3D Printed SiOC(N) Ceramic Scaffolds for Bone Tissue Regeneration: Improved Osteogenic Differentiation of Human Bone Marrow-Derived Mesenchymal Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
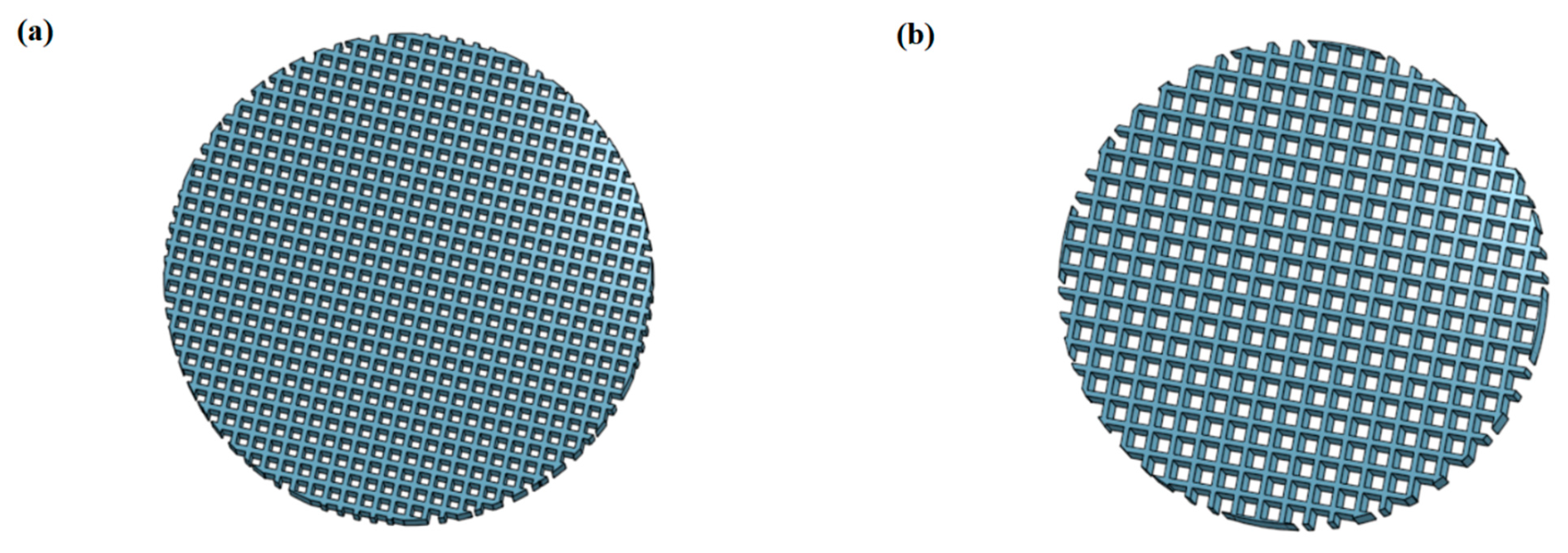
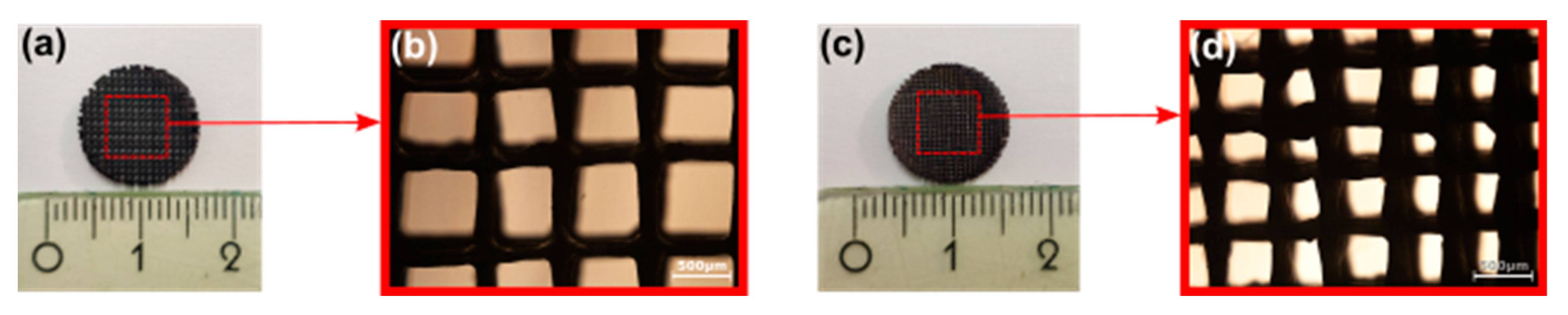
2.1. Preparation of Ceramic Scaffolds
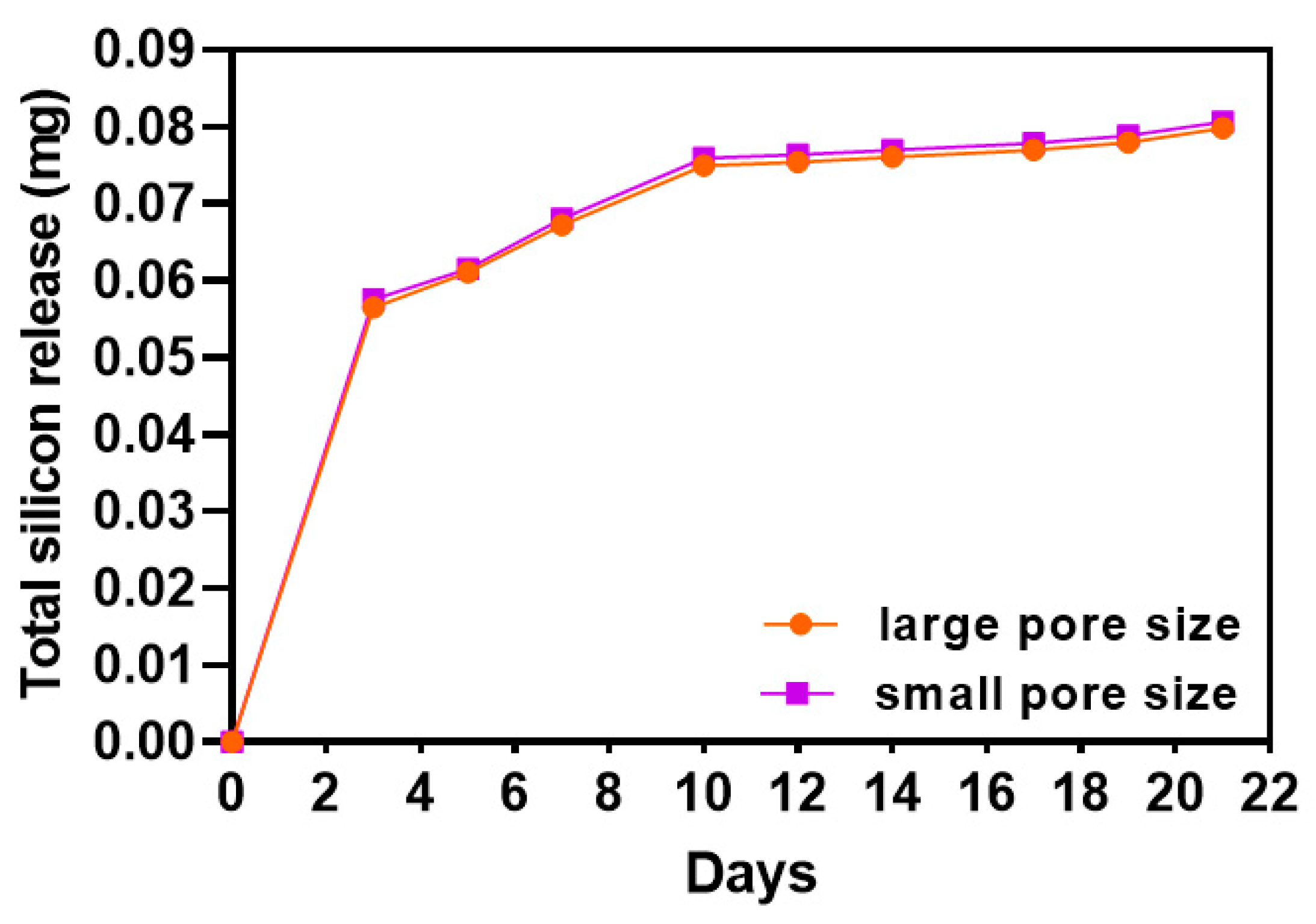
2.2. Silicon Release
2.3. Cell Culture
2.4. Cell Seeding and Differentiation
2.5. AlamarBlue Assay
2.6. DNA Quantification Assay
2.7. Alkaline Phosphatase (ALP) Activity Assay
2.8. RNA Isolation
2.9. cDNA Synthesis
2.10. Gene Expression by Quantitative Real-Time PCR (RT-qPCR)
2.11. Cell Morphology, Distribution, and Immunofluorescence Staining
2.12. Statistical Analysis
3. Results
3.1. Structural Characterization of SiOC(N) Ceramic Scaffolds
3.2. Silicon Iron Release of SiOC(N) Ceramic Scaffolds
3.3. Characterization of Proliferation, Metabolism of hMSCs during Osteogenic Differentiation
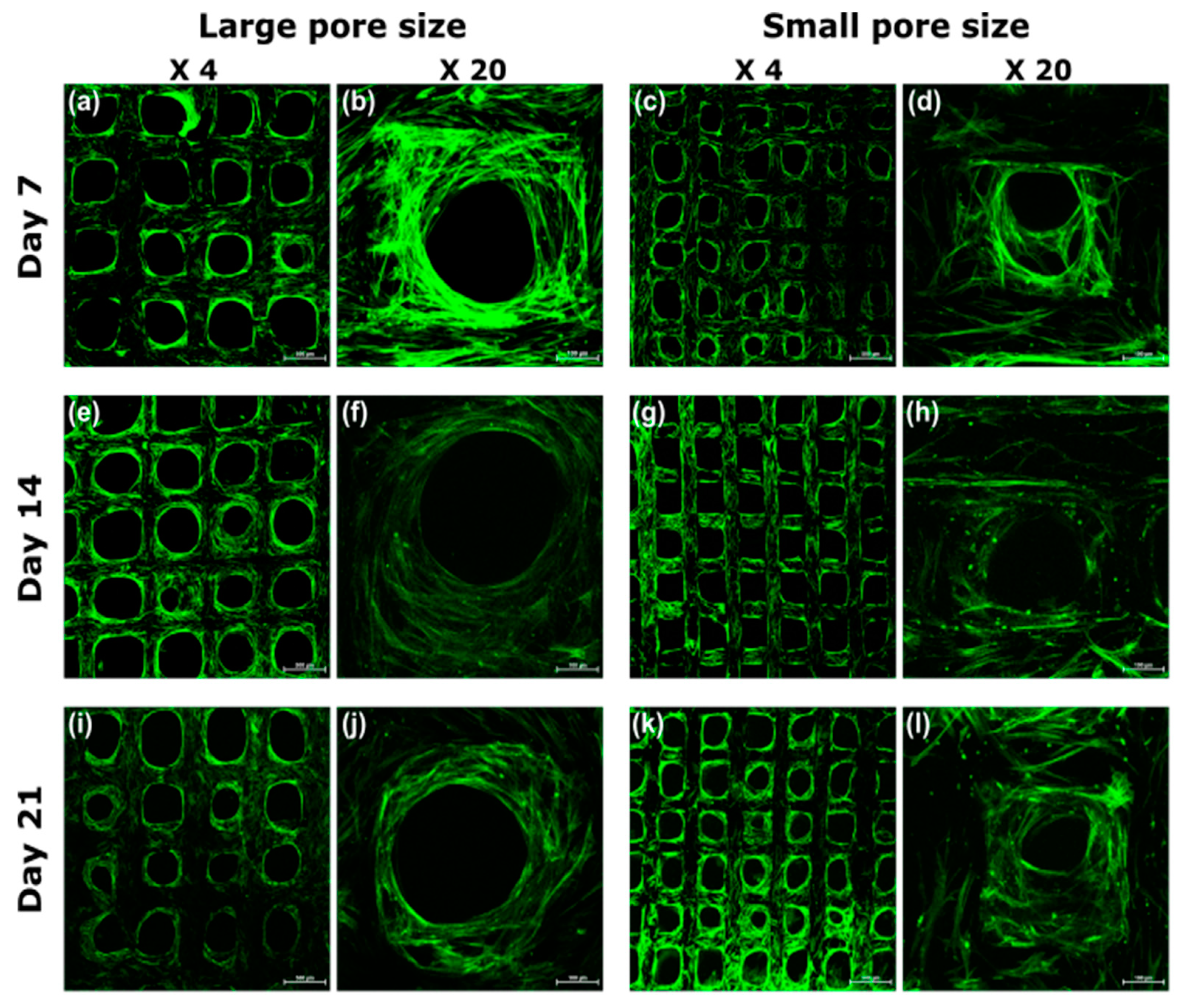
3.4. Visualization of Cell Morphology and Distribution by Confocal Laser Scanning Microscopy
3.5. Characterization of ALP Activity of hMSCs during Osteogenic Differentiation
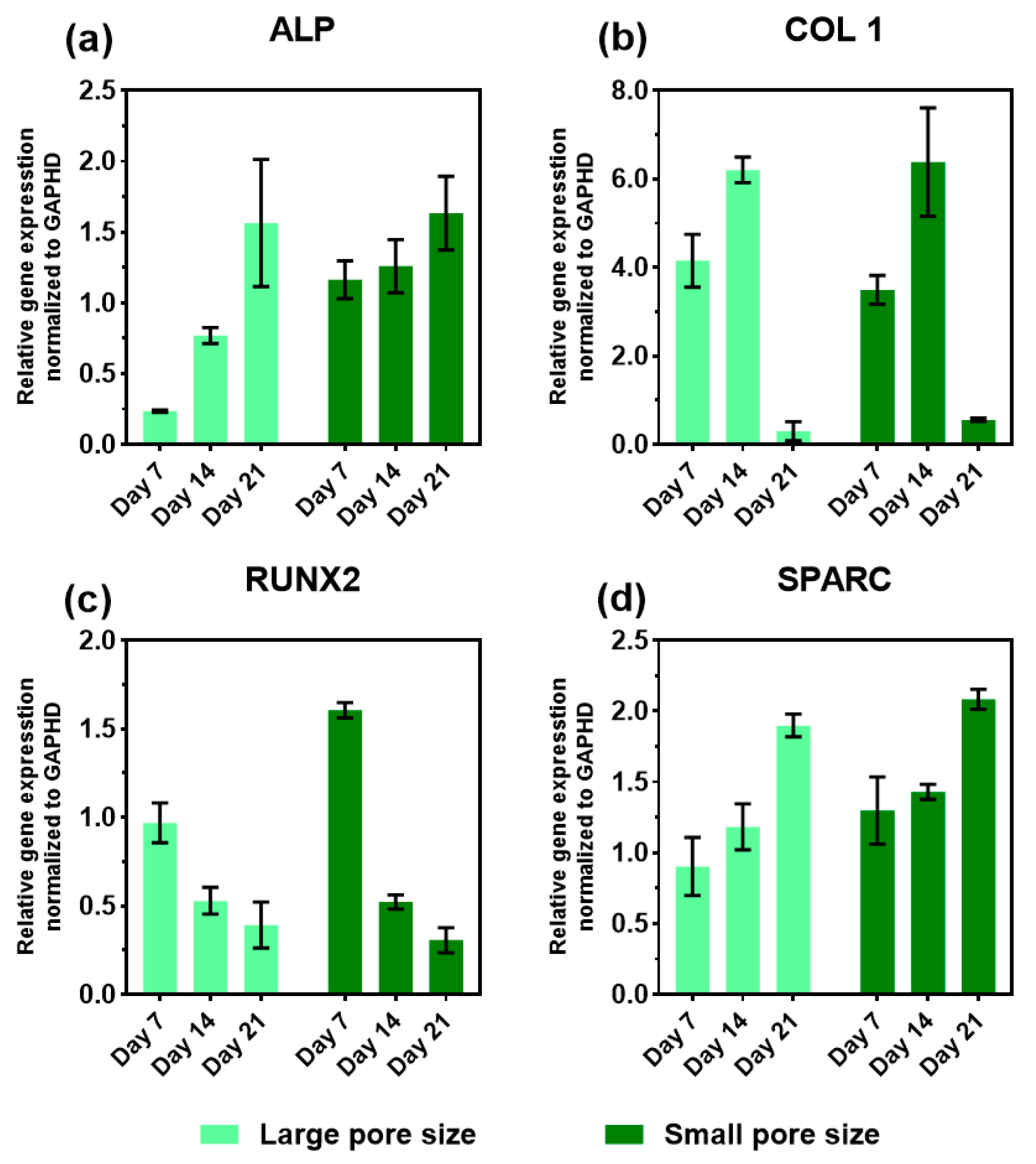
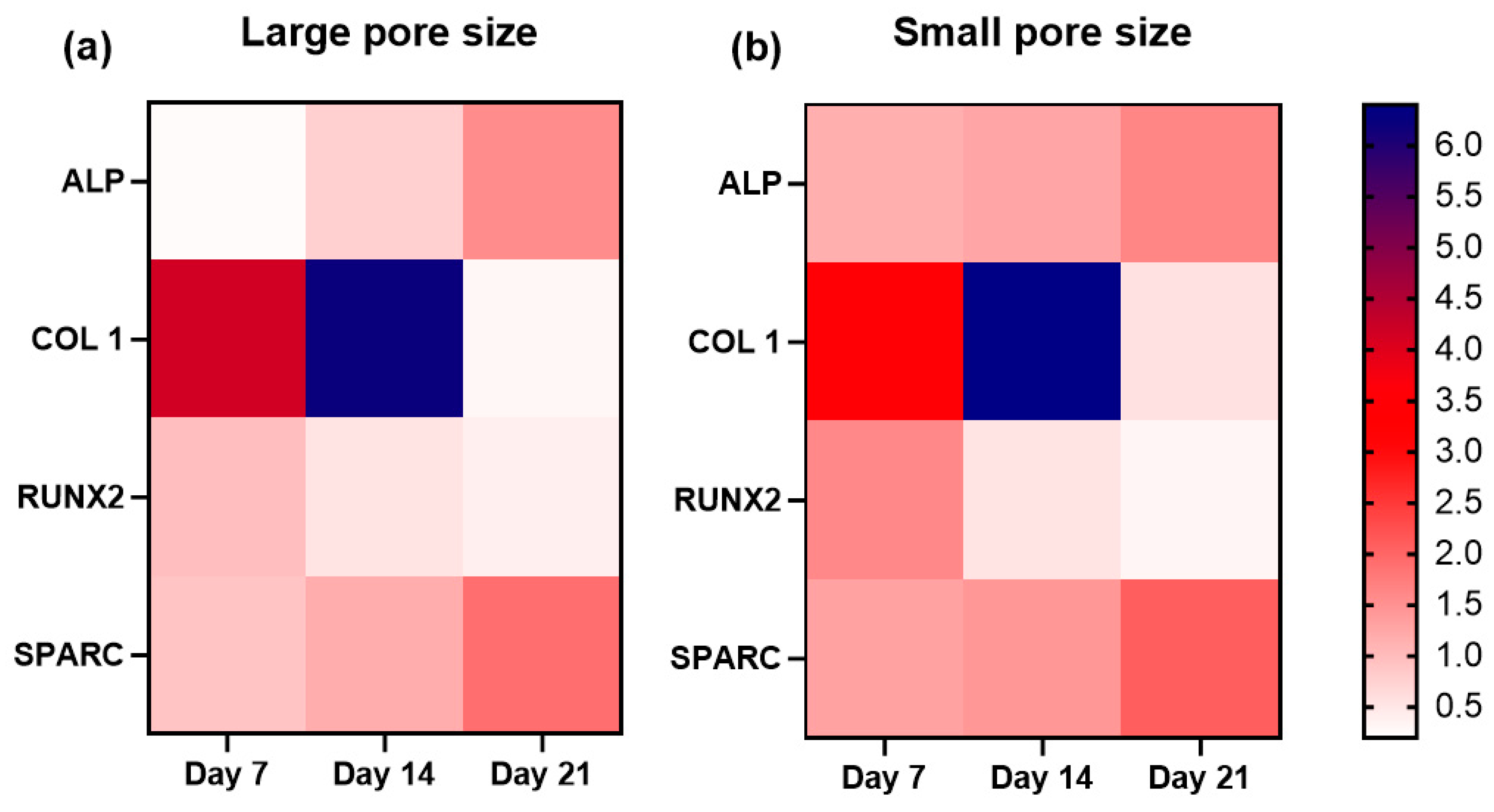
3.6. Expression of Osteogenic Marker Genes in the Presence of the SiOC(N) Ceramic Scaffold
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hing, K.A.; Revell, P.A.; Smith, N.; Buckland, T. Effect of Silicon Level on Rate, Quality and Progression of Bone Healing within Silicate-Substituted Porous Hydroxyapatite Scaffolds. Biomaterials 2006, 27, 5014–5026. [Google Scholar] [CrossRef] [PubMed]
- Casarrubios, L.; Gómez-Cerezo, N.; Sánchez-Salcedo, S.; Feito, M.J.; Serrano, M.C.; Saiz-Pardo, M.; Ortega, L.; de Pablo, D.; Díaz-Güemes, I.; Fernández-Tomé, B.; et al. Silicon Substituted Hydroxyapatite/VEGF Scaffolds Stimulate Bone Regeneration in Osteoporotic Sheep. Acta Biomater. 2020, 101, 544–553. [Google Scholar] [CrossRef]
- Kamboj, N.; Aghayan, M.; Rodrigo-Vazquez, C.S.; Rodríguez, M.A.; Hussainova, I. Novel Silicon-Wollastonite Based Scaffolds for Bone Tissue Engineering Produced by Selective Laser Melting. Ceram. Int. 2019, 45, 24691–24701. [Google Scholar] [CrossRef]
- Du, X.; Fu, S.; Zhu, Y. 3D Printing of Ceramic-Based Scaffolds for Bone Tissue Engineering: An Overview. J. Mater. Chem. B 2018, 6, 4397–4412. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Fiume, E. 3D Printing of Hierarchical Scaffolds Based on Mesoporous Bioactive Glasses (MBGs)—Fundamentals and Applications. Materials 2020, 13, 1688. [Google Scholar] [CrossRef] [Green Version]
- Arora, M.; Arora, E. The Promise of Silicon: Bone Regeneration and Increased Bone Density. J. Arthrosc. Jt. Surg. 2017, 4, 103–105. [Google Scholar] [CrossRef]
- Götz, W.; Tobiasch, E.; Witzleben, S.; Schulze, M. Effects of Silicon Compounds on Biomineralization, Osteogenesis, and Hard Tissue Formation. Pharmaceutics 2019, 11, 117. [Google Scholar] [CrossRef] [Green Version]
- Amaral, M.; Costa, M.A.; Lopes, M.A.; Silva, R.F.; Santos, J.D.; Fernandes, M.H. Si3N4-Bioglass Composites Stimulate the Proliferation of MG63 Osteoblast-like Cells and Support the Osteogenic Differentiation of Human Bone Marrow Cells. Biomaterials 2002, 23, 4897–4906. [Google Scholar] [CrossRef]
- Li, R.; Ying, B.; Wei, Y.; Xing, H.; Qin, Y.; Li, D. Comparative Evaluation of Sr-Incorporated Calcium Phosphate and Calcium Silicate as Bioactive Osteogenesis Coating Orthopedics Applications. Colloids Surf. A Physicochem. Eng. Asp. 2020, 600, 124834. [Google Scholar] [CrossRef]
- Gomes, P.S.; Botelho, C.; Lopes, M.A.; Santos, J.D.; Fernandes, M.H. Evaluation of Human Osteoblastic Cell Response to Plasma-Sprayed Silicon-Substituted Hydroxyapatite Coatings over Titanium Substrates. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 94, 337–346. [Google Scholar] [CrossRef]
- Liu, X.; Xie, Y.; Ding, C.; Chu, P.K. Early Apatite Deposition and Osteoblast Growth on Plasma-Sprayed Dicalcium Silicate Coating. J. Biomed. Mater. Res. Part A 2005, 74A, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, Y.; Xia, L.; Zhao, C.; Chen, L.; Yi, D.; Chang, J.; Huang, L.; Zheng, X.; Zhu, H.; et al. Fabrication of Nano-Structured Calcium Silicate Coatings with Enhanced Stability, Bioactivity and Osteogenic and Angiogenic Activity. Colloids Surf. B Biointerfaces 2015, 126, 358–366. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, Y.-Y.; Cui, F.-Z.; Zhang, S.-M.; Ruan, D.-K. A Study on a Tissue-Engineered Bone Using RhBMP-2 Induced Periosteal Cells with a Porous Nano-Hydroxyapatite/Collagen/Poly(l-Lactic Acid) Scaffold. Biomed. Mater. 2006, 1, 56–62. [Google Scholar] [CrossRef]
- Barrère, F.; Mahmood, T.A.; de Groot, K.; van Blitterswijk, C.A. Advanced Biomaterials for Skeletal Tissue Regeneration: Instructive and Smart Functions. Mater. Sci. Eng. R Rep. 2008, 59, 38–71. [Google Scholar] [CrossRef]
- Zeltinger, J.; Sherwood, J.K.; Graham, D.A.; Müeller, R.; Griffith, L.G. Effect of Pore Size and Void Fraction on Cellular Adhesion, Proliferation, and Matrix Deposition. Tissue Eng. 2001, 7, 557–572. [Google Scholar] [CrossRef]
- O’Brien, F.J.; Harley, B.A.; Yannas, I.V.; Gibson, L.J. The Effect of Pore Size on Cell Adhesion in Collagen-GAG Scaffolds. Biomaterials 2005, 26, 433–441. [Google Scholar] [CrossRef]
- Kuboki, Y.; Jin, Q.; Takita, H. Geometry of Carriers Controlling Phenotypic Expression in BMP-Induced Osteogenesis and Chondrogenesis. J. Bone Jt. Surg. 2001, 83, S105. [Google Scholar] [CrossRef]
- Hulbert, S.F.; Young, F.A.; Mathews, R.S.; Klawitter, J.J.; Talbert, C.D.; Stelling, F.H. Potential of Ceramic Materials as Permanently Implantable Skeletal Prostheses. J. Biomed. Mater. Res. 1970, 4, 433–456. [Google Scholar] [CrossRef]
- Chen, G.; Dong, C.; Yang, L.; Lv, Y. 3D Scaffolds with Different Stiffness but the Same Microstructure for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2015, 7, 15790–15802. [Google Scholar] [CrossRef] [PubMed]
- Breuls, R.G.M.; Jiya, T.U.; Smit, T.H. Scaffold Stiffness Influences Cell Behavior: Opportunities for Skeletal Tissue Engineering. Open Orthop. J. 2008, 2, 103–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mastrogiacomo, M.; Muraglia, A.; Komlev, V.; Peyrin, F.; Rustichelli, F.; Crovace, A.; Cancedda, R. Tissue Engineering of Bone: Search for a Better Scaffold. Orthod. Craniofacial Res. 2005, 8, 277–284. [Google Scholar] [CrossRef]
- Chan, B.P.; Leong, K.W. Scaffolding in Tissue Engineering: General Approaches and Tissue-Specific Considerations. Eur. Spine J. 2008, 17, 467–479. [Google Scholar] [CrossRef] [Green Version]
- Hollister, S.J. Porous Scaffold Design for Tissue Engineering. Nat. Mater 2005, 4, 518–524. [Google Scholar] [CrossRef]
- Boccaccio, A.; Ballini, A.; Pappalettere, C.; Tullo, D.; Cantore, S.; Desiate, A. Finite Element Method (FEM), Mechanobiology and Biomimetic Scaffolds in Bone Tissue Engineering. Int. J. Biol. Sci. 2011, 7, 112–132. [Google Scholar] [CrossRef]
- Kelly, D.J.; Prendergast, P.J. Prediction of the Optimal Mechanical Properties for a Scaffold Used in Osteochondral Defect Repair. Tissue Eng. 2006, 12, 2509–2519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandino, C.; Lacroix, D. A Dynamical Study of the Mechanical Stimuli and Tissue Differentiation within a CaP Scaffold Based on Micro-CT Finite Element Models. Biomech. Model Mechanobiol. 2011, 10, 565–576. [Google Scholar] [CrossRef]
- Williams, J.M.; Adewunmi, A.; Schek, R.M.; Flanagan, C.L.; Krebsbach, P.H.; Feinberg, S.E.; Hollister, S.J.; Das, S. Bone Tissue Engineering Using Polycaprolactone Scaffolds Fabricated via Selective Laser Sintering. Biomaterials 2005, 26, 4817–4827. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhou, P.; Cheng, X.; Xie, Y.; Liang, C.; Li, C.; Xu, S. Selective Laser Sintering Fabrication of Nano-Hydroxyapatite/Poly--ε-Caprolactone Scaffolds for Bone Tissue Engineering Applications. IJN 2013, 8, 4197–4213. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Yeatts, A.; Dean, D.; Fisher, J.P. Stereolithographic Bone Scaffold Design Parameters: Osteogenic Differentiation and Signal Expression. Tissue Eng. Part B Rev. 2010, 16, 523–539. [Google Scholar] [CrossRef] [Green Version]
- Zein, I.; Hutmacher, D.W.; Tan, K.C.; Teoh, S.H. Fused Deposition Modeling of Novel Scaffold Architectures for Tissue Engineering Applications. Biomaterials 2002, 23, 1169–1185. [Google Scholar] [CrossRef]
- Do, A.-V.; Khorsand, B.; Geary, S.M.; Salem, A.K. 3D Printing of Scaffolds for Tissue Regeneration Applications. Adv. Healthc. Mater. 2015, 4, 1742–1762. [Google Scholar] [CrossRef] [Green Version]
- Jones, R.; Haufe, P.; Sells, E.; Iravani, P.; Olliver, V.; Palmer, C.; Bowyer, A. RepRap—The Replicating Rapid Prototyper. Robotica 2011, 29, 177–191. [Google Scholar] [CrossRef] [Green Version]
- Bowyer, A. 3D Printing and Humanity’s First Imperfect Replicator. 3D Print. Addit. Manuf. 2014, 1, 4–5. [Google Scholar] [CrossRef]
- Zhang, B.; Seong, B.; Nguyen, V.; Byun, D. 3D Printing of High-Resolution PLA-Based Structures by Hybrid Electrohydrodynamic and Fused Deposition Modeling Techniques. J. Micromech. Microeng. 2016, 26, 025015. [Google Scholar] [CrossRef]
- Colombo, P.; Mera, G.; Riedel, R.; Sorarù, G.D. Polymer-Derived Ceramics: 40 Years of Research and Innovation in Advanced Ceramics. J. Am. Ceram. Soc. 2010, 93, 1805–1837. [Google Scholar] [CrossRef]
- Santhosh, B.; Vakifahmetoglu, C.; Ionescu, E.; Reitz, A.; Albert, B.; Sorarù, G.D. Processing and Thermal Characterization of Polymer Derived SiCN(O) and SiOC Reticulated Foams. Ceram. Int. 2020, 46, 5594–5601. [Google Scholar] [CrossRef]
- Vakifahmetoglu, C.; Semerci, T.; Gurlo, A.; Soraru, G.D. Polymer Derived Ceramic Aerogels. Curr. Opin. Solid State Mater. Sci. 2021, 25, 100936. [Google Scholar] [CrossRef]
- Arango-Ospina, M.; Xie, F.; Gonzalo-Juan, I.; Riedel, R.; Ionescu, E.; Boccaccini, A.R. Review: Silicon Oxycarbide Based Materials for Biomedical Applications. Appl. Mater. Today 2020, 18, 100482. [Google Scholar] [CrossRef]
- Vakifahmetoglu, C.; Zeydanli, D.; Ozalp, V.C.; Borsa, B.A.; Soraru, G.D. Hierarchically Porous Polymer Derived Ceramics: A Promising Platform for Multidrug Delivery Systems. Mater. Des. 2018, 140, 37–44. [Google Scholar] [CrossRef]
- Kulkarni, A.; Pearce, J.; Yang, Y.; Motta, A.; Sorarù, G.D. SiOC(N) Cellular Structures with Dense Struts by Integrating Fused Filament Fabrication 3D Printing with Polymer-Derived Ceramics. Adv. Eng. Mater. 2021, 2100535. [Google Scholar] [CrossRef]
- Kulkarni, A.; Sorarù, G.D.; Pearce, J.M. Polymer-Derived SiOC Replica of Material Extrusion-Based 3-D Printed Plastics. Addit. Manuf. 2020, 32, 100988. [Google Scholar] [CrossRef] [Green Version]
- Woern, A.L.; Pearce, J.M. Distributed Manufacturing of Flexible Products: Technical Feasibility and Economic Viability. Technologies 2017, 5, 71. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Gao, Y. The Manufacture of 3D Printing of Medical Grade TPU. Prog. Addit. Manuf. 2017, 2, 117–123. [Google Scholar] [CrossRef]
- Open-Source Foundation. Available online: https://osf.io/grv8m/ (accessed on 11 November 2021).
- Stein, G.S.; Lian, J.B.; Owen, T.A. Relationship of Cell Growth to the Regulation of Tissue-Specific Gene Expression during Osteoblast Differentiation. FASEB J. 1990, 4, 3111–3123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bicer, M.; Cottrell, G.S.; Widera, D. Impact of 3D Cell Culture on Bone Regeneration Potential of Mesenchymal Stromal Cells. Stem Cell Res. Ther. 2021, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Mayer, H.; Bertram, H.; Lindenmaier, W.; Korff, T.; Weber, H.; Weich, H. Vascular Endothelial Growth Factor (VEGF-A) Expression in Human Mesenchymal Stem Cells: Autocrine and Paracrine Role on Osteoblastic and Endothelial Differentiation. J. Cell. Biochem. 2005, 95, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.B.; Bernhardt, A.; Lode, A.; Heinemann, C.; Thieme, S.; Hanke, T.; Varma, H.; Gelinsky, M.; John, A. A Bioactive Triphasic Ceramic-Coated Hydroxyapatite Promotes Proliferation and Osteogenic Differentiation of Human Bone Marrow Stromal Cells. J. Biomed. Mater. Res. Part A 2009, 90A, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Botelho, C.M.; Brooks, R.A.; Best, S.M.; Lopes, M.A.; Santos, J.D.; Rushton, N.; Bonfield, W. Human Osteoblast Response to Silicon-Substituted Hydroxyapatite. J. Biomed. Mater. Res. Part A 2006, 79A, 723–730. [Google Scholar] [CrossRef]
- Komori, T. Regulation of Osteoblast Differentiation by Transcription Factors. J. Cell. Biochem. 2006, 99, 1233–1239. [Google Scholar] [CrossRef]
- Amiri, B.; Ghollasi, M.; Shahrousvand, M.; Kamali, M.; Salimi, A. Osteoblast Differentiation of Mesenchymal Stem Cells on Modified PES-PEG Electrospun Fibrous Composites Loaded with Zn2SiO4 Bioceramic Nanoparticles. Differentiation 2016, 92, 148–158. [Google Scholar] [CrossRef]
- Zur Nieden, N.I.; Kempka, G.; Ahr, H.J. In Vitro Differentiation of Embryonic Stem Cells into Mineralized Osteoblasts. Differentiation 2003, 71, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Aubin, J.E.; Triffitt, J.T. Chapter 4—Mesenchymal Stem Cells and Osteoblast Differentiation. In Principles of Bone Biology, 2nd ed.; Bilezikian, J.P., Raisz, L.G., Rodan, G.A., Eds.; Academic Press: San Diego, CA, USA, 2002; pp. 59–81. ISBN 978-0-12-098652-1. [Google Scholar]
- Fuchs, S.; Jiang, X.; Schmidt, H.; Dohle, E.; Ghanaati, S.; Orth, C.; Hofmann, A.; Motta, A.; Migliaresi, C.; Kirkpatrick, C.J. Dynamic Processes Involved in the Pre-Vascularization of Silk Fibroin Constructs for Bone Regeneration Using Outgrowth Endothelial Cells. Biomaterials 2009, 30, 1329–1338. [Google Scholar] [CrossRef] [PubMed]


| Code | Gene | Primer | Catalog No. |
|---|---|---|---|
| ALP | Alkaline phosphatase | ALPL, human | qHsaCID0010031 |
| COL1 | Collagen type I | COL1A1, human | qHsaCED0043248 |
| RUNX2 | Runt-related transcription factor 2 | RUNX2, human | qHsaCED0044067 |
| SPARC | Osteonectin | SPARC, human | qHsaCID0010332 |
| GAPHD | glyceraldehyde-3 phosphate dehydrogenase | GAPDH, human | qHsaCED0038674 |
| Large Pore Size | Small Pore Size | |
|---|---|---|
| Diameter (mm) | 13 | 13 |
| Thickness (mm) | 1.5 | 1.5 |
| Pore size (μm) | 500 | 300 |
| Theoretical total surface area (mm2) | 1077.00 | 1358.84 |
| Theoretical top/bottom surface area (mm2) | 53.88 | 73.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Kulkarni, A.; Soraru, G.D.; Pearce, J.M.; Motta, A. 3D Printed SiOC(N) Ceramic Scaffolds for Bone Tissue Regeneration: Improved Osteogenic Differentiation of Human Bone Marrow-Derived Mesenchymal Stem Cells. Int. J. Mol. Sci. 2021, 22, 13676. https://doi.org/10.3390/ijms222413676
Yang Y, Kulkarni A, Soraru GD, Pearce JM, Motta A. 3D Printed SiOC(N) Ceramic Scaffolds for Bone Tissue Regeneration: Improved Osteogenic Differentiation of Human Bone Marrow-Derived Mesenchymal Stem Cells. International Journal of Molecular Sciences. 2021; 22(24):13676. https://doi.org/10.3390/ijms222413676
Chicago/Turabian StyleYang, Yuejiao, Apoorv Kulkarni, Gian Domenico Soraru, Joshua M. Pearce, and Antonella Motta. 2021. "3D Printed SiOC(N) Ceramic Scaffolds for Bone Tissue Regeneration: Improved Osteogenic Differentiation of Human Bone Marrow-Derived Mesenchymal Stem Cells" International Journal of Molecular Sciences 22, no. 24: 13676. https://doi.org/10.3390/ijms222413676
APA StyleYang, Y., Kulkarni, A., Soraru, G. D., Pearce, J. M., & Motta, A. (2021). 3D Printed SiOC(N) Ceramic Scaffolds for Bone Tissue Regeneration: Improved Osteogenic Differentiation of Human Bone Marrow-Derived Mesenchymal Stem Cells. International Journal of Molecular Sciences, 22(24), 13676. https://doi.org/10.3390/ijms222413676








