S100B Protein as a Therapeutic Target in Multiple Sclerosis: The S100B Inhibitor Arundic Acid Protects from Chronic Experimental Autoimmune Encephalomyelitis
Abstract
1. Introduction
2. Results
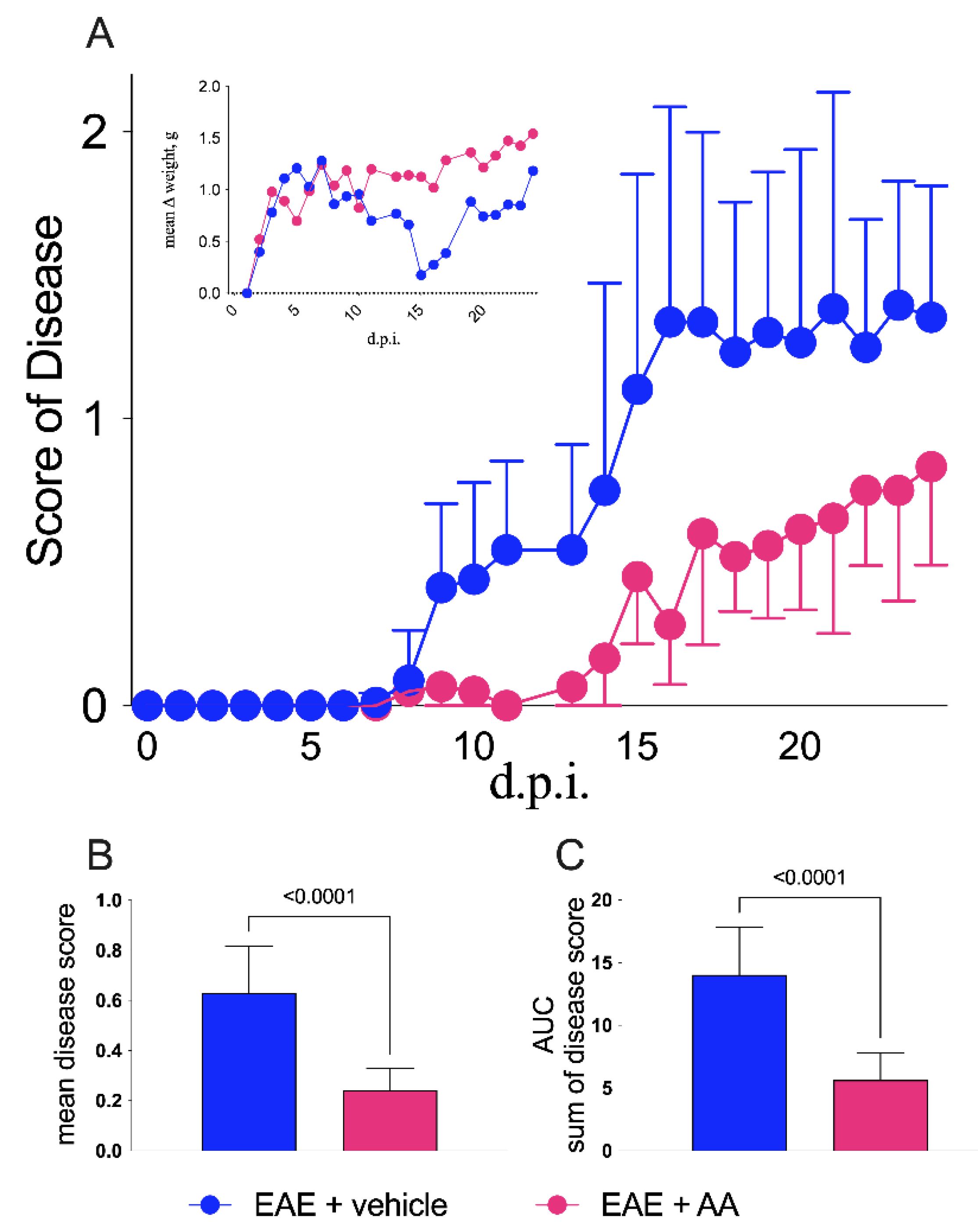
2.1. Clinical Results
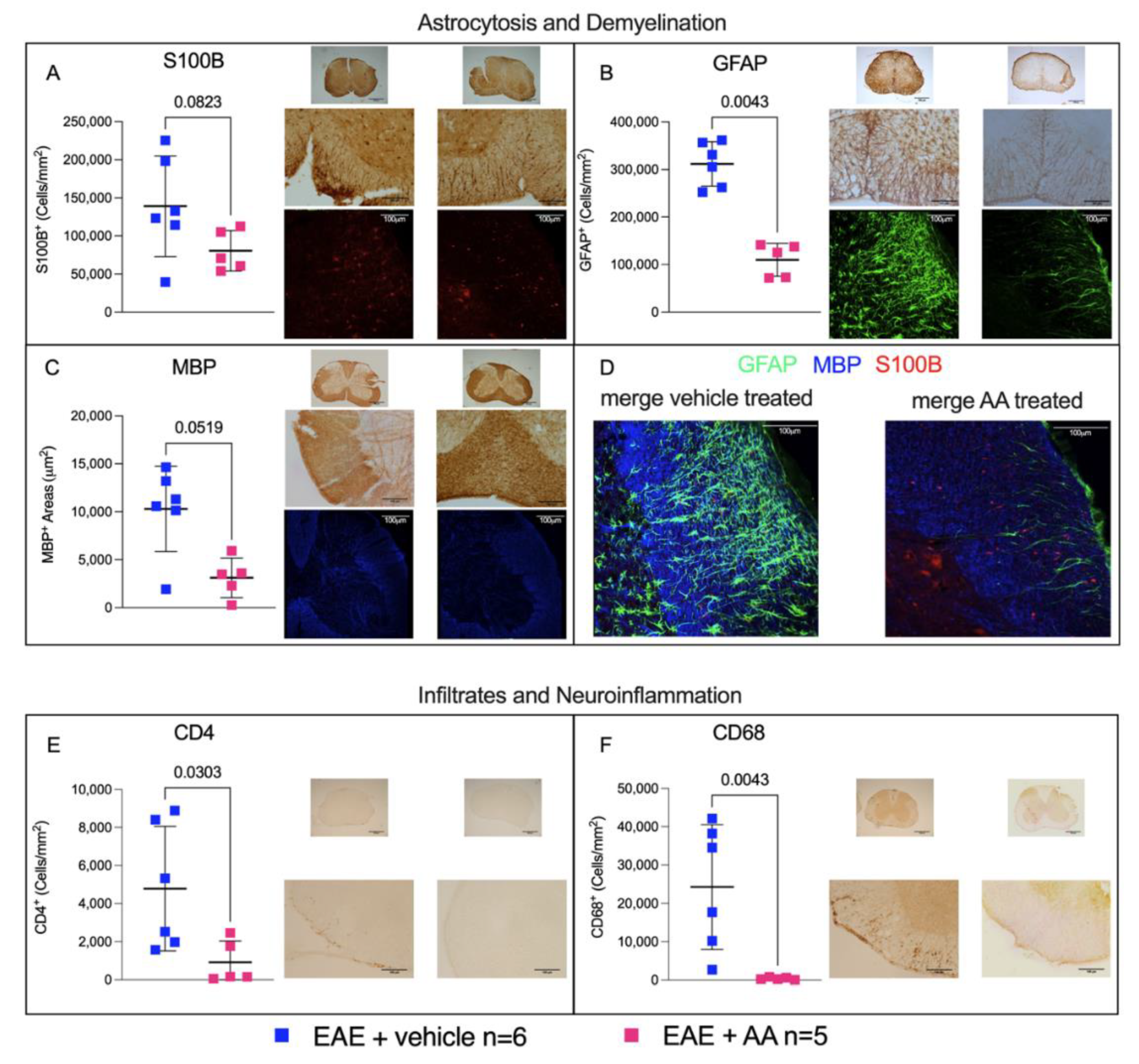
2.2. Modulation by AA of Astrocytosis and Demyelination during EAE
2.3. Impact of AA on the CNS Histologic Immune Infiltrates and on Neuroinflammation during EAE
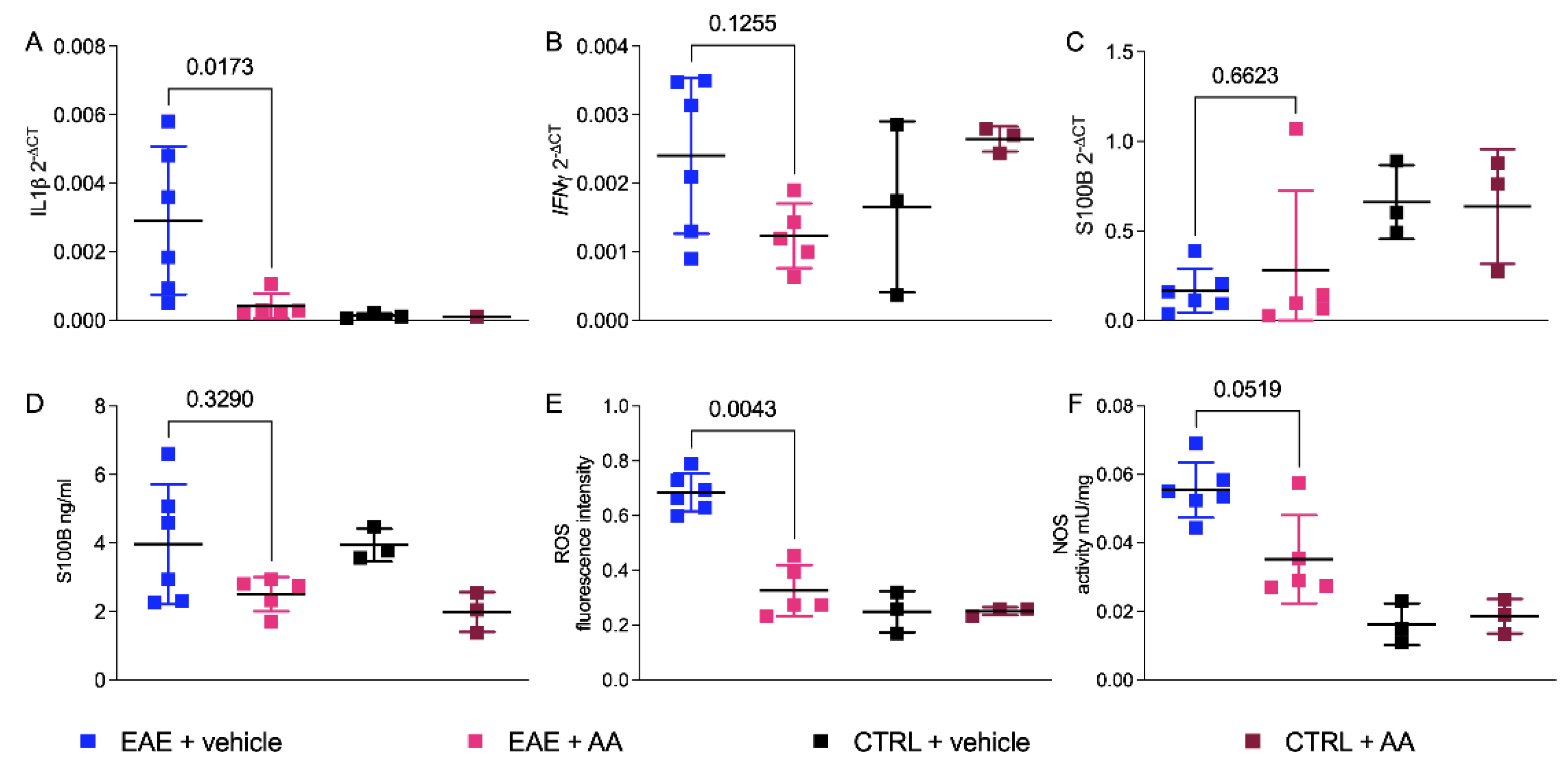
2.4. Regulation of Proinflammatory Cytokines and Enzymatic Oxidative Reactivity by AA
3. Discussion
4. Materials and Methods
4.1. Animal Procedure
4.2. RT-qPCR Assay
4.3. S100B ELISA Assay
4.4. Oxidative Stress Enzymes Activity
4.5. Immunohistochemistry and Immunofluorescence
4.6. ImageJ Immunohistochemical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Magyari, M.; Sorensen, P.S. The Changing Course of Multiple Sclerosis: Rising Incidence, Change in Geographic Distribution, Disease Course, and Prognosis. Curr. Opin. Neurol. 2019, 32, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Kaunzner, U.W.; Al-Kawaz, M.; Gauthier, S.A. Defining Disease Activity and Response to Therapy in MS. Curr. Treat Options Neurol. 2017, 19, 20. [Google Scholar] [CrossRef]
- Michetti, F.; Corvino, V.; Geloso, M.C.; Lattanzi, W.; Bernardini, C.; Serpero, L.; Gazzolo, D. The S100B Protein in Biological Fluids: More than a Lifelong Biomarker of Brain Distress. J. Neurochem. 2012, 120, 644–659. [Google Scholar] [CrossRef]
- Michetti, F.; D’Ambrosi, N.; Toesca, A.; Puglisi, M.A.; Serrano, A.; Marchese, E.; Corvino, V.; Geloso, M.C. The S100B Story: From Biomarker to Active Factor in Neural Injury. J. Neurochem. 2019, 148, 168–187. [Google Scholar] [CrossRef] [PubMed]
- Michetti, F.; Di Sante, G.; Clementi, M.E.; Sampaolese, B.; Casalbore, P.; Volonté, C.; Romano Spica, V.; Parnigotto, P.P.; Di Liddo, R.; Amadio, S.; et al. Growing Role of S100B Protein as a Putative Therapeutic Target for Neurological- and Nonneurological-Disorders. Neurosci. Biobehav. Rev. 2021, 127, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Michetti, F.; Massaro, A.; Murazio, M. The Nervous System-Specific S-100 Antigen in Cerebrospinal Fluid of Multiple Sclerosis Patients. Neurosci. Lett. 1979, 11, 171–175. [Google Scholar] [CrossRef]
- Barateiro, A.; Afonso, V.; Santos, G.; Cerqueira, J.J.; Brites, D.; van Horssen, J.; Fernandes, A. S100B as a Potential Biomarker and Therapeutic Target in Multiple Sclerosis. Mol. Neurobiol. 2016, 53, 3976–3991. [Google Scholar] [CrossRef]
- Petzold, A.; Eikelenboom, M.J.; Gveric, D.; Keir, G.; Chapman, M.; Lazeron, R.H.C.; Cuzner, M.L.; Polman, C.H.; Uitdehaag, B.M.J.; Thompson, E.J.; et al. Markers for Different Glial Cell Responses in Multiple Sclerosis: Clinical and Pathological Correlations. Brain 2002, 125, 1462–1473. [Google Scholar] [CrossRef]
- Yan, S.S.; Wu, Z.-Y.; Zhang, H.P.; Furtado, G.; Chen, X.; Yan, S.F.; Schmidt, A.M.; Brown, C.; Stern, A.; LaFaille, J.; et al. Suppression of Experimental Autoimmune Encephalomyelitis by Selective Blockade of Encephalitogenic T-Cell Infiltration of the Central Nervous System. Nat. Med. 2003, 9, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Santos, G.; Barateiro, A.; Gomes, C.M.; Brites, D.; Fernandes, A. Impaired Oligodendrogenesis and Myelination by Elevated S100B Levels during Neurodevelopment. Neuropharmacology 2018, 129, 69–83. [Google Scholar] [CrossRef]
- Wilder, P.T.; Varney, K.M.; Weber, D.J. Targeting S100 Calcium-Binding Proteins with Small Molecule Inhibitors. In Methods in Molecular Biology; Heizmann, C.W., Ed.; Humana Press: Totowa, NJ, USA, 2019; Volume 1929, pp. 291–310. ISBN 978-1-4939-9029-0. [Google Scholar]
- Charpentier, T.H.; Wilder, P.T.; Liriano, M.A.; Varney, K.M.; Pozharski, E.; MacKerell, A.D.; Coop, A.; Toth, E.A.; Weber, D.J. Divalent Metal Ion Complexes of S100B in the Absence and Presence of Pentamidine. J. Mol. Biol. 2008, 382, 56–73. [Google Scholar] [CrossRef] [PubMed]
- Di Sante, G.; Amadio, S.; Sampaolese, B.; Valentini, M.; Volonté, C.; Casalbore, P.; Ria, F.; Michetti, F. The S100B Inhibitor Pentamidine Ameliorates Clinical Score and Neuropathology of Relapsing—Remitting Multiple Sclerosis Mouse Model. Cells 2020, 9, 748. [Google Scholar] [CrossRef]
- Tateishi, N.; Mori, T.; Kagamiishi, Y.; Satoh, S.; Katsube, N.; Morikawa, E.; Morimoto, T.; Matsui, T.; Asano, T. Astrocytic Activation and Delayed Infarct Expansion after Permanent Focal Ischemia in Rats. Part II. J. Cereb. Blood Flow Metab. 2002, 22, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Glatigny, S.; Bettelli, E. Experimental Autoimmune Encephalomyelitis (EAE) as Animal Models of Multiple Sclerosis (MS). Cold Spring Harb. Perspect. Med. 2018, 8, a028977. [Google Scholar] [CrossRef]
- Lublin, F.D.; Reingold, S.C. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis* Defining the Clinical Course of Multiple Sclerosis: Results of an International Survey. Neurology 1996, 46, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Antel, J.; Antel, S.; Caramanos, Z.; Arnold, D.L.; Kuhlmann, T. Primary Progressive Multiple Sclerosis: Part of the MS Disease Spectrum or Separate Disease Entity? Acta Neuropathol. 2012, 123, 627–638. [Google Scholar] [CrossRef]
- Ohtani, R.; Tomimoto, H.; Wakita, H.; Kitaguchi, H.; Nakaji, K.; Takahashi, R. Expression of S100 Protein and Protective Effect of Arundic Acid on the Rat Brain in Chronic Cerebral Hypoperfusion. Brain Res. 2007, 1135, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, H.; Kaito, T.; Hashimoto, K.; Kushioka, J.; Okada, R.; Tsukazaki, H.; Kodama, J.; Bal, Z.; Ukon, Y.; Takenaka, S.; et al. Administration of ONO-2506 Suppresses Neuropathic Pain after Spinal Cord Injury by Inhibition of Astrocytic Activation. Spine J. 2019, 19, 1434–1442. [Google Scholar] [CrossRef] [PubMed]
- Burrows, D.J.; McGown, A.; Jain, S.A.; De Felice, M.; Ramesh, T.M.; Sharrack, B.; Majid, A. Animal Models of Multiple Sclerosis: From Rodents to Zebrafish. Mult. Scler. 2019, 25, 306–324. [Google Scholar] [CrossRef] [PubMed]
- Steiner, J.; Bernstein, H.-G.; Bielau, H.; Berndt, A.; Brisch, R.; Mawrin, C.; Keilhoff, G.; Bogerts, B. Evidence for a Wide Extra-Astrocytic Distribution of S100B in Human Brain. BMC Neurosci. 2007, 8, 2. [Google Scholar] [CrossRef]
- Kuerten, S.; Kostova-Bales, D.A.; Frenzel, L.P.; Tigno, J.T.; Tary-Lehmann, M.; Angelov, D.N.; Lehmann, P.V. MP4- and MOG:35-55-Induced EAE in C57BL/6 Mice Differentially Targets Brain, Spinal Cord and Cerebellum. J. Neuroimmunol. 2007, 189, 31–40. [Google Scholar] [CrossRef]
- Hagmeyer, S.; Romão, M.A.; Cristóvão, J.S.; Vilella, A.; Zoli, M.; Gomes, C.M.; Grabrucker, A.M. Distribution and Relative Abundance of S100 Proteins in the Brain of the APP23 Alzheimer’s Disease Model Mice. Front. Neurosci. 2019, 13, 640. [Google Scholar] [CrossRef] [PubMed]
- Cree, B.A.C.; Arnold, D.L.; Chataway, J.; Chitnis, T.; Fox, R.J.; Pozo Ramajo, A.; Murphy, N.; Lassmann, H. Secondary Progressive Multiple Sclerosis: New Insights. Neurology 2021, 97, 378–388. [Google Scholar] [CrossRef]
- Mori, T.; Town, T.; Tan, J.; Yada, N.; Horikoshi, Y.; Yamamoto, J.; Shimoda, T.; Kamanaka, Y.; Tateishi, N.; Asano, T. Arundic Acid Ameliorates Cerebral Amyloidosis and Gliosis in Alzheimer Transgenic Mice. J. Pharmacol. Exp. Ther. 2006, 318, 571–578. [Google Scholar] [CrossRef]
- Kato, H.; Kurosaki, R.; Oki, C.; Araki, T. Arundic Acid, an Astrocyte-Modulating Agent, Protects Dopaminergic Neurons against MPTP Neurotoxicity in Mice. Brain Res. 2004, 1030, 66–73. [Google Scholar] [CrossRef]
- Asano, T.; Mori, T.; Shimoda, T.; Shinagawa, R.; Satoh, S.; Yada, N.; Katsumata, S.; Matsuda, S.; Kagamiishi, Y.; Tateishi, N. Arundic Acid (ONO-2506) Ameliorates Delayed Ischemic Brain Damage by Preventing Astrocytic Overproduction of S100B. Curr. Drug Targets CNS Neurol. Disord. 2005, 4, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, J.L.; Neves, J.D.; Vizuete, A.F.; Aristimunha, D.; Pedroso, T.A.; Sanches, E.F.; Gonçalves, C.A.; Netto, C.A. Arundic Acid (ONO-2506), an Inhibitor of S100B Protein Synthesis, Prevents Neurological Deficits and Brain Tissue Damage Following Intracerebral Hemorrhage in Male Wistar Rats. Neuroscience 2020, 440, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, J.L.; Neves, J.D.; Nicola, F.; Vizuete, A.F.; Sanches, E.F.; Gonçalves, C.A.; Netto, C.A. Arundic Acid (ONO-2506) Attenuates Neuroinflammation and Prevents Motor Impairment in Rats with Intracerebral Hemorrhage. Cell Mol. Neurobiol. 2020. [Google Scholar] [CrossRef]
- Wajima, D.; Nakagawa, I.; Nakase, H.; Yonezawa, T. Neuroprotective Effect of Suppression of Astrocytic Activation by Arundic Acid on Brain Injuries in Rats with Acute Subdural Hematomas. Brain Res. 2013, 1519, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Hanada, M.; Shinjo, R.; Miyagi, M.; Yasuda, T.; Tsutsumi, K.; Sugiura, Y.; Imagama, S.; Ishiguro, N.; Matsuyama, Y. Arundic Acid (ONO-2506) Inhibits Secondary Injury and Improves Motor Function in Rats with Spinal Cord Injury. J. Neurol. Sci. 2014, 337, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Higashino, H.; Niwa, A.; Satou, T.; Ohta, Y.; Hashimoto, S.; Tabuchi, M.; Ooshima, K. Immunohistochemical Analysis of Brain Lesions Using S100B and Glial Fibrillary Acidic Protein Antibodies in Arundic Acid- (ONO-2506) Treated Stroke-Prone Spontaneously Hypertensive Rats. J. Neural. Transm. 2009, 116, 1209–1219. [Google Scholar] [CrossRef][Green Version]
- Mari, C.; Odorcyk, F.K.; Sanches, E.F.; Wartchow, K.M.; Martini, A.P.; Nicola, F.; Zanotto, C.; Wyse, A.T.; Gonçalves, C.A.; Netto, C.A. Arundic Acid Administration Protects Astrocytes, Recovers Histological Damage and Memory Deficits Induced by Neonatal Hypoxia Ischemia in Rats. Int. J. Dev. Neurosci. 2019, 76, 41–51. [Google Scholar] [CrossRef]
- Vizuete, A.F.K.; Hansen, F.; Negri, E.; Leite, M.C.; de Oliveira, D.L.; Gonçalves, C.-A. Effects of Dexamethasone on the Li-Pilocarpine Model of Epilepsy: Protection against Hippocampal Inflammation and Astrogliosis. J. Neuroinflamm. 2018, 15, 68. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, R.; Giambanco, I.; Donato, R. S100B/RAGE-Dependent Activation of Microglia via NF-KappaB and AP-1 Co-Regulation of COX-2 Expression by S100B, IL-1beta and TNF-Alpha. Neurobiol. Aging 2010, 31, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, A.; Aviles Reyes, R.X.; Angelo, M.F.; Reines, A.G.; Ramos, A.J. S100B Alters Neuronal Survival and Dendrite Extension via RAGE-Mediated NF-ΚB Signaling. J. Neurochem. 2011, 117, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Klegeris, A. Regulation of Neuroimmune Processes by Damage- and Resolution-Associated Molecular Patterns. Neural. Regen. Res. 2021, 16, 423. [Google Scholar] [CrossRef] [PubMed]
- Absinta, M.; Maric, D.; Gharagozloo, M.; Garton, T.; Smith, M.D.; Jin, J.; Fitzgerald, K.C.; Song, A.; Liu, P.; Lin, J.-P.; et al. A Lymphocyte-Microglia-Astrocyte Axis in Chronic Active Multiple Sclerosis. Nature 2021, 597, 709–714. [Google Scholar] [CrossRef]
- das Neves, S.P.; Sousa, J.C.; Sousa, N.; Cerqueira, J.J.; Marques, F. Altered Astrocytic Function in Experimental Neuroinflammation and Multiple Sclerosis. Glia 2021, 69, 1341–1368. [Google Scholar] [CrossRef]
- Fernandes, R.A.; Ingle, A.B. Arundic Acid a Potential Neuroprotective Agent: Biological Development and Syntheses. Curr. Med. Chem. 2013, 20, 2315–2329. [Google Scholar] [CrossRef]
- Hartman, K.G.; McKnight, L.E.; Liriano, M.A.; Weber, D.J. The Evolution of S100B Inhibitors for the Treatment of Malignant Melanoma. Future Med. Chem. 2013, 5, 97–109. [Google Scholar] [CrossRef]
- Cirillo, C.; Capoccia, E.; Iuvone, T.; Cuomo, R.; Sarnelli, G.; Steardo, L.; Esposito, G. S100B Inhibitor Pentamidine Attenuates Reactive Gliosis and Reduces Neuronal Loss in a Mouse Model of Alzheimer’s Disease. Biomed. Res. Int. 2015, 2015, 508342. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, F.; Seguella, L.; Gigli, S.; Hanieh, P.N.; Del Favero, E.; Cantù, L.; Pesce, M.; Sarnelli, G.; Marianecci, C.; Esposito, G.; et al. InPentasomes: An Innovative Nose-to-Brain Pentamidine Delivery Blunts MPTP Parkinsonism in Mice. J. Control. Release 2019, 294, 17–26. [Google Scholar] [CrossRef]
- Valori, C.F.; Possenti, A.; Brambilla, L.; Rossi, D. Challenges and Opportunities of Targeting Astrocytes to Halt Neurodegenerative Disorders. Cells 2021, 10, 2019. [Google Scholar] [CrossRef] [PubMed]
- Aharoni, R.; Eilam, R.; Arnon, R. Astrocytes in Multiple Sclerosis—Essential Constituents with Diverse Multifaceted Functions. Int. J. Mol. Sci. 2021, 22, 5904. [Google Scholar] [CrossRef]
- Lo, C.H.; Skarica, M.; Mansoor, M.; Bhandarkar, S.; Toro, S.; Pitt, D. Astrocyte Heterogeneity in Multiple Sclerosis: Current Understanding and Technical Challenges. Front. Cell. Neurosci. 2021, 15, 726479. [Google Scholar] [CrossRef] [PubMed]
- Stromnes, I.M.; Goverman, J.M. Active Induction of Experimental Allergic Encephalomyelitis. Nat. Protoc. 2006, 1, 1810–1819. [Google Scholar] [CrossRef] [PubMed]
- Nicolò, C.; Sali, M.; Di Sante, G.; Geloso, M.C.; Signori, E.; Penitente, R.; Uniyal, S.; Rinaldi, M.; Ingrosso, L.; Fazio, V.M.; et al. Mycobacterium Smegmatis Expressing a Chimeric Protein MPT64-Proteolipid Protein (PLP) 139-151 Reorganizes the PLP-Specific T Cell Repertoire Favoring a CD8-Mediated Response and Induces a Relapsing Experimental Autoimmune Encephalomyelitis. J. Immunol. 2010, 184, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Penitente, R.; Nicolò, C.; Van den Elzen, P.; Di Sante, G.; Agrati, C.; Aloisi, F.; Sercarz, E.E.; Ria, F. Administration of PLP 139–151 Primes T Cells Distinct from Those Spontaneously Responsive In Vitro to This Antigen. J. Immunol. 2008, 180, 6611–6622. [Google Scholar] [CrossRef] [PubMed]
- Marchese, E.; Valentini, M.; Sante, G.D.; Cesari, E.; Adinolfi, A.; Corvino, V.; Ria, F.; Sette, C.; Geloso, M.C. Alternative Splicing of Neurexins 1-3 Is Modulated by Neuroinflammation in the Prefrontal Cortex of a Murine Model of Multiple Sclerosis. Exp. Neurol. 2020, 335, 113497. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.D.; Karpus, W.J. Experimental Autoimmune Encephalomyelitis in the Mouse. Curr. Protoc. Immunol. 2007. [Google Scholar] [CrossRef] [PubMed]
- Piermattei, A.; Migliara, G.; Di Sante, G.; Foti, M.; Hayrabedyan, S.B.; Papagna, A.; Geloso, M.C.; Corbi, M.; Valentini, M.; Sgambato, A.; et al. Toll-Like Receptor 2 Mediates In Vivo Pro- and Anti-Inflammatory Effects of Mycobacterium Tuberculosis and Modulates Autoimmune Encephalomyelitis. Front. Immunol. 2016, 7, 191. [Google Scholar] [CrossRef] [PubMed]
- Di Sante, G.; Migliara, G.; Valentini, M.; Delogu, G.; Ria, F. Regulation of and Regulation by CD 44: A Paradigm Complex Regulatory Network. Int. Trends Immun. 2013, 1, 33–42. [Google Scholar]
- Pandolfi, F.; Cianci, R.; Casciano, F.; Pagliari, D.; De Pasquale, T.; Landolfi, R.; Di Sante, G.; Kurnick, J.T.; Ria, F. Skewed T-Cell Receptor Repertoire: More than a Marker of Malignancy, a Tool to Dissect the Immunopathology of Inflammatory Diseases. J. Biol. Regul. Homeost. Agents 2011, 25, 153–161. [Google Scholar]
- Fu, C.; Zhu, X.; Xu, P.; Li, Y. Pharmacological Inhibition of USP7 Promotes Antitumor Immunity and Contributes to Colon Cancer Therapy. Onco. Targets Ther. 2019, 12, 609–617. [Google Scholar] [CrossRef]
- Xu, Z.; Fouda, A.Y.; Lemtalsi, T.; Shosha, E.; Rojas, M.; Liu, F.; Patel, C.; Caldwell, R.W.; Narayanan, S.P.; Caldwell, R.B. Retinal Neuroprotection from Optic Nerve Trauma by Deletion of Arginase. Front. Neurosci. 2018, 12, 970. [Google Scholar] [CrossRef]
- Cao, L.; Malon, J.T. Anti-Nociceptive Role of CXCL1 in a Murine Model of Peripheral Nerve Injury-Induced Neuropathic Pain. Neuroscience 2018, 372, 225–236. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camponeschi, C.; De Carluccio, M.; Amadio, S.; Clementi, M.E.; Sampaolese, B.; Volonté, C.; Tredicine, M.; Romano Spica, V.; Di Liddo, R.; Ria, F.; et al. S100B Protein as a Therapeutic Target in Multiple Sclerosis: The S100B Inhibitor Arundic Acid Protects from Chronic Experimental Autoimmune Encephalomyelitis. Int. J. Mol. Sci. 2021, 22, 13558. https://doi.org/10.3390/ijms222413558
Camponeschi C, De Carluccio M, Amadio S, Clementi ME, Sampaolese B, Volonté C, Tredicine M, Romano Spica V, Di Liddo R, Ria F, et al. S100B Protein as a Therapeutic Target in Multiple Sclerosis: The S100B Inhibitor Arundic Acid Protects from Chronic Experimental Autoimmune Encephalomyelitis. International Journal of Molecular Sciences. 2021; 22(24):13558. https://doi.org/10.3390/ijms222413558
Chicago/Turabian StyleCamponeschi, Chiara, Maria De Carluccio, Susanna Amadio, Maria Elisabetta Clementi, Beatrice Sampaolese, Cinzia Volonté, Maria Tredicine, Vincenzo Romano Spica, Rosa Di Liddo, Francesco Ria, and et al. 2021. "S100B Protein as a Therapeutic Target in Multiple Sclerosis: The S100B Inhibitor Arundic Acid Protects from Chronic Experimental Autoimmune Encephalomyelitis" International Journal of Molecular Sciences 22, no. 24: 13558. https://doi.org/10.3390/ijms222413558
APA StyleCamponeschi, C., De Carluccio, M., Amadio, S., Clementi, M. E., Sampaolese, B., Volonté, C., Tredicine, M., Romano Spica, V., Di Liddo, R., Ria, F., Michetti, F., & Di Sante, G. (2021). S100B Protein as a Therapeutic Target in Multiple Sclerosis: The S100B Inhibitor Arundic Acid Protects from Chronic Experimental Autoimmune Encephalomyelitis. International Journal of Molecular Sciences, 22(24), 13558. https://doi.org/10.3390/ijms222413558










