Aldose Reductase and the Polyol Pathway in Schwann Cells: Old and New Problems
Abstract
1. Introduction
2. Physiological and Pathological Roles of AR in Schwann Cells
2.1. Possible Functions of AR in Schwann Cells under Non-Diabetic Conditions
2.1.1. Responses to Hyperosmotic Stress
2.1.2. Aldehyde Detoxification
2.1.3. Steroid Metabolism
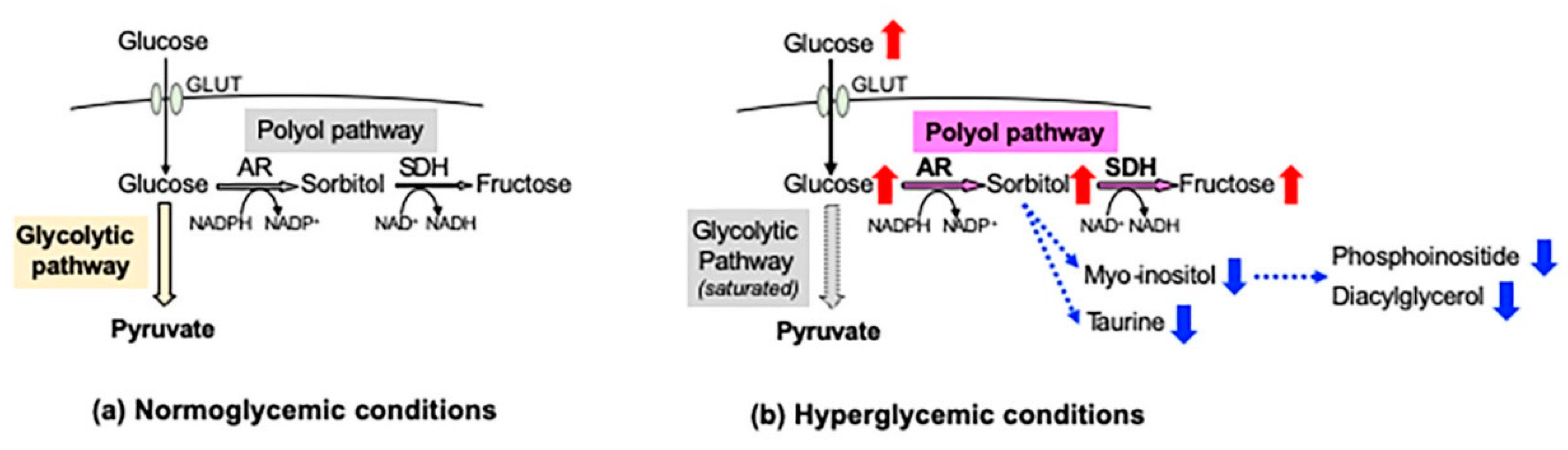
2.2. The Polyol Pathway as a Major Pathogenic Factor of DPN
2.2.1. Increased Sorbitol Contents
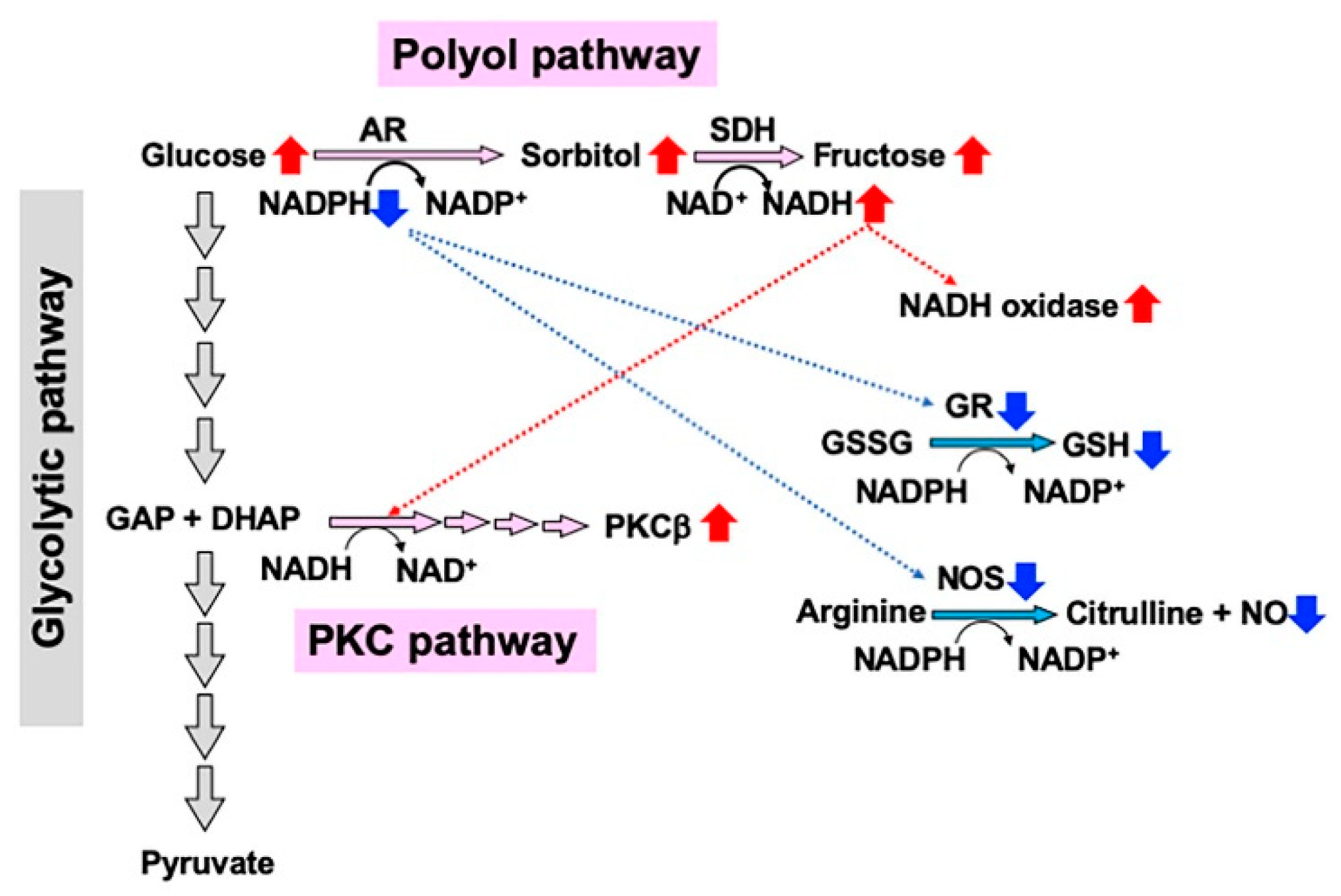
2.2.2. Redox State Changes
2.2.3. Formation of Dicarbonyl Compounds and Advanced Glycation Endproducts (AGEs)
2.2.4. PKC Activity Abnormalities
2.2.5. Epalrestat as a Pathogenesis-Based Medicine for DPN
3. IMS32 Schwann Cells as a Useful Tool to Study AR and the Polyol Pathway under Diabetic Conditions
3.1. IMS32 Cells Have Been Utilized for the Study of DPN
3.2. IMS32 Cells Are Suitable for Exploring AR/Polyol Pathway-Related Abnormalities in DPN
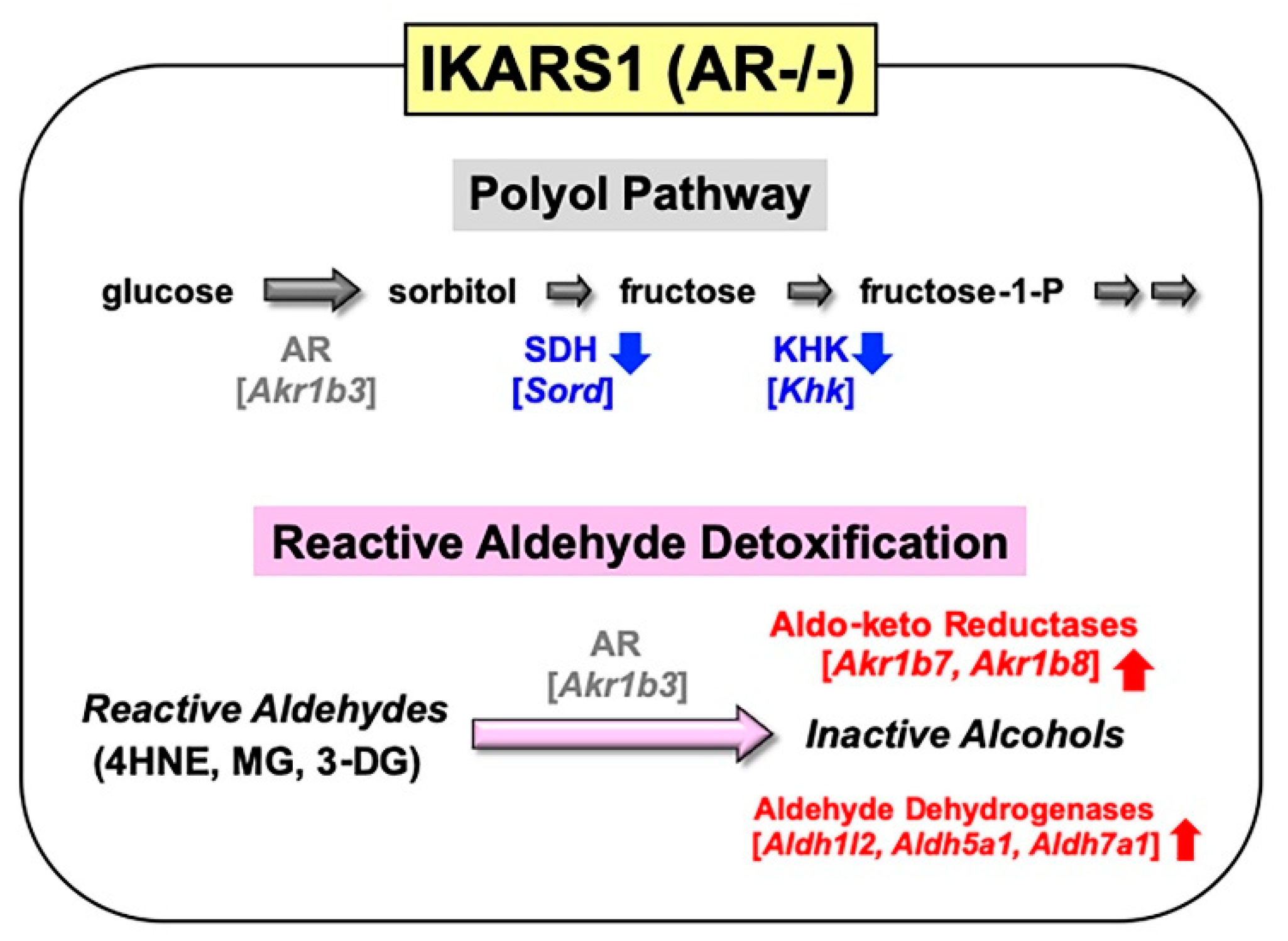
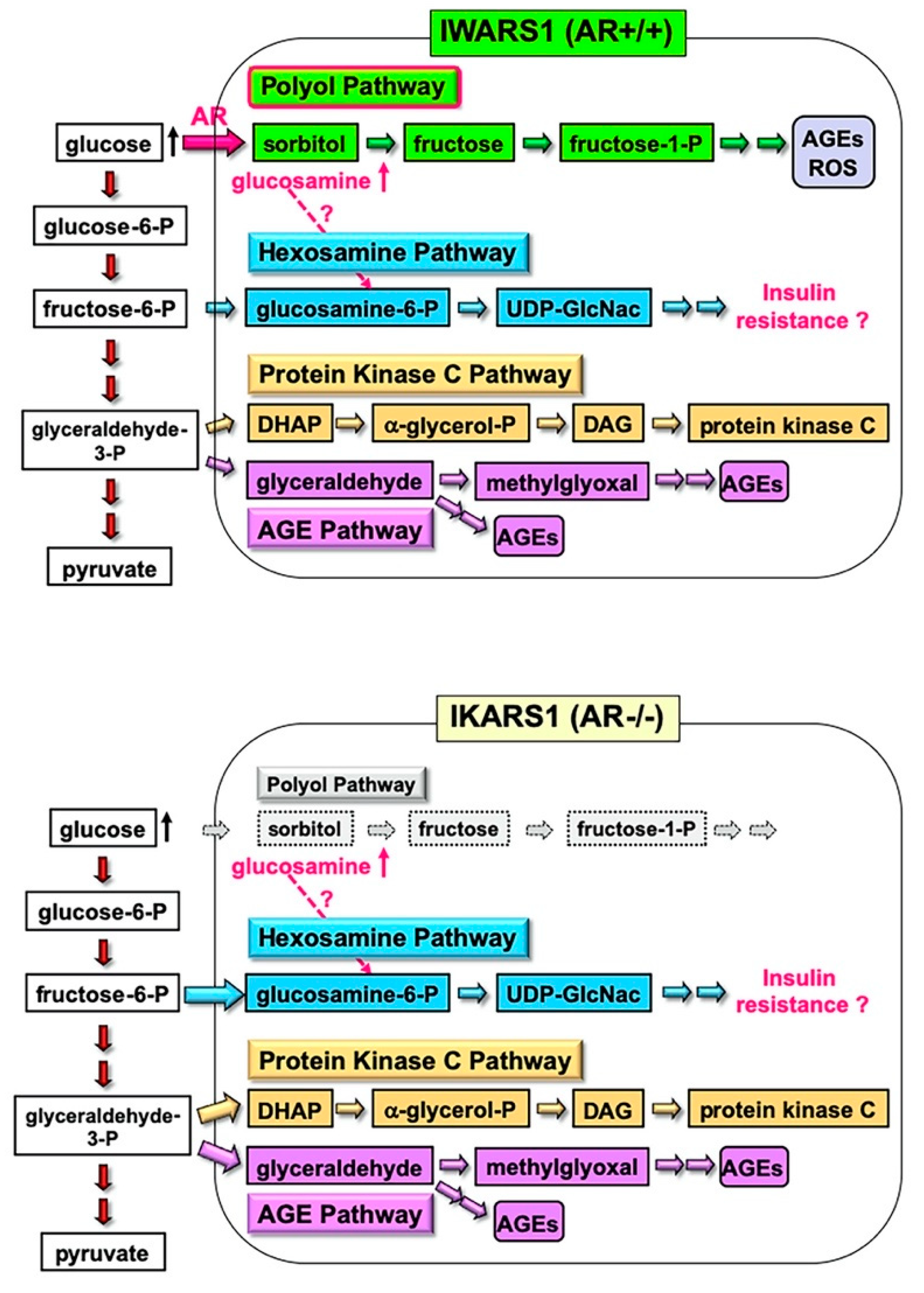
4. Establishment of an AR-Deficient Schwann Cell Line IKARS1
4.1. Establishment and Characterization of IKARS1 Cells
4.2. Establishment of IWARS1 Cells and Future Studies with IKARS1 and IWARS1
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKR | Aldo-keto reductases |
| ALDH | Aldehyde dehydrogenase |
| AR | Aldose reductase |
| 3-DG | 3-Deoxyglucosone |
| DPN | Diabetic peripheral neuropathy |
| DRG | Dorsal root ganglion |
| GDNF | Glial cell line-derived neurotrophic factor |
| 4HNE | 6-Hydroxy-2-nonenal |
| IKARS1 | Immortalized knockout aldose reductase Schwann cells 1 |
| IMS32 | Immortalized mouse Schwann cells 32 |
| IWARS1 | Immortalized wild-type aldose reductase Schwann cells 1 |
| KHK | Ketohexokinase |
| MG | Methylglyoxal |
| NADPH | Reduced nicotinamide adenosine dinucleotide phosphate |
| NGF | Nerve growth factor |
| p75NTR | p75 low-affinity neurotrophin receptor |
| PKC | Protein kinase C |
| PNS | Peripheral nervous system |
| SDH | Sorbitol dehydrogenase |
References
- Hers, H.G. The mechanism of the transformation of glucose in fructose in the seminal vesicles. Biochem. Biophys. Acta 1956, 22, 202–203. [Google Scholar] [CrossRef]
- Yagihashi, S. Glucotoxic Mechanisms and Related Therapeutic Approaches. Int. Rev. Neurobiol. 2016, 127, 121–149. [Google Scholar] [CrossRef] [PubMed]
- González, R.G.; Barnett, P.; Aguayo, J.; Cheng, H.M.; Chylack, L.T. Direct measurement of polyol pathway activity in the ocular lens. Diabetes 1984, 33, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Yabe-Nishimura, C. Aldose reductase in glucose toxicity: A potential target for the prevention of diabetic complications. Pharmacol. Rev. 1998, 50, 21–33. [Google Scholar] [PubMed]
- Grewal, A.S.; Bhardwaj, S.; Pandita, D.; Lather, V.; Sekhon, B.S. Updates on Aldose Reductase Inhibitors for Management of Diabetic Complications and Non-diabetic Diseases. Mini-Rev. Med. Chem. 2015, 16, 120–162. [Google Scholar] [CrossRef]
- Hotta, N.; Kawamori, R.; Fukuda, M.; Shigeta, Y. The Aldose Reductase Inhibitor–Diabetes Complications Trial Study Group Long-term clinical effects of epalrestat, an aldose reductase inhibitor, on progression of diabetic neuropathy and other microvascular complications: Multivariate epidemiological analysis based on patient background factors and severity of diabetic neuropathy. Diabet. Med. 2012, 29, 1529–1533. [Google Scholar] [CrossRef]
- Ludvigson, M.A.; Sorenson, R.L. Immunohistochemical localization of aldose reductase. I. Enzyme purification and antibody preparation--localization in peripheral nerve, artery, and testis. Diabetes 1980, 29, 438–449. [Google Scholar] [CrossRef]
- Sango, K.; Mizukami, H.; Horie, H.; Yagihashi, S. Impaired Axonal Regeneration in Diabetes. Perspective on the Underlying Mechanism from In Vivo and In Vitro Experimental Studies. Front. Endocrinol. 2017, 8, 12. [Google Scholar] [CrossRef]
- Vincent, A.M.; Callaghan, B.C.; Smith, A.L.; Feldman, E.L. Diabetic neuropathy: Cellular mechanisms as therapeutic targets. Nat. Rev. Neurol. 2011, 7, 573–583. [Google Scholar] [CrossRef]
- Song, Z.; Fu, D.T.W.; Chan, Y.-S.; Leung, S.Y.; Chung, S.S.M.; Chung, S.K. Transgenic mice overexpressing aldose reductase in Schwann cells show more severe nerve conduction velocity deficit and oxidative stress under hyperglycemic stress. Mol. Cell. Neurosci. 2003, 23, 638–647. [Google Scholar] [CrossRef]
- Ho, E.C.; Lam, K.S.L.; Chen, Y.S.; Yip, J.C.; Arvindakshan, M.; Yamagishi, S.-I.; Yagihashi, S.; Oates, P.J.; Ellery, C.A.; Chung, S.S.; et al. Aldose Reductase-Deficient Mice Are Protected From Delayed Motor Nerve Conduction Velocity, Increased c-Jun NH2-Terminal Kinase Activation, Depletion of Reduced Glutathione, Increased Superoxide Accumulation, and DNA Damage. Diabetes 2006, 55, 1946–1953. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, H.; Osonoi, S.; Takaku, S.; Yamagishi, S.-I.; Ogasawara, S.; Sango, K.; Chung, S.; Yagihashi, S. Role of glucosamine in development of diabetic neuropathy independent of the aldose reductase pathway. Brain Commun. 2020, 2, fcaa168. [Google Scholar] [CrossRef] [PubMed]
- Niimi, N.; Yako, H.; Takaku, S.; Kato, H.; Matsumoto, T.; Nishito, Y.; Watabe, K.; Ogasawara, S.; Mizukami, H.; Yagihashi, S.; et al. A spontaneously immortalized Schwann cell line from aldose reductase-deficient mice as a useful tool for studying polyol pathway and aldehyde metabolism. J. Neurochem. 2018, 144, 710–722. [Google Scholar] [CrossRef] [PubMed]
- Watabe, K.; Fukuda, T.; Tanaka, J.; Honda, H.; Toyohara, K.; Sakai, O. Spontaneously immortalized adult mouse Schwann cells secrete autocrine and paracrine growth-promoting activities. J. Neurosci. Res. 1995, 41, 279–290. [Google Scholar] [CrossRef]
- Sango, K.; Suzuki, T.; Yanagisawa, H.; Takaku, S.; Hirooka, H.; Tamura, M.; Watabe, K. High glucose-induced activation of the polyol pathway and changes of gene expression profiles in immortalized adult mouse Schwann cells IMS32. J. Neurochem. 2006, 98, 446–458. [Google Scholar] [CrossRef]
- Sango, K.; Yanagisawa, H.; Takaku, S.; Kawakami, E.; Watabe, K. Immortalized Adult Rodent Schwann Cells as In Vitro Models to Study Diabetic Neuropathy. Exp. Diabetes Res. 2011, 2011, 1–9. [Google Scholar] [CrossRef]
- Jez, J.M.; Bennett, M.J.; Schlegel, B.P.; Lewis, M.; Penning, T.M. Comparative anatomy of the aldo–keto reductase superfamily. Biochem. J. 1997, 326, 625–636. [Google Scholar] [CrossRef]
- Pastel, E.; Pointud, J.-C.; Volat, F.; Martinez, A.; Lefrançois-Martinez, A.-M. Aldo-Keto Reductases 1B in Endocrinology and Metabolism. Front. Pharmacol. 2012, 3, 148. [Google Scholar] [CrossRef]
- Cao, D.; Fan, S.T.; Chung, S.S.M. Identification and Characterization of a Novel Human Aldose Reductase-like Gene. J. Biol. Chem. 1998, 273, 11429–11435. [Google Scholar] [CrossRef]
- Joshi, A.; Rajput, S.; Wang, C.; Ma, J.; Cao, D. Murine aldo-keto reductase family 1 subfamily B: Identification of AKR1B8 as an ortholog of human AKR1B10. Biol. Chem. 2010, 391, 1371–1378. [Google Scholar] [CrossRef]
- Salabei, J.K.; Li, X.-P.; Petrash, J.M.; Bhatnagar, A.; Barski, O.A. Functional expression of novel human and murine AKR1B genes. Chem. Interact. 2011, 191, 177–184. [Google Scholar] [CrossRef]
- MacLeod, A.K.; Kelly, V.P.; Higgins, L.G.; Kelleher, M.; Price, S.A.; Bigley, A.L.; Betton, G.R.; Hayes, J.D. Expression and Localization of Rat Aldo-Keto Reductases and Induction of the 1B13 and 1D2 Isoforms by Phenolic Antioxidants. Drug Metab. Dispos. 2009, 38, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Flynn, T.G. Aldehyde reductases: Monomeric NADPH-dependent oxidoreductases with multifunctional potential. Biochem. Pharmacol. 1982, 31, 2705–2712. [Google Scholar] [CrossRef]
- Steffgen, J.; Kampfer, K.; Grupp, C.; Langenberg, C.; Müller, G.A.; Grunewald, R.W. Osmoregulation of aldose reductase and sorbitol dehydrogenase in cultivated interstitial cells of rat renal inner medulla. Nephrol. Dial. Transplant. 2003, 18, 2255–2261. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aida, K.; Ikegishi, Y.; Chen, J.; Tawata, M.; Ito, S.; Maeda, S.; Onaya, T. Disruption of Aldose Reductase Gene (Akr1b1) Causes Defect in Urinary Concentrating Ability and Divalent Cation Homeostasis. Biochem. Biophys. Res. Commun. 2000, 277, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Tam, W.Y.; Tam, S.; Guo, H.; Wu, X.; Li, G.; Chau, J.F.L.; Klein, J.D.; Chung, S.K.; Sands, J.M.; et al. Genetic restoration of aldose reductase to the collecting tubules restores maturation of the urine concentrating mechanism. Am. J. Physiol. Physiol. 2006, 291, F186–F195. [Google Scholar] [CrossRef] [PubMed]
- Mizisin, A.P.; Li, L.; Perello, M.; Freshwater, J.D.; Kalichman, M.W.; Roux, L.; Calcutt, N.A. Polyol pathway and osmoregulation in JS1 Schwann cells grown in hyperglycemic and hyperosmotic conditions. Am. J. Physiol. 1996, 270, F90–F97. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Mizuno, K.; Yashima, S.; Watanabe, K.; Taniko, K.; Yabe-Nishimura, C. Characterization of polyol pathway in Schwann cells isolated from adult rat sciatic nerves. J. Neurosci. Res. 1999, 57, 495–503. [Google Scholar] [CrossRef]
- Sango, K.; Saito, H.; Takano, M.; Tokashiki, A.; Inoue, S.; Horie, H. Cultured adult animal neurons and schwann cells give us new insights into diabetic neuropathy. Curr. Diabetes Rev. 2006, 2, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Barski, O.A.; Tipparaju, S.M.; Bhatnagar, A. The Aldo-Keto Reductase Superfamily and its Role in Drug Metabolism and Detoxification. Drug Metab. Rev. 2008, 40, 553–624. [Google Scholar] [CrossRef]
- Kolb, N.S.; Hunsaker, L.A.; Jagt, D.L.V. Aldose reductase-catalyzed reduction of acrolein: Implications in cyclophosphamide toxicity. Mol. Pharmacol. 1994, 45, 797–801. [Google Scholar] [PubMed]
- Corso, A.; Cappiello, M.; Mura, U. From a Dull Enzyme to Something Else: Facts and Perspectives Regarding Aldose Reductase. Curr. Med. Chem. 2008, 15, 1452–1461. [Google Scholar] [CrossRef]
- Sango, K.; Yanagisawa, H.; Kato, K.; Kato, N.; Hirooka, H.; Watabe, K. Differential Effects of High Glucose and Methylglyoxal on Viability and Polyol Metabolism in Immortalized Adult Mouse Schwann Cells. Open Diabetes J. 2008, 1, 1–11. [Google Scholar] [CrossRef]
- Roglio, I.; Giatti, S.; Pesaresi, M.; Bianchi, R.; Cavaletti, G.; Lauria, G.; Garcia-Segura, L.-M.; Melcangi, R.C. Neuroactive steroids and peripheral neuropathy. Brain Res. Rev. 2008, 57, 460–469. [Google Scholar] [CrossRef]
- Mitro, N.; Cermenati, G.; Brioschi, E.; Abbiati, F.; Audano, M.; Giatti, S.; Crestani, M.; De Fabiani, E.; Azcoitia, I.; Garcia-Segura, L.M.; et al. Neuroactive steroid treatment modulates myelin lipid profile in diabetic peripheral neuropathy. J. Steroid Biochem. Mol. Biol. 2014, 143, 115–121. [Google Scholar] [CrossRef]
- Colciago, A.; Negri-Cesi, P.; Celotti, F. Pathogenesis of diabetic neuropathy—Do hyperglycemia and aldose reductase inhibitors affect neuroactive steroid formation in the rat sciatic nerves? Exp. Clin. Endocrinol. Diabetes 2002, 110, 22–26. [Google Scholar] [CrossRef]
- Schüttert, J.B.; Fiedler, G.M.; Grupp, C.; Blaschke, S.; Grunewald, R.W.; Sch, G.M.F.J.B. Sorbitol transport in rat renal inner medullary interstitial cells. Kidney Int. 2002, 61, 1407–1415. [Google Scholar] [CrossRef]
- Oates, P.J. Aldose Reductase, Still a Compelling Target for Diabetic Neuropathy. Curr. Drug Targets 2008, 9, 14–36. [Google Scholar] [CrossRef]
- Stevens, M.J.; Lattimer, S.A.; Kamijo, M.; Van Huysen, C.; Sima, A.A.F.; Greene, D.A. Osmotically-induced nerve taurine depletion and the compatible osmolyte hypothesis in experimental diabetic neuropathy in the rat. Diabetologia 1993, 36, 608–614. [Google Scholar] [CrossRef]
- Flier, J.S.; Underhill, L.H.; Greene, D.A.; Lattimer, S.A.; Sima, A.A. Sorbitol, Phosphoinositides, and Sodium-Potassium-ATPase in the Pathogenesis of Diabetic Complications. N. Engl. J. Med. 1987, 316, 599–606. [Google Scholar] [CrossRef]
- Raccah, D.; Coste, T.C.; Cameron, N.E.; Dufayet, D.; Vague, P.; Hohman, T.C. Effect of the aldose reductase inhibitor tolrestat on nerve conduction velocity, Na/K ATPase activity, and polyols in red blood cells, sciatic nerve, kidney cortex, and kidney medulla of diabetic rats. J. Diabetes Complicat. 1998, 12, 154–162. [Google Scholar] [CrossRef]
- Kamiya, H.; Nakamura, J.; Hamada, Y.; Nakashima, E.; Naruse, K.; Kato, K.; Yasuda, Y.; Hotta, N. Polyol pathway and protein kinase C activity of rat Schwannoma cells. Diabetes/Metab. Res. Rev. 2002, 19, 131–139. [Google Scholar] [CrossRef]
- Askwith, T.; Zeng, W.; Eggo, M.C.; Stevens, M.J. Taurine reduces nitrosative stress and nitric oxide synthase expression in high glucose-exposed human Schwann cells. Exp. Neurol. 2012, 233, 154–162. [Google Scholar] [CrossRef]
- Jakaria; Azam, S.; Haque, E.; Jo, S.-H.; Uddin, S.; Kim, I.-S.; Choi, D.-K. Taurine and its analogs in neurological disorders: Focus on therapeutic potential and molecular mechanisms. Redox Biol. 2019, 24, 101223. [Google Scholar] [CrossRef]
- Ramana, K.V. Aldose reductase: New insights for an old enzyme. Biomol. Concepts 2011, 2, 103–114. [Google Scholar] [CrossRef]
- Lee, A.Y.W.; Chung, S.S.M. Contributions of polyol pathway to oxidative stress in diabetic cataract. FASEB J. 1999, 13, 23–30. [Google Scholar] [CrossRef]
- Obrosova, I.G.; Fathallah, L.; Lang, H.J. Interaction between osmotic and oxidative stress in diabetic precataractous lens: Studies with a sorbitol dehydrogenase inhibitor. Biochem. Pharmacol. 1999, 58, 1945–1954. [Google Scholar] [CrossRef]
- Yamagishi, S.-I.; Uehara, K.; Otsuki, S.; Yagihashi, S. Differential influence of increased polyol pathway on protein kinase C expressions between endoneurial and epineurial tissues in diabetic mice. J. Neurochem. 2003, 87, 497–507. [Google Scholar] [CrossRef]
- Hamada, Y.; Araki, N.; Koh, N.; Nakamura, J.; Horiuchi, S.; Hotta, N. Rapid Formation of Advanced Glycation End Products by Intermediate Metabolites of Glycolytic Pathway and Polyol Pathway. Biochem. Biophys. Res. Commun. 1996, 228, 539–543. [Google Scholar] [CrossRef]
- Liu, J.; Wang, R.; Desai, K.M.; Wu, L. Upregulation of aldolase B and overproduction of methylglyoxal in vascular tissues from rats with metabolic syndrome. Cardiovasc. Res. 2011, 92, 494–503. [Google Scholar] [CrossRef]
- Sekido, H.; Suzuki, T.; Jomori, T.; Takeuchi, M.; Yabe-Nishimura, C.; Yagihashi, S. Reduced cell replication and induction of apoptosis by advanced glycation end products in rat Schwann cells. Biochem. Biophys. Res. Commun. 2004, 320, 241–248. [Google Scholar] [CrossRef]
- Yu, T.; Li, L.; Chen, T.; Liu, Z.; Liu, H.; Li, Z. Erythropoietin Attenuates Advanced Glycation Endproducts-Induced Toxicity of Schwann Cells In Vitro. Neurochem. Res. 2015, 40, 698–712. [Google Scholar] [CrossRef]
- Fukunaga, M.; Miyata, S.; Liu, B.F.; Miyazaki, H.; Hirota, Y.; Higo, S.; Hamada, Y.; Ueyama, S.; Kasuga, M. Methylglyoxal induces apoptosis through activation of p38 MAPK in rat Schwann cells. Biochem. Biophys. Res. Commun. 2004, 320, 689–695. [Google Scholar] [CrossRef]
- Tsukamoto, M.; Sango, K.; Niimi, N.; Yanagisawa, H.; Watabe, K.; Utsunomiya, K. Upregulation of galectin-3 in immortalized Schwann cells IFRS1 under diabetic conditions. Neurosci. Res. 2015, 92, 80–85. [Google Scholar] [CrossRef]
- Mastrocola, R.; Nigro, D.; Cento, A.S.; Chiazza, F.; Collino, M.; Aragno, M. High-fructose intake as risk factor for neurodegeneration: Key role for carboxy methyllysine accumulation in mice hippocampal neurons. Neurobiol. Dis. 2016, 89, 65–75. [Google Scholar] [CrossRef]
- Gugliucci, A. Formation of Fructose-Mediated Advanced Glycation End Products and Their Roles in Metabolic and Inflammatory Diseases. Adv. Nutr. 2017, 8, 54–62. [Google Scholar] [CrossRef]
- Kato, K.; Feldman, E.L.; Nakamura, J. Chapter 9. Pathogenesis of Diabetic Neuropathy from the Point of View of Schwann Cell Abnormalities. In Schwann Cell Development and Pathology; Sango, K., Yamauchi, J., Eds.; Springer: Tokyo, Japan, 2014; pp. 135–146. [Google Scholar]
- Petrash, J.M. All in the family: Aldose reductase and closely related aldo-keto reductases. Cell Mol. Life Sci. 2004, 61, 737–749. [Google Scholar] [CrossRef]
- Nakamura, J.; Kasuya, Y.; Hamada, Y.; Nakashima, E.; Naruse, K.; Yasuda, Y.; Kato, K.; Hotta, N. Glucose-induced hyperproliferation of cultured rat aortic smooth muscle cells through polyol pathway hyperactivity. Diabetologia 2001, 44, 480–487. [Google Scholar] [CrossRef]
- Ramana, K.V.; Bhatnagar, A.; Srivastava, S.K. Inhibition of aldose reductase attenuates TNF-α-induced expression of adhesion molecules in endothelial cells. FASEB J. 2004, 18, 1209–1218. [Google Scholar] [CrossRef]
- Yorek, M.A. Vascular Impairment of Epineurial Arterioles of the Sciatic Nerve: Implications for Diabetic Peripheral Neuropathy. Rev. Diabet. Stud. 2015, 12, 13–28. [Google Scholar] [CrossRef]
- Boulton, A.J.M. Diabetic neuropathy: Classification, measurement and treatment. Curr. Opin. Endocrinol. Diabetes Obes. 2007, 14, 141–145. [Google Scholar] [CrossRef]
- Gardiner, N.J.; Freeman, O.J. Can Diabetic Neuropathy Be Modeled In Vitro? Int. Rev. Neurobiol. 2016, 127, 53–87. [Google Scholar] [CrossRef]
- De Vries, G.H.; Boullerne, A.I. Glial Cell Lines: An Overview. Neurochem. Res. 2010, 35, 1978–2000. [Google Scholar] [CrossRef]
- Ota, K.; Nakamura, J.; Li, W.; Kozakae, M.; Watarai, A.; Nakamura, N.; Yasuda, Y.; Nakashima, E.; Naruse, K.; Watabe, K.; et al. Metformin prevents methylglyoxal-induced apoptosis of mouse Schwann cells. Biochem. Biophys. Res. Commun. 2007, 357, 270–275. [Google Scholar] [CrossRef]
- Tosaki, T.; Kamiya, H.; Yasuda, Y.; Naruse, K.; Kato, K.; Kozakae, M.; Nakamura, N.; Shibata, T.; Hamada, Y.; Nakashima, E.; et al. Reduced NGF secretion by Schwann cells under the high glucose condition decreases neurite outgrowth of DRG neurons. Exp. Neurol. 2008, 213, 381–387. [Google Scholar] [CrossRef]
- Kim, N.; Kim, S.-H.; Kim, Y.-J.; Kim, J.-K.; Nam, M.-K.; Rhim, H.; Yoon, S.K.; Choi, S.-Z.; Son, M.; Kim, S.-Y.; et al. Neurotrophic activity of DA-9801, a mixture extract of Dioscorea japonica Thunb. and Dioscorea nipponica Makino, in vitro. J. Ethnopharmacol. 2011, 137, 312–319. [Google Scholar] [CrossRef]
- Kim, E.S.; Isoda, F.; Kurland, I.; Mobbs, C. Glucose-Induced Metabolic Memory in Schwann Cells: Prevention by PPAR Agonists. Endocrinology 2013, 154, 3054–3066. [Google Scholar] [CrossRef]
- Hao, W.; Tashiro, S.; Hasegawa, T.; Sato, Y.; Kobayashi, T.; Tando, T.; Katsuyama, E.; Fujie, A.; Watanabe, R.; Morita, M.; et al. Hyperglycemia Promotes Schwann Cell De-differentiation and De-myelination via Sorbitol Accumulation and Igf1 Protein Down-regulation. J. Biol. Chem. 2015, 290, 17106–17115. [Google Scholar] [CrossRef]
- Cinci, L.; Corti, F.; Mannelli, L.D.C.; Micheli, L.; Zanardelli, M.; Ghelardini, C. Oxidative, Metabolic, and Apoptotic Responses of Schwann Cells to High Glucose Levels. J. Biochem. Mol. Toxicol. 2015, 29, 274–279. [Google Scholar] [CrossRef]
- Min, S.H.; Kim, J.H.; Kang, Y.M.; Lee, S.H.; Oh, B.-M.; Han, K.-S.; Zhang, M.; Kim, H.S.; Moon, W.K.; Lee, H.; et al. Transplantation of human mobilized mononuclear cells improved diabetic neuropathy. J. Endocrinol. 2018, 239, 277–287. [Google Scholar] [CrossRef]
- Tatsumi, Y.; Kato, A.; Sango, K.; Himeno, T.; Kondo, M.; Kato, Y.; Kamiya, H.; Nakamura, J.; Kato, K. Omega-3 polyunsaturated fatty acids exert anti-oxidant effects through the nuclear factor (erythroid-derived 2)-related factor 2 pathway in immortalized mouse Schwann cells. J. Diabetes Investig. 2018, 10, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Tatsumi, Y.; Yako, H.; Sango, K.; Himeno, T.; Kondo, M.; Kato, Y.; Kamiya, H.; Nakamura, J.; Kato, K. Recurrent short-term hypoglycemia and hyperglycemia induce apoptosis and oxidative stress via the ER stress response in immortalized adult mouse Schwann (IMS32) cells. Neurosci. Res. 2019, 147, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Ohsawa, Y.; Zhenghua, L.; Yamamura, K.-I.; Sunada, Y. The transthyretin gene is expressed in Schwann cells of peripheral nerves. Brain Res. 2010, 1348, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Wiese, S.; Funk, N.; Chittka, A.; Rossoll, W.; Bömmel, H.; Watabe, K.; Wegner, M.; Sendtner, M. Sox10 regulates ciliary neurotrophic factor gene expression in Schwann cells. Proc. Natl. Acad. Sci. USA 2006, 103, 7871–7876. [Google Scholar] [CrossRef]
- Hashimoto, M.; Ishii, K.; Nakamura, Y.; Watabe, K.; Kohsaka, S.; Akazawa, C. Neuroprotective effect of sonic hedgehog up-regulated in Schwann cells following sciatic nerve injury. J. Neurochem. 2008, 107, 918–927. [Google Scholar] [CrossRef]
- Sango, K.; Tokashiki, A.; Ajiki, K.; Horie, M.; Kawano, H.; Watabe, K.; Horie, H.; Kadoya, T. Synthesis, localization and externalization of galectin-1 in mature dorsal root ganglion neurons and Schwann cells. Eur. J. Neurosci. 2004, 19, 55–64. [Google Scholar] [CrossRef]
- Hotta, N.; Kawamori, R.; Atsumi, Y.; Baba, M.; Kishikawa, H.; Nakamura, J.; Oikawa, S.; Yamada, N.; Yasuda, H.; Shigeta, Y.; et al. Stratified analyses for selecting appropriate target patients with diabetic peripheral neuropathy for long-term treatment with an aldose reductase inhibitor, epalrestat. Diabet. Med. 2008, 25, 818–825. [Google Scholar] [CrossRef]
- Yagihashi, S. Pathology and pathogenetic mechanisms of diabetic neuropathy. Diabetes Metab. Rev. 1995, 11, 193–225. [Google Scholar] [CrossRef]
- Yagihashi, S.; Yamagishi, S.-I.; Wada, R.-I.; Baba, M.; Hohman, T.C.; Yabe-Nishimura, C.; Kokai, Y. Neuropathy in diabetic mice overexpressing human aldose reductase and effects of aldose reductase inhibitor. Brain 2001, 124, 2448–2458. [Google Scholar] [CrossRef]
- Yagihashi, S.; Yamagishi, S.; Wada, R.; Sugimoto, K.; Baba, M.; Wong, H.G.; Fujimoto, J.; Nishimura, C.; Kokai, Y. Galactosemic neuropathy in transgenic mice for human aldose reductase. Diabetes 1996, 45, 56–59. [Google Scholar] [CrossRef]
- Sekiguchi, K.; Kohara, N.; Baba, M.; Komori, T.; Naito, Y.; Imai, T.; Satoh, J.; Yamaguchi, Y.; Hamatani, T. Aldose reductase inhibitor ranirestat significantly improves nerve conduction velocity in diabetic polyneuropathy: A randomized double-blind placebo-controlled study in Japan. J. Diabetes Investig. 2018, 10, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Sango, K.; Tsukamoto, M.; Utsunomiya, K.; Watabe, K. Chapter 10. Spontaneously Immortalized Adult Rodent Schwann Cells as Valuable Tools for the Study of Peripheral Nerve Degeneration and Regeneration. In Schwann Cell Development and Pathology; Sango, K., Yamauchi, J., Eds.; Springer: Tokyo, Japan, 2014; pp. 147–170. [Google Scholar]
- Singh, M.; Kapoor, A.; Bhatnagar, A. Oxidative and reductive metabolism of lipid-peroxidation derived carbonyls. Chem. Interact. 2015, 234, 261–273. [Google Scholar] [CrossRef] [PubMed]
| Name | Epalrestat (Kinedak®) | Fidarestat (SNK-860) | Ranirestat (AS-3201) |
|---|---|---|---|
| Molecular Formula | C15H13NO3S2 | C12H10FN3O4 | C17H11BrFN3O4 |
| Molecular Weight | 319.4 | 279.2 | 420.2 |
| International Union of Pure and Applied Chemistry (IUPAC) Name | 2-[(5Z)-5-[(E)-2-methyl-3-phenylprop-2-enylidene]-4-oxo-2-sulfanylidene-1,3-thiazolidin-3-yl]acetic acid | (2S,4S)-6-fluoro-2’,5’-dioxospiro[2 ,3-dihydrochromene-4,4’-imidazolidine]-2-carboxamide | (3R)-2’-[(4-bromo-2-fluorophenyl)methyl] spiro[pyrrolidine-3,4’-pyrrolo[1,2-a]pyrazine]-1’,2,3’,5-tetrone |
| Chemical Structure | 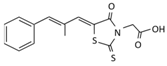 | 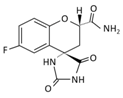 | 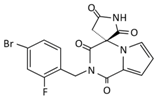 |
| Current Status | It is commercially available in Japan | Its development was terminated | Its development was terminated |
| References | Major Findings |
|---|---|
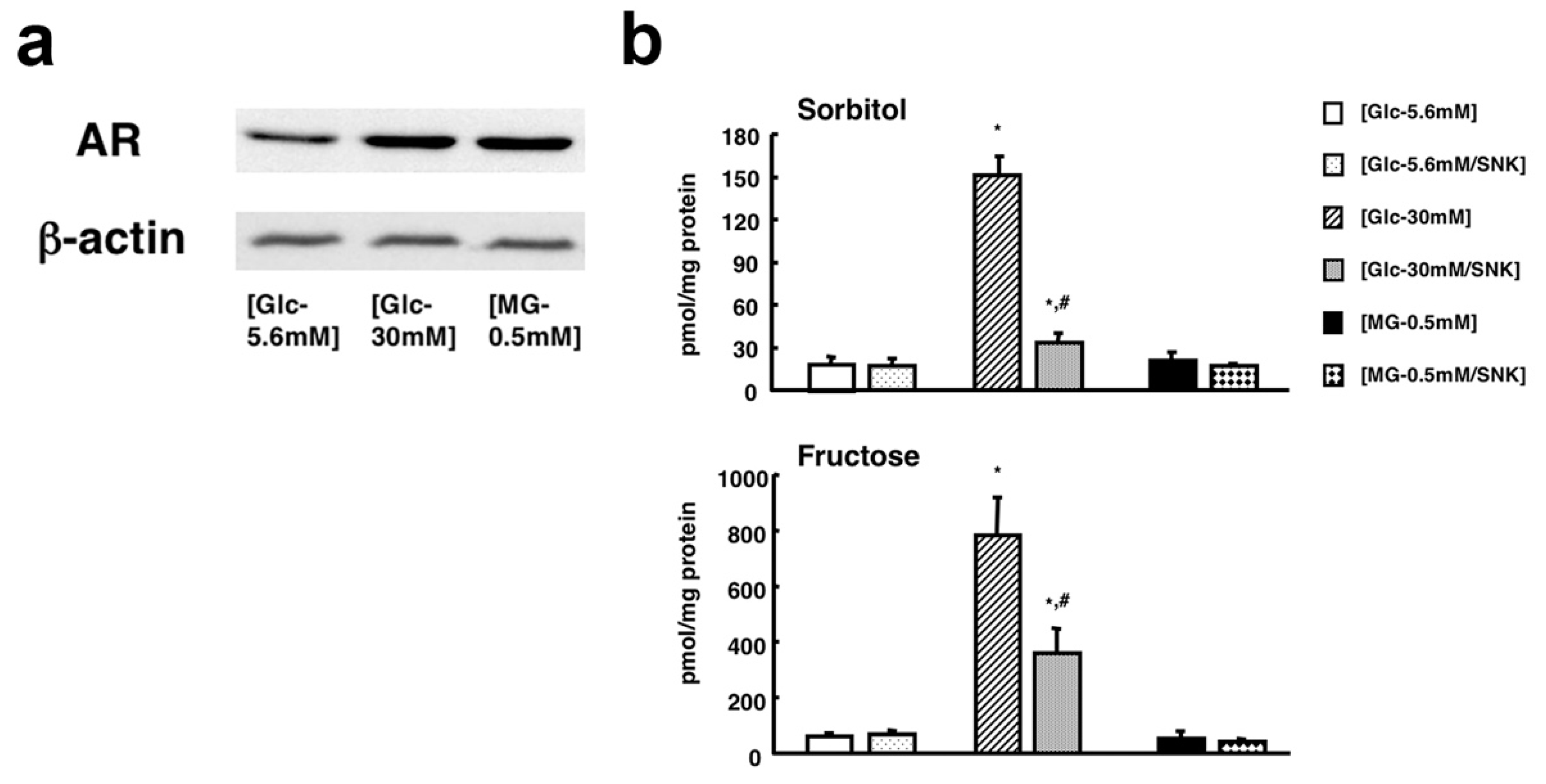
| Sango et al. (2006) [15] | High-glucose (≥30 mM) conditions increased AR mRNA/protein expression and the intracellular contents of sorbitol and fructose. |
| Ota et al. (2007) [65] | Metformin inhibited MG-induced apoptosis via JNK signaling pathway. |
| Sango et al. (2008) [33] | Both high-glucose and MG-induced upregulation of AR and oxidative stress markers (4-hydroxy-2-nonenal, acrolein and hexanoyl lysine). |
| Tosaki et al. (2008) [66] | Hyperglycemic insults inhibited nerve growth factor (NGF) secretion from IMS32 cells, being a cause of reduced neurite outgrowth activity of the conditioned media. |
| Kim et al. (2011) [67] | A mixture extract of Dioscorea japonica Thunb and Dioscorea nipponica Makino exerted neurite outgrowth-promoting activity on dorsal root ganglion (DRG) neurons, but not NGF induction effects on primary cultured and IMS32 Schwann cells. |
| Kim et al. (2013) [68] | Long-term (>8 wk) hyperglycemic insults up-regulated the expression of genes that promote glycolytic pathway and down-regulated the expression of genes involved in fatty acid metabolism, pentose–phosphate pathway and TCA cycle. |
| Hao et al. (2015) [69] | Hyperglycemic insults induced Schwann cell de-differentiation and suppressed insulin-like growth factor 1 expression via polyol pathway hyperactivity. |
| Cinci et al. (2015) [70] | Hyperglycemic insults enhanced AR expression, lipid peroxidation, and caspase-3 activity in a time-dependent manner (2 days < 7 days < 14 days). |
| Min et al. (2018) [71] | Human mobilized mononuclear cells (hMNC) restored DPN in STZ-mice and enhanced the expression of myelin protein zero in co-cultured IMS32 cells through hepatocyte growth factor-paracrine activity. |
| Tatsumi et al. (2019) [72] | Omega-3 polyunsaturated fatty acids alleviated oxidative stress-induced cell death by activating the antioxidant enzymes through the Nrf2 pathway. |
| Kato et al. (2019) [73] | Recurrent short-term hypoglycemic (2.5 mM) and hyperglycemic (25 mM) insults induced apoptosis and oxidative stress via the ER stress response. |
| Mizukami et al. (2020) [12] | Glucosamine induced IMS32 cell death via insulin signaling impairment and ATP depletion. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niimi, N.; Yako, H.; Takaku, S.; Chung, S.K.; Sango, K. Aldose Reductase and the Polyol Pathway in Schwann Cells: Old and New Problems. Int. J. Mol. Sci. 2021, 22, 1031. https://doi.org/10.3390/ijms22031031
Niimi N, Yako H, Takaku S, Chung SK, Sango K. Aldose Reductase and the Polyol Pathway in Schwann Cells: Old and New Problems. International Journal of Molecular Sciences. 2021; 22(3):1031. https://doi.org/10.3390/ijms22031031
Chicago/Turabian StyleNiimi, Naoko, Hideji Yako, Shizuka Takaku, Sookja K. Chung, and Kazunori Sango. 2021. "Aldose Reductase and the Polyol Pathway in Schwann Cells: Old and New Problems" International Journal of Molecular Sciences 22, no. 3: 1031. https://doi.org/10.3390/ijms22031031
APA StyleNiimi, N., Yako, H., Takaku, S., Chung, S. K., & Sango, K. (2021). Aldose Reductase and the Polyol Pathway in Schwann Cells: Old and New Problems. International Journal of Molecular Sciences, 22(3), 1031. https://doi.org/10.3390/ijms22031031







