Recent Advances in the Inhibition of the IL-4 Cytokine Pathway for the Treatment of Allergen-Induced Asthma
Abstract
:1. Introduction
2. IL-4 and IL-13 Pathways
3. Other Interleukin Pathways
4. IL-4 Pathway Inhibition
5. IL-13 Inhibition
6. STAT6 Inhibition
7. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dierick, B.J.H.; van der Molen, T.; Flokstra-de Blok, B.M.J.; Muraro, A.; Postma, M.J.; Kocks, J.W.H.; van Boven, J.F.M. Burden and socioeconomics of asthma, allergic rhinitis, atopic dermatitis and food allergy. Expert Rev. Pharmacoecon. Outcomes Res. 2020, 20, 437–453. [Google Scholar] [CrossRef]
- Igea, J.M. The history of the idea of allergy. Allergy 2013, 68, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Global Asthma Network. The Global Asthma Report 2018. Available online: http://globalasthmareport.org/ (accessed on 20 February 2021).
- Baldacci, S.; Maio, S.; Cerrai, S.; Sarno, G.; Baïz, N.; Simoni, M.; Annesi-Maesano, I.; Viegi, G.; HEALS Study. Allergy and asthma: Effects of the exposure to particulate matter and biological allergens. Respir. Med. 2015, 109, 1089–1104. [Google Scholar] [CrossRef] [Green Version]
- CDC COVID-19 Response Team. Coronavirus Disease 2019 in Children—United States, 12 February–2 April 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Abrams, E.M.; Sinha, I.; Fernandes, R.M.; Hawcutt, D.B. Pediatric asthma and COVID-19: The known, the unknown, and the controversial. Pediatr. Pulmonol. 2020, 55, 3573–3578. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhi, Y.; Ying, S. COVID-19 and Asthma: Reflection During the Pandemic. Clin. Rev. Allergy Immunol. 2020, 59, 78–88. [Google Scholar] [CrossRef]
- Mims, J.W. Asthma: Definitions and pathophysiology. Int. Forum Allergy Rhinol. 2015, 5 (Suppl. 1), S2–S6. [Google Scholar] [CrossRef]
- Bagnasco, D.; Ferrando, M.; Varricchi, G.; Passalacqua, G.; Canonica, G.W. A Critical Evaluation of Anti-IL-13 and Anti-IL-4 Strategies in Severe Asthma. Int. Arch. Allergy Immunol. 2016, 170, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.W.; Campbell, J.D.; Ghushchyan, V.H.; Globe, G. Outcomes before and after treatment escalation to Global Initiative for Asthma steps 4 and 5 in severe asthma. Ann. Allergy Asthma Immunol. 2015, 114, 462–469. [Google Scholar] [CrossRef]
- Bateman, E.D.; Boushey, H.A.; Bousquet, J.; Busse, W.W.; Clark, T.J.; Pauwels, R.A.; Pedersen, S.E.; GOAL Investigators Group. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am. J. Respir. Crit. Care Med. 2004, 170, 836–844. [Google Scholar] [CrossRef] [Green Version]
- Gieseck, R.L.; Wilson, M.S.; Wynn, T.A. Type 2 immunity in tissue repair and fibrosis. Nat. Rev. Immunol. 2018, 18, 62–76. [Google Scholar] [CrossRef]
- Bao, K.; Reinhardt, R.L. The differential expression of IL-4 and IL-13 and its impact on type-2 immunity. Cytokine 2015, 75, 25–37. [Google Scholar] [CrossRef] [Green Version]
- Busse, W.W. Biological treatments for severe asthma: A major advance in asthma care. Allergol. Int. 2019, 68, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J. T helper 2 (Th2) cell differentiation, type 2 innate lymphoid cell (ILC2) development and regulation of interleukin-4 (IL-4) and IL-13 production. Cytokine 2015, 75, 14–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antczak, A.; Domanska-Senderowska, D.; Gorski, P.; Pastuszak-Lewandoska, D.; Nielepkowicz-Gozdzinska, A.; Szewczyk, K.; Kurmanowska, Z.; Kiszałkiewicz, J.; Brzeziańska-Lasota, E. Analysis of changes in expression of IL-4/IL-13/STAT6 pathway and correlation with the selected clinical parameters in patients with atopic asthma. Int. J. Immunopathol. Pharmacol. 2016, 29, 195–204. [Google Scholar] [CrossRef] [Green Version]
- Kelly-Welch, A.; Hanson, E.M.; Keegan, A.D. Interleukin-4 (IL-4) pathway. Sci. Signal. 2005, 2005, cm9. [Google Scholar] [CrossRef]
- Sheikh, F.; Dickensheets, H.; Pedras-Vasconcelos, J.; Ramalingam, T.; Helming, L.; Gordon, S.; Donnelly, R.P. The Interleukin-13 Receptor-α1 Chain Is Essential for Induction of the Alternative Macrophage Activation Pathway by IL-13 but Not IL-4. J. Innate Immun. 2015, 7, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Kelly-Welch, A.; Hanson, E.M.; Keegan, A.D. Interleukin-13 (IL-13) pathway. Sci. Signal. 2005, 2005, cm8. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.J.; Wang, S.H.; Wu, C.C.; Su, Y.A.; Chiang, C.Y.; Lai, C.H.; Wang, T.H.; Cheng, T.-L.; Kuo, J.-Y.; Hsu, T.-C.; et al. IL-4 and IL-13 Promote Proliferation of Mammary Epithelial Cells through STAT6 and IRS-1. Int. J. Mol. Sci. 2021, 22, 12008. [Google Scholar] [CrossRef]
- Liu, C.; Richard, K.; Wiggins, M.; Zhu, X.; Conrad, D.H.; Song, W. CD23 can negatively regulate B-cell receptor signaling. Sci. Rep. 2016, 6, 25629. [Google Scholar] [CrossRef] [Green Version]
- Kneitz, C.; Goller, M.; Seggewiss, R.; Yaman, A.; Serfling, E.; Tony, H.P. STAT6 and the regulation of CD23 expression in B-chronic lymphocytic leukemia. Leuk. Res. 2000, 24, 331–337. [Google Scholar] [CrossRef]
- Walford, H.H.; Doherty, T.A. STAT6 and lung inflammation. JAK-STAT 2013, 2, e25301. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.; Ravi, A.; Southworth, T. CRTH2 antagonists in asthma: Current perspectives. Clin. Pharmacol. 2017, 9, 165–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauder, A.J.; McKenzie, A.N.J.; Sanderson, C.J. Interleukin-5. In Encyclopedia of Hormones; Henry, H.L., Norman, A.W., Eds.; Academic Press: New York, NY, USA, 2003; pp. 422–429. [Google Scholar]
- Pelaia, C.; Paoletti, G.; Puggioni, F.; Racca, F.; Pelaia, G.; Canonica, G.W.; Heffler, E. Interleukin-5 in the Pathophysiology of Severe Asthma. Front. Physiol. 2019, 10, 1514. [Google Scholar] [CrossRef]
- Greenfeder, S.; Umland, S.P.; Cuss, F.M.; Chapman, R.W.; Egan, R.W. Th2 cytokines and asthma. The role of interleukin-5 in allergic eosinophilic disease. Respir. Res. 2001, 2, 71–79. [Google Scholar] [CrossRef]
- Wenzel, S.; Castro, M.; Corren, J.; Maspero, J.; Wang, L.; Zhang, B.; Pirozzi, G.; Sutherland, E.R.; Evans, R.R.; Joish, V.N.; et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: A randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet 2016, 388, 31–44. [Google Scholar] [CrossRef]
- Castro, M.; Corren, J.; Pavord, I.D.; Maspero, J.; Wenzel, S.; Rabe, K.F.; Busse, W.W.; Ford, L.; Sher, L.; FitzGerald, J.M.; et al. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. N. Engl. J. Med. 2018, 378, 2486–2496. [Google Scholar] [CrossRef]
- McGregor, M.C.; Krings, J.G.; Nair, P.; Castro, M. Role of Biologics in Asthma. Am. J. Respir. Crit. Care Med. 2019, 199, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Santini, G.; Mores, N.; Malerba, M.; Mondino, C.; Anzivino, R.; Macis, G.; Montuschi, P. Dupilumab for the treatment of asthma. Expert Opin. Investig. Drugs 2017, 26, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Hart, T.K.; Blackburn, M.N.; Brigham-Burke, M.; Dede, K.; Al-Mahdi, N.; Zia-Amirhosseini, P.; Cook, R.M. Preclinical efficacy and safety of pascolizumab (SB 240683): A humanized anti-interleukin-4 antibody with therapeutic potential in asthma. Clin. Exp. Immunol. 2002, 130, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Long, A.A. Monoclonal antibodies and other biologic agents in the treatment of asthma. MAbs 2009, 1, 237–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antoniu, S.A. Pitrakinra, a dual IL-4/IL-13 antagonist for the potential treatment of asthma and eczema. Curr. Opin. Investig. Drugs 2010, 11, 1286–1294. [Google Scholar]
- Slager, R.E.; Otulana, B.A.; Hawkins, G.A.; Yen, Y.P.; Peters, S.P.; Wenzel, S.E.; Meyers, D.A.; Bleecker, R. IL-4 receptor polymorphisms predict reduction in asthma exacerbations during response to an anti-IL-4 receptor alpha antagonist. J. Allergy Clin. Immunol. 2012, 130, 516–522.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pennington, E.J.; Wechsler, M.E.; Ortega, V.E. Chapter 9—Pharmacogenomics and Applications to Asthma Management. In Personalizing Asthma Management for the Clinician; Szefler, S.J., Holguin, F., Wechsler, M.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 97–112. [Google Scholar]
- Loh, T.Y.; Hsiao, J.L.; Shi, V.Y. Therapeutic Potential of Lebrikizumab in the Treatment of Atopic Dermatitis. J. Asthma Allergy 2020, 13, 109–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corren, J.; Lemanske, R.F.; Hanania, N.A.; Korenblat, P.E.; Parsey, M.V.; Arron, J.R.; Harris, J.M.; Scheerens, H.; Wu, L.C.; Su, Z.; et al. Lebrikizumab treatment in adults with asthma. N. Engl. J. Med. 2011, 365, 1088–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanania, N.A.; Korenblat, P.; Chapman, K.R.; Bateman, E.D.; Kopecky, P.; Paggiaro, P.; Yokoyama, A.; Olsson, J.; Gray, S.; Holweg, C.T.; et al. Efficacy and safety of lebrikizumab in patients with uncontrolled asthma (LAVOLTA I and LAVOLTA II): Replicate, phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir. Med. 2016, 4, 781–796. [Google Scholar] [CrossRef]
- Hua, F.; Ribbing, J.; Reinisch, W.; Cataldi, F.; Martin, S. A pharmacokinetic comparison of anrukinzumab, an anti- IL-13 monoclonal antibody, among healthy volunteers, asthma and ulcerative colitis patients. Br. J. Clin. Pharmacol. 2015, 80, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Reinisch, W.; Panes, J.; Khurana, S.; Toth, G.; Hua, F.; Comer, G.M.; Hinz, M.; Page, K.; O’Toole, M.; McDonnell Moorehead, T.; et al. Anrukinzumab, an anti-interleukin 13 monoclonal antibody, in active UC: Efficacy and safety from a phase IIa randomised multicentre study. Gut 2015, 64, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Apter, A.J. The tralokinumab story: Nothing is ever simple. J. Allergy Clin. Immunol. 2019, 143, 1336–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panettieri, R.A., Jr.; Wang, M.; Braddock, M.; Bowen, K.; Colice, G. Tralokinumab for the treatment of severe, uncontrolled asthma: The ATMOSPHERE clinical development program. Immunotherapy 2018, 10, 473–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ClinicalTrials.gov. Tralokinumab in Combination with Topical Corticosteroids for Moderate to Severe Atopic Dermatitis—ECZTRA 3. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT03363854 (accessed on 16 November 2021).
- Oh, C.K.; Geba, G.P.; Molfino, N. Investigational therapeutics targeting the IL-4/IL-13/STAT-6 pathway for the treatment of asthma. Eur. Respir. Rev. 2010, 19, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Stolzenberger, S.; Haake, M.; Duschl, A. Specific inhibition of interleukin-4-dependent Stat6 activation by an intracellularly delivered peptide. Eur. J. Biochem. 2001, 268, 4809–4814. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Shi, W.; Zheng, H. Inhibition of STAT6/Anoctamin-1 Activation Suppresses Proliferation and Invasion of Gastric Cancer Cells. Cancer Biother. Radiopharm. 2018, 33, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Ramirez, Y.; Colly, V.; Villanueva Gonzalez, G.; Leon-Cabrera, S. Signal transducer and activator of transcription 6 as a target in colon cancer therapy. Oncol. Lett. 2020, 20, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Rodríguez, M.G.; Sánchez-Barrera, C.Á.; Callejas, B.E.; García-Castillo, V.; Beristain-Terrazas, D.L.; Delgado-Buenrostro, N.L.; Chirino, Y.I.; León-Cabrera, S.A.; Rodriguez-Sosa, M.; Gutierrez-cirlos, E.B.; et al. Use of STAT6 Phosphorylation Inhibitor and Trimethylglycine as New Adjuvant Therapies for 5-Fluorouracil in Colitis-Associated Tumorigenesis. Int. J. Mol. Sci. 2020, 21, 2130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sparrow, E.; Friede, M.; Sheikh, M.; Torvaldsen, S. Therapeutic antibodies for infectious diseases. Bull. World Health Organ. 2017, 95, 235–237. [Google Scholar] [CrossRef]
- Zimmermann, M.; Rind, D.; Chapman, R.; Kumar, V.; Kahn, S.; Carlson, J. Economic Evaluation of Dupilumab for Moderate-to-Severe Atopic Dermatitis: A Cost-Utility Analysis. J. Drugs Dermatol. 2018, 17, 750–756. [Google Scholar] [PubMed]
- Anselmo, A.C.; Gokarn, Y.; Mitragotri, S. Non-invasive delivery strategies for biologics. Nat. Rev. Drug Discov. 2019, 18, 19–40. [Google Scholar] [CrossRef]
- Saeed, A.F.; Wang, R.; Ling, S.; Wang, S. Antibody Engineering for Pursuing a Healthier Future. Front. Microbiol. 2017, 8, 495. [Google Scholar] [CrossRef] [Green Version]
- Matucci, A.; Vultaggio, A.; Danesi, R. The use of intravenous versus subcutaneous monoclonal antibodies in the treatment of severe asthma: A review. Respir. Res. 2018, 19, 154. [Google Scholar] [CrossRef]
- Drucker, D.J. Advances in oral peptide therapeutics. Nat. Rev. Drug Discov. 2020, 19, 277–289. [Google Scholar] [CrossRef] [PubMed]
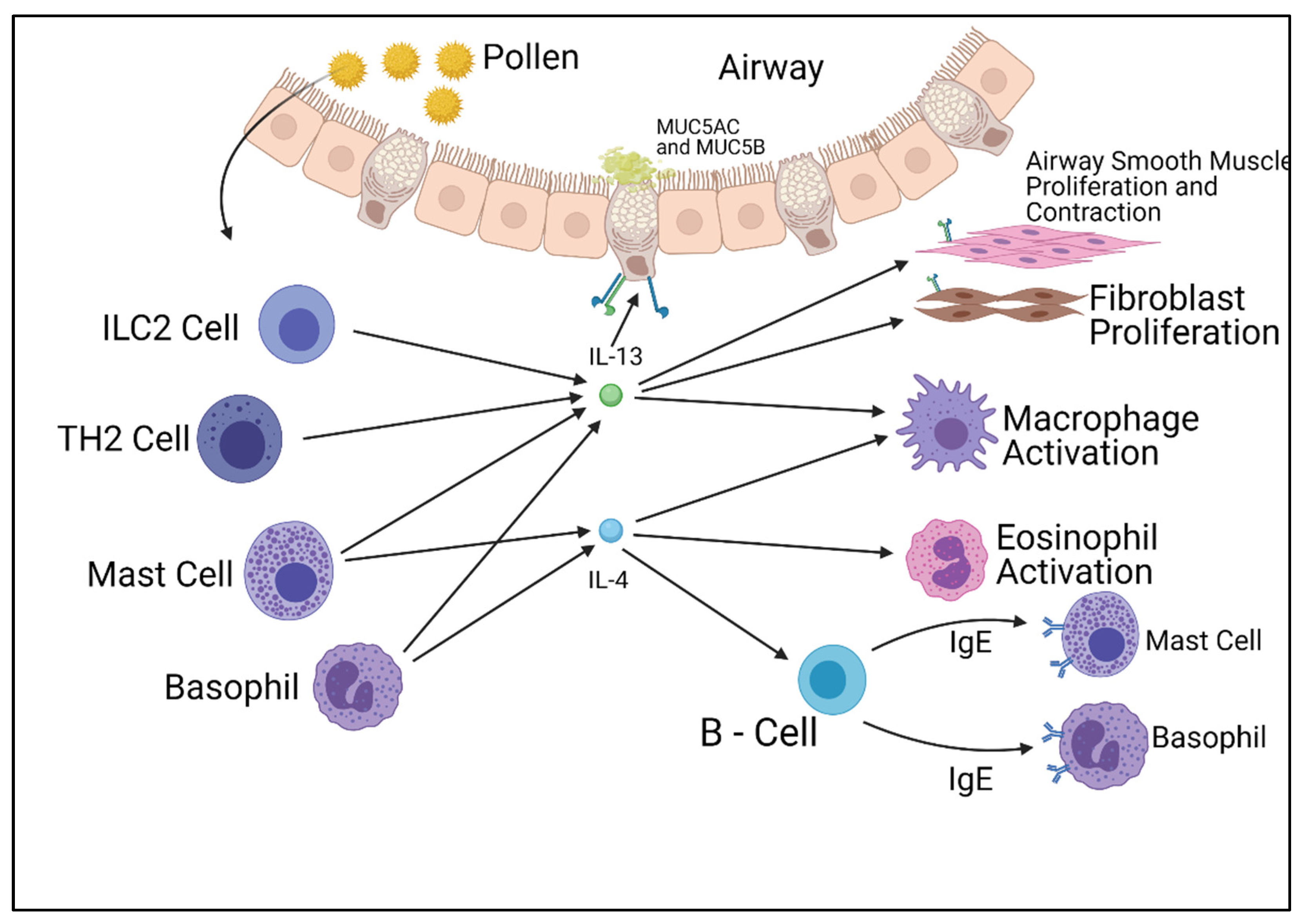
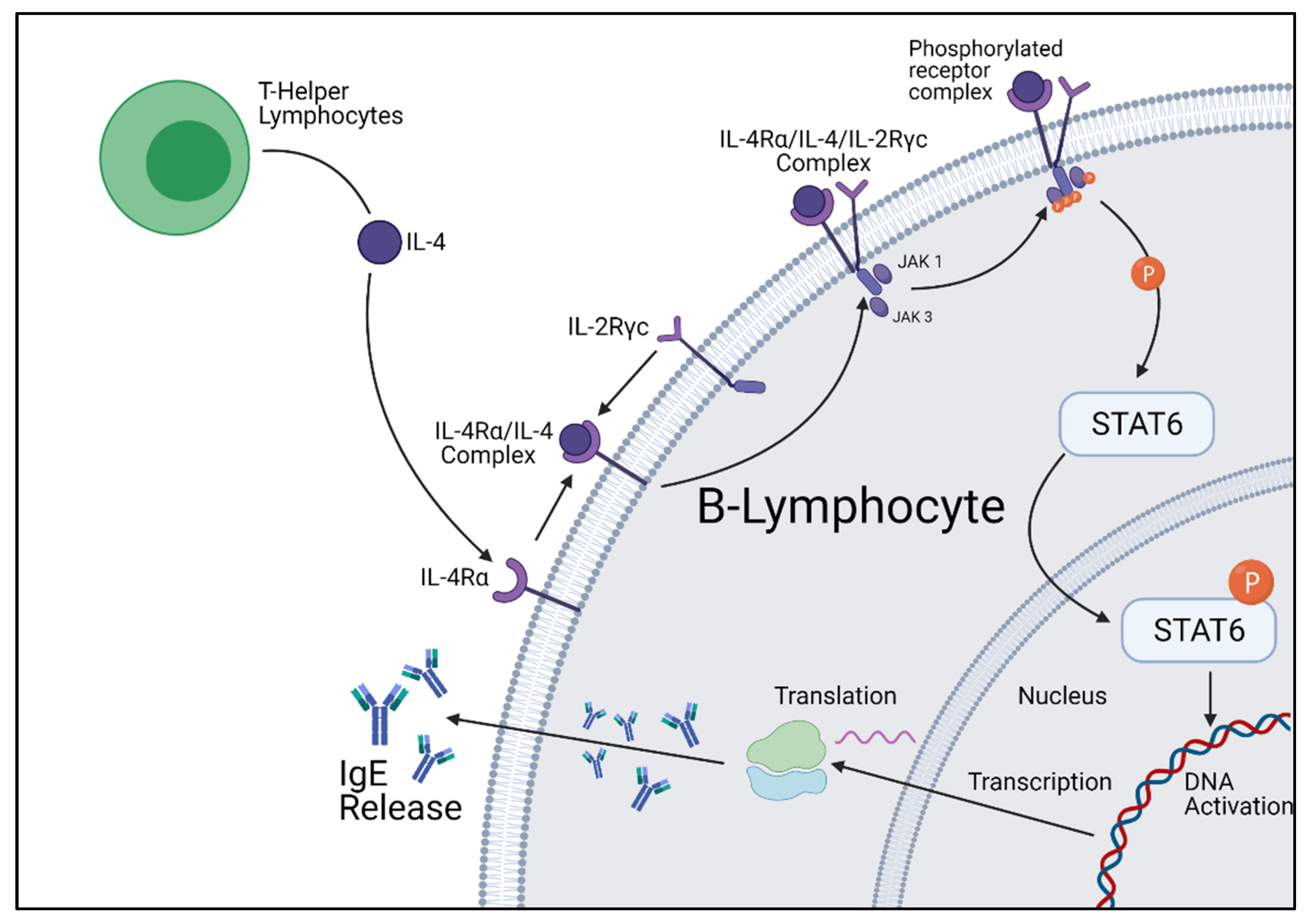
| Name | Inhibitory Target | Description | Reference |
|---|---|---|---|
| Dupilumab | IL-4Rα | Monoclonal antibody targeting the IL-14Rα receptor, thereby inhibiting both the IL-4 and IL-13 pathways. Currently approved in the US for treatment of atopic dermatitis. Under investigation for asthma treatment. | [29,30,31] |
| Pascolizumab | IL-4 cytokine | Humanized monoclonal antibody targeting the IL-4 cytokine. Binding to the cytokine inhibits prevents receptor binding, preventing the downstream effects of the IL-4 pathway. | [32,33] |
| Pitrakinra | IL-4α | Synthetic protein targeting the IL-4Rα receptor. Like dupilumab, pitrakinra inhibits both the IL-4 and IL-13 pathways, though clinical trials have shown little efficacy, leading to an investigation into the IL-4Rα genes. | [34,35,36] |
| Lebrikizumab | IL-13 cytokine | Monoclonal antibody that targets IL-13 cytokines, thereby blocking the downstream pathway. Trials are still being conducted, with inconsistent results reported. | [37,38,39] |
| Anrukisumab | IL-13 cytokine | Monoclonal antibody that also targets the IL-13 cytokine, like lebrikizumab, although it is aimed at the treatment of ulcerative colitis. Having only undergone phase I trials, more study is required. | [40,41] |
| Tralokinumab | IL-13 | Monoclonal antibody that targets the IL-13 cytokine. Having recently undergone phase III trials, tralokinumab has shown promising results in the treatment of atopic dermatitis. | [42,43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massey, O.; Suphioglu, C. Recent Advances in the Inhibition of the IL-4 Cytokine Pathway for the Treatment of Allergen-Induced Asthma. Int. J. Mol. Sci. 2021, 22, 13655. https://doi.org/10.3390/ijms222413655
Massey O, Suphioglu C. Recent Advances in the Inhibition of the IL-4 Cytokine Pathway for the Treatment of Allergen-Induced Asthma. International Journal of Molecular Sciences. 2021; 22(24):13655. https://doi.org/10.3390/ijms222413655
Chicago/Turabian StyleMassey, Oliver, and Cenk Suphioglu. 2021. "Recent Advances in the Inhibition of the IL-4 Cytokine Pathway for the Treatment of Allergen-Induced Asthma" International Journal of Molecular Sciences 22, no. 24: 13655. https://doi.org/10.3390/ijms222413655
APA StyleMassey, O., & Suphioglu, C. (2021). Recent Advances in the Inhibition of the IL-4 Cytokine Pathway for the Treatment of Allergen-Induced Asthma. International Journal of Molecular Sciences, 22(24), 13655. https://doi.org/10.3390/ijms222413655







