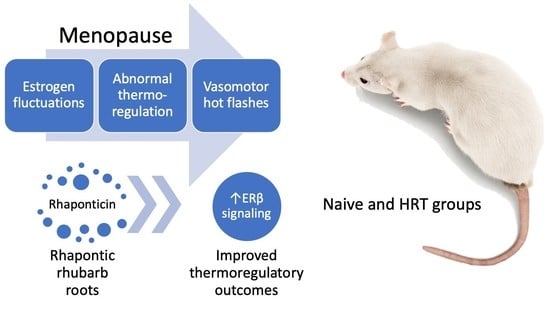
Rheum rhaponticum Root Extract Improves Vasomotor Menopausal Symptoms and Estrogen-Regulated Targets in Ovariectomized Rat Model
Abstract
1. Introduction
2. Results
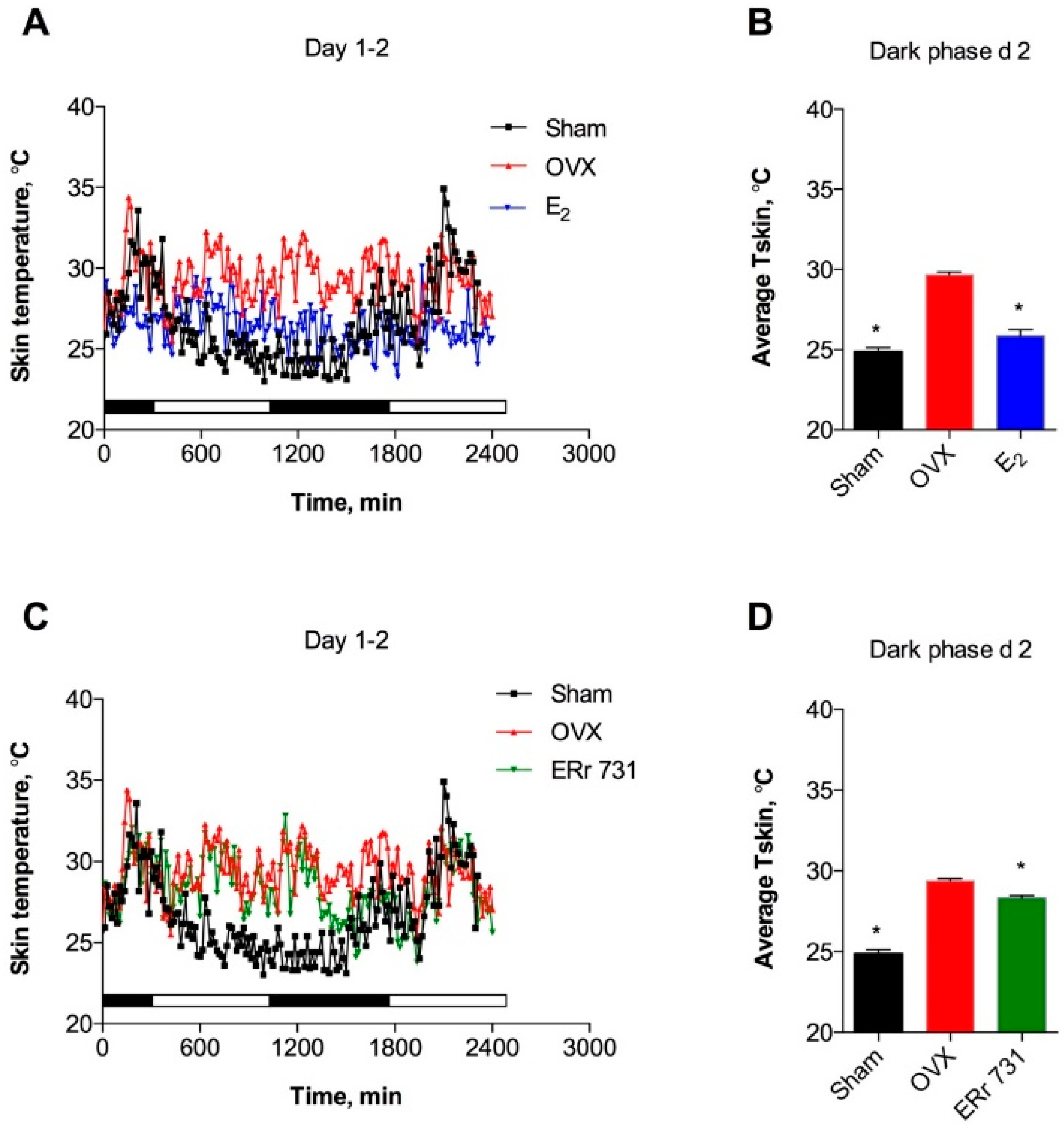
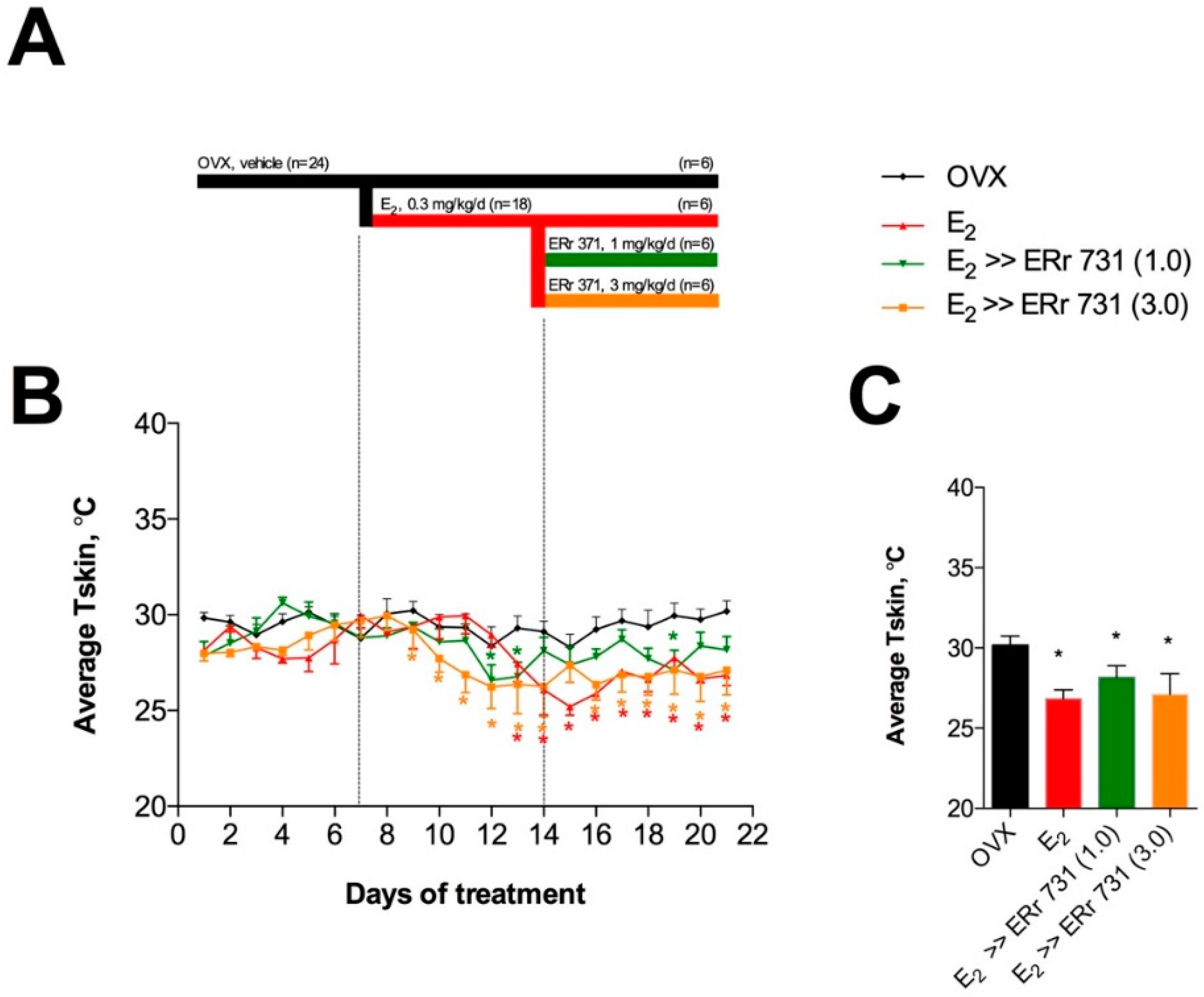
2.1. ERr 731 and E2 Treatments Lower Tskin in OVX Rat Model
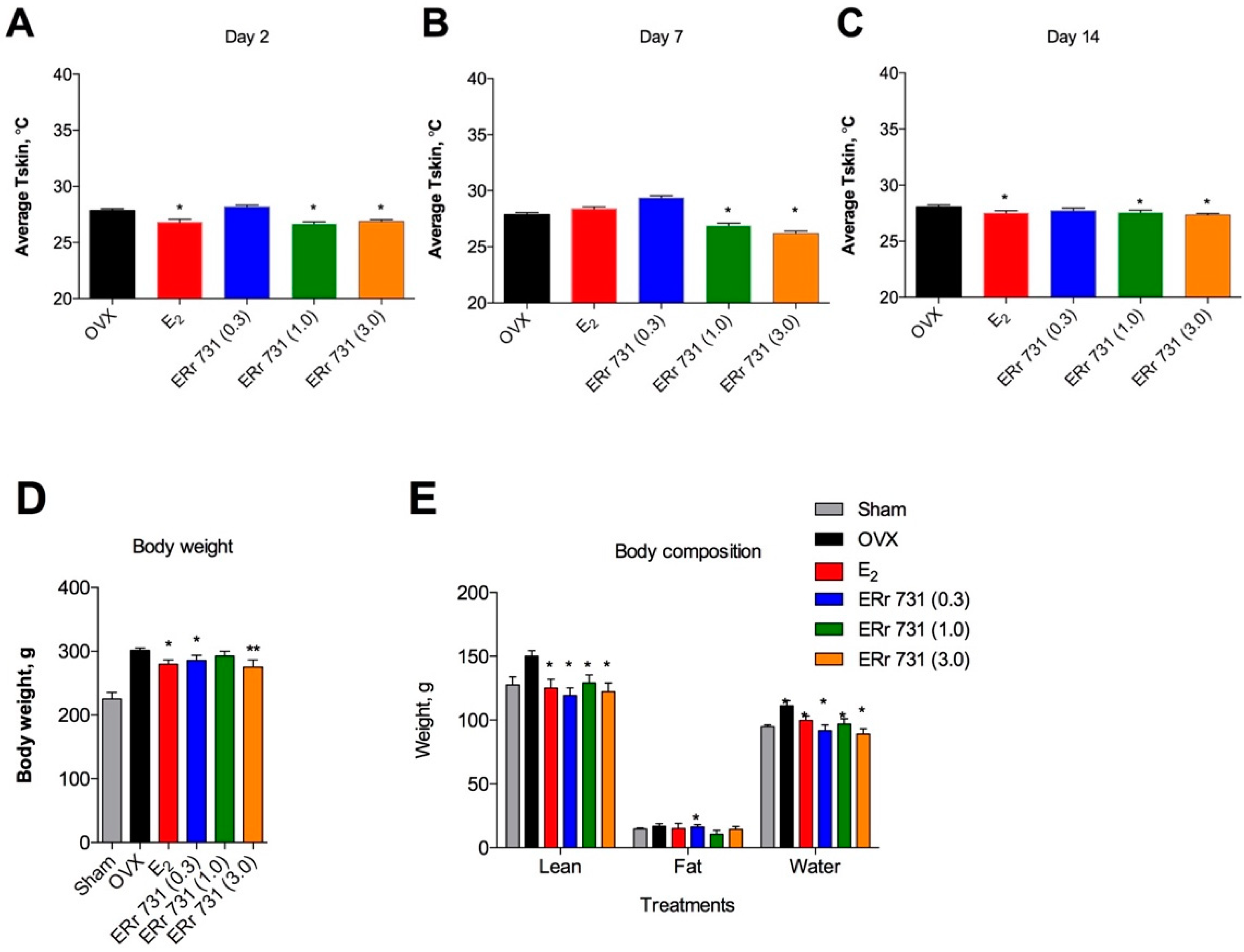
2.2. Dose-Dependent Effects of ERr 731 Treatment
2.3. Replacement Effects of ERr 731 in OVX Rats Receiving E2
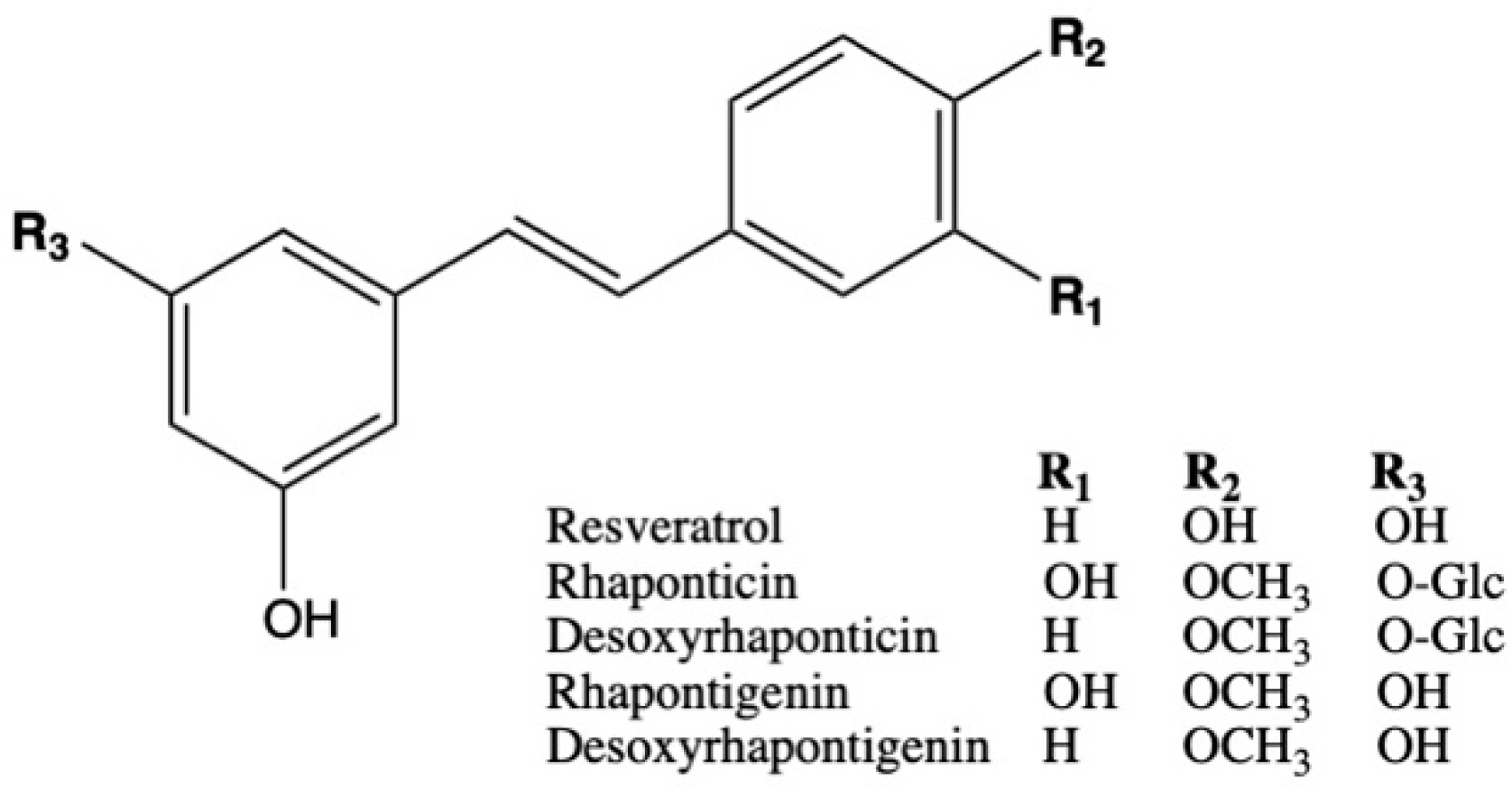
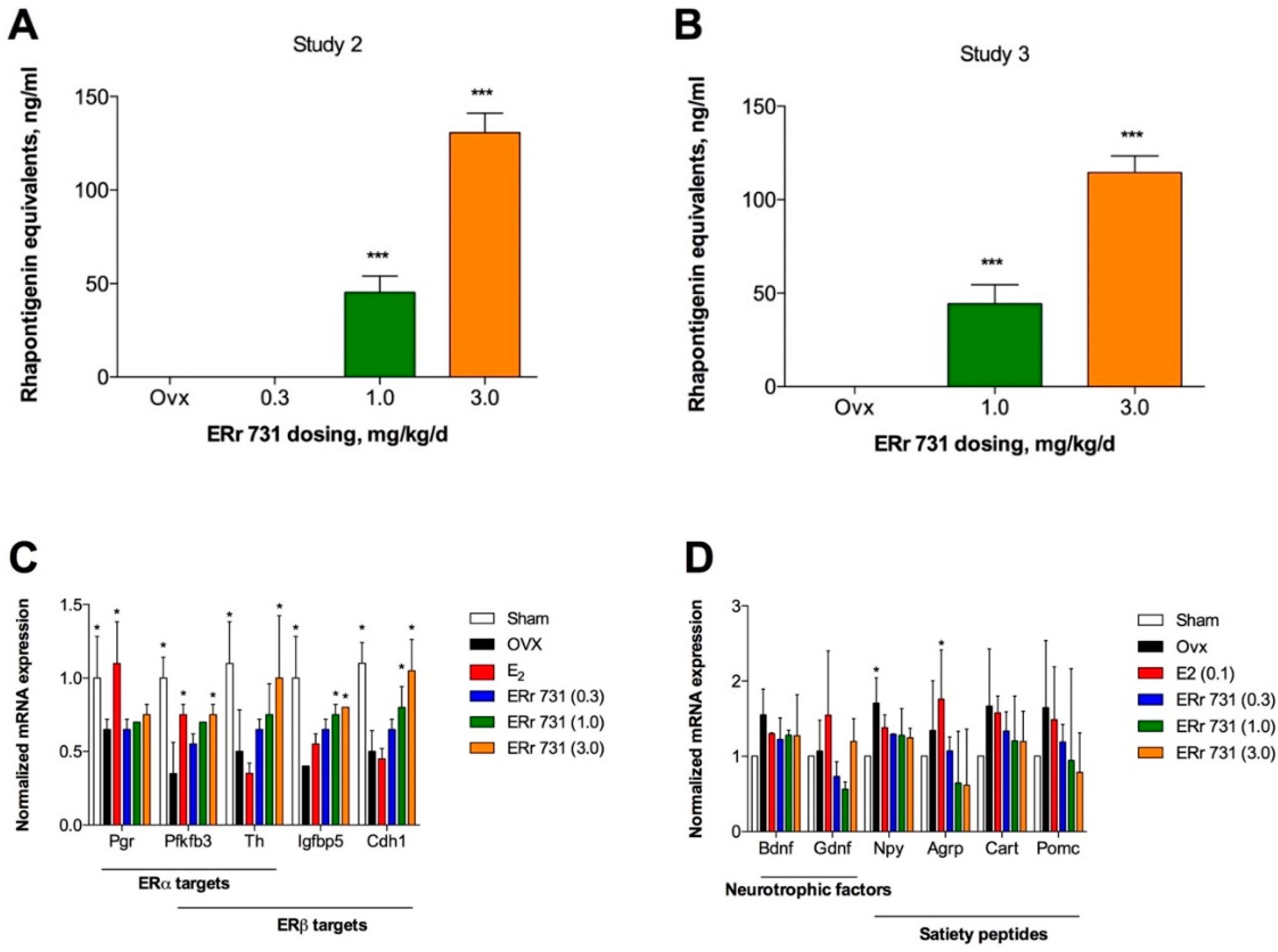
2.4. Plasma Levels of Rhubarb Extract Bioactives
2.5. Hypothalamic Gene Expression Profiles for ERα/β Target Genes and Peptides
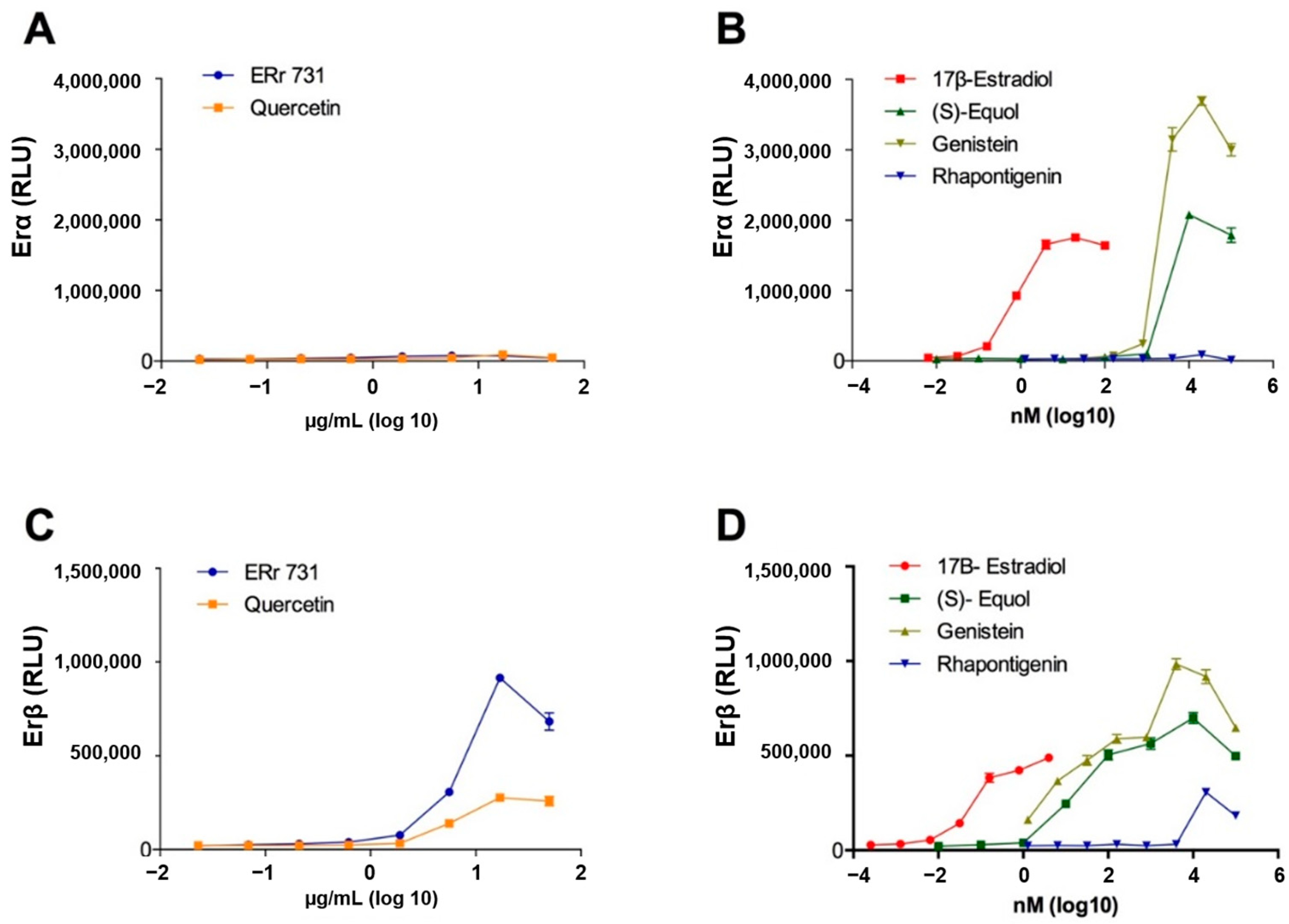
2.6. ERr 731 Shows a Selective ERβ Agonist Activity In Vitro
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Monitoring of Tail Skin Temperature Tskin
4.3. Animal Study 1: Model Validation
4.4. Animal Study 2: Dose Response
4.5. Animal Study 3: Replacement Therapy
4.6. Quantification of Plasma Rhaponticin
4.7. Gene Expression Studies
4.8. Estrogen Receptor Activity
4.9. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| E2 | 17β-estradiol |
| ERr 731 | Siberian rhubarb Rheum rhaponticum L. root extract |
| ERα/β | Estrogen receptor α/β |
| HRT | Hormone replacement therapy |
| OVX | Ovariectomized |
| Tskin | Skin temperature |
References
- Shapiro, M. Menopause Practice. Can. Fam. Physician 2012, 58, 989. [Google Scholar]
- Van Dijk, G.M.; Kavousi, M.; Troup, J.; Franco, O.H. Health Issues for Menopausal Women: The Top 11 Conditions Have Common Solutions. Maturitas 2015, 80, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Sassarini, J.; Lumsden, M.A. Oestrogen Replacement in Postmenopausal Women. Age Ageing 2015, 44, 551–558. [Google Scholar] [CrossRef]
- Nelson, H.D.; Vesco, K.K.; Haney, E.; Fu, R.; Nedrow, A.; Miller, J.; Nicolaidis, C.; Walker, M.; Humphrey, L. Nonhormonal Therapies for Menopausal Hot Flashes: Systematic Review and Meta-Analysis. JAMA 2006, 295, 2057–2071. [Google Scholar] [CrossRef] [PubMed]
- Geller, S.E.; Studee, L. Botanical and Dietary Supplements for Menopausal Symptoms: What Works, What Does Not. J. Womens Health 2005, 14, 634–649. [Google Scholar] [CrossRef]
- Cvoro, A.; Paruthiyil, S.; Jones, J.O.; Tzagarakis-Foster, C.; Clegg, N.J.; Tatomer, D.; Medina, R.T.; Tagliaferri, M.; Schaufele, F.; Scanlan, T.S.; et al. Selective Activation of Estrogen Receptor-Beta Transcriptional Pathways by an Herbal Extract. Endocrinology 2007, 148, 538–547. [Google Scholar] [CrossRef]
- Heger, M.; Ventskovskiy, B.M.; Borzenko, I.; Kneis, K.C.; Rettenberger, R.; Kaszkin-Bettag, M.; Heger, P.W. Efficacy and Safety of a Special Extract of Rheum rhaponticum (ERr 731) in Perimenopausal Women with Climacteric Complaints: A 12-Week Randomized, Double-Blind, Placebo-Controlled Trial. Menopause 2006, 13, 744–759. [Google Scholar] [CrossRef]
- Kaszkin-Bettag, M.; Ventskovskiy, B.M.; Kravchenko, A.; Rettenberger, R.; Richardson, A.; Heger, P.W.; Heger, M. The Special Extract ERr 731 of the Roots of Rheum rhaponticum Decreases Anxiety and Improves Health State and General Well-Being in Perimenopausal Women. Menopause 2007, 14, 270–283. [Google Scholar] [CrossRef]
- Wober, J.; Möller, F.; Richter, T.; Unger, C.; Weigt, C.; Jandausch, A.; Zierau, O.; Rettenberger, R.; Kaszkin-Bettag, M.; Vollmer, G. Activation of Estrogen Receptor-Beta by a Special Extract of Rheum rhaponticum (ERr 731), Its Aglycones and Structurally Related Compounds. J. Steroid Biochem. Mol. Biol. 2007, 107, 191–201. [Google Scholar] [CrossRef]
- Papke, A.; Kretzschmar, G.; Zierau, O.; Kaszkin-Bettag, M.; Vollmer, G. Effects of the Special Extract ERr 731® from Rheum rhaponticum on Estrogen-Regulated Targets in the Uterotrophy Model of Ovariectomized Rats. J. Steroid Biochem. Mol. Biol. 2009, 117, 176–184. [Google Scholar] [CrossRef]
- Kaszkin-Bettag, M.; Richardson, A.; Rettenberger, R.; Heger, P.W. Long-Term Toxicity Studies in Dogs Support the Safety of the Special Extract ERr 731 from the Roots of Rheum rhaponticum. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2008, 46, 1608–1618. [Google Scholar] [CrossRef] [PubMed]
- Hasper, I.; Ventskovskiy, B.M.; Rettenberger, R.; Heger, P.W.; Riley, D.S.; Kaszkin-Bettag, M. Long-Term Efficacy and Safety of the Special Extract ERr 731 of Rheum rhaponticum in Perimenopausal Women with Menopausal Symptoms. Menopause 2009, 16, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Kaszkin-Bettag, M.; Beck, S.; Richardson, A.; Heger, P.W.; Beer, A.-M. Efficacy of the Special Extract ERr 731 from Rhapontic Rhubarb for Menopausal Complaints: A 6-Month Open Observational Study. Altern. Ther. Health Med. 2008, 14, 32–38. [Google Scholar] [PubMed]
- Kaszkin-Bettag, M.; Ventskovskiy, B.M.; Solskyy, S.; Beck, S.; Hasper, I.; Kravchenko, A.; Rettenberger, R.; Richardson, A.; Heger, P.W. Confirmation of the Efficacy of ERr 731 in Perimenopausal Women with Menopausal Symptoms. Altern. Ther. Health Med. 2009, 15, 24–34. [Google Scholar]
- Chang, J.-L.; Montalto, M.B.; Heger, P.W.; Thiemann, E.; Rettenberger, R.; Wacker, J. Rheum rhaponticum Extract (ERr 731): Postmarketing Data on Safety Surveillance and Consumer Complaints. Integr. Med. Encinitas Calif. 2016, 15, 34–39. [Google Scholar]
- Romanovsky, A.A.; Ivanov, A.I.; Shimansky, Y.P. Selected Contribution: Ambient Temperature for Experiments in Rats: A New Method for Determining the Zone of Thermal Neutrality. J. Appl. Physiol. 2002, 92, 2667–2679. [Google Scholar] [CrossRef]
- Williams, H.; Dacks, P.A.; Rance, N.E. An Improved Method for Recording Tail Skin Temperature in the Rat Reveals Changes during the Estrous Cycle and Effects of Ovarian Steroids. Endocrinology 2010, 151, 5389–5394. [Google Scholar] [CrossRef][Green Version]
- Dacks, P.A.; Rance, N.E. Effects of Estradiol on the Thermoneutral Zone and Core Temperature in Ovariectomized Rats. Endocrinology 2010, 151, 1187–1193. [Google Scholar] [CrossRef]
- Casper, R.F.; Yen, S.S. Neuroendocrinology of Menopausal Flushes: An Hypothesis of Flush Mechanism. Clin. Endocrinol. 1985, 22, 293–312. [Google Scholar] [CrossRef]
- Winer, J.; Jung, C.K.S.; Shackel, I.; Williams, P.M. Development and Validation of Real-Time Quantitative Reverse Transcriptase–Polymerase Chain Reaction for Monitoring Gene Expression in Cardiac Myocytesin Vitro. Anal. Biochem. 1999, 270, 41–49. [Google Scholar] [CrossRef]
- Richardson, D.; Shepherd, S.; Tyra, J. Age-Related Potentiation of the Cutaneous Capillary Blood Flow Response to Heat Stress. Microcirc. Endothelium. Lymph. 1991, 7, 305–323. [Google Scholar]
- Thompson, G.E.; Stevenson, J.A. A Sex Difference in the Temperature Response of Rats To Exercise. Can. J. Physiol. Pharmacol. 1965, 43, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Tamura, M.; Hayashi, M.; Katsuura, Y.; Tanabe, H.; Ohta, T.; Komoriya, K. Elevation of Tail Skin Temperature in Ovariectomized Rats in Relation to Menopausal Hot Flushes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 278, R863–R869. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose Translation from Animal to Human Studies Revisited. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Teekachunhatean, S.; Mattawanon, N.; Khunamornpong, S. Short-Term Isoflavone Intervention in the Treatment of Severe Vasomotor Symptoms after Surgical Menopause: A Case Report and Literature Review. Case Rep. Obstet. Gynecol. 2015, 2015. [Google Scholar] [CrossRef]
- Ghazanfarpour, M.; Sadeghi, R.; Roudsari, R.L.; Najmabadi, K.M.; Bazaz, M.M.; Abdolahian, S.; Khadivzadeh, T. Effects of Red Clover on Hot Flash and Circulating Hormone Concentrations in Menopausal Women: A Systematic Review and Meta-Analysis. Avicenna J. Phytomed. 2015, 5, 498–511. [Google Scholar]
- Borrelli, F.; Ernst, E. Black Cohosh (Cimicifuga racemosa) for Menopausal Symptoms: A Systematic Review of Its Efficacy. Pharmacol. Res. 2008, 58, 8–14. [Google Scholar] [CrossRef]
- Cone, R.D.; Cowley, M.A.; Butler, A.A.; Fan, W.; Marks, D.L.; Low, M.J. The Arcuate Nucleus as a Conduit for Diverse Signals Relevant to Energy Homeostasis. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2001, 25 (Suppl. 5), S63–S67. [Google Scholar] [CrossRef]
- Komarnytsky, S.; Esposito, D.; Rathinasabapathy, T.; Poulev, A.; Raskin, I. Effects of Pregnane Glycosides on Food Intake Depend on Stimulation of the Melanocortin Pathway and BDNF in an Animal Model. J. Agric. Food Chem. 2013, 61, 1841–1849. [Google Scholar] [CrossRef][Green Version]
- Esposito, D.; Komarnytsky, S.; Shapses, S.; Raskin, I. Anabolic Effect of Plant Brassinosteroid. FASEB J. 2011, 25, 3708–3719. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, Y.; Yang, X. A Simple and Rapid Spectrofluorimetric Method for Determining the Pharmacokinetics and Metabolism of Rhaponticin in Rat Plasma, Feces and Urine Using a Cerium Probe. Lumin. J. Biol. Chem. Lumin. 2013, 28, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Shanle, E.K.; Zhao, Z.; Hawse, J.; Wisinski, K.; Keles, S.; Yuan, M.; Xu, W. Research Resource: Global Identification of Estrogen Receptor β Target Genes in Triple Negative Breast Cancer Cells. Mol. Endocrinol. 2013, 27, 1762–1775. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilson, M.; Konda, V.; Heidt, K.; Rathinasabapathy, T.; Desai, A.; Komarnytsky, S. Rheum rhaponticum Root Extract Improves Vasomotor Menopausal Symptoms and Estrogen-Regulated Targets in Ovariectomized Rat Model. Int. J. Mol. Sci. 2021, 22, 1032. https://doi.org/10.3390/ijms22031032
Wilson M, Konda V, Heidt K, Rathinasabapathy T, Desai A, Komarnytsky S. Rheum rhaponticum Root Extract Improves Vasomotor Menopausal Symptoms and Estrogen-Regulated Targets in Ovariectomized Rat Model. International Journal of Molecular Sciences. 2021; 22(3):1032. https://doi.org/10.3390/ijms22031032
Chicago/Turabian StyleWilson, Mickey, Veera Konda, Kathryn Heidt, Thirumurugan Rathinasabapathy, Anuradha Desai, and Slavko Komarnytsky. 2021. "Rheum rhaponticum Root Extract Improves Vasomotor Menopausal Symptoms and Estrogen-Regulated Targets in Ovariectomized Rat Model" International Journal of Molecular Sciences 22, no. 3: 1032. https://doi.org/10.3390/ijms22031032
APA StyleWilson, M., Konda, V., Heidt, K., Rathinasabapathy, T., Desai, A., & Komarnytsky, S. (2021). Rheum rhaponticum Root Extract Improves Vasomotor Menopausal Symptoms and Estrogen-Regulated Targets in Ovariectomized Rat Model. International Journal of Molecular Sciences, 22(3), 1032. https://doi.org/10.3390/ijms22031032