A Comparative Study of Koizumi and Longa Methods of Intraluminal Filament Middle Cerebral Artery Occlusion in Rats: Early Corticosterone and Inflammatory Response in the Hippocampus and Frontal Cortex
Abstract
1. Introduction
2. Results
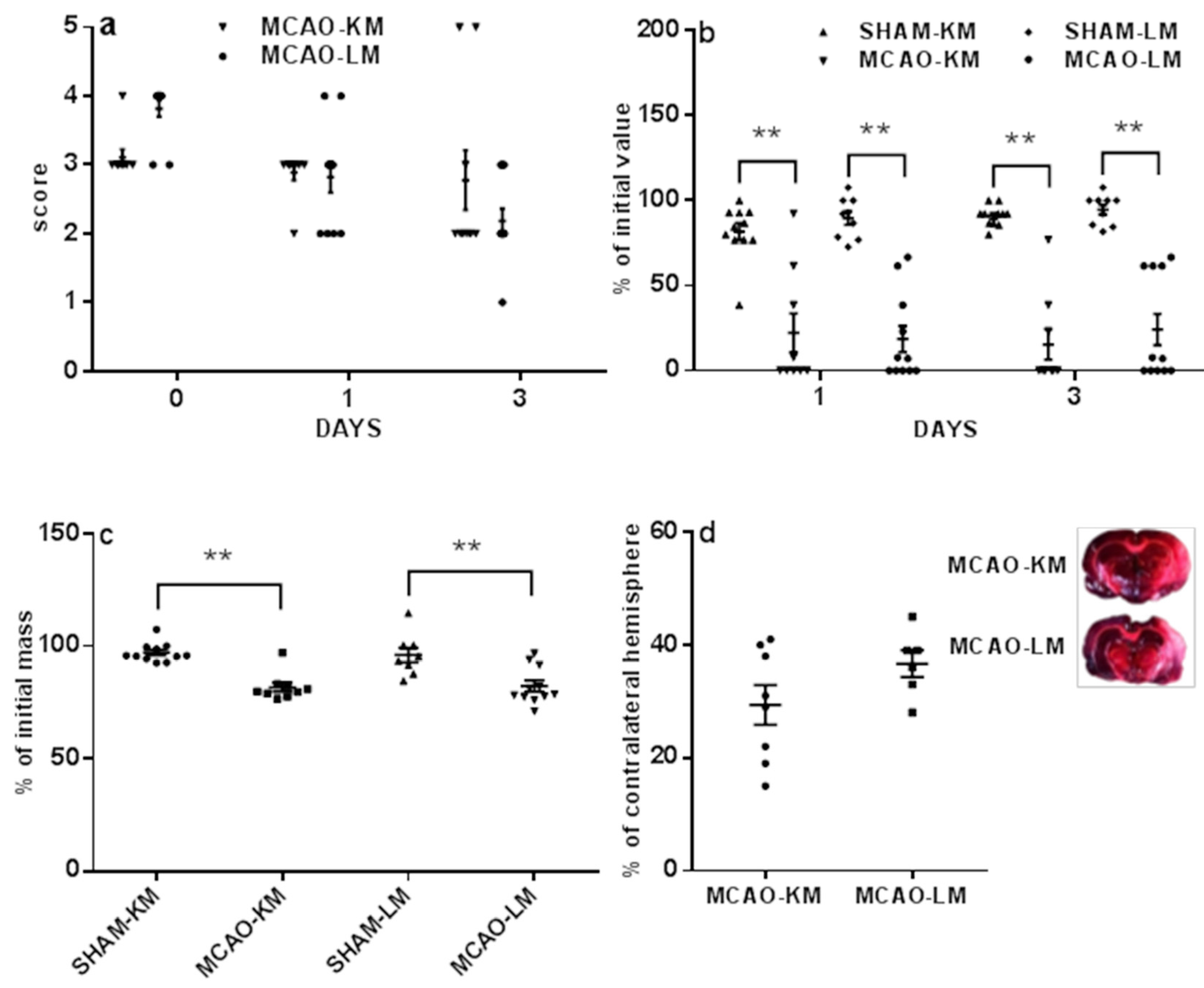
2.1. Mortality, Neurological Deficits, and Brain Infarct Volume
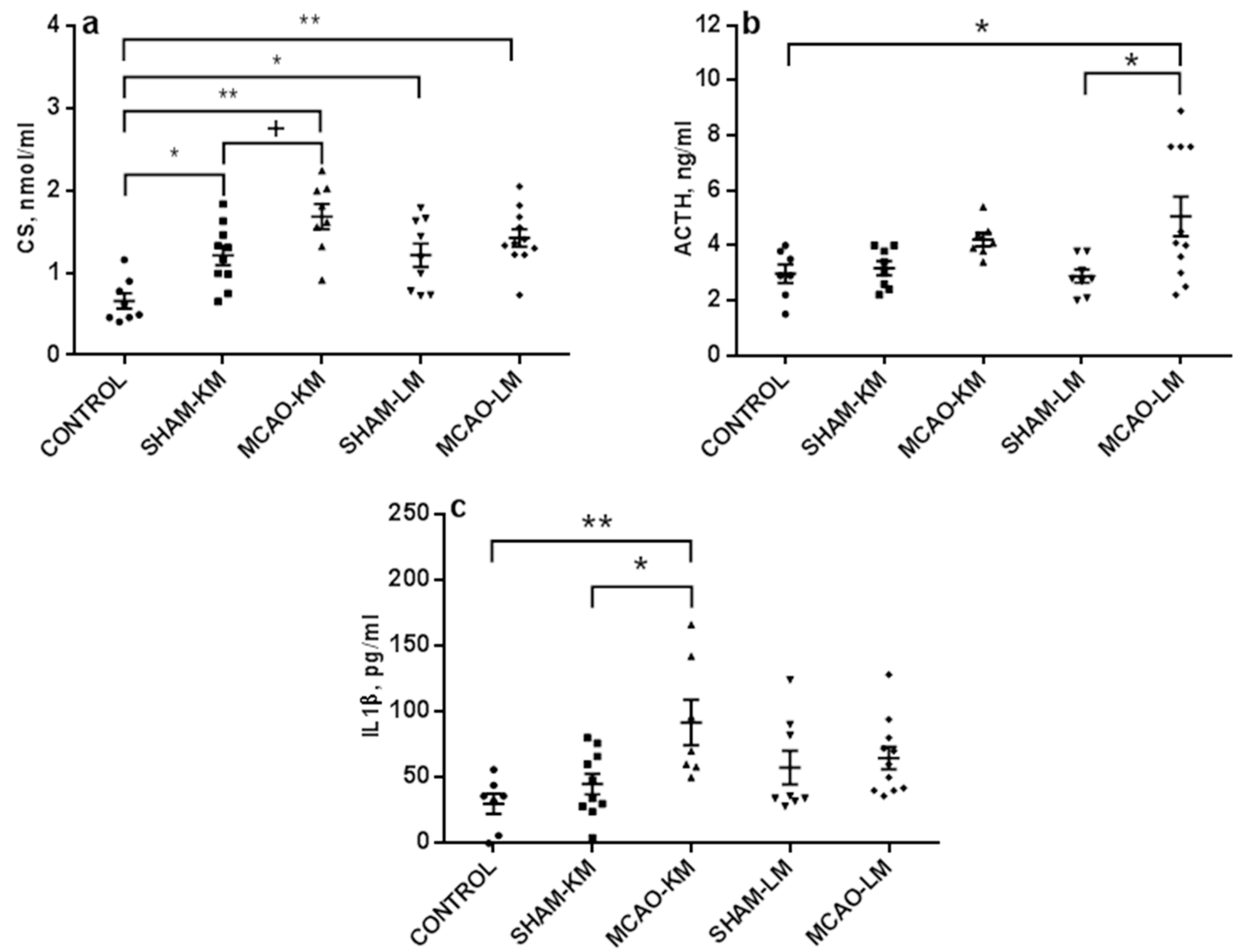
2.2. Corticosterone and Pro-Inflammatory Interleukins in Blood Serum
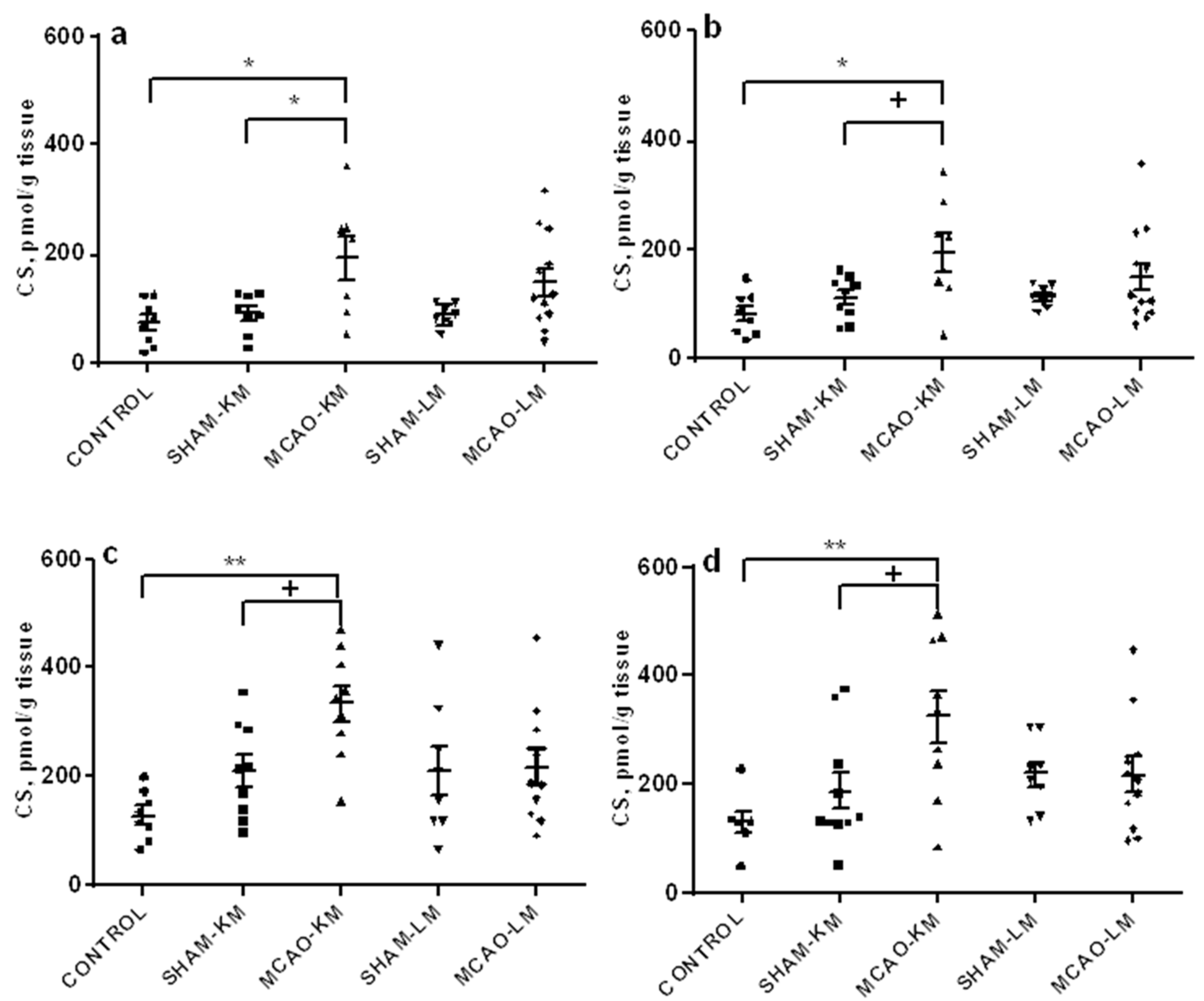
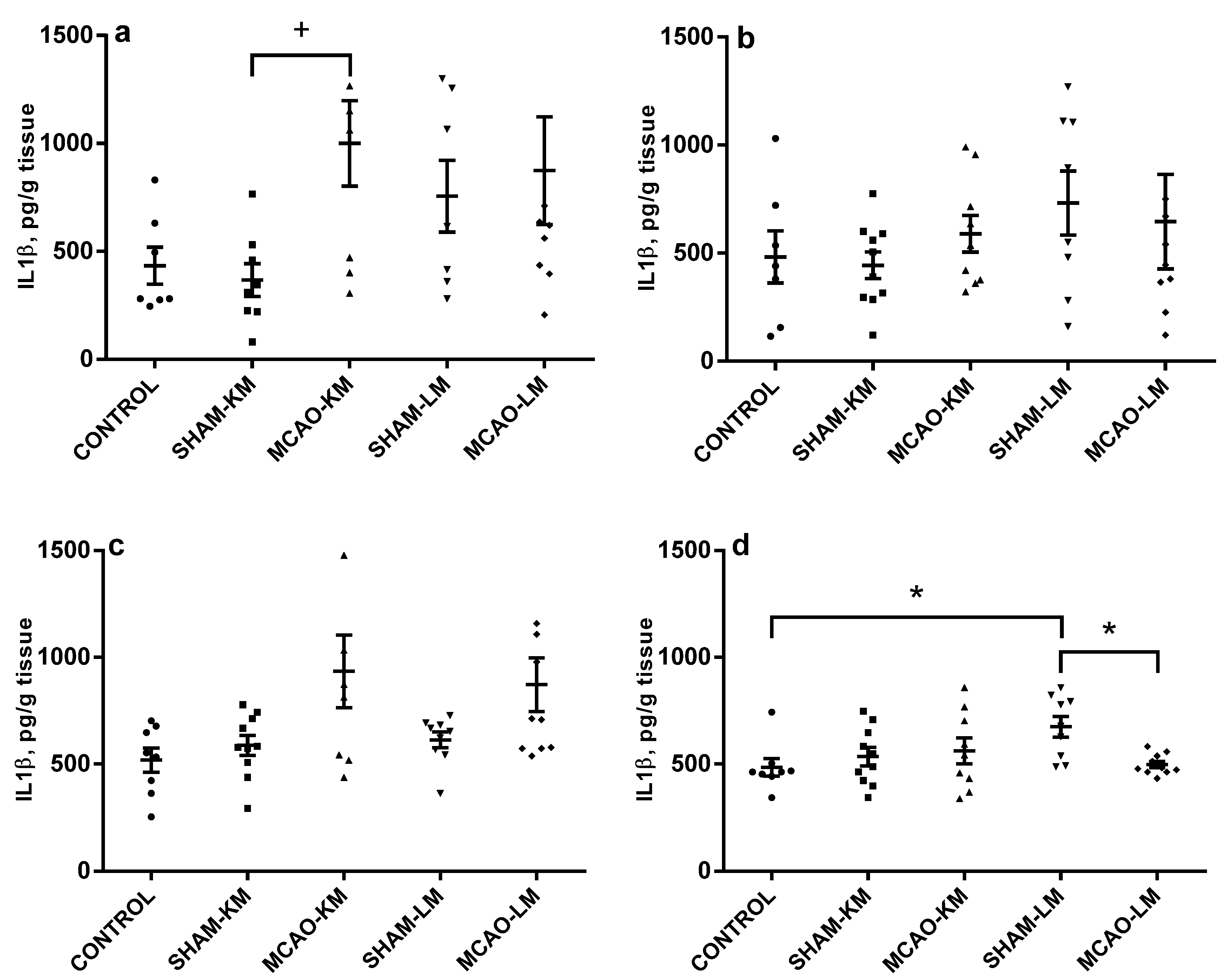
2.3. Corticosterone and Pro-Inflammatory Interleukins in the Hippocampus and Frontal Cortex
3. Discussion
3.1. HPA Axis, Stress Response, and Brain Ischemia
3.2. HPA Axis, Brain Ischemia, and Neuroinflammation
3.3. Ischemic Stroke and Distant Hippocampal Damage
3.4. Rodent MCAO Models Are Not Identical
4. Materials and Methods
4.1. Environment and Housing
4.2. Modeling Ischemic Stroke
4.3. Assessment of Neurological Deficits
- Five-point neurological scale. Motor and behavioral changes were assessed using a 0–5 point grading scale [52] at 1 h following MCA occlusion, and daily prior to sacrifice. This standard test used conventionally to assess the efficiency of focal brain ischemia in rodents, is based on a 5-point behavioral scale and allows to evaluate the functional state of the contralateral foreleg of the rat, the presence of turns and circulation in the contralateral side, as well as the mobility of the animal: 0, no deficit; 1. failure to extend right forepaw fully; 2. decreased grip of right forelimb while tail pulled; 3. spontaneous circling or walking to contralateral side; 4. walks only when stimulated with depressed level of consciousness; 5. unresponsive to stimulation.
- Tongue protrusion test. This test indicates the ability of the rat to lick peanut butter out from a thin full glass cylinder left in the cage overnight [53]. The length of the butter pile, from the beginning of the cylinder to the level of the remaining butter, shows the ability of the animal to protrude its tongue, which is impaired during the acute phase of MCAO.
4.4. Collection of Biomaterial
4.5. Enzyme-Linked Immunosorbent Assays (ELISA)
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FC | frontal cortex |
| HPA | hypothalamic–pituitary–adrenal |
| MCAO | middle cerebral artery occlusion |
| MCAO-KM | middle cerebral artery occlusion, Koizumi et al. model |
| MCAO-LM | middle cerebral artery occlusion, Longa et al. model |
| ELISA | enzyme-linked immunosorbent assay |
| IL1β | interleukin-1beta |
| IL6 | interleukin-6 |
| TNFα | tumor necrosis factor-alpha |
References
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G.; et al. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef]
- Gulyaeva, N.V. Functional Neurochemistry of the Ventral and Dorsal Hippocampus: Stress, Depression, Dementia and Remote Hippocampal Damage. Neurochem. Res. 2019, 44, 1306–1322. [Google Scholar] [CrossRef]
- Dhir, N.; Medhi, B.; Prakash, A.; Goyal, M.K.; Modi, M.; Mohindra, S. Pre-clinical to Clinical Translational Failures and Current Status of Clinical Trials in Stroke Therapy: A Brief Review. Curr. Neuropharmacol. 2020, 18, 596–612. [Google Scholar] [CrossRef] [PubMed]
- Levard, D.; Buendia, I.; Lanquetin, A.; Glavan, M.; Vivien, D.; Rubio, M. Filling the gaps on stroke research: Focus on inflammation and immunity. Brain Behav. Immun. 2021, 91, 649–667. [Google Scholar] [CrossRef]
- Lyden, P.D. Cerebroprotection for Acute Ischemic Stroke: Looking Ahead. Stroke 2021, 52, 3033–3044. [Google Scholar] [CrossRef] [PubMed]
- Narayan, S.K.; Grace Cherian, S.; Babu Phaniti, P.; Babu Chidambaram, S.; Rachel Vasanthi, A.H.; Arumugam, M. Preclinical animal studies in ischemic stroke:Challenges and some solutions. Animal Model Exp. Med. 2021, 4, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Wasan, H.; Reeta, K.H. Preclinical Stroke Research and Translational Failure: A Bird’s Eye View on Preventable Variables. Cell. Mol. Neurobiol. 2021, 1–15. [Google Scholar] [CrossRef]
- Candelario-Jalil, E.; Paul, S. Impact of aging and comorbidities on ischemic stroke outcomes in preclinical animal models: A translational perspective. Exp. Neurol. 2020, 335, 113494. [Google Scholar] [CrossRef]
- Mizuma, A.; Yenari, M.A. Clinical perspectives on ischemic stroke. Exp. Neurol. 2021, 338, 113599. [Google Scholar] [CrossRef] [PubMed]
- Gulyaeva, N.V.; Onufriev, M.V.; Moiseeva, Y.V. Ischemic stroke, glucocorticoids and remote hippocampal damage: A translational outlook and implications for modeling. Front. Neurosci. 2021, in press. [Google Scholar] [CrossRef]
- Gulyaeva, N.V. Biochemical Mechanisms and Translational Relevance of Hippocampal Vulnerability to Distant Focal Brain Injury: The Price of Stress Response. Biochemistry 2019, 84, 1306–1328. [Google Scholar] [CrossRef] [PubMed]
- Yavas, E.; Gonzalez, S.; Fanselow, M.S. Interactions between the hippocampus, prefrontal cortex, and amygdala support complex learning and memory. F1000Research 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Chao, O.Y.; de Souza Silva, M.A.; Yang, Y.M.; Huston, J.P. The medial prefrontal cortex—hippocampus circuit that integrates information of object, place and time to construct episodic memory in rodents: Behavioral, anatomical and neurochemical properties. Neurosci. Biobehav. Rev. 2020, 113, 373–407. [Google Scholar] [CrossRef]
- Koizumi, J.; Nakazawa, T.; Ooneda, G. Experimental studies of ischemic brain edema. I. A new experimental model of cerebral embolism in rats in which recirculation can be introduced in the ischemic area. Jpn. J. Stroke 1986, 8, 1–8. [Google Scholar] [CrossRef]
- Longa, E.Z.; Weinstein, P.R.; Carlson, S.; Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989, 20, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Kasatkina, M.Y.; Moiseeva, Y.V.; Onufriev, M.V.; Gulyaeva, N.V. Experimental Middle Cerebral Artery Occlusion (MCAO) for Studying Hippocampus–Associated Mechanisms of Post-Stroke Disorders: A Comparative Study of Two Models. In Proceedings of the FENS Regional Meeting 2021 Book of Abstracts, Krakow, Poland, 25–27 August 2021; p. 166. [Google Scholar]
- Smith, H.K.; Russell, J.M.; Granger, D.N.; Gavins, F.N. Critical differences between two classical surgical approaches for middle cerebral artery occlusion-induced stroke in mice. J. Neurosci. Methods. 2015, 249, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.P.; Wright, A.L.; Tan, R.P.; Gladbach, A.; Ittner, L.M.; Vissel, B.A. Comparative Study of Variables Influencing Ischemic Injury in the Longa and Koizumi Methods of Intraluminal Filament Middle Cerebral Artery Occlusion in Mice. PLoS ONE 2016, 11, e0148503. [Google Scholar] [CrossRef] [PubMed]
- Fassbender, K.; Schmidt, R.; Mössner, R.; Daffertshofer, M.; Hennerici, M. Pattern of activation of the hypothalamic-pituitary-adrenal axis in acute stroke. Relation to acute confusional state, extent of brain damage, and clinical outcome. Stroke 1994, 25, 1105–1108. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.; Olsson, T.; Carlberg, B.; Karlsson, K.; Fagerlund, M. Hypercortisolism after stroke–partly cytokine-mediated? J. Neurol. Sci. 1997, 147, 43–47. [Google Scholar] [CrossRef]
- Olsson, T. Urinary free cortisol excretion shortly after ischaemic stroke. J. Intern. Med. 1990, 228, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Montellano, F.A.; Ungethüm, K.; Ramiro, L.; Nacu, A.; Hellwig, S.; Fluri, F.; Whiteley, W.N.; Bustamante, A.; Montaner, J.; Heuschmann, P.U. Role of Blood-Based Biomarkers in Ischemic Stroke Prognosis: A Systematic Review. Stroke 2021, 52, 543–551. [Google Scholar] [CrossRef]
- Robertson, D.A.; Beattie, J.E.; Reid, I.C.; Balfour, D.J. Regulation of corticosteroid receptors in the rat brain: The role of serotonin and stress. Eur. J. Neurosci. 2005, 21, 1511–1520. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Packan, D.R.; Vale, W.W. Glucocorticoid toxicity in the hippocampus: In vitro demonstration. Brain Res. 1988, 453, 367–371. [Google Scholar] [CrossRef]
- Jacobson, L.; Sapolsky, R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr. Rev. 1991, 12, 118–134. [Google Scholar] [CrossRef]
- Sapolsky, R.M. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch. Gen. Psychiatry 2000, 57, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Sarabdjitsingh, R.A.; Meijer, O.C.; Schaaf, M.J.; de Kloet, E.R. Subregion-specific differences in translocation patterns of mineralocorticoid and glucocorticoid receptors in rat hippocampus. Brain Res. 2009, 1249, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Szczudlik, A.; Dziedzic, T.; Bartus, S.; Slowik, A.; Kieltyka, A. Serum interleukin-6 predicts cortisol release in acute stroke patients. J. Endocrinol. Investig. 2004, 27, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Slowik, A.; Turaj, W.; Pankiewicz, J.; Dziedzic, T.; Szermer, P.; Szczudlik, A. Hypercortisolemia in acute stroke is related to the inflammatory response. J. Neurol. Sci. 2002, 196, 27–32. [Google Scholar] [CrossRef]
- Davies, C.A.; Loddick, S.A.; Toulmond, S.; Stroemer, R.P.; Hunt, J.; Rothwell, N.J. The progression and topographic distribution of interleukin-1beta expression after permanent middle cerebral artery occlusion in the rat. J. Cereb. Blood Flow Metab. 1999, 19, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Block, F.; Peters, M.; Nolden-Koch, M. Expression of IL-6 in the ischemic penumbra. NeuroReport 2000, 11, 963–967. [Google Scholar] [CrossRef]
- Dunn, A.J. Cytokine activation of the HPA axis. Ann. N. Y. Acad. Sci. 2000, 917, 608–617. [Google Scholar] [CrossRef]
- John, C.D.; Buckingham, J.C. Cytokines: Regulation of the hypothalamo-pituitary-adrenocortical axis. Curr. Opin. Pharmacol. 2003, 3, 78–84. [Google Scholar] [CrossRef]
- McCoy, M.K.; Tansey, M.G. TNF signaling inhibition in the CNS: Implications for normal brain function and neurodegenerative disease. J. Neuroinflamm. 2008, 5, 45. [Google Scholar] [CrossRef]
- Simi, A.; Tsakiri, N.; Wang, P.; Rothwell, N.J. Interleukin-1 and inflammatory neurodegeneration. Biochem. Soc. Trans. 2007, 35, 1122–1126. [Google Scholar] [CrossRef]
- Whiteley, W.; Jackson, C.; Lewis, S.; Lowe, G.; Rumley, A.; Sandercock, P.; Wardlaw, J.; Dennis, M.; Sudlow, C. Inflammatory markers and poor outcome after stroke: A prospective cohort study and systematic review of interleukin-6. PLoS Med. 2009, 6, e1000145. [Google Scholar] [CrossRef]
- Caso, J.R.; Lizasoain, I.; Lorenzo, P.; Moro, M.A.; Leza, J.C. The role of tumor necrosis factor-alpha in stress-induced worsening of cerebral ischemia in rats. Neuroscience 2006, 142, 59–69. [Google Scholar] [CrossRef]
- Caso, J.R.; Moro, M.A.; Lorenzo, P.; Lizasoain, I.; Leza, J.C. Involvement of IL-1beta in acute stress-induced worsening of cerebral ischaemia in rats. Eur. Neuropsychopharmacol. 2007, 1, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Block, F.; Dihné, M.; Loos, M. Inflammation in areas of remote changes following focal brain lesion. Prog. Neurobiol. 2005, 75, 342–365. [Google Scholar] [CrossRef] [PubMed]
- Butler, T.L.; Kassed, C.A.; Sanberg, P.R.; Willing, A.E.; Pennypacker, K.R. Neurodegeneration in the rat hippocampus and striatum after middle cerebral artery occlusion. Brain Res. 2002, 929, 252–260. [Google Scholar] [CrossRef]
- Gulyaeva, N.V. Stress-Associated Molecular and Cellular Hippocampal Mechanisms Common for Epilepsy and Comorbid Depressive Disorders. Biochemistry 2021, 86, 641–656. [Google Scholar] [CrossRef] [PubMed]
- Podgorny, O.V.; Gulyaeva, N.V. Glucocorticoid-mediated mechanisms of hippocampal damage: Contribution of subgranular neurogenesis. J. Neurochem. 2021, 157, 370–392. [Google Scholar] [CrossRef]
- Milot, M.R.; Plamondon, H. Changes in HPA reactivity and noradrenergic functions regulate spatial memory impairments at delayed time intervals following cerebral ischemia. Horm. Behav. 2011, 59, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Kadar, T.; Dachir, S.; Shukitt-Hale, B.; Levy, A. Sub-regional hippocampal vulnerability in various animal models leading to cognitive dysfunction. J. Neural Transm. 1998, 105, 987–1004. [Google Scholar] [CrossRef]
- Rami, A.; Rabié, A.; Winckler, J. Synergy between chronic corticosterone treatment and cerebral ischemia in producing damage in noncalbindinergic neurons. Exp. Neurol. 1998, 149, 439–446. [Google Scholar] [CrossRef]
- Onufriev, M.V.; Freiman, S.V.; Moiseeva, Y.V.; Stepanichev, M.Y.; Lazareva, N.A.; Gulyaeva, N.V. Accumulation of corticosterone and interleukin-1β in the hippocampus after focal ischemic damage of the neocortex: Selective vulnerability of the ventral hippocampus. Neurochem. J. 2017, 11, 236–241. [Google Scholar] [CrossRef]
- Ruggiero, R.N.; Rossignoli, M.T.; Marques, D.B.; de Sousa, B.M.; Romcy-Pereira, R.N.; Lopes-Aguiar, C.; Leite, J.P. Neuromodulation of Hippocampal-Prefrontal Cortical SynapticPlasticity and Functional Connectivity: Implications for Neuropsychiatric Disorders. Front. Cell. Neurosci. 2021, 15, 732360. [Google Scholar] [CrossRef] [PubMed]
- Sampath, D.; Sathyanesan, M.; Newton, S.S. Cognitive dysfunction in major depression and Alzheimer’s disease is associated with hippocampal-prefrontal cortex dysconnectivity. Neuropsychiatr. Dis. Treat. 2017, 13, 1509–1519. [Google Scholar] [CrossRef]
- Lapin, I.P. Only controls: Effect of handling, sham injection, and intraperitoneal injection of saline on behavior of mice in an elevated plus-maze. J. Pharmacol. Toxicol. Methods 1995, 34, 73–77. [Google Scholar] [CrossRef]
- Freiman, S.V.; Onufriev, M.V.; Stepanichev, M.Y.; Moiseeva, Y.V.; Lazareva, N.A.; Gulyaeva, N.V. The stress effects of a single injection of isotonic saline solution: Systemic (blood) and central (frontal cortex and dorsal and ventral hippocampus). Neurochem. J. 2016, 10, 115–119. [Google Scholar] [CrossRef]
- Werner, W.; Sallmon, H.; Leder, A.; Lippert, S.; Reutzel-Selke, A.; Morgül, M.H.; Jonas, S.; Dame, C.; Neuhaus, P.; Iacomini, J.; et al. Independent effects of sham laparotomy and anesthesia on hepatic microRNA expression in rats. BMC Res Notes 2014, 7, 702. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hunter, A.J.; Hatcher, J.; Virley, D.; Nelson, P.; Irving, E.; Hadingham, S.J.; Parsons, A.A. Functional assessments in mice and rats after focal stroke. Neuropharmacology 2000, 39, 806–816. [Google Scholar] [CrossRef]
- Gulyaeva, N.; Thompson, C.; Shinohara, N.; Lazareva, N.; Onufriev, M.; Stepanichev, M.; Moiseeva, Y.; Fliss, H.; Hakim, A.M. Tongue protrusion: A simple test for neurological recovery in rats following focal cerebral ischemia. J. Neurosci. Meth. 2003, 125, 183–193. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onufriev, M.V.; Moiseeva, Y.V.; Zhanina, M.Y.; Lazareva, N.A.; Gulyaeva, N.V. A Comparative Study of Koizumi and Longa Methods of Intraluminal Filament Middle Cerebral Artery Occlusion in Rats: Early Corticosterone and Inflammatory Response in the Hippocampus and Frontal Cortex. Int. J. Mol. Sci. 2021, 22, 13544. https://doi.org/10.3390/ijms222413544
Onufriev MV, Moiseeva YV, Zhanina MY, Lazareva NA, Gulyaeva NV. A Comparative Study of Koizumi and Longa Methods of Intraluminal Filament Middle Cerebral Artery Occlusion in Rats: Early Corticosterone and Inflammatory Response in the Hippocampus and Frontal Cortex. International Journal of Molecular Sciences. 2021; 22(24):13544. https://doi.org/10.3390/ijms222413544
Chicago/Turabian StyleOnufriev, Mikhail V., Yulia V. Moiseeva, Marina Y. Zhanina, Natalia A. Lazareva, and Natalia V. Gulyaeva. 2021. "A Comparative Study of Koizumi and Longa Methods of Intraluminal Filament Middle Cerebral Artery Occlusion in Rats: Early Corticosterone and Inflammatory Response in the Hippocampus and Frontal Cortex" International Journal of Molecular Sciences 22, no. 24: 13544. https://doi.org/10.3390/ijms222413544
APA StyleOnufriev, M. V., Moiseeva, Y. V., Zhanina, M. Y., Lazareva, N. A., & Gulyaeva, N. V. (2021). A Comparative Study of Koizumi and Longa Methods of Intraluminal Filament Middle Cerebral Artery Occlusion in Rats: Early Corticosterone and Inflammatory Response in the Hippocampus and Frontal Cortex. International Journal of Molecular Sciences, 22(24), 13544. https://doi.org/10.3390/ijms222413544






