Current Advancements of Plant-Derived Agents for Triple-Negative Breast Cancer Therapy through Deregulating Cancer Cell Functions and Reprogramming Tumor Microenvironment
Abstract
1. Introduction
2. Plant-Derived Compounds Inhibit Cell Proliferation, Tumor Growth/Metastasis, and Induction of Programmed Cell Death in TNBC
2.1. Phenolics
2.2. Terpenoids
2.3. Alkaloids
2.4. Other Phytocompounds/Extracts
3. Plant-Derived Compounds Reprogram Cellular Metabolisms and Associated Proteins and Signaling Pathways in Drug Sensitive/Resistant TNBC
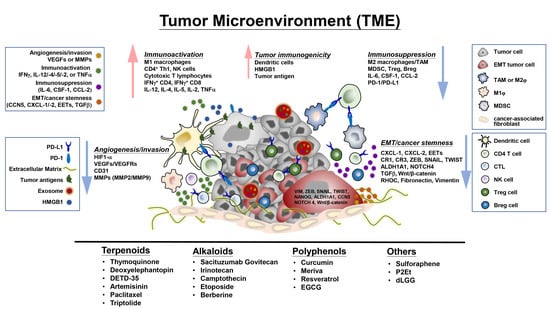
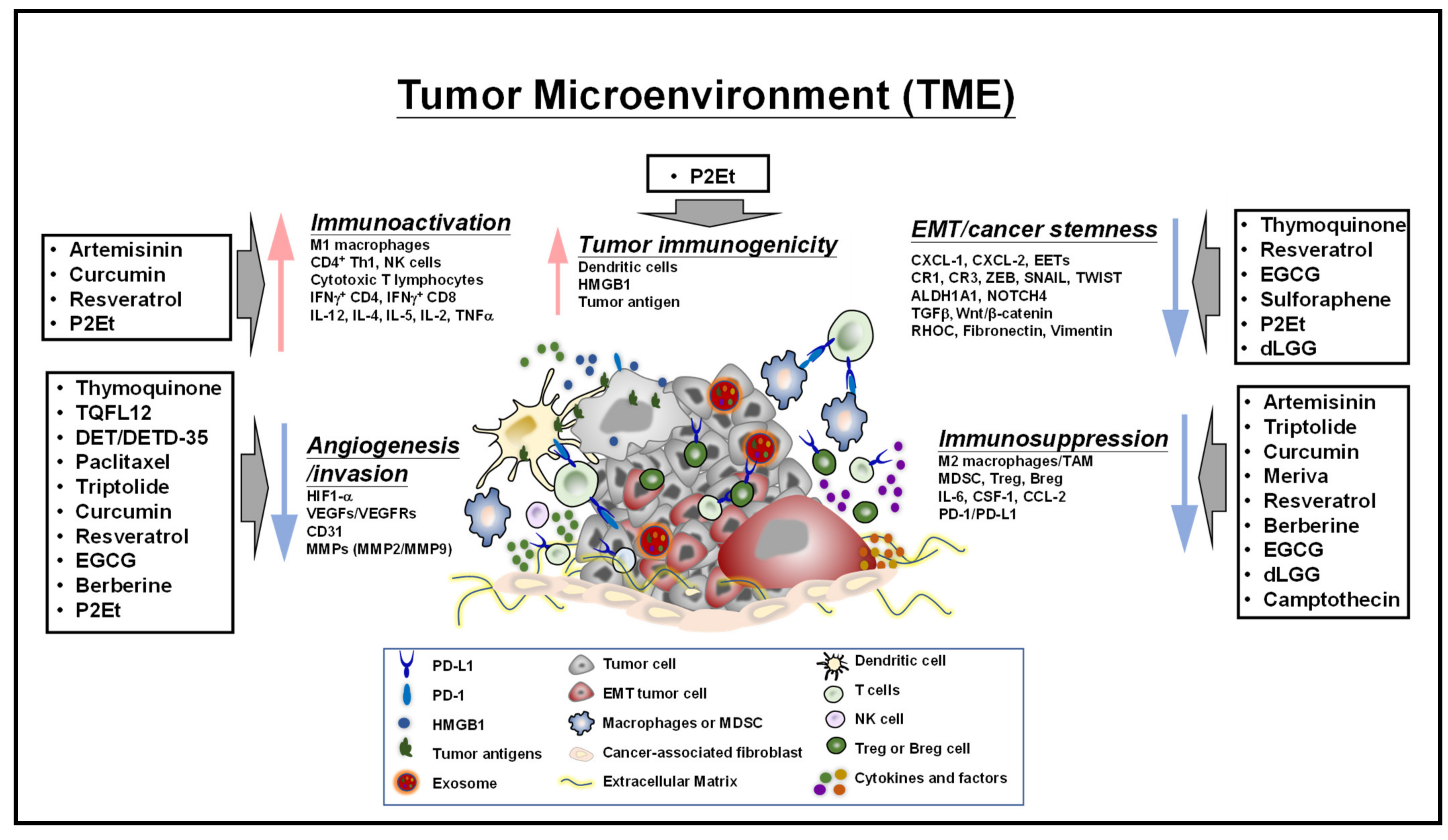
4. Plant-Derived Compounds Educate the Tumor Microenvironment and Immune Checkpoints Activity-Associated Signaling Molecules and Pathways
4.1. Regulation of Tumor-Infiltrating Cells, Tumor Cell-Immune Cell Interactions, and Associated Signaling Molecules in the TME
4.2. Regulation of Immune Checkpoint Expression and Activity
4.3. Effects on Exosomes, Epithelial-Mesenchymal Transition and Extracellular Matrix
5. Highlights of Some Clinical Trial Studies for Plant-Derived Drugs against Breast Cancers
6. Current Challenges and Future Prospects for Development of Phytoagents for TNBC Therapy
| Compound Alone or in Combination | Molecular Mechanisms/Targets | Preclinical Animal Model | Ref. |
|---|---|---|---|
| Terpenoids | |||
| Monoterpenoids Thymoquinone | ↑p-p38, ROS↑PARP cleavage, TUNEL ↓XIAP, survivin, Bcl-xL, Bcl-2 ↓Ki67, tumor growth | Subcutaneous injection of MDA-MB-231 in nude mice | [56] |
| Thymoquinone in liposomal nanoparticles | ↓eEF-2K, Src/FAK, Akt/NF-κB ↑miR-603, ↓cell proliferation ↓migration and tumor growth | Orthotopic injection of MDA-MB-231 and MDA-MB-436 in nude mice | [55] |
| Thymoquinone-loaded, hyaluronic acid-conjugated copolymer nanoparticles | ↑miRNA-361 ↓Rac1, RhoA, VEGF-A ↓vascularization | Orthotopic injection of 4T1 tumor model in BALB/c mice | [57] |
| Thymoquinone + Doxorubicin | ↓XIAP, surviving, Bcl-xL, Bcl-2 ↑TUNEL ↓Ki67, tumor growth | Subcutaneous injection of MDA-MB-231 in nude mice | [56] |
| TQFL12 | ↑stabilize AMPKα ↑p-acetyl-CoA, apoptosis ↓cell growth, migration, invasion | Orthotopic injection of 4T1 tumor model in BALB/c mice | [58] |
| Sesquiterpene lactones DET/DETD-35 | ↑G2/M cell-cycle arrest, cell apoptosis ↓migration, invasion, motility ↑cytoplasmic vacuolation ↑exosome release/affect exosomal proteins ↑p-ERK, p-JNK, p-p38 ↑ubiquitinated protein accumulation ↑ER stress-mediated paraptosis and apoptosis, ROS, LC3, | Orthotopic/lung metastatic MDA-MB-231 tumor model in NOD/SCID mice | [63,217] |
| DETD-35 + paclitaxel | ↓VEGF, COX-2, Ki67 ↑caspase-3 ↓metastatic pulmonary foci | Lung metastatic MDA-MB-231 tumor model in NOD/SCID mice | [62] |
| Artemisinin | ↓TGF-β mRNA levels, MDSC, Treg cells ↑TNFα mRNA levels, Tbet ↑CD4+ IFN-γ+ T cells ↑cytotoxic T lymphocytes ↓tumor growth ↑survival | Orthotopic 4T1 tumor model in BALB/c mice | [189] |
| Artemisinin Artemisinin-loaded biotin-PEG-PCL polymers | ↑BAX, ratio of BAX/Bcl-2 ↓tumor growth ↑BAX, ratio of BAX/Bcl-2;↓Bcl-2 ↓tumor growth | 4T1 tumor model in BALB/c mice | [67] |
| Artesunate + irinotecan in phosphatidylcholine-based liposomes | ↓tumor growth | 4T1 tumor model in BALB/c mice | [70] |
| Dihydroartemisinin + docetaxel in a pH-sensitive nanoparticle delivery system | ↑ROS, p53, cytochrome c release;↓Bcl-2 ↑caspase-3 ↓mitochondrial membrane potential ↓tumor growth;↓metastasis | Orthotopic injection of 4T1 tumor model in BALB/c mice | [66] |
| Dihydroartemisinin Dihydroartemisinin+ docetaxel in disulfide-linked nanoparticle delivery system | ↑early apoptosis ↑cell cycle arrest ↓tumor growth ↑cell cycle arrest ↑early apoptosis ↑sustained release, circulating time ↓migration, tumor growth ↑prolonged survival | Orthotopic injection of 4T1 tumor model in BALB/c mice | [65] |
| Diterpenoids Paclitaxel Paclitaxel + tyrosine kinases inhibitor (E-3810) | ↑caspase-3/7 activity ↑ECM remodeling, ↓tumor growth ↑caspase-3/7 activity ↑ECM remodeling, MMP-9 ↓tumor growth | Subcutaneous injection of MDA-MB-231 and MX-1 tumor model in nude mice | [222] |
| Triptolide | ↓HMGB1, TLR4, p-NF-κB ↓cell viability, clonogenic ability ↓Tumor growth | Subcutaneous injection of MDA-MB-231 tumor model in nude mice | [76] |
| ↓CD206, Arginase-1, CD204 ↓M2 TAM ↓anti-inflammatory cytokines ↓tumor growth | Orthotopic injection of 4T1 tumor model in BALB/c mice | [190] | |
| ↓VEGF-A, angiogenesis ↓ERK1/2, HIF1-α ↓tumor growth, tumor cell proliferation | Orthotopic injection of MDA-MB-231 tumor model in nude mice | [196] | |
| Polyphenols | |||
| Curcumin | ↑miR181b ↓CXCL-1 and CXCL-2 ↓lung metastasis | Intracardiac injection of human MDA-MB-231 cells in immunodeficient mice | [184] |
| ↓Ki67 ↓VEGFR2/3 ↓micro-vessel density | Subcutaneous injection of human MDA-MB-231 cells in immunodeficient mice | [194] | |
| Curcumin (before tumor inoculation) + Listeria-Mage-b vaccine (therapeutic immunization) | ↓IL-6 by MDSCs in tumor/blood ↑IL-12 by MDSCs in blood ↑IFNγ by CD4+ and CD8+ T cells in blood | Orthotopic injection of 4T1 cells in BALB/c mice | [182] |
| Curcumin + metformin | ↑tumor apoptosis ↑Serum IL-4 | Subcutaneous injection of EMT6/P cells in BALB/c mice | [183] |
| Curcumin + arabinogalactan | ↓Ki67 ↑p53 | Subcutaneous injection of 4T1 cells in BALB/c mice | [30] |
| Curcumin + calcitriol | ↓micro-vessel density | Subcutaneous injection of MBCDF-T cells in nude mice | [195] |
| Meriva administered after cryoablation | ↓IL-6 | Orthotopic injection of 4T1 cells in BALB/c mice | [185] |
| Resveratrol | ↓lung nodules ↓plasma MMP-9 | Intravenous injection of 4T1 cells to develop lung metastasis in BALB/c mice | [219] |
| ↓MMP-2, MMP-9, vimentin, snail1, slug ↑E-cadherin | Orthotopic injection of human MDA-MB-231 cells in a xenograft model | [210] | |
| ↑IFNγ and IL-2, M1 TAM in the lung ↑lung-filtrating CD4+ and CD8+ T cells ↑perforin/granzyme on splenic CD8+ T cells ↓PD-1 on pulmonary CD4+ and CD8+ T cells | Intravenous injection of 4T1 cells to develop lung metastasis in BALB/c mice | [200] | |
| ↓Bregs, TGFβ, Treg | Orthotopic injection of 4T1 cells in BALB/c mice | [186] | |
| ↓FASN expression ↓lipid synthesis | Orthotopic injection of human MDA-MB-231 cells in nude mice | [130] | |
| a 5-LOX inhibitor ↓COX-2 and MMP-9 expression | Rats treated with DMBA to induce mammary cancer | [43,44] | |
| ↑LC3-II, Beclin1 and Atg 7 in BCSCs ↓Wnt/β-catenin signaling pathway in BCSCs | Orthotopic injection of human SUM159 cells in NOD/SCID mice | [46] | |
| Resveratrol + tamoxifen | ↓acetylated STAT3 ↑ER-α gene expression | Subcutaneous injection of human MDA-MB-231 cells in nude mice | [42] |
| Resveratrol + cisplatin | ↓p-AKT, p-PI3K, Smad2, Smad3, p-JNK, p-ERK, and NF-κB in tumor tissues | Orthotopic injection of human MDA-MB-231 cells in a xenograft model | [211] |
| EGCG | ↓CD44+ BCSCs ↓VEGF ↓MMP-2 ↑Caspase-3 | Rats treated with 7,12 dimethylbenzanthracene (DMBA) to induce mammary cancer | [48] |
| ↓RNA levels of cyclin D1 (CCND1), RHOC, fibronectin (FN1), E-cadherin (CDH1), vimentin (VIM) and BCL-XL ↓VEGF expression ↓tumor sphere formation | Orthotopic injection of human ALDH-positive SUM-149 cells in NOD/SCID mice | [49] | |
| ↓CSF-1, CCL-2, IL-6, and TGFβ ↓infiltration of M2 TAM | Subcutaneous injection of 4T1 cells in BALB/c mice | [216] | |
| ↓tumor glucose and lactic acid levels ↓tumor VEGF | Subcutaneous injection of 4T1 cells in BALB/c mice | [121] | |
| ↑CCN5 expression ↓EMT, stemness | Subcutaneous injection of human MDA-MB-231 cells in nude mice | [214] | |
| EGCG + taxol | ↑tumor apoptosis ↓tumor GRP78, JNK phosphorylation | Murine breast 4T1 cells in BALB/c mice | [153] |
| EGCG + cetuximab | ↓FASN activity | Orthotopic injection of sensitive and chemoresistant TNBC cells | [129] |
| Alkaloids | |||
| Sacituzumab Govitecan + PARP inhibitors (olaparib or talazoparib) | ↑γ-H2AX | Subcutaneous injection of human BRCA1/2-mutated or—wild-type TNBC cells in nude mice | [86] |
| Camptothecin + doxorubicin | ↓M2-like TAMs | Orthotopic injection of 4T1 cells in BALB/c mice | [187] |
| Camptothecin-loaded nanoparticle displaying cetuximab | ↓Ki67 | Orthotopic injection of bone-metastatic MDA-MB-231 cells in NSG mice | [77] |
| bevacizumab + CRLX101 (a nanoparticle–drug conjugate containing camptothecin) | ↓HIF1α ↓hypoxia | Orthotopic injection of highly aggressive variant MDA-MB-231 cells (LM2-4) in SCID mice | [79] |
| Etoposide + TMU-35435 | ↑LC3, γ-H2AX, caspase-3 | Orthotopic injection of 4T1 cells in BALB/c mice | [91] |
| Etoposide + TRAIL | ↑DR5 expression ↑PARP, caspases and p53 expressions | Orthotopic injection of human MDA-MB-231 cells in a xenograft model | [93] |
| Berberine | ↓TGF-β1 ↓MMP-2 | Orthotopic injection of MDA-MB-231 or 4T1 cells in mice | [220] |
| ↓Ki67 ↑caspase-9 | Orthotopic injection of MDA-MB-231 cells in nude mice | [221] | |
| Berberine binds to VASP Secondary structure of VASP changes ↓actin polymerization | Subcutaneous injection of human MDA-MB-231 cells in nude mice | [98] | |
| ↓NF-κB, IL-1β, IL-6 and TNFα ↓PCNA | Rats treated with DMBA to induce mammary cancer | [232] | |
| Berberine + anti-DR5 antibody | ↑caspase-3 ↑PARP | Orthotopic injection of 4T1 cells in BALB/c mice | [99] |
| co-loaded liposome of berberine and doxorubicin | ↓cardiotoxicity ↓tumor | Subcutaneous injection of 4T1 cells in BALB/c mice | [188] |
| Plant extracts/other phytocompounds | |||
| Sulforaphene | ↓cell proliferation ↓cyclin B1, Cdc2 ↑G2/M phase arrest, Egr1 | Orthotopic injection of MDA-MB-453 tumor model in nude mice | [100] |
| ↓CRIPTO-1/TDGF1 ↓CRIPTO-3/TDGF1P3 ↓Nanog, ALDH1A1, Wnt3, Notch 4 | Orthotopic injection of MDA-MB-231 tumor model in nude mice | [101] | |
| Sulforaphene Sulforaphene + doxorubicin | ↓cell growth, HDAC6;↑autophagy ↑membrane translocation ↑acetylation modification of PTEN synergistic inhibition on MDA-MB-231 xenografts growth. | Orthotopic injection of MDA-MB-231 tumor model in nude mice | [102] |
| Sulforaphene Sulforaphene + docetaxel | ↓NF-κB p65 translocation;↓p52 ↓mammosphere formation ↓taxane-induced ALDH+ cell enrichment ↓primary tumor volume ↓secondary tumor formation | Orthotopic injection of SUM149 tumor model in NOD/SCID mice | [103] |
| P2Et (Caesalpinia spinosa extract) | ↑mitochondrial membrane potential loss ↑phosphatidylserine externalization ↑caspase 3 activation, IL-6, MCP-1 ↑DNA fragmentation ↓clonogenic capacity of 4T1 cells ↓tumor growth, spleen metastasis | Orthotopic injection of 4T1 tumor model in BALB/c mice | [104] |
| ↑calreticulin, ATP secretion ↑HMGB1 translocation ↑IL-2, TNFα, IL-4, IL-5 ↑IFNγ-producing CD4+ and CD8+ T cells | Orthotopic injection of 4T1 tumor model in BALB/c mice | [191] | |
| ↓cell viability, proliferation ↓tumor growth | Orthotopic injection of MDA-MB-468 tumor model in NSG mice | [107] | |
| Prophylactic therapy of P2Et | ↑CD4+ T, CD8+ T, NK, DC ↑Treg, MDSC, plasma IL-6 | orthotopic injection of 4T1 tumor model in BALB/c mice | [192] |
| P2Et + antiPD-L1 | ↓tumor growth, granulocytes | orthotopic injection of 4T1 tumor model in BALB/c mice | [193] |
| Compound Alone or in Combination | Molecular Mechanisms/Targets | Treatment Results | Phase; Intervention | Ref. |
|---|---|---|---|---|
| Terpenoids | ||||
| Artesunate as add-on therapy | Anticancer ↓TGF-β mRNA levels, MDSC, Treg cells ↑TNFα mRNA levels, Tbet ↑CD4+ IFN-γ+ T cells ↑cytotoxic T lymphocytes ↓tumor growth ↑survival | The pharmacokinetics of artesunate and its metabolites—dihydroartemisinin was well described by a combined drug-metabolite model. The saliva sampling for artesunate monitoring of dihydroartemisinin was suggested. | ARTIC-M33/2 Metastatic breast cancer patients (phase I, n = 23) 100, 150, or 200 mg oral artesunate daily as add-on therapy to their guideline-based oncological therapy. | [189,226] |
| Anticancer ↓TGF-β mRNA levels, MDSC, Treg cells ↑TNFα mRNA levels, Tbet ↑CD4+ IFN-γ+ T cells ↑cytotoxic T lymphocytes ↓tumor growth ↑survival | The continuous intake of artesunate for 4 weeks in doses up to 200 mg daily was well tolerated in test patients. However, a temporary dose-limiting vertigo was observed in three patients. | ARTIC-M33/2 Metastatic breast cancer patients (phase I, n = 23) 100, 150, or 200 mg oral artesunate daily as add-on therapy to their guideline-based oncological therapy. | [189,227] | |
| Anticancer ↓TGF-β mRNA levels, MDSC, Treg cells ↑TNFα mRNA levels, Tbet ↑CD4+ IFN-γ+ T cells ↑cytotoxic T lymphocytes ↓tumor growth ↑survival n | 200 mg/d are recommended for phase II/III trials. | ARTIC-M33/2 Metastatic breast cancer patients (phase I, n = 23) 100, 150, or 200 mg oral artesunate daily as add-on therapy to their guideline-based oncological therapy. | [189,228] | |
| Anticancer ↓TGF-β mRNA levels, MDSC, Treg cells ↑TNFα mRNA levels, Tbet ↑CD4+ IFN-γ+ T cells ↑cytotoxic T lymphocytes ↓tumor growth ↑survival | In 13 patients with metastatic breast cancer, up to 200 mg/d long-term oral artesunate in up to 1115 cumulative treatment days (cumulative doses up to 167.3 g) did not result in any major safety concerns. | ARTIC-M33/2 Metastatic breast cancer patients (phase I, n = 23) 100, 150, or 200 mg oral artesunate daily as add-on therapy to their guideline-based oncological therapy. | [189,229] | |
| Paclitaxel + Atezolizumab (anti-PD-L1) | Targeting microtubule and PD-L1 | The median OS of 25.4 months (19.6–30.7 months) with Paclitaxel + Atezolizumab (n = 185) and 17.9 months (13.6–20.3 months) with Paclitaxel + Placebo + nP (n = 184) in PD-L1 IC-positive population (n = 369). | Metastatic TNBC Patients (Phase III); nab-paclitaxel (100 mg/m2 of body surface area on days 1, 8, and 15 of every 28-day cycle) was combined with either placebo (n = 451) or atezolizumab (840 mg on days 1 and 15 of each cycle, n = 451). | [17] |
| Paclitaxel + iniparib (PARP inhibitor) | Targeting microtubule and PARP | pCR rate was similar among the three arms (21, 22, and 19% for PTX, PWI, and PTI, respectively). pCR in breast and axilla (21, 17, and 19%); best overall response in the breast (60, 61, and 63%); and breast conservation rate (53, 54, and 50%). | 141 TNBC patients with Stage II-IIIA TNBC were randomly assigned to receive paclitaxel (80 mg/m2, d1; n = 47) alone (PTX) or in combination with iniparib, either once-weekly (PTW (11.2 mg/kg, d1; n = 46) or twice-weekly (PTI) (5.6 mg/kg, d1, 4; n = 48) for 12 weeks. | [224] |
| Paclitaxel + Tigatuzumab (anti-DR5) | Targeting microtubule and DR5 ROCK1 gene pathway activation | 3 CR, 8 PR; 1 almost CR, 11 SD, and 17 PD in the combination arm (ORR, 28%). No CRs, 8 PRs, 4 SDs, and 9 PDs in the Paclitaxel arm (ORR, 38%). There was a numerical increase in CRs and several patients had prolonged PFS in the combination arm. | TBNC patients (Phase II) A treatment cycle was defined as 4 weeks. Patients received intravenous nab- aclitaxel on days 1, 8, and 15 (100 mg/m2) at 28 days interval with (n = 39) or without (n = 21)) Tigatuzumab intravenously on days 1 and 15 of every cycle (10 mg/kg loading dose followed by 5 mg/kg every other week). | [225] |
| Polyphenols | ||||
| Curcumin + docetaxel | ↓carcinoembryonic antigen ↓VEGF ↓P-glycoprotein (P-gp, MDR1) | Five patients had PR, and three patients had SD at least 6 w after the last cycle of treatment. ORR was up to 50%. no progressive disease was observed. | Metastatic breast cancer patients (phase I, n = 14) docetaxel (IV 100 mg/m2) every 3 week on day 1 for 6 cycles + curcumin (p.o. 500 mg/day) for 7 consecutive days by cycle | [155] |
| Alkaloids | ||||
| Sacituzumab Govitecan | Targeting TOP1 in the Trop-2-positive cells | Median PFS was 5.5 months, and median OS was 13 months. ORR was 33%. | refractory metastatic TNBC patients (phase I/II, n = 108) 10 mg/kg, intravenously on days 1 and 8 of each 21-day cycle | [83] |
| Median PFS was 5.6 months, and median OS was 12.1 months. ORR was 35%. | Metastatic TNBC patients (phase III, n = 468) 10 mg/kg, intravenously on days 1 and 8 of each 21-day cycle | [82] | ||
| Irinotecan + iniparib (PARP inhibitor) | Targeting TOP1 and PARP | Median OS was 7.8 months. Intracranial RR was 12%, while intracranial CBR was 27%. | TNBC patients with new or progressive brain metastases (phase II, n = 37) Irinotecan 125 mg/m2 intravenously (IV) on days 1 and 8 of each 21 day cycle. Iniparib was dosed at 5.6 or 8 mg/kg IV on days 1, 4, 8, 11 of each 21 day cycle. | [81] |
| Etoposide | Targeting topoisomerase II | ORR was 25%. Nine patients achieved SD for more than 24 weeks and CBR was 53%. The median PFS and OS were 5 (range, 1.5–17.0 months) and 16 months (range, 3.0–51.0 months), respectively. | Metastatic breast cancer patients (phase I, n = 32) 60 mg/m2/d on days 1–10, followed by 11 days of rest | [88] |
| Seven (9.3%) patients achieved PR and 29 (38.7%) had SD. Nine patients (12%) had SD for >24 weeks and the CBR was 21.3% (16/75). The median PFS was 4.5 (range, 1.3–7.7) months. | Metastatic breast cancer patients (phase II, n = 75) 60 mg/m2/d on days 1–10, followed by 11 days of rest | [89] | ||
| Median PFS was 4 months, CBR was 18% (overall response rate 4%), and median OS from the start of treatment was 11 months. | Metastatic breast cancer patients (phase II, n = 75) 50 mg/day in 20-day cycles with 1-week of rest | [90] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hwang, K.T.; Kim, J.; Jung, J.; Chang, J.H.; Chai, Y.J.; Oh, S.W.; Oh, S.; Kim, Y.A.; Park, S.B.; Hwang, K.R. Impact of breast cancer subtypes on prognosis of women with operable invasive breast cancer: A population-based study using SEER database. Clin. Cancer Res. 2019, 25, 1970–1979. [Google Scholar] [CrossRef] [PubMed]
- Lips, E.H.; Mulder, L.; Oonk, A.; van der Kolk, L.E.; Hogervorst, F.B.; Imholz, A.L.; Wesseling, J.; Rodenhuis, S.; Nederlof, P.M. Triple-negative breast cancer: BRCAness and concordance of clinical features with BRCA1-mutation carriers. Br. J. Cancer 2013, 108, 2172–2177. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.S.; Lindahl, T. Role of poly(ADP-ribose) formation in DNA repair. Nature 1992, 356, 356–358. [Google Scholar] [CrossRef] [PubMed]
- Benafif, S.; Hall, M. An update on PARP inhibitors for the treatment of cancer. Onco Targets Ther. 2015, 8, 519–528. [Google Scholar]
- Afghahi, A.; Timms, K.M.; Vinayak, S.; Jensen, K.C.; Kurian, A.W.; Carlson, R.W.; Chang, P.J.; Schackmann, E.; Hartman, A.R.; Ford, J.M.; et al. Tumor BRCA1 reversion mutation arising during neoadjuvant platinum-based chemotherapy in triple-negative breast cancer is associated with therapy resistance. Clin. Cancer Res. 2017, 23, 3365–3370. [Google Scholar] [CrossRef]
- Loi, S.; Pommey, S.; Haibe-Kains, B.; Beavis, P.A.; Darcy, P.K.; Smyth, M.J.; Stagg, J. CD73 promotes anthracycline resistance and poor prognosis in triple negative breast cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 11091–11096. [Google Scholar] [CrossRef]
- O’Reilly, E.A.; Gubbins, L.; Sharma, S.; Tully, R.; Guang, M.H.; Weiner-Gorzel, K.; McCaffrey, J.; Harrison, M.; Furlong, F.; Kell, M.; et al. The fate of chemoresistance in triple negative breast cancer (TNBC). BBA Clin. 2015, 3, 257–275. [Google Scholar] [CrossRef]
- Wein, L.; Loi, S. Mechanisms of resistance of chemotherapy in early-stage triple negative breast cancer (TNBC). Breast 2017, 34 (Suppl. 1), S27–S30. [Google Scholar] [CrossRef]
- McCann, K.E.; Hurvitz, S.A.; McAndrew, N. Advances in targeted therapies for triple-negative breast cancer. Drugs 2019, 79, 1217–1230. [Google Scholar] [CrossRef]
- Wang, D.Y.; Jiang, Z.; Ben-David, Y.; Woodgett, J.R.; Zacksenhaus, E. Molecular stratification within triple-negative breast cancer subtypes. Sci. Rep. 2019, 9, 19107. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef]
- Basho, R.K.; Gilcrease, M.; Murthy, R.K.; Helgason, T.; Karp, D.D.; Meric-Bernstam, F.; Hess, K.R.; Herbrich, S.M.; Valero, V.; Albarracin, C.; et al. Targeting the PI3K/AKT/mTOR pathway for the treatment of mesenchymal triple-negative breast cancer: Evidence from a phase 1 trial of mTOR inhibition in combination with liposomal doxorubicin and bevacizumab. JAMA Oncol. 2017, 3, 509–515. [Google Scholar] [CrossRef]
- Nanda, R.; Chow, L.Q.; Dees, E.C.; Berger, R.; Gupta, S.; Geva, R.; Pusztai, L.; Pathiraja, K.; Aktan, G.; Cheng, J.D.; et al. Pembrolizumab in patients with advanced triple-negative breast cancer: Phase Ib KEYNOTE-012 study. J. Clin. Oncol. 2016, 34, 2460–2467. [Google Scholar] [CrossRef]
- Adams, S.; Schmid, P.; Rugo, H.S.; Winer, E.P.; Loirat, D.; Awada, A.; Cescon, D.W.; Iwata, H.; Campone, M.; Nanda, R.; et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: Cohort a of the phase II KEYNOTE-086 study. Ann. Oncol. 2019, 30, 397–404. [Google Scholar] [CrossRef]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Dieras, V.; Hegg, R.; Im, S.A.; Shaw Wright, G.; et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. New Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef]
- Dirix, L.Y.; Takacs, I.; Jerusalem, G.; Nikolinakos, P.; Arkenau, H.T.; Forero-Torres, A.; Boccia, R.; Lippman, M.E.; Somer, R.; Smakal, M.; et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: A phase 1b JAVELIN solid tumor study. Breast Cancer Res. Treat. 2018, 167, 671–686. [Google Scholar] [CrossRef]
- Emens, L.A.; Adams, S.; Barrios, C.H.; Dieras, V.; Iwata, H.; Loi, S.; Rugo, H.S.; Schneeweiss, A.; Winer, E.P.; Patel, S.; et al. First-line atezolizumab plus nab-paclitaxel for unresectable, locally advanced, or metastatic triple-negative breast cancer: IMpassion130 final overall survival analysis. Ann. Oncol. 2021, 32, 983–993. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Sahin Kavakli, H.; Koca, C.; Alici, O. Antioxidant effects of curcumin in spinal cord injury in rats. Ulus. Travma Acil Cerrahi Derg. 2011, 17, 14–18. [Google Scholar] [CrossRef]
- Zhang, N.; Li, H.; Jia, J.; He, M. Anti-inflammatory effect of curcumin on mast cell-mediated allergic responses in ovalbumin-induced allergic rhinitis mouse. Cell. Immunol. 2015, 298, 88–95. [Google Scholar] [CrossRef]
- Bachmeier, B.E.; Killian, P.H.; Melchart, D. The role of curcumin in prevention and management of metastatic disease. Int. J. Mol. Sci. 2018, 19, 1716. [Google Scholar] [CrossRef] [PubMed]
- Bielak-Zmijewska, A.; Grabowska, W.; Ciolko, A.; Bojko, A.; Mosieniak, G.; Bijoch, L.; Sikora, E. The role of curcumin in the modulation of ageing. Int. J. Mol. Sci. 2019, 20, 1239. [Google Scholar] [CrossRef] [PubMed]
- Mollazadeh, H.; Cicero, A.F.G.; Blesso, C.N.; Pirro, M.; Majeed, M.; Sahebkar, A. Immune modulation by curcumin: The role of interleukin-10. Crit. Rev. Food Sci. Nutr. 2019, 59, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Rowe, D.L.; Ozbay, T.; O’Regan, R.M.; Nahta, R. Modulation of the BRCA1 protein and induction of apoptosis in triple negative breast cancer cell lines by the polyphenolic compound curcumin. Breast Cancer 2009, 3, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Vinod, B.S.; Antony, J.; Nair, H.H.; Puliyappadamba, V.T.; Saikia, M.; Narayanan, S.S.; Bevin, A.; Anto, R.J. Mechanistic evaluation of the signaling events regulating curcumin-mediated chemosensitization of breast cancer cells to 5-fluorouracil. Cell Death Dis. 2013, 4, e505. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Karin, M. NF-kappaB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef]
- Aggarwal, S.; Ichikawa, H.; Takada, Y.; Sandur, S.K.; Shishodia, S.; Aggarwal, B.B. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Mol. Pharmacol. 2006, 69, 195–206. [Google Scholar] [CrossRef]
- Chiu, T.L.; Su, C.C. Curcumin inhibits proliferation and migration by increasing the Bax to Bcl-2 ratio and decreasing NF-kappaBp65 expression in breast cancer MDA-MB-231 cells. Int. J. Mol. Med. 2009, 23, 469–475. [Google Scholar]
- Bento, J.F.; Noleto, G.R.; de Oliveira Petkowicz, C.L. Isolation of an arabinogalactan from endopleura uchi bark decoction and its effect on hela cells. Carbohydr. Polym. 2014, 101, 871–877. [Google Scholar] [CrossRef]
- Moghtaderi, H.; Sepehri, H.; Attari, F. Combination of arabinogalactan and curcumin induces apoptosis in breast cancer cells in vitro and inhibits tumor growth via overexpression of p53 level in vivo. Biomed. Pharmacother. 2017, 88, 582–594. [Google Scholar] [CrossRef]
- Ko, J.H.; Sethi, G.; Um, J.Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The role of resveratrol in cancer therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.; Fong, H.H.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef]
- Holmes-McNary, M.; Baldwin, A.S., Jr. Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of the IkappaB kinase. Cancer Res. 2000, 60, 3477–3483. [Google Scholar]
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Pourbagher-Shahri, A.M.; Samarghandian, S. STAT3 pathway as a molecular target for resveratrol in breast cancer treatment. Cancer Cell Int. 2021, 21, 468. [Google Scholar] [CrossRef]
- Stunkel, W.; Campbell, R.M. Sirtuin 1 (SIRT1): The misunderstood HDAC. J. Biomol. Screen. 2011, 16, 1153–1169. [Google Scholar] [CrossRef]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.L.; et al. Small molecule activators of sirtuins extend saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef]
- Nie, Y.; Erion, D.M.; Yuan, Z.; Dietrich, M.; Shulman, G.I.; Horvath, T.L.; Gao, Q. STAT3 inhibition of gluconeogenesis is downregulated by SirT1. Nat. Cell Biol. 2009, 11, 492–500. [Google Scholar] [CrossRef]
- Wieczorek, M.; Ginter, T.; Brand, P.; Heinzel, T.; Kramer, O.H. Acetylation modulates the STAT signaling code. Cytokine Growth Factor Rev. 2012, 23, 293–305. [Google Scholar] [CrossRef]
- Yu, H.; Pardoll, D.; Jove, R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat. Rev. Cancer 2009, 9, 798–809. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, H.Y.; Marzec, M.; Raghunath, P.N.; Nagasawa, T.; Wasik, M.A. STAT3- and DNA methyltransferase 1-mediated epigenetic silencing of SHP-1 tyrosine phosphatase tumor suppressor gene in malignant T lymphocytes. Proc. Natl. Acad. Sci. USA 2005, 102, 6948–6953. [Google Scholar] [CrossRef]
- Brinkman, J.A.; El-Ashry, D. ER re-expression and re-sensitization to endocrine therapies in ER-negative breast cancers. J. Mammary Gland Biol. Neoplasia 2009, 14, 67–78. [Google Scholar] [CrossRef]
- Lee, H.; Zhang, P.; Herrmann, A.; Yang, C.; Xin, H.; Wang, Z.; Hoon, D.S.; Forman, S.J.; Jove, R.; Riggs, A.D.; et al. Acetylated STAT3 is crucial for methylation of tumor-suppressor gene promoters and inhibition by resveratrol results in demethylation. Proc. Natl. Acad. Sci. USA 2012, 109, 7765–7769. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, M.; Das, S.; Janarthan, M.; Ramachandran, H.K.; Chatterjee, M. Role of 5-lipoxygenase in resveratrol mediated suppression of 7,12-dimethylbenz(alpha)anthracene-induced mammary carcinogenesis in rats. Eur. J. Pharmacol. 2011, 668, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Bueso-Ramos, C.; Aggarwal, B.B. Suppression of 7,12-dimethylbenz(alpha)anthracene-induced mammary carcinogenesis in rats by resveratrol: Role of nuclear factor-kappaB, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Res. 2002, 62, 4945–4954. [Google Scholar] [PubMed]
- Tharmapalan, P.; Mahendralingam, M.; Berman, H.K.; Khokha, R. Mammary stem cells and progenitors: Targeting the roots of breast cancer for prevention. EMBO J. 2019, 38, e100852. [Google Scholar] [CrossRef]
- Fu, Y.; Chang, H.; Peng, X.; Bai, Q.; Yi, L.; Zhou, Y.; Zhu, J.; Mi, M. Resveratrol inhibits breast cancer stem-like cells and induces autophagy via suppressing Wnt/beta-catenin signaling pathway. PLoS ONE 2014, 9, e102535. [Google Scholar]
- Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Alhumaydhi, F.A.; Alsahli, M.A.; Rahmani, A.H. Potential therapeutic targets of epigallocatechin gallate (EGCG), the most abundant catechin in green tea, and its role in the therapy of various types of cancer. Molecules 2020, 25, 3146. [Google Scholar] [CrossRef]
- Abd El-Rahman, S.S.; Shehab, G.; Nashaat, H. Epigallocatechin-3-gallate: The prospective targeting of cancer stem cells and preventing metastasis of chemically-induced mammary cancer in rats. Am. J. Med. Sci. 2017, 354, 54–63. [Google Scholar] [CrossRef]
- Mineva, N.D.; Paulson, K.E.; Naber, S.P.; Yee, A.S.; Sonenshein, G.E. Epigallocatechin-3-gallate inhibits stem-like inflammatory breast cancer cells. PLoS ONE 2013, 8, e73464. [Google Scholar] [CrossRef]
- Pan, X.; Zhao, B.; Song, Z.; Han, S.; Wang, M. Estrogen receptor-alpha36 is involved in epigallocatechin-3-gallate induced growth inhibition of ER-negative breast cancer stem/progenitor cells. J. Pharmacol. Sci. 2016, 130, 85–93. [Google Scholar] [CrossRef]
- Zhang, X.T.; Kang, L.G.; Ding, L.; Vranic, S.; Gatalica, Z.; Wang, Z.Y. A positive feedback loop of ER-alpha36/EGFR promotes malignant growth of ER-negative breast cancer cells. Oncogene 2011, 30, 770–780. [Google Scholar] [CrossRef]
- Deng, H.; Zhang, X.T.; Wang, M.L.; Zheng, H.Y.; Liu, L.J.; Wang, Z.Y. Er-alpha36-mediated rapid estrogen signaling positively regulates er-positive breast cancer stem/progenitor cells. PLoS ONE 2014, 9, e88034. [Google Scholar]
- Chung, S.S.; Vadgama, J.V. Curcumin and epigallocatechin gallate inhibit the cancer stem cell phenotype via down-regulation of STAT3-NF-kappaB signaling. Anticancer Res. 2015, 35, 39–46. [Google Scholar]
- Wei, W.; Tweardy, D.J.; Zhang, M.; Zhang, X.; Landua, J.; Petrovic, I.; Bu, W.; Roarty, K.; Hilsenbeck, S.G.; Rosen, J.M.; et al. STAT3 signaling is activated preferentially in tumor-initiating cells in claudin-low models of human breast cancer. Stem Cells 2014, 32, 2571–2582. [Google Scholar] [CrossRef]
- Kabil, N.; Bayraktar, R.; Kahraman, N.; Mokhlis, H.A.; Calin, G.A.; Lopez-Berestein, G.; Ozpolat, B. Thymoquinone inhibits cell proliferation, migration, and invasion by regulating the elongation factor 2 kinase (eEF-2K) signaling axis in triple-negative breast cancer. Breast Cancer Res. Treat. 2018, 171, 593–605. [Google Scholar] [CrossRef]
- Woo, C.C.; Hsu, A.; Kumar, A.P.; Sethi, G.; Tan, K.H. Thymoquinone inhibits tumor growth and induces apoptosis in a breast cancer xenograft mouse model: The role of p38 MAPK and ROS. PLoS ONE 2013, 8, e75356. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Ghosh, A.; Maiti, S.; Ahir, M.; Debnath, G.H.; Gupta, P.; Bhattacharjee, M.; Ghosh, S.; Chattopadhyay, S.; Mukherjee, P.; et al. Delivery of thymoquinone through hyaluronic acid-decorated mixed Pluronic® nanoparticles to attenuate angiogenesis and metastasis of triple-negative breast cancer. J. Control. Release 2020, 322, 357–374. [Google Scholar] [CrossRef]
- Wei, C.; Zou, H.; Xiao, T.; Liu, X.; Wang, Q.; Cheng, J.; Fu, S.; Peng, J.; Xie, X.; Fu, J. TQFL12, a novel synthetic derivative of TQ, inhibits triple-negative breast cancer metastasis and invasion through activating AMPK/ACC pathway. J. Cell Mol. Med. 2021, 25, 10101–10110. [Google Scholar] [CrossRef]
- Huang, C.C.; Lo, C.P.; Chiu, C.Y.; Shyur, L.F. Deoxyelephantopin, a novel multifunctional agent, suppresses mammary tumour growth and lung metastasis and doubles survival time in mice. Br. J. Pharmacol. 2010, 159, 856–871. [Google Scholar] [CrossRef]
- Lee, W.L.; Shyur, L.F. Deoxyelephantopin impedes mammary adenocarcinoma cell motility by inhibiting calpain-mediated adhesion dynamics and inducing reactive oxygen species and aggresome formation. Free Radic. Biol. Med. 2012, 52, 1423–1436. [Google Scholar] [CrossRef]
- Lee, W.L.; Wen, T.N.; Shiau, J.Y.; Shyur, L.F. Differential proteomic profiling identifies novel molecular targets of paclitaxel and phytoagent deoxyelephantopin against mammary adenocarcinoma cells. J. Proteome Res. 2010, 9, 237–253. [Google Scholar] [CrossRef]
- Nakagawa-Goto, K.; Chen, J.Y.; Cheng, Y.T.; Lee, W.L.; Takeya, M.; Saito, Y.; Lee, K.H.; Shyur, L.F. Novel sesquiterpene lactone analogues as potent anti-breast cancer agents. Mol. Oncol. 2016, 10, 921–937. [Google Scholar] [CrossRef]
- Shiau, J.Y.; Nakagawa-Goto, K.; Lee, K.H.; Shyur, L.F. Phytoagent deoxyelephantopin derivative inhibits triple negative breast cancer cell activity by inducing oxidative stress-mediated paraptosis-like cell death. Oncotarget 2017, 8, 56942–56958. [Google Scholar] [CrossRef]
- Slezakova, S.; Ruda-Kucerova, J. Anticancer activity of artemisinin and its derivatives. Anticancer Res. 2017, 37, 5995–6003. [Google Scholar]
- Li, N.; Guo, W.; Li, Y.; Zuo, H.; Zhang, H.; Wang, Z.; Zhao, Y.; Yang, F.; Ren, G.; Zhang, S. Construction and anti-tumor activities of disulfide-linked docetaxel-dihydroartemisinin nanoconjugates. Colloids Surf. B Biointerfaces 2020, 191, 111018. [Google Scholar] [CrossRef]
- Tao, J.; Diao, L.; Chen, F.; Shen, A.; Wang, S.; Jin, H.; Cai, D.; Hu, Y. pH-sensitive nanoparticles codelivering docetaxel and dihydroartemisinin effectively treat breast cancer by enhancing reactive oxidative species-mediated mitochondrial apoptosis. Mol. Pharm. 2021, 18, 74–86. [Google Scholar] [CrossRef]
- Nosrati, H.; Barzegari, P.; Danafar, H.; Kheiri Manjili, H. Biotin-functionalized copolymeric PEG-PCL micelles for in vivo tumour-targeted delivery of artemisinin. Artif. Cells Nanomed. Biotechnol. 2019, 47, 104–114. [Google Scholar] [CrossRef]
- Natesan, S.; Ponnusamy, C.; Sugumaran, A.; Chelladurai, S.; Shanmugam Palaniappan, S.; Palanichamy, R. Artemisinin loaded chitosan magnetic nanoparticles for the efficient targeting to the breast cancer. Int. J. Biol. Macromol. 2017, 104, 1853–1859. [Google Scholar] [CrossRef]
- Pirali, M.; Taheri, M.; Zarei, S.; Majidi, M.; Ghafouri, H. Artesunate, as a HSP70 ATPase activity inhibitor, induces apoptosis in breast cancer cells. Int. J. Biol. Macromol. 2020, 164, 3369–3375. [Google Scholar] [CrossRef]
- He, W.; Du, Y.; Wang, T.; Wang, J.; Cheng, L.; Li, X. Dimeric artesunate-phosphatidylcholine-based liposomes for irinotecan delivery as a combination therapy approach. Mol. Pharm. 2021, 18, 3862–3870. [Google Scholar] [CrossRef] [PubMed]
- Riganti, C.; Doublier, S.; Viarisio, D.; Miraglia, E.; Pescarmona, G.; Ghigo, D.; Bosia, A. Artemisinin induces doxorubicin resistance in human colon cancer cells via calcium-dependent activation of HIF-1alpha and P-glycoprotein overexpression. Br. J. Pharmacol. 2009, 156, 1054–1066. [Google Scholar] [CrossRef] [PubMed]
- Bachmeier, B.; Fichtner, I.; Killian, P.H.; Kronski, E.; Pfeffer, U.; Efferth, T. Development of resistance towards artesunate in MDA-MB-231 human breast cancer cells. PLoS ONE 2011, 6, e20550. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gallego-Jara, J.; Lozano-Terol, G.; Sola-Martinez, R.A.; Canovas-Diaz, M.; de Diego Puente, T. A compressive review about Taxol®: History and future challenges. Molecules 2020, 25, 5986. [Google Scholar] [CrossRef]
- Ateba, S.B.; Mvondo, M.A.; Ngeu, S.T.; Tchoumtchoua, J.; Awounfack, C.F.; Njamen, D.; Krenn, L. Natural terpenoids against female breast cancer: A 5-year recent research. Curr. Med. Chem. 2018, 25, 3162–3213. [Google Scholar] [CrossRef]
- Abu Samaan, T.M.; Samec, M.; Liskova, A.; Kubatka, P.; Busselberg, D. Paclitaxel’s mechanistic and clinical effects on breast cancer. Biomolecules 2019, 9, 789. [Google Scholar] [CrossRef]
- Jiang, W.; Chen, M.; Xiao, C.; Yang, W.; Qin, Q.; Tan, Q.; Liang, Z.; Liao, X.; Mao, A.; Wei, C. Triptolide suppresses growth of breast cancer by targeting HMGB1 in vitro and in vivo. Biol. Pharm. Bull. 2019, 42, 892–899. [Google Scholar] [CrossRef]
- Landgraf, M.; Lahr, C.A.; Kaur, I.; Shafiee, A.; Sanchez-Herrero, A.; Janowicz, P.W.; Ravichandran, A.; Howard, C.B.; Cifuentes-Rius, A.; McGovern, J.A.; et al. Targeted camptothecin delivery via silicon nanoparticles reduces breast cancer metastasis. Biomaterials 2020, 240, 119791. [Google Scholar] [CrossRef]
- Rapisarda, A.; Melillo, G. Overcoming disappointing results with antiangiogenic therapy by targeting hypoxia. Nat. Rev. Clin. Oncol. 2012, 9, 378–390. [Google Scholar] [CrossRef]
- Pham, E.; Yin, M.; Peters, C.G.; Lee, C.R.; Brown, D.; Xu, P.; Man, S.; Jayaraman, L.; Rohde, E.; Chow, A.; et al. Preclinical efficacy of bevacizumab with CRLX101, an investigational nanoparticle-drug conjugate, in treatment of metastatic triple-negative breast cancer. Cancer Res. 2016, 76, 4493–4503. [Google Scholar] [CrossRef]
- Efferth, T.; Fu, Y.J.; Zu, Y.G.; Schwarz, G.; Konkimalla, V.S.; Wink, M. Molecular target-guided tumor therapy with natural products derived from traditional chinese medicine. Curr. Med. Chem. 2007, 14, 2024–2032. [Google Scholar] [CrossRef]
- Anders, C.; Deal, A.M.; Abramson, V.; Liu, M.C.; Storniolo, A.M.; Carpenter, J.T.; Puhalla, S.; Nanda, R.; Melhem-Bertrandt, A.; Lin, N.U.; et al. TBCRC 018: Phase II study of iniparib in combination with irinotecan to treat progressive triple negative breast cancer brain metastases. Breast Cancer Res. Treat. 2014, 146, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab Govitecan in metastatic triple-negative breast cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Mayer, I.A.; Vahdat, L.T.; Tolaney, S.M.; Isakoff, S.J.; Diamond, J.R.; O’Shaughnessy, J.; Moroose, R.L.; Santin, A.D.; Abramson, V.G.; et al. Sacituzumab Govitecan-hziy in refractory metastatic triple-negative breast cancer. N. Engl. J. Med. 2019, 380, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Tolaney, S.M.; Punie, K.; Loirat, D.; Oliveira, M.; Kalinsky, K.; Zelnak, A.; Aftimos, P.; Dalenc, F.; Sardesai, S.; et al. Biomarker analyses in the Phase III ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer. Ann. Oncol. 2021, 32, 1148–1156. [Google Scholar] [CrossRef]
- Kuo, L.J.; Yang, L.X. Gamma-H2AX—A novel biomarker for DNA double-strand breaks. In Vivo 2008, 22, 305–309. [Google Scholar]
- Cardillo, T.M.; Sharkey, R.M.; Rossi, D.L.; Arrojo, R.; Mostafa, A.A.; Goldenberg, D.M. Synthetic lethality exploitation by an anti- Trop-2-SN-38 antibody-drug conjugate, IMMU-132, plus PARP inhibitors in BRCA1/2-wild-type triple-negative breast cancer. Clin. Cancer Res. 2017, 23, 3405–3415. [Google Scholar] [CrossRef]
- Najar, I.A.; Johri, R.K. Pharmaceutical and pharmacological approaches for bioavailability enhancement of etoposide. J. Biosci. 2014, 39, 139–144. [Google Scholar] [CrossRef]
- Yuan, P.; Xu, B.H.; Wang, J.Y.; Ma, F.; Fan, Y.; Li, Q.; Zhang, P. Oral etoposide monotherapy is effective for metastatic breast cancer with heavy prior therapy. Chin. Med. J. 2012, 125, 775–779. [Google Scholar]
- Yuan, P.; Di, L.; Zhang, X.; Yan, M.; Wan, D.; Li, L.; Zhang, Y.; Cai, J.; Dai, H.; Zhu, Q.; et al. Efficacy of oral etoposide in pretreated metastatic breast cancer: A multicenter Phase 2 study. Medicine 2015, 94, e774. [Google Scholar] [CrossRef]
- Valabrega, G.; Berrino, G.; Milani, A.; Aglietta, M.; Montemurro, F. A retrospective analysis of the activity and safety of oral etoposide in heavily pretreated metastatic breast cancer patients. Breast J. 2015, 21, 241–245. [Google Scholar] [CrossRef]
- Wu, Y.H.; Hong, C.W.; Wang, Y.C.; Huang, W.J.; Yeh, Y.L.; Wang, B.J.; Wang, Y.J.; Chiu, H.W. A novel histone deacetylase inhibitor tMU-35435 enhances etoposide cytotoxicity through the proteasomal degradation of DNA-PKcs in triple-negative breast cancer. Cancer Lett. 2017, 400, 79–88. [Google Scholar] [CrossRef]
- Deng, D.; Shah, K. TRAIL of hope meeting resistance in cancer. Trends Cancer 2020, 6, 989–1001. [Google Scholar] [CrossRef]
- Das, S.; Tripathi, N.; Siddharth, S.; Nayak, A.; Nayak, D.; Sethy, C.; Bharatam, P.V.; Kundu, C.N. Etoposide and doxorubicin enhance the sensitivity of triple negative breast cancers through modulation of TRAIL-DR5 axis. Apoptosis 2017, 22, 1205–1224. [Google Scholar] [CrossRef]
- Yao, M.; Fan, X.; Yuan, B.; Takagi, N.; Liu, S.; Han, X.; Ren, J.; Liu, J. Berberine inhibits NLRP3 inflammasome pathway in human triple-negative breast cancer MDA-MB-231 cell. BMC Complement. Altern. Med. 2019, 19, 216. [Google Scholar] [CrossRef]
- Karki, R.; Man, S.M.; Kanneganti, T.D. Inflammasomes and cancer. Cancer Immunol. Res. 2017, 5, 94–99. [Google Scholar] [CrossRef]
- Guo, B.; Fu, S.; Zhang, J.; Liu, B.; Li, Z. Targeting inflammasome/IL-1 pathways for cancer immunotherapy. Sci. Rep. 2016, 6, 36107. [Google Scholar] [CrossRef]
- Carmona, G.; Perera, U.; Gillett, C.; Naba, A.; Law, A.L.; Sharma, V.P.; Wang, J.; Wyckoff, J.; Balsamo, M.; Mosis, F.; et al. Lamellipodin promotes invasive 3D cancer cell migration via regulated interactions with Ena/VASP and SCAR/WAVE. Oncogene 2016, 35, 5155–5169. [Google Scholar] [CrossRef]
- Su, K.; Hu, P.; Wang, X.; Kuang, C.; Xiang, Q.; Yang, F.; Xiang, J.; Zhu, S.; Wei, L.; Zhang, J. Tumor suppressor berberine binds VASP to inhibit cell migration in basal-like breast cancer. Oncotarget 2016, 7, 45849–45862. [Google Scholar] [CrossRef]
- Refaat, A.; Abdelhamed, S.; Yagita, H.; Inoue, H.; Yokoyama, S.; Hayakawa, Y.; Saiki, I. Berberine enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in breast cancer. Oncol. Lett. 2013, 6, 840–844. [Google Scholar] [CrossRef]
- Yang, M.; Teng, W.; Qu, Y.; Wang, H.; Yuan, Q. Sulforaphene inhibits triple negative breast cancer through activating tumor suppressor Egr1. Breast Cancer Res. Treat. 2016, 158, 277–286. [Google Scholar] [CrossRef]
- Castro, N.P.; Rangel, M.C.; Merchant, A.S.; MacKinnon, G.; Cuttitta, F.; Salomon, D.S.; Kim, Y.S. Sulforaphane suppresses the growth of triple-negative breast cancer stem-like cells in vitro and in vivo. Cancer Prev. Res. 2019, 12, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wang, F.; Liu, Y.; Wang, S.; Li, X.; Huang, Y.; Xia, Y.; Cao, C. Sulforaphane induces autophagy by inhibition of HDAC6-mediated PTEN activation in triple negative breast cancer cells. Life Sci. 2018, 213, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Burnett, J.P.; Lim, G.; Li, Y.; Shah, R.B.; Lim, R.; Paholak, H.J.; McDermott, S.P.; Sun, L.; Tsume, Y.; Bai, S.; et al. Sulforaphane enhances the anticancer activity of taxanes against triple negative breast cancer by killing cancer stem cells. Cancer Lett. 2017, 394, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Uruena, C.; Mancipe, J.; Hernandez, J.; Castaneda, D.; Pombo, L.; Gomez, A.; Asea, A.; Fiorentino, S. Gallotannin-rich Caesalpinia spinosa fraction decreases the primary tumor and factors associated with poor prognosis in a murine breast cancer model. BMC Complement. Altern. Med. 2013, 13, 74. [Google Scholar] [CrossRef]
- Sandoval, T.A.; Uruena, C.P.; Llano, M.; Gomez-Cadena, A.; Hernandez, J.F.; Sequeda, L.G.; Loaiza, A.E.; Barreto, A.; Li, S.; Fiorentino, S. Standardized extract from Caesalpinia spinosa is cytotoxic over cancer stem cells and enhance anticancer activity of doxorubicin. Am. J. Chin. Med. 2016, 44, 1693–1717. [Google Scholar] [CrossRef]
- Kitagawa, S.; Nabekura, T.; Kamiyama, S.; Takahashi, T.; Nakamura, Y.; Kashiwada, Y.; Ikeshiro, Y. Effects of alkyl gallates on P-glycoprotein function. Biochem. Pharmacol. 2005, 70, 1262–1266. [Google Scholar] [CrossRef]
- Uruena, C.; Sandoval, T.A.; Lasso, P.; Tawil, M.; Barreto, A.; Torregrosa, L.; Fiorentino, S. Evaluation of chemotherapy and P2Et extract combination in ex-vivo derived tumor mammospheres from breast cancer patients. Sci. Rep. 2020, 10, 19639. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Germain, N.; Dhayer, M.; Boileau, M.; Fovez, Q.; Kluza, J.; Marchetti, P. Lipid metabolism and resistance to anticancer treatment. Biology 2020, 9, 474. [Google Scholar] [CrossRef]
- Bronte, V.; Serafini, P.; Mazzoni, A.; Segal, D.M.; Zanovello, P. L-arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends Immunol. 2003, 24, 302–306. [Google Scholar] [CrossRef]
- Pavlova, N.N.; Thompson, C.B. The emerging hallmarks of cancer metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Chen, X.S.; Li, L.Y.; Guan, Y.D.; Yang, J.M.; Cheng, Y. Anticancer strategies based on the metabolic profile of tumor cells: Therapeutic targeting of the Warburg effect. Acta Pharmacol. Sin. 2016, 37, 1013–1019. [Google Scholar] [CrossRef]
- Pelicano, H.; Zhang, W.; Liu, J.; Hammoudi, N.; Dai, J.; Xu, R.H.; Pusztai, L.; Huang, P. Mitochondrial dysfunction in some triple-negative breast cancer cell lines: Role of mTOR pathway and therapeutic potential. Breast Cancer Res. 2014, 16, 434. [Google Scholar] [CrossRef]
- Brown, R.S.; Wahl, R.L. Overexpression of Glut-1 glucose transporter in human breast cancer. An immunohistochemical study. Cancer 1993, 72, 2979–2985. [Google Scholar] [CrossRef]
- Younes, M.; Brown, R.W.; Mody, D.R.; Fernandez, L.; Laucirica, R. GLUT1 expression in human breast carcinoma: Correlation with known prognostic markers. Anticancer Res. 1995, 15, 2895–2898. [Google Scholar]
- Hussein, Y.R.; Bandyopadhyay, S.; Semaan, A.; Ahmed, Q.; Albashiti, B.; Jazaerly, T.; Nahleh, Z.; Ali-Fehmi, R. GLUT-1 expression correlates with basal-like breast cancer. Transl. Oncol. 2011, 4, 321–327. [Google Scholar] [CrossRef]
- Xiao, H.; Wang, J.; Yan, W.; Cui, Y.; Chen, Z.; Gao, X.; Wen, X.; Chen, J. Glut1 regulates cell glycolysis and proliferation in prostate cancer. Prostate 2018, 78, 86–94. [Google Scholar] [CrossRef]
- Wang, T.; Liu, H.; Lian, G.; Zhang, S.Y.; Wang, X.; Jiang, C. HIF1alpha-induced glycolysis metabolism is essential to the activation of inflammatory macrophages. Mediat. Inflamm. 2017, 2017, 9029327. [Google Scholar] [CrossRef]
- Robey, I.F.; Lien, A.D.; Welsh, S.J.; Baggett, B.K.; Gillies, R.J. Hypoxia-inducible factor-1alpha and the glycolytic phenotype in tumors. Neoplasia 2005, 7, 324–330. [Google Scholar] [CrossRef]
- Wei, R.; Mao, L.; Xu, P.; Zheng, X.; Hackman, R.M.; Mackenzie, G.G.; Wang, Y. Suppressing glucose metabolism with epigallocatechin-3-gallate (EGCG) reduces breast cancer cell growth in preclinical models. Food Funct. 2018, 9, 5682–5696. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, X.; Xie, X.; Ye, F.; Chen, B.; Song, C.; Tang, H.; Xie, X. High expressions of LDHA and AMPK as prognostic biomarkers for breast cancer. Breast 2016, 30, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhao, Y.; Ding, Y.; Liu, H.; Liu, Z.; Fodstad, O.; Riker, A.I.; Kamarajugadda, S.; Lu, J.; Owen, L.B.; et al. Warburg effect in chemosensitivity: Targeting lactate dehydrogenase-A re-sensitizes taxol-resistant cancer cells to taxol. Mol. Cancer 2010, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Menendez, J.A.; Lupu, R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat. Rev. Cancer 2007, 7, 763–777. [Google Scholar] [CrossRef]
- Costea, T.; Vlad, O.C.; Miclea, L.C.; Ganea, C.; Szollosi, J.; Mocanu, M.M. Alleviation of multidrug resistance by flavonoid and non-flavonoid compounds in breast, lung, colorectal and prostate cancer. Int. J. Mol. Sci. 2020, 21, 401. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Zhang, J.T. A new mechanism of drug resistance in breast cancer cells: Fatty acid synthase overexpression-mediated palmitate overproduction. Mol. Cancer Ther. 2008, 7, 263–270. [Google Scholar] [CrossRef]
- Wu, X.; Qin, L.; Fako, V.; Zhang, J.T. Molecular mechanisms of fatty acid synthase (FASN)-mediated resistance to anti-cancer treatments. Adv. Biol. Regul. 2014, 54, 214–221. [Google Scholar] [CrossRef]
- Giro-Perafita, A.; Sarrats, A.; Perez-Bueno, F.; Oliveras, G.; Buxo, M.; Brunet, J.; Vinas, G.; Miquel, T.P. Fatty acid synthase expression and its association with clinico-histopathological features in triple-negative breast cancer. Oncotarget 2017, 8, 74391–74405. [Google Scholar] [CrossRef]
- Giro-Perafita, A.; Palomeras, S.; Lum, D.H.; Blancafort, A.; Vinas, G.; Oliveras, G.; Perez-Bueno, F.; Sarrats, A.; Welm, A.L.; Puig, T. Preclinical evaluation of fatty acid synthase and EGFR inhibition in triple-negative breast cancer. Clin. Cancer Res. 2016, 22, 4687–4697. [Google Scholar] [CrossRef]
- Pandey, P.R.; Okuda, H.; Watabe, M.; Pai, S.K.; Liu, W.; Kobayashi, A.; Xing, F.; Fukuda, K.; Hirota, S.; Sugai, T.; et al. Resveratrol suppresses growth of cancer stem-like cells by inhibiting fatty acid synthase. Breast Cancer Res. Treat. 2011, 130, 387–398. [Google Scholar] [CrossRef]
- Furuhashi, M.; Hotamisligil, G.S. Fatty acid-binding proteins: Role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discov. 2008, 7, 489–503. [Google Scholar] [CrossRef]
- Guaita-Esteruelas, S.; Bosquet, A.; Saavedra, P.; Guma, J.; Girona, J.; Lam, E.W.; Amillano, K.; Borras, J.; Masana, L. Exogenous FABP4 increases breast cancer cell proliferation and activates the expression of fatty acid transport proteins. Mol. Carcinog. 2017, 56, 208–217. [Google Scholar] [CrossRef]
- Di-Poi, N.; Tan, N.S.; Michalik, L.; Wahli, W.; Desvergne, B. Antiapoptotic role of PPARbeta in keratinocytes via transcriptional control of the Akt1 signaling pathway. Mol. Cell 2002, 10, 721–733. [Google Scholar] [CrossRef]
- Liu, R.Z.; Godbout, R. An amplified fatty acid-binding protein gene cluster in prostate cancer: Emerging roles in lipid metabolism and metastasis. Cancers 2020, 12, 3823. [Google Scholar] [CrossRef]
- Kannan-Thulasiraman, P.; Seachrist, D.D.; Mahabeleshwar, G.H.; Jain, M.K.; Noy, N. Fatty acid-binding protein 5 and PPARbeta/delta are critical mediators of epidermal growth factor receptor-induced carcinoma cell growth. J. Biol. Chem. 2010, 285, 19106–19115. [Google Scholar] [CrossRef]
- Powell, C.A.; Nasser, M.W.; Zhao, H.; Wochna, J.C.; Zhang, X.; Shapiro, C.; Shilo, K.; Ganju, R.K. Fatty acid binding protein 5 promotes metastatic potential of triple negative breast cancer cells through enhancing epidermal growth factor receptor stability. Oncotarget 2015, 6, 6373–6385. [Google Scholar] [CrossRef]
- Peters, J.M.; Foreman, J.E.; Gonzalez, F.J. Dissecting the role of peroxisome proliferator-activated receptor-beta/delta (PPARbeta/delta) in colon, breast, and lung carcinogenesis. Cancer Metastasis Rev. 2011, 30, 619–640. [Google Scholar] [CrossRef]
- Liu, R.Z.; Graham, K.; Glubrecht, D.D.; Germain, D.R.; Mackey, J.R.; Godbout, R. Association of FABP5 expression with poor survival in triple-negative breast cancer: Implication for retinoic acid therapy. Am. J. Pathol. 2011, 178, 997–1008. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.; Koo, J.S. Differential expression of lipid metabolism-related proteins in different breast cancer subtypes. PLoS ONE 2015, 10, e0119473. [Google Scholar] [CrossRef]
- Apaya, M.K.; Hsiao, P.W.; Yang, Y.C.; Shyur, L.F. Deregulating the CYP2C19/epoxy-eicosatrienoic acid-associated FABP4/FABP5 signaling network as a therapeutic approach for metastatic triple-negative breast cancer. Cancers 2020, 12, 199. [Google Scholar] [CrossRef]
- Thulasiraman, P.; McAndrews, D.J.; Mohiudddin, I.Q. Curcumin restores sensitivity to retinoic acid in triple negative breast cancer cells. BMC Cancer 2014, 14, 724. [Google Scholar] [CrossRef]
- Panigrahy, D.; Kaipainen, A.; Greene, E.R.; Huang, S. Cytochrome p450-derived eicosanoids: The neglected pathway in cancer. Cancer Metastasis Rev. 2010, 29, 723–735. [Google Scholar] [CrossRef]
- Apaya, M.K.; Shiau, J.Y.; Liao, G.S.; Liang, Y.J.; Chen, C.W.; Yang, H.C.; Chu, C.H.; Yu, J.C.; Shyur, L.F. Integrated omics-based pathway analyses uncover CYP epoxygenase-associated networks as theranostic targets for metastatic triple negative breast cancer. J. Exp. Clin. Cancer Res. 2019, 38, 187. [Google Scholar] [CrossRef]
- Du, S.; Wagner, N.; Wagner, K.D. The emerging role of PPARbeta/delta in tumor angiogenesis. PPAR Res. 2020, 2020, 3608315. [Google Scholar] [CrossRef] [PubMed]
- Forootan, F.S.; Forootan, S.S.; Gou, X.; Yang, J.; Liu, B.; Chen, D.; Al Fayi, M.S.; Al-Jameel, W.; Rudland, P.S.; Hussain, S.A.; et al. Fatty acid activated PPARgamma promotes tumorigenicity of prostate cancer cells by up regulating VEGF via PPAR responsive elements of the promoter. Oncotarget 2016, 7, 9322–9339. [Google Scholar] [CrossRef] [PubMed]
- Varghese, E.; Samuel, S.M.; Liskova, A.; Samec, M.; Kubatka, P.; Busselberg, D. Targeting glucose metabolism to overcome resistance to anticancer chemotherapy in breast cancer. Cancers 2020, 12, 2252. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, K.; Hana, D.; Chou, J.T.; Singh, C.; Mackiewicz, A.; Kaczmarek, M. Aspects of the tumor microenvironment involved in immune resistance and drug resistance. Front. Immunol. 2021, 12, 656364. [Google Scholar] [CrossRef]
- Dan, V.M.; Raveendran, R.S.; Baby, S. Resistance to intervention: Paclitaxel in breast cancer. Mini. Rev. Med. Chem. 2021, 21, 1237–1268. [Google Scholar] [CrossRef]
- Cao, L.; Zhou, Y.; Li, X.; Lin, S.; Tan, Z.; Guan, F. Integrating transcriptomics, proteomics, glycomics and glycoproteomics to characterize paclitaxel resistance in breast cancer cells. J. Proteom. 2021, 243, 104266. [Google Scholar] [CrossRef]
- Song, Y.; Li, W.; Peng, X.; Xie, J.; Li, H.; Tan, G. Inhibition of autophagy results in a reversal of taxol resistance in nasopharyngeal carcinoma by enhancing taxol-induced caspase-dependent apoptosis. Am. J. Transl. Res. 2017, 9, 1934–1942. [Google Scholar]
- Gonzalez-Reyes, S.; Marin, L.; Gonzalez, L.; Gonzalez, L.O.; del Casar, J.M.; Lamelas, M.L.; Gonzalez-Quintana, J.M.; Vizoso, F.J. Study of TLR3, TLR4 and TLR9 in breast carcinomas and their association with metastasis. BMC Cancer 2010, 10, 665. [Google Scholar] [CrossRef]
- Mikula-Pietrasik, J.; Witucka, A.; Pakula, M.; Uruski, P.; Begier-Krasinska, B.; Niklas, A.; Tykarski, A.; Ksiazek, K. Comprehensive review on how platinum- and taxane-based chemotherapy of ovarian cancer affects biology of normal cells. Cell. Mol. Life Sci. 2019, 76, 681–697. [Google Scholar] [CrossRef]
- Luo, T.; Wang, J.; Yin, Y.; Hua, H.; Jing, J.; Sun, X.; Li, M.; Zhang, Y.; Jiang, Y. (−)-Epigallocatechin gallate sensitizes breast cancer cells to paclitaxel in a murine model of breast carcinoma. Breast Cancer Res. 2010, 12, R8. [Google Scholar] [CrossRef]
- Narayanan, S.; Mony, U.; Vijaykumar, D.K.; Koyakutty, M.; Paul-Prasanth, B.; Menon, D. Sequential release of epigallocatechin gallate and paclitaxel from PLGA-casein core/shell nanoparticles sensitizes drug-resistant breast cancer cells. Nanomedicine 2015, 11, 1399–1406. [Google Scholar] [CrossRef]
- Bayet-Robert, M.; Kwiatkowski, F.; Leheurteur, M.; Gachon, F.; Planchat, E.; Abrial, C.; Mouret-Reynier, M.A.; Durando, X.; Barthomeuf, C.; Chollet, P. Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol. Ther. 2010, 9, 8–14. [Google Scholar] [CrossRef]
- Balkwill, F.R.; Capasso, M.; Hagemann, T. The tumor microenvironment at a glance. J. Cell Sci. 2012, 125, 5591–5596. [Google Scholar] [CrossRef]
- O’Donnell, J.S.; Teng, M.W.L.; Smyth, M.J. Cancer immunoediting and resistance to t cell-based immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 151–167. [Google Scholar] [CrossRef]
- Mittal, D.; Gubin, M.M.; Schreiber, R.D.; Smyth, M.J. New insights into cancer immunoediting and its three component phases--elimination, equilibrium and escape. Curr. Opin. Immunol. 2014, 27, 16–25. [Google Scholar] [CrossRef]
- Place, A.E.; Jin Huh, S.; Polyak, K. The microenvironment in breast cancer progression: Biology and implications for treatment. Breast Cancer Res. 2011, 13, 227. [Google Scholar] [CrossRef]
- Nagarajan, D.; McArdle, S.E.B. Immune landscape of breast cancers. Biomedicines 2018, 6, 20. [Google Scholar] [CrossRef]
- Denkert, C.; von Minckwitz, G.; Darb-Esfahani, S.; Lederer, B.; Heppner, B.I.; Weber, K.E.; Budczies, J.; Huober, J.; Klauschen, F.; Furlanetto, J.; et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: A pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018, 19, 40–50. [Google Scholar] [CrossRef]
- Sidaway, P. Immunoscore provides a more accurate prognosis. Nat. Rev. Clin. Oncol. 2018, 15, 471. [Google Scholar] [CrossRef] [PubMed]
- Gruosso, T.; Gigoux, M.; Manem, V.S.K.; Bertos, N.; Zuo, D.; Perlitch, I.; Saleh, S.M.I.; Zhao, H.; Souleimanova, M.; Johnson, R.M.; et al. Spatially distinct tumor immune microenvironments stratify triple-negative breast cancers. J. Clin. Investig. 2019, 129, 1785–1800. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, Q.; Ye, J.; Wang, F.; Zhang, Y.; Wevers, E.; Schwartz, T.; Hunborg, P.; Varvares, M.A.; Hoft, D.F.; et al. Tumor-infiltrating gammadelta T lymphocytes predict clinical outcome in human breast cancer. J. Immunol. 2012, 189, 5029–5036. [Google Scholar] [CrossRef] [PubMed]
- Kresovich, J.K.; O’Brien, K.M.; Xu, Z.; Weinberg, C.R.; Sandler, D.P.; Taylor, J.A. Prediagnostic immune cell profiles and breast cancer. JAMA Netw. Open 2020, 3, e1919536. [Google Scholar] [CrossRef]
- Choi, J.; Gyamfi, J.; Jang, H.; Koo, J.S. The role of tumor-associated macrophage in breast cancer biology. Histol. Histopathol. 2018, 33, 133–145. [Google Scholar]
- Condamine, T.; Gabrilovich, D.I. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2011, 32, 19–25. [Google Scholar] [CrossRef]
- Zhang, B.; Yin, C.; Li, H.; Shi, L.; Liu, N.; Sun, Y.; Lu, S.; Liu, Y.; Sun, L.; Li, X.; et al. Nir1 promotes invasion of breast cancer cells by binding to chemokine (C-C motif) ligand 18 through the PI3K/Akt/GSK3beta/Snail signalling pathway. Eur. J. Cancer 2013, 49, 3900–3913. [Google Scholar] [CrossRef]
- Zhao, C.; Zheng, S.; Yan, Z.; Deng, Z.; Wang, R.; Zhang, B. CCL18 promotes the invasion and metastasis of breast cancer through Annexin A2. Oncol. Rep. 2020, 43, 571–580. [Google Scholar] [CrossRef]
- Patel, D.A.; Xi, J.; Luo, J.; Hassan, B.; Thomas, S.; Ma, C.X.; Campian, J.L. Neutrophil-to-lymphocyte ratio as a predictor of survival in patients with triple-negative breast cancer. Breast Cancer Res. Treat. 2019, 174, 443–452. [Google Scholar] [CrossRef]
- Helfen, A.; Roth, J.; Ng, T.; Eisenblaetter, M. In vivo imaging of pro- and antitumoral cellular components of the tumor microenvironment. J. Nucl. Med. 2018, 59, 183–188. [Google Scholar] [CrossRef]
- Anders, C.; Carey, L.A. Understanding and treating triple-negative breast cancer. Oncology 2008, 22, 1233–1239. [Google Scholar]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of exosomes in cancer. J. Clin. Investig. 2016, 126, 1208–1215. [Google Scholar] [CrossRef]
- Sun, D.; Zhuang, X.; Zhang, S.; Deng, Z.B.; Grizzle, W.; Miller, D.; Zhang, H.G. Exosomes are endogenous nanoparticles that can deliver biological information between cells. Adv. Drug Deliv. Rev. 2013, 65, 342–347. [Google Scholar] [CrossRef]
- Jain, R.K. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science 2005, 307, 58–62. [Google Scholar] [CrossRef]
- Cooke, V.G.; LeBleu, V.S.; Keskin, D.; Khan, Z.; O’Connell, J.T.; Teng, Y.; Duncan, M.B.; Xie, L.; Maeda, G.; Vong, S.; et al. Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by met signaling pathway. Cancer Cell 2012, 21, 66–81. [Google Scholar] [CrossRef]
- Chu, D.T.; Phuong, T.N.T.; Tien, N.L.B.; Tran, D.K.; Nguyen, T.T.; Thanh, V.V.; Quang, T.L.; Minh, L.B.; Pham, V.H.; Ngoc, V.T.N.; et al. The effects of adipocytes on the regulation of breast cancer in the tumor microenvironment: An update. Cells 2019, 8, 857. [Google Scholar] [CrossRef]
- De Backer, O.; Verheyden, A.M.; Martin, B.; Godelaine, D.; De Plaen, E.; Brasseur, R.; Avner, P.; Boon, T. Structure, chromosomal location, and expression pattern of three mouse genes homologous to the human MAGE genes. Genomics 1995, 28, 74–83. [Google Scholar] [CrossRef]
- Curigliano, G.; Viale, G.; Ghioni, M.; Jungbluth, A.A.; Bagnardi, V.; Spagnoli, G.C.; Neville, A.M.; Nole, F.; Rotmensz, N.; Goldhirsch, A. Cancer-testis antigen expression in triple-negative breast cancer. Ann. Oncol. 2011, 22, 98–103. [Google Scholar] [CrossRef]
- Kim, S.H.; Castro, F.; Gonzalez, D.; Maciag, P.C.; Paterson, Y.; Gravekamp, C. Mage-b vaccine delivered by recombinant Listeria monocytogenes is highly effective against breast cancer metastases. Br. J. Cancer 2008, 99, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Ramos, I.; Asafu-Adjei, D.; Quispe-Tintaya, W.; Chandra, D.; Jahangir, A.; Zang, X.; Aggarwal, B.B.; Gravekamp, C. Curcumin improves the therapeutic efficacy of Listeria(at)-Mage-b vaccine in correlation with improved t-cell responses in blood of a triple-negative breast cancer model 4T1. Cancer Med. 2013, 2, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Falah, R.R.; Talib, W.H.; Shbailat, S.J. Combination of metformin and curcumin targets breast cancer in mice by angiogenesis inhibition, immune system modulation and induction of p53 independent apoptosis. Ther. Adv. Med. Oncol. 2017, 9, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Kronski, E.; Fiori, M.E.; Barbieri, O.; Astigiano, S.; Mirisola, V.; Killian, P.H.; Bruno, A.; Pagani, A.; Rovera, F.; Pfeffer, U.; et al. miR181b is induced by the chemopreventive polyphenol curcumin and inhibits breast cancer metastasis via down-regulation of the inflammatory cytokines CXCL1 and -2. Mol. Oncol. 2014, 8, 581–595. [Google Scholar] [CrossRef]
- Chandra, D.; Jahangir, A.; Cornelis, F.; Rombauts, K.; Meheus, L.; Jorcyk, C.L.; Gravekamp, C. Cryoablation and Meriva have strong therapeutic effect on triple-negative breast cancer. Oncoimmunology 2016, 5, e1049802. [Google Scholar] [CrossRef]
- Lee-Chang, C.; Bodogai, M.; Martin-Montalvo, A.; Wejksza, K.; Sanghvi, M.; Moaddel, R.; de Cabo, R.; Biragyn, A. Inhibition of breast cancer metastasis by resveratrol-mediated inactivation of tumor-evoked regulatory B cells. J. Immunol. 2013, 191, 4141–4151. [Google Scholar] [CrossRef]
- Pusuluri, A.; Krishnan, V.; Wu, D.; Shields, C.W.t.; Wang, L.W.; Mitragotri, S. Role of synergy and immunostimulation in design of chemotherapy combinations: An analysis of doxorubicin and camptothecin. Bioeng. Transl. Med. 2019, 4, e10129. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Y.; Zhang, Y.; Wang, X.; Gao, X.; Liu, Y.; Zhang, X.; He, Z.; Wang, D.; Wang, Y. Ratiometric delivery of doxorubicin and berberine by liposome enables superior therapeutic index than Doxil®. Asian J. Pharm. Sci. 2020, 15, 385–396. [Google Scholar] [CrossRef]
- Cao, Y.; Feng, Y.H.; Gao, L.W.; Li, X.Y.; Jin, Q.X.; Wang, Y.Y.; Xu, Y.Y.; Jin, F.; Lu, S.L.; Wei, M.J. Artemisinin enhances the anti-tumor immune response in 4T1 breast cancer cells in vitro and in vivo. Int. Immunopharmacol. 2019, 70, 110–116. [Google Scholar] [CrossRef]
- Li, H.; Li, L.; Mei, H.; Pan, G.; Wang, X.; Huang, X.; Wang, T.; Jiang, Z.; Zhang, L.; Sun, L. Antitumor properties of triptolide: Phenotype regulation of macrophage differentiation. Cancer Biol. Ther. 2020, 21, 178–188. [Google Scholar] [CrossRef]
- Uruena, C.; Gomez, A.; Sandoval, T.; Hernandez, J.; Li, S.; Barreto, A.; Fiorentino, S. Multifunctional T lymphocytes generated after therapy with an antitumor gallotanin-rich normalized fraction are related to primary tumor size reduction in a breast cancer model. Integr. Cancer Ther. 2015, 14, 468–483. [Google Scholar] [CrossRef]
- Lasso, P.; Gomez-Cadena, A.; Uruena, C.; Donda, A.; Martinez-Usatorre, A.; Barreto, A.; Romero, P.; Fiorentino, S. Prophylactic vs. Therapeutic treatment with P2Et polyphenol-rich extract has opposite effects on tumor growth. Front. Oncol 2018, 8, 356. [Google Scholar] [CrossRef]
- Lasso, P.; Gomez-Cadena, A.; Uruena, C.; Donda, A.; Martinez-Usatorre, A.; Romero, P.; Barreto, A.; Fiorentino, S. An immunomodulatory gallotanin-rich fraction from Caesalpinia spinosa enhances the therapeutic effect of anti-PD-L1 in melanoma. Front. Immunol. 2020, 11, 584959. [Google Scholar] [CrossRef]
- Ferreira, L.C.; Arbab, A.S.; Jardim-Perassi, B.V.; Borin, T.F.; Varma, N.R.; Iskander, A.S.; Shankar, A.; Ali, M.M.; Zuccari, D.A. Effect of curcumin on pro-angiogenic factors in the xenograft model of breast cancer. Anti-Cancer Agents Med. Chem. 2015, 15, 1285–1296. [Google Scholar] [CrossRef]
- Garcia-Quiroz, J.; Garcia-Becerra, R.; Santos-Cuevas, C.; Ramirez-Nava, G.J.; Morales-Guadarrama, G.; Cardenas-Ochoa, N.; Segovia-Mendoza, M.; Prado-Garcia, H.; Ordaz-Rosado, D.; Avila, E.; et al. Synergistic antitumorigenic activity of calcitriol with curcumin or resveratrol is mediated by angiogenesis inhibition in triple negative breast cancer xenografts. Cancers 2019, 11, 1739. [Google Scholar] [CrossRef]
- Liu, H.; Tang, L.; Li, X.; Li, H. Triptolide inhibits vascular endothelial growth factor-mediated angiogenesis in human breast cancer cells. Exp. Ther. Med. 2018, 16, 830–836. [Google Scholar] [CrossRef]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef]
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr. Opin. Immunol. 2012, 24, 207–212. [Google Scholar] [CrossRef]
- Dongre, A.; Rashidian, M.; Reinhardt, F.; Bagnato, A.; Keckesova, Z.; Ploegh, H.L.; Weinberg, R.A. Epithelial-to-mesenchymal transition contributes to immunosuppression in breast carcinomas. Cancer Res. 2017, 77, 3982–3989. [Google Scholar] [CrossRef]
- Han, X.; Zhao, N.; Zhu, W.; Wang, J.; Liu, B.; Teng, Y. Resveratrol attenuates TNBC lung metastasis by down-regulating PD-1 expression on pulmonary t cells and converting macrophages to M1 phenotype in a murine tumor model. Cell Immunol. 2021, 368, 104423. [Google Scholar] [CrossRef]
- Verdura, S.; Cuyas, E.; Cortada, E.; Brunet, J.; Lopez-Bonet, E.; Martin-Castillo, B.; Bosch-Barrera, J.; Encinar, J.A.; Menendez, J.A. Resveratrol targets PD-L1 glycosylation and dimerization to enhance antitumor T-cell immunity. Aging 2020, 12, 8–34. [Google Scholar] [CrossRef]
- Li, C.W.; Lim, S.O.; Chung, E.M.; Kim, Y.S.; Park, A.H.; Yao, J.; Cha, J.H.; Xia, W.; Chan, L.C.; Kim, T.; et al. Eradication of triple-negative breast cancer cells by targeting glycosylated PD-L1. Cancer Cell 2018, 33, 187–201.e110. [Google Scholar] [CrossRef]
- Hu, Y.; Manasrah, B.K.; McGregor, S.M.; Lera, R.F.; Norman, R.X.; Tucker, J.B.; Scribano, C.M.; Yan, R.E.; Humayun, M.; Wisinski, K.B.; et al. Paclitaxel induces micronucleation and activates pro-inflammatory cGAS-STING signaling in triple-negative breast cancer. Mol. Cancer Ther. 2021, 20, 2553–2567. [Google Scholar] [CrossRef]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef]
- Sui, H.; Zhu, L.; Deng, W.; Li, Q. Epithelial-mesenchymal transition and drug resistance: Role, molecular mechanisms, and therapeutic strategies. Oncol. Res. Treat. 2014, 37, 584–589. [Google Scholar] [CrossRef]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug resistance in cancer: An overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef]
- Guo, W.; Keckesova, Z.; Donaher, J.L.; Shibue, T.; Tischler, V.; Reinhardt, F.; Itzkovitz, S.; Noske, A.; Zurrer-Hardi, U.; Bell, G.; et al. Slug and Sox9 cooperatively determine the mammary stem cell state. Cell 2012, 148, 1015–1028. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Becker, A.; Zimmer, A.; Lu, J.; Buettner, R.; Kirfel, J. SNAI1-mediated epithelial-mesenchymal transition confers chemoresistance and cellular plasticity by regulating genes involved in cell death and stem cell maintenance. PLoS ONE 2013, 8, e66558. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhou, Q.M.; Lu, Y.Y.; Zhang, H.; Chen, Q.L.; Zhao, M.; Su, S.B. Resveratrol inhibits the migration and metastasis of MDA-MB-231 human breast cancer by reversing TGF-beta1-induced epithelial-mesenchymal transition. Molecules 2019, 24, 1131. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.D.; Sun, Y.; Zhou, W.J.; Xie, X.Z.; Zhou, Q.M.; Lu, Y.Y.; Su, S.B. Resveratrol enhances inhibition effects of cisplatin on cell migration and invasion and tumor growth in breast cancer MDA-MB-231 cell models in vivo and in vitro. Molecules 2021, 26, 2204. [Google Scholar] [CrossRef]
- Ferrand, N.; Gnanapragasam, A.; Dorothee, G.; Redeuilh, G.; Larsen, A.K.; Sabbah, M. Loss of WISP2/CCN5 in estrogen-dependent MCF7 human breast cancer cells promotes a stem-like cell phenotype. PLoS ONE 2014, 9, e87878. [Google Scholar] [CrossRef]
- Sarkar, S.; Ghosh, A.; Banerjee, S.; Maity, G.; Das, A.; Larson, M.A.; Gupta, V.; Haque, I.; Tawfik, O.; Banerjee, S.K. CCN5/WISP-2 restores ER-alpha proportional, variant in normal and neoplastic breast cells and sensitizes triple negative breast cancer cells to tamoxifen. Oncogenesis 2017, 6, e340. [Google Scholar] [CrossRef]
- Das, A.; Haque, I.; Ray, P.; Ghosh, A.; Dutta, D.; Quadir, M.; De, A.; Gunewardena, S.; Chatterjee, I.; Banerjee, S.; et al. Ccn5 activation by free or encapsulated EGCG is required to render triple-negative breast cancer cell viability and tumor progression. Pharmacol. Res. Perspect. 2021, 9, e00753. [Google Scholar] [CrossRef]
- Whiteside, T.L. Exosomes and tumor-mediated immune suppression. J. Clin. Investig. 2016, 126, 1216–1223. [Google Scholar] [CrossRef]
- Jang, J.Y.; Lee, J.K.; Jeon, Y.K.; Kim, C.W. Exosome derived from epigallocatechin gallate treated breast cancer cells suppresses tumor growth by inhibiting tumor-associated macrophage infiltration and M2 polarization. BMC Cancer 2013, 13, 421. [Google Scholar] [CrossRef]
- Shiau, J.Y.; Chang, Y.Q.; Nakagawa-Goto, K.; Lee, K.H.; Shyur, L.F. Phytoagent deoxyelephantopin and its derivative inhibit triple negative breast cancer cell activity through ROS-mediated exosomal activity and protein functions. Front. Pharmacol. 2017, 8, 398. [Google Scholar] [CrossRef]
- Quintero-Fabian, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argaez, V.; Lara-Riegos, J.; Ramirez-Camacho, M.A.; Alvarez-Sanchez, M.E. Role of matrix metalloproteinases in angiogenesis and cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef]
- Lee, H.S.; Ha, A.W.; Kim, W.K. Effect of resveratrol on the metastasis of 4T1 mouse breast cancer cells in vitro and in vivo. Nutr. Res. Pract. 2012, 6, 294–300. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.; You, D.; Jeong, Y.; Jeon, M.; Yu, J.; Kim, S.W.; Nam, S.J.; Lee, J.E. Berberine suppresses cell motility through downregulation of TGF-beta1 in triple negative breast cancer cells. Cell Physiol. Biochem. 2018, 45, 795–807. [Google Scholar] [CrossRef]
- Zhao, Y.; Jing, Z.; Lv, J.; Zhang, Z.; Lin, J.; Cao, X.; Zhao, Z.; Liu, P.; Mao, W. Berberine activates caspase-9/cytochrome c-mediated apoptosis to suppress triple-negative breast cancer cells in vitro and in vivo. Biomed. Pharmacother. 2017, 95, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Bello, E.; Taraboletti, G.; Colella, G.; Zucchetti, M.; Forestieri, D.; Licandro, S.A.; Berndt, A.; Richter, P.; D’Incalci, M.; Cavalletti, E.; et al. The tyrosine kinase inhibitor E-3810 combined with paclitaxel inhibits the growth of advanced-stage triple-negative breast cancer xenografts. Mol. Cancer. Ther. 2013, 12, 131–140. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lehmann, B.D.; Pietenpol, J.A. Clinical implications of molecular heterogeneity in triple negative breast cancer. Breast 2015, 24 (Suppl. 2), S36–S40. [Google Scholar] [CrossRef] [PubMed]
- Llombart-Cussac, A.; Bermejo, B.; Villanueva, C.; Delaloge, S.; Morales, S.; Balmana, J.; Amillano, K.; Bonnefoi, H.; Casas, A.; Manso, L.; et al. Solti neoparp: A phase II randomized study of two schedules of iniparib plus paclitaxel versus paclitaxel alone as neoadjuvant therapy in patients with triple-negative breast cancer. Breast Cancer Res. Treat. 2015, 154, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Forero-Torres, A.; Varley, K.E.; Abramson, V.G.; Li, Y.; Vaklavas, C.; Lin, N.U.; Liu, M.C.; Rugo, H.S.; Nanda, R.; Storniolo, A.M.; et al. TBCRC 019: A phase II trial of nanoparticle albumin-bound paclitaxel with or without the anti-death receptor 5 monoclonal antibody tigatuzumab in patients with triple-negative breast cancer. Clin. Cancer Res. 2015, 21, 2722–2729. [Google Scholar] [CrossRef] [PubMed]
- Ericsson, T.; Blank, A.; von Hagens, C.; Ashton, M.; Abelo, A. Population pharmacokinetics of artesunate and dihydroartemisinin during long-term oral administration of artesunate to patients with metastatic breast cancer. Eur. J. Clin. Pharmacol. 2014, 70, 1453–1463. [Google Scholar] [CrossRef]
- Konig, M.; von Hagens, C.; Hoth, S.; Baumann, I.; Walter-Sack, I.; Edler, L.; Sertel, S. Investigation of ototoxicity of artesunate as add-on therapy in patients with metastatic or locally advanced breast cancer: New audiological results from a prospective, open, uncontrolled, monocentric phase I study. Cancer Chemother. Pharmacol. 2016, 77, 413–427. [Google Scholar] [CrossRef]
- Von Hagens, C.; Walter-Sack, I.; Goeckenjan, M.; Osburg, J.; Storch-Hagenlocher, B.; Sertel, S.; Elsasser, M.; Remppis, B.A.; Edler, L.; Munzinger, J.; et al. Prospective open uncontrolled phase I study to define a well-tolerated dose of oral artesunate as add-on therapy in patients with metastatic breast cancer (ARTIC-M33/2). Breast Cancer Res. Treat. 2017, 164, 359–369. [Google Scholar] [CrossRef]
- Von Hagens, C.; Walter-Sack, I.; Goeckenjan, M.; Storch-Hagenlocher, B.; Sertel, S.; Elsasser, M.; Remppis, B.A.; Munzinger, J.; Edler, L.; Efferth, T.; et al. Long-term add-on therapy (compassionate use) with oral artesunate in patients with metastatic breast cancer after participating in a phase I study (ARTIC-M33/2). Phytomedicine 2019, 54, 140–148. [Google Scholar] [CrossRef]
- Bayat Mokhtari, R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef]
- Chen, H.M.; Sun, L.; Pan, P.Y.; Wang, L.H.; Chen, S.H. Nutrient supplements from selected botanicals mediated immune modulation of the tumor microenvironment and antitumor mechanism. Cancer Immunol. Immunother. 2021, 70, 3435–3449. [Google Scholar] [CrossRef]
- Karnam, K.C.; Ellutla, M.; Bodduluru, L.N.; Kasala, E.R.; Uppulapu, S.K.; Kalyankumarraju, M.; Lahkar, M. Preventive effect of berberine against DMBA-induced breast cancer in female Sprague Dawley rats. Biomed. Pharmacother. 2017, 92, 207–214. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.-N.; Chen, H.-M.; Shyur, L.-F. Current Advancements of Plant-Derived Agents for Triple-Negative Breast Cancer Therapy through Deregulating Cancer Cell Functions and Reprogramming Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 13571. https://doi.org/10.3390/ijms222413571
Wu T-N, Chen H-M, Shyur L-F. Current Advancements of Plant-Derived Agents for Triple-Negative Breast Cancer Therapy through Deregulating Cancer Cell Functions and Reprogramming Tumor Microenvironment. International Journal of Molecular Sciences. 2021; 22(24):13571. https://doi.org/10.3390/ijms222413571
Chicago/Turabian StyleWu, Tai-Na, Hui-Ming Chen, and Lie-Fen Shyur. 2021. "Current Advancements of Plant-Derived Agents for Triple-Negative Breast Cancer Therapy through Deregulating Cancer Cell Functions and Reprogramming Tumor Microenvironment" International Journal of Molecular Sciences 22, no. 24: 13571. https://doi.org/10.3390/ijms222413571
APA StyleWu, T.-N., Chen, H.-M., & Shyur, L.-F. (2021). Current Advancements of Plant-Derived Agents for Triple-Negative Breast Cancer Therapy through Deregulating Cancer Cell Functions and Reprogramming Tumor Microenvironment. International Journal of Molecular Sciences, 22(24), 13571. https://doi.org/10.3390/ijms222413571