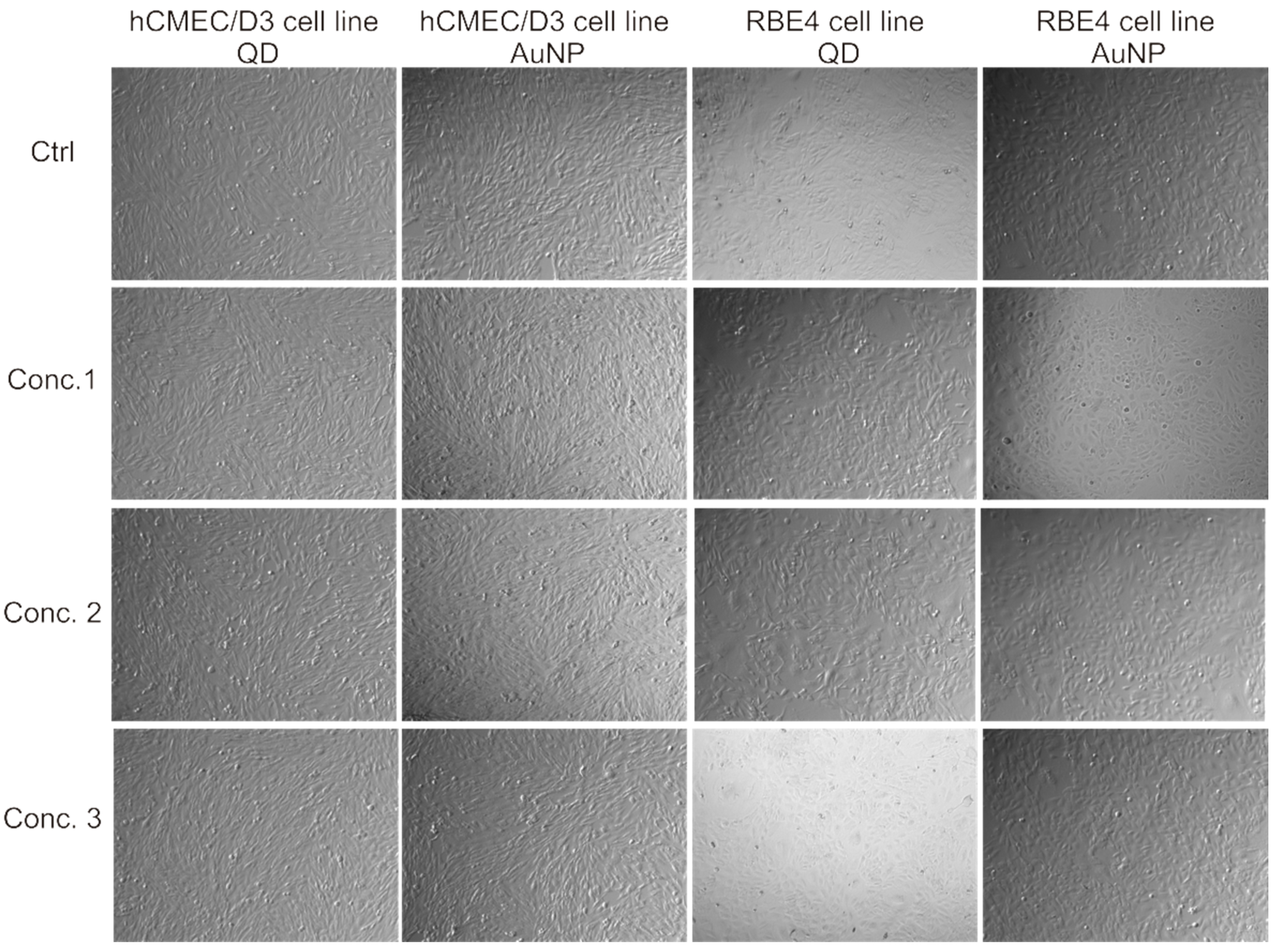
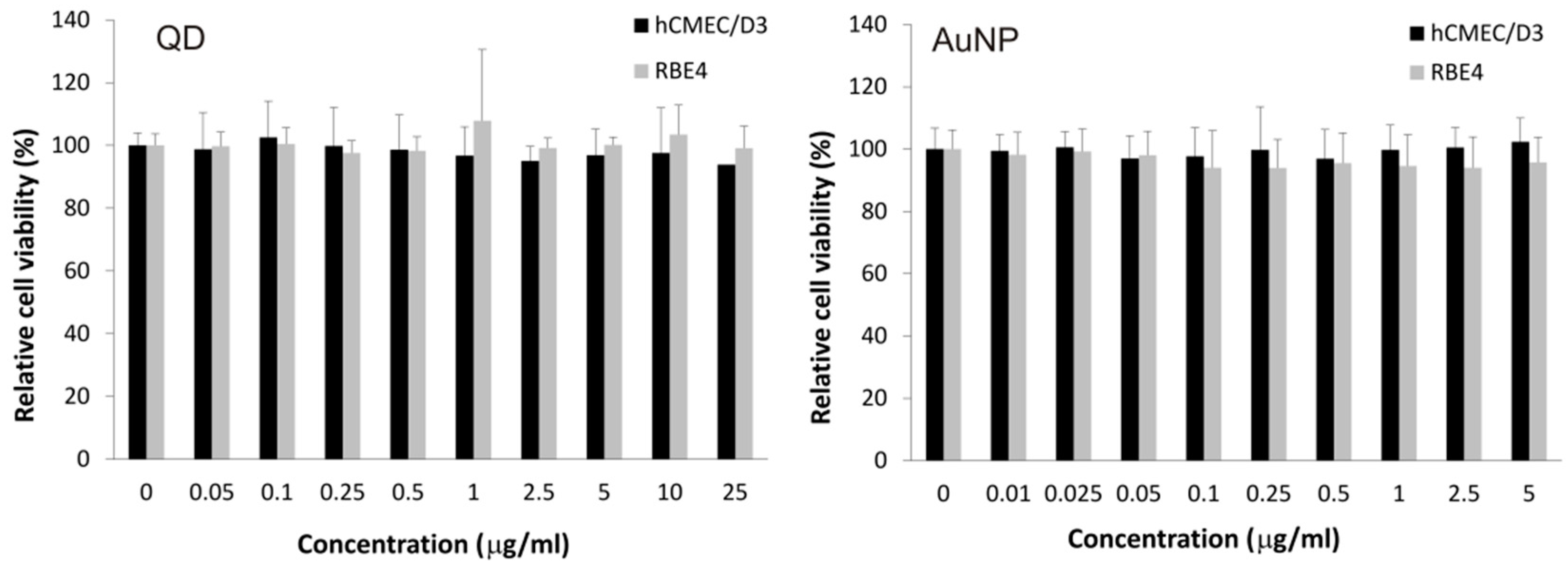
2.1. Assessing Toxicity of Nanoparticles on hCMEC/D3 Cells
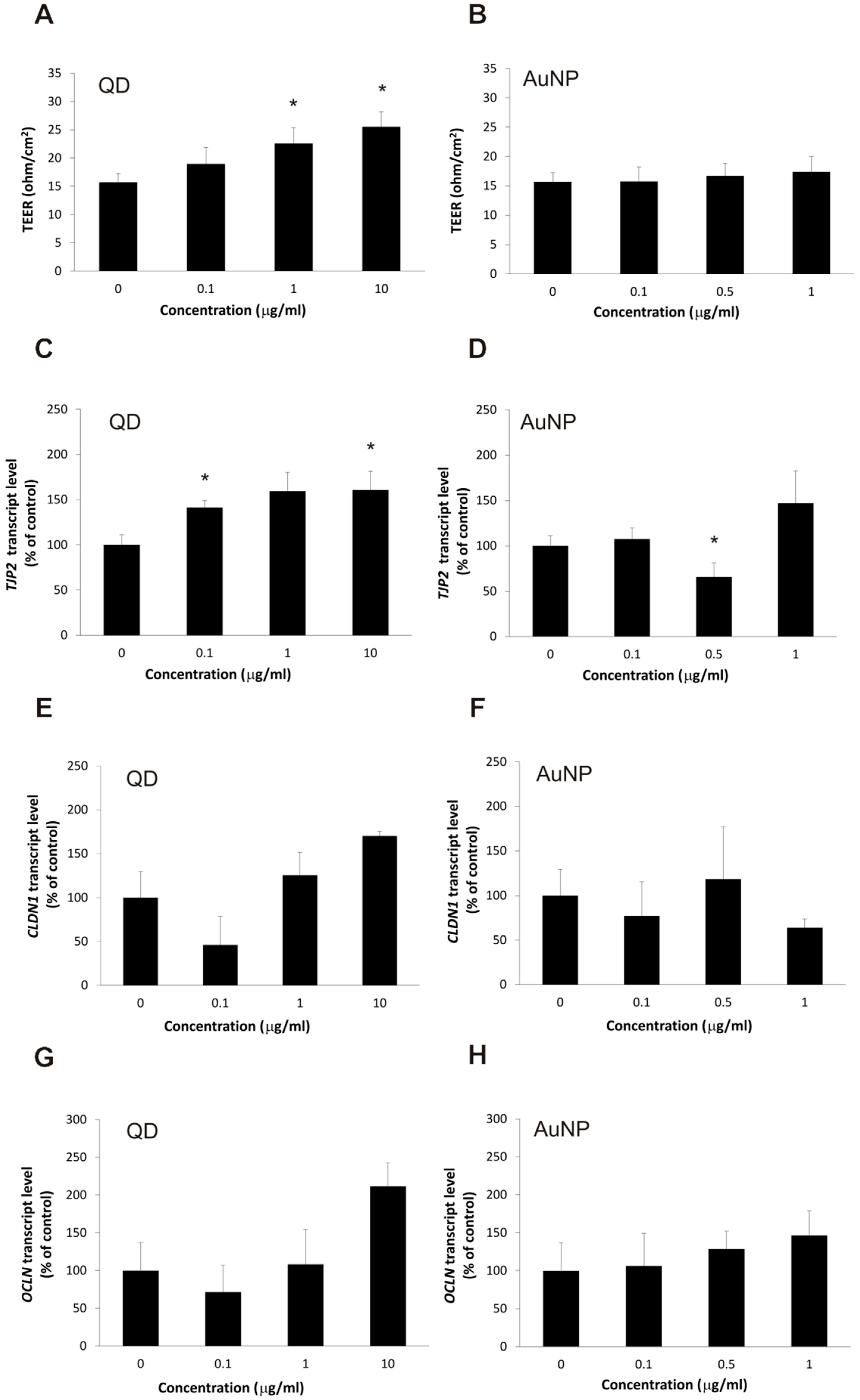
2.2. The Impact of the Nanoparticles on Barrier Phenotype Properties in Brain Microvascular Endothelial Cells
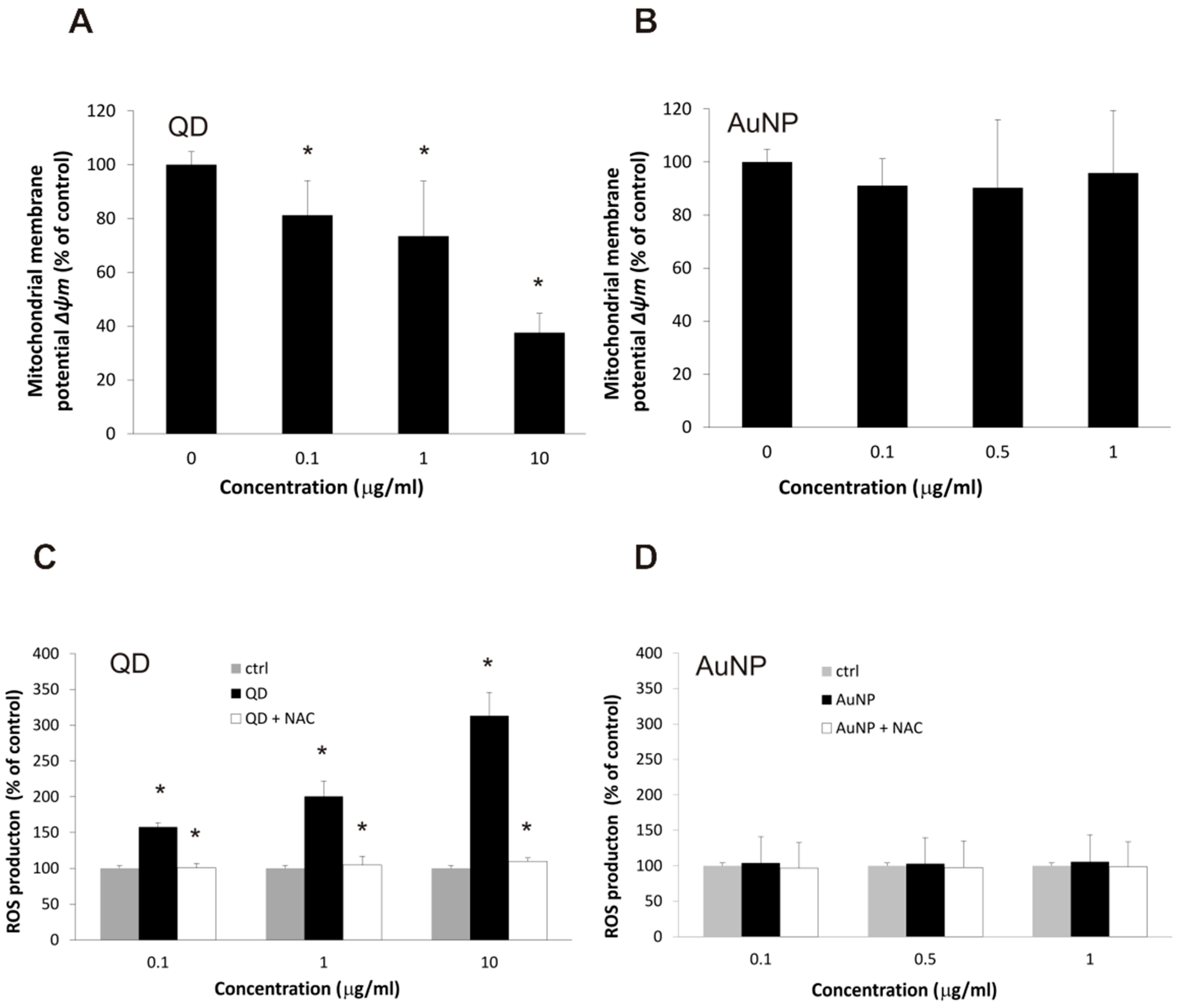
2.3. Changes in Cellular Respiration and Redox Homeostasis Caused by Nanoparticles
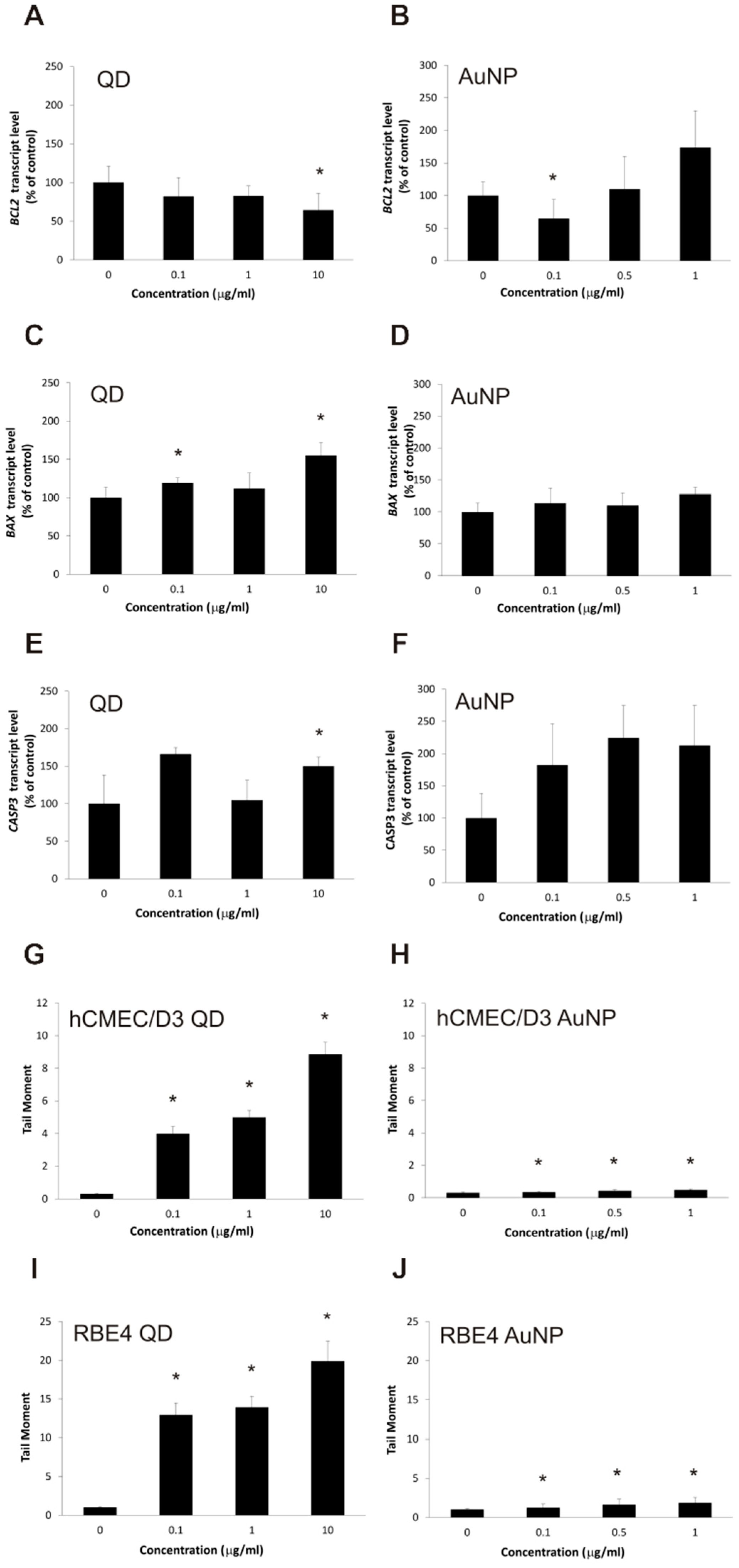
2.4. Apoptotic Changes in Brain Microvascular Endothelial Cells after Exposure to Nanoparticles
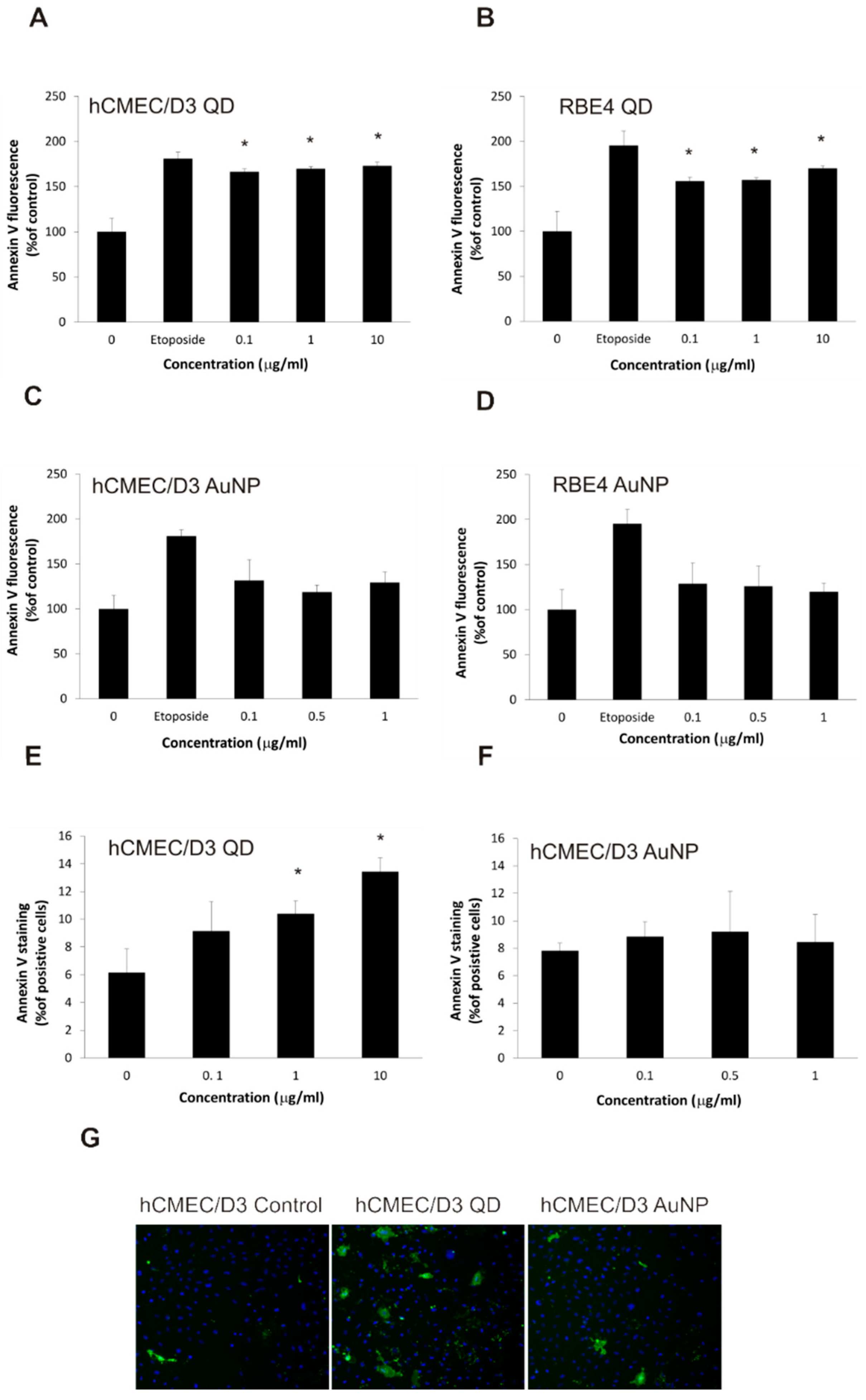
2.5. Nanoparticles Cause Changes in Plasma Membrane Asymmetry Related to Apoptosis
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture
4.3. Cell Treatment
4.4. Cytotoxicity Assay
4.5. TEER Measurement
4.6. Quantitative Real-Time RT-PCR
4.7. Estimation of Mitochondrial Membrane Potential (Δψm)
4.8. Determination of Reactive Oxygen Species Production
4.9. Comet Assay
4.10. Annexin V Binding Assay
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| BAX | BCL2 Associated X |
| BCL2 | B-cell lymphoma 2 |
| DKK1 | Dickkopf-related protein 1 |
| DKK3 | Dickkopf-related protein 3 |
| DMSO | Dimethyl Sulfoxide |
| EDTA | Ethylenediaminetetraacetic acid |
| HBSS | Hank’s balanced salt solution |
| hTERT | Human telomerase reverse transcriptase |
| LRP5 | LDL Receptor Related Protein 5 |
| LRP6 | LDL Receptor Related Protein 6 |
| MEM | Minimum Essential Medium |
| PBS | Phosphate buffered saline |
| TJP2 | Tight Junction Protein 2 |
References
- Daneman, R. The blood-brain barrier in health and disease. Ann. Neurol. 2012, 72, 648–672. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V. The Blood-Brain Barrier in Health and Chronic Neurodegenerative Disorders. Neuron 2008, 57, 178–201. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Prat, A. The Blood–Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef] [PubMed]
- Selvan, S.T.; Padmanabhan, P.; Gulyás, B.Z. Nanotechnology-Based Diagnostics and Therapy for Pathogen-Related Infections in the CNS. ACS Chem. Neurosci. 2019, 11, 2371–2377. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.; Martin, U.; Byrne, M. Recent Advances in Delivery through the Blood-Brain Barrier. Curr. Top. Med. Chem. 2014, 14, 1148–1160. [Google Scholar] [CrossRef] [PubMed]
- Logothetidis, S. Nanostructured Materials and Their Applications; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Singh, A.V.; Laux, P.; Luch, A.; Sudrik, C.; Wiehr, S.; Wild, A.-M.; Santomauro, G.; Bill, J.; Sitti, M. Review of emerging concepts in nanotoxicology: Opportunities and challenges for safer nanomaterial design. Toxicol. Mech. Methods 2019, 29, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Bishop, B.P.; Thompson, N.L.; Hildahl, K.; Dang, B.; Mironchuk, O.; Chen, N.; Aoki, R.; Holmberg, V.C.; Nance, E. Quantum dot cellular uptake and toxicity in the developing brain: Implications for use as imaging probes. Nanoscale Adv. 2019, 1, 3424–3442. [Google Scholar] [CrossRef]
- Orlando, A.; Colombo, M.; Prosperi, D.; Corsi, F.; Panariti, A.; Rivolta, I.; Masserini, M.; Cazzaniga, E. Evaluation of gold nanoparticles biocompatibility: A multiparametric study on cultured endothelial cells and macrophages. J. Nanoparticle Res. 2016, 18. [Google Scholar] [CrossRef]
- Simkó, M.; Mattsson, M.-O. Interactions between Nanosized Materials and the Brain. Curr. Med. Chem. 2014, 21, 4200–4214. [Google Scholar] [CrossRef]
- Ceña, V.; Játiva, P. Nanoparticle crossing of blood–brain barrier: A road to new therapeutic approaches to central nervous system diseases. Nanomedicine 2018, 13, 1513–1516. [Google Scholar] [CrossRef]
- Yu, Q.-J.; Li, M.-C.; Tao, H.; Wang, X. Targeting brain microvascular endothelial cells: A therapeutic approach to neuroprotection against stroke. Neural Regen. Res. 2015, 10, 1882–1891. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, C.; Cavalli, R.; Panciani, P.P.; Battaglia, L. Overcoming the Blood–Brain Barrier: Successes and Challenges in Developing Nanoparticle-Mediated Drug Delivery Systems for the Treatment of Brain Tumours. Int. J. Nanomed. 2020, 15, 2999–3022. [Google Scholar] [CrossRef] [PubMed]
- Engin, A.B.; Engin, A. Nanoparticles and neurotoxicity: Dual response of glutamatergic receptors. Sleep Deprivation Cogn. 2019, 245, 281–303. [Google Scholar]
- Sadowska-Bartosz, I.; Bartosz, G. Redox nanoparticles: Synthesis, properties and perspectives of use for treatment of neuro-degenerative diseases. J. Nanobiotechnol. 2018, 16, 87. [Google Scholar] [CrossRef]
- Chen, I.-C.; Hsiao, I.-L.; Lin, H.-C.; Wu, C.-H.; Chuang, C.-Y.; Huang, Y.-J. Influence of silver and titanium dioxide nanoparticles on in vitro blood-brain barrier permeability. Environ. Toxicol. Pharmacol. 2016, 47, 108–118. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, C.; Chang, Q.; Deng, Q.; Yang, X.; Wu, Y. Study of the neurotoxicity of indoor airborne nanoparticles based on a 3D human blood-brain barrier chip. Environ. Int. 2020, 143, 105598. [Google Scholar] [CrossRef]
- Weksler, B.; Subileau, E.A.; Perrière, N.; Charneau, P.; Holloway, K.; Leveque, M.; Tricoire-Leignel, H.; Nicotra, A.; Bourdoulous, S.; Turowski, P.; et al. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005, 19, 1872–1874. [Google Scholar] [CrossRef]
- Weksler, B.; Romero, I.A.; Couraud, P.-O. The hCMEC/D3 cell line as a model of the human blood brain barrier. Fluids Barriers CNS 2013, 10, 16. [Google Scholar] [CrossRef]
- Kania, K.D.; Wijesuriya, H.C.; Hladky, S.B.; Barrand, M.A. Beta amyloid effects on expression of multidrug efflux transporters in brain endothelial cells. Brain Res. 2011, 1418, 1–11. [Google Scholar] [CrossRef]
- Pichla, M.; Pulaski, Ł.; Kania, K.D.; Stefaniuk, I.; Cieniek, B.; Pieńkowska, N.; Bartosz, G.; Sadowska-Bartosz, I. Nitroxide Radical-Containing Redox Nanoparticles Protect Neuroblastoma SH-SY5Y Cells against 6-Hydroxydopamine Toxicity. Oxidative Med. Cell. Longev. 2020, 2020, 9260748. [Google Scholar] [CrossRef]
- Chen, D.; Monteiro-Riviere, N.A.; Zhang, L.W. Intracellular imaging of quantum dots, gold, and iron oxide nanoparticles with associated endocytic pathways. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 9, e1419. [Google Scholar] [CrossRef] [PubMed]
- Bejgum, B.C.; Donovan, M.D. Uptake and Transport of Ultrafine Nanoparticles (Quantum Dots) in the Nasal Mucosa. Mol. Pharm. 2021, 18, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, B.; Xu, F.; Wang, X.; Liu, G.; Zheng, L.; Zhao, J.; Zhang, X. RETRACTED: Functional quantum dot-siRNA nanoplexes to regulate chondrogenic differentiation of mesenchymal stem cells. Acta Biomater. 2016, 46, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, Q.; Cui, M.; Pang, S.; Wang, J.; Liu, Y.; Xie, L. Probing Temperature- and pH-Dependent Binding between Quantum Dots and Bovine Serum Albumin by Fluorescence Correlation Spectroscopy. Nanomaterials 2017, 7, 93. [Google Scholar] [CrossRef]
- Liao, C.; Jin, Y.; Li, Y.; Tjong, S.C. Interactions of Zinc Oxide Nanostructures with Mammalian Cells: Cytotoxicity and Photocatalytic Toxicity. Int. J. Mol. Sci. 2020, 21, 6305. [Google Scholar] [CrossRef]
- Liu, J.; Katahara, J.; Li, G.; Coe-Sullivan, S.; Hurt, R.H. Degradation Products from Consumer Nanocomposites: A Case Study on Quantum Dot Lighting. Environ. Sci. Technol. 2012, 46, 3220–3227. [Google Scholar] [CrossRef]
- Mo, D.; Hu, L.; Zeng, G.; Chen, G.; Wan, J.; Yu, Z.; Huang, Z.; He, K.; Zhang, C.; Cheng, M. Cadmium-containing quantum dots: Properties, applications, and toxicity. Appl. Microbiol. Biotechnol. 2017, 101, 2713–2733. [Google Scholar] [CrossRef]
- Atha, D.H.; Nagy, A.; Steinbrück, A.; Dennis, A.; Hollingsworth, J.A.; Dua, V.; Iyer, R.; Nelson, B.C. Quantifying engineered nanomaterial toxicity: Comparison of common cytotoxicity and gene expression measurements. J. Nanobiotechnol. 2017, 15, 79. [Google Scholar] [CrossRef]
- Wang, J.; Liu, R.; Liuac, B. Cadmium-containing Quantum Dots: Current Perspectives on Their Application as Nanomedicine and Toxicity Concerns. Mini Rev. Med. Chem. 2016, 16, 905–916. [Google Scholar] [CrossRef]
- Nikazar, S.; Sivasankarapillai, V.S.; Rahdar, A.; Gasmi, S.; Anumol, P.S.; Shanavas, M.S. Revisiting the cytotoxicity of quantum dots: An in-depth overview. Biophys. Rev. 2020, 12, 703–718. [Google Scholar] [CrossRef]
- Yan, M.; Zhang, Y.; Xu, K.; Fu, T.; Qin, H.; Zheng, X. An in vitro study of vascular endothelial toxicity of CdTe quantum dots. Toxicol. 2011, 282, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, Y.; Kong, L.; Xue, Y.; Tang, M. Threshold Dose of Three Types of Quantum Dots (QDs) Induces Oxidative Stress Triggers DNA Damage and Apoptosis in Mouse Fibroblast L929 Cells. Int. J. Environ. Res. Public Health 2015, 12, 13435–13454. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.C.; Willmore, W.G.; Tayabali, A.F. Cadmium telluride quantum dots cause oxidative stress leading to extrinsic and intrinsic apoptosis in hepatocellular carcinoma HepG2 cells. Toxicology 2013, 306, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.; Li, Y.-P.; Mei, P.; Chen, W.; Jiang, F.-L.; Li, X. Size Effects on the Interaction of QDs with the Mitochondrial Membrane In Vitro. J. Membr. Biol. 2016, 249, 757–767. [Google Scholar] [CrossRef]
- Hao, M.; Liu, R. Molecular mechanism of CAT and SOD activity change under MPA-CdTe quantum dots induced oxidative stress in the mouse primary hepatocytes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 220, 117104. [Google Scholar] [CrossRef]
- Katubi, K.M.; Alzahrani, F.M.; Ali, D.; Alarifi, S. Dose- and duration-dependent cytotoxicity and genotoxicity in human hepato carcinoma cells due to CdTe QDs exposure. Hum. Exp. Toxicol. 2019, 38, 914–926. [Google Scholar] [CrossRef]
- Liang, X.; Wu, T.; Wang, Y.; Wei, T.; Zou, L.; Bai, C.; Liu, N.; Zhang, T.; Xue, Y.; Tang, M. CdTe and CdTe@ZnS quantum dots induce IL-1ß-mediated inflammation and pyroptosis in microglia. Toxicol. Vitr. 2020, 65, 104827. [Google Scholar] [CrossRef]
- Larner, S.F.; Wang, J.; Goodman, J.; Altman, M.B.O.; Xin, M.; Wang, K.K.W. In Vitro Neurotoxicity Resulting from Exposure of Cultured Neural Cells to Several Types of Nanoparticles. J. Cell Death 2017, 10. [Google Scholar] [CrossRef]
- Li, H.; Li, M.; Shih, W.Y.; Lelkes, P.I.; Shih, W.-H. Cytotoxicity Tests of Water Soluble ZnS and CdS Quantum Dots. J. Nanosci. Nanotechnol. 2011, 11, 3543–3551. [Google Scholar] [CrossRef]
- Panzarini, E.; Mariano, S.; Carata, E.; Mura, F.; Rossi, M.; Dini, L. Intracellular Transport of Silver and Gold Nanoparticles and Biological Responses: An Update. Int. J. Mol. Sci. 2018, 19, 1305. [Google Scholar] [CrossRef]
- Norouzi, M. Gold Nanoparticles in Glioma Theranostics. Pharmacol. Res. 2020, 156, 104753. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Du, Y. Cadmium and Its Neurotoxic Effects. Oxidative Med. Cell. Longev. 2013, 2013, 898034. [Google Scholar] [CrossRef] [PubMed]
- Mezzaroba, L.; Alfieri, D.F.; Simão, A.N.C.; Reiche, E.M.V. The role of zinc, copper, manganese and iron in neurodegenerative diseases. NeuroToxicology 2019, 74, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Xu, Y.-M.; Wu, D.-D.; Yao, Y.; Liang, Z.-L.; Tan, H.W.; Lau, A.T.Y. Acute and chronic cadmium telluride quantum dots-exposed human bronchial epithelial cells: The effects of particle sizes on their cytotoxicity and carcinogenicity. Biochem. Biophys. Res. Commun. 2018, 495, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Lamson, N.G.; Berger, A.; Fein, K.C.; Whitehead, K.A. A ionic particles inable the oral delivery of proteins by enhancing intes-tinal permeability. Nat. Biomed. Eng. 2020, 4, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Bell, I.R.; Ives, J.A.; Wayne, B.J. Nonlinear Effects of Nanoparticles: Biological Variability from Hormetic Doses, Small Particle Sizes, and Dynamic Adaptive Interactions. Dose Response 2013, 12, 202–232. [Google Scholar] [CrossRef]
- Liu, C.; Wu, J.; Zou, M.-H. Activation of AMP-activated protein kinase alleviates High-glucose-induced dysfunction of brain microvascular endothelial cell tight-junction dynamics. Free. Radic. Biol. Med. 2012, 53, 1213–1221. [Google Scholar] [CrossRef]
- Zhao, Z.; Hu, J.; Gao, X.; Liang, H.; Liu, Z. Activation of AMPK attenuates lipopolysaccharide-impaired integrity and function of blood–brain barrier in human brain microvascular endothelial cells. Exp. Mol. Pathol. 2014, 97, 386–392. [Google Scholar] [CrossRef]
- Song, J.; Choi, S.-M.; Whitcomb, D.J.; Kim, B.C. Adiponectin controls the apoptosis and the expression of tight junction proteins in brain endothelial cells through AdipoR1 under beta amyloid toxicity. Cell Death Dis. 2017, 8, e3102. [Google Scholar] [CrossRef]
- Roux, F.; Durieu-Trautmann, O.; Chaverot, N.; Claire, M.; Mailly, P.; Bourre, J.-M.; Strosberg, A.D.; Couraud, P.-O. Regulation of gamma-glutamyl transpeptidase and alkaline phosphatase activities in immortalized rat brain microvessel endothelial cells. J. Cell. Physiol. 1994, 159, 101–113. [Google Scholar] [CrossRef]
- Tai, L.M.; Reddy, P.S.; Lopez-Ramirez, M.A.; Davies, H.A.; Male, A.D.K.; Loughlin, A.J.; Romero, I.A. Polarized P-glycoprotein expression by the immortalised human brain endothelial cell line, hCMEC/D3, restricts apical-to-basolateral permeability to rhodamine 123. Brain Res. 2009, 1292, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Szwed, M.; Kania, K.D.; Jozwiak, Z. Relationship between therapeutic efficacy of doxorubicin-transferrin conjugate and expression of P-glycoprotein in chronic erythromyeloblastoid leukemia cells sensitive and resistant to doxorubicin. Cell. Oncol. 2014, 37, 421–428. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Szwed, M.; Kania, K.D.; Jóźwiak, Z. Changes in the activity of antioxidant barrier after treatment of K562 and CCRF-CEM cell lines with doxorubicin–transferrin conjugate. Biochimie 2014, 107, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Szwed, M.; Kania, K.D.; Jóźwiak, Z. Assessment of pro-apoptotic activity of doxorubicin–transferrin conjugate in cells derived from human solid tumors. Int. J. Biochem. Cell Biol. 2016, 70, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Końca, K.; Lankoff, A.; Banasik, A.; Lisowska, H.; Kuszewski, T.; Góźdź, S.; Koza, Z.; Wojcik, A. A cross-platform public domain PC image-analysis program for the comet assay. Mutat. Res. Toxicol. Environ. Mutagen. 2003, 534, 15–20. [Google Scholar] [CrossRef]
- Kania, K.; Matławska-Wąsowska, K.; Osiecka, R.; Jóźwiak, Z. Analysis of aclarubicin-induced cell death in human fibroblasts. Cell Biol. Int. 2007, 31, 1049–1056. [Google Scholar] [CrossRef]
- Wagner, W.; Kania, K.D.; Blauz, A.; Ciszewski, W.M. The lactate receptor (HCAR1/GPR81) contributes to doxorubicin chemo-resistance via ABCB1 transporter up-regulation in human cervical cancer HeLa cells. J. Physiol. Pharmacol. 2017, 68, 555–564. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kania, K.D.; Wagner, W.; Pułaski, Ł. CdSe/ZnS Core-Shell-Type Quantum Dot Nanoparticles Disrupt the Cellular Homeostasis in Cellular Blood–Brain Barrier Models. Int. J. Mol. Sci. 2021, 22, 1068. https://doi.org/10.3390/ijms22031068
Kania KD, Wagner W, Pułaski Ł. CdSe/ZnS Core-Shell-Type Quantum Dot Nanoparticles Disrupt the Cellular Homeostasis in Cellular Blood–Brain Barrier Models. International Journal of Molecular Sciences. 2021; 22(3):1068. https://doi.org/10.3390/ijms22031068
Chicago/Turabian StyleKania, Katarzyna Dominika, Waldemar Wagner, and Łukasz Pułaski. 2021. "CdSe/ZnS Core-Shell-Type Quantum Dot Nanoparticles Disrupt the Cellular Homeostasis in Cellular Blood–Brain Barrier Models" International Journal of Molecular Sciences 22, no. 3: 1068. https://doi.org/10.3390/ijms22031068
APA StyleKania, K. D., Wagner, W., & Pułaski, Ł. (2021). CdSe/ZnS Core-Shell-Type Quantum Dot Nanoparticles Disrupt the Cellular Homeostasis in Cellular Blood–Brain Barrier Models. International Journal of Molecular Sciences, 22(3), 1068. https://doi.org/10.3390/ijms22031068





