4-O-Glucosylation of Trichothecenes by Fusarium Species: A Phase II Xenobiotic Metabolism for t-Type Trichothecene Producers
Abstract
1. Introduction
2. Results and Discussion
2.1. Feeding of d-Type Trichothecenes to t-Type Trichothecene Producers
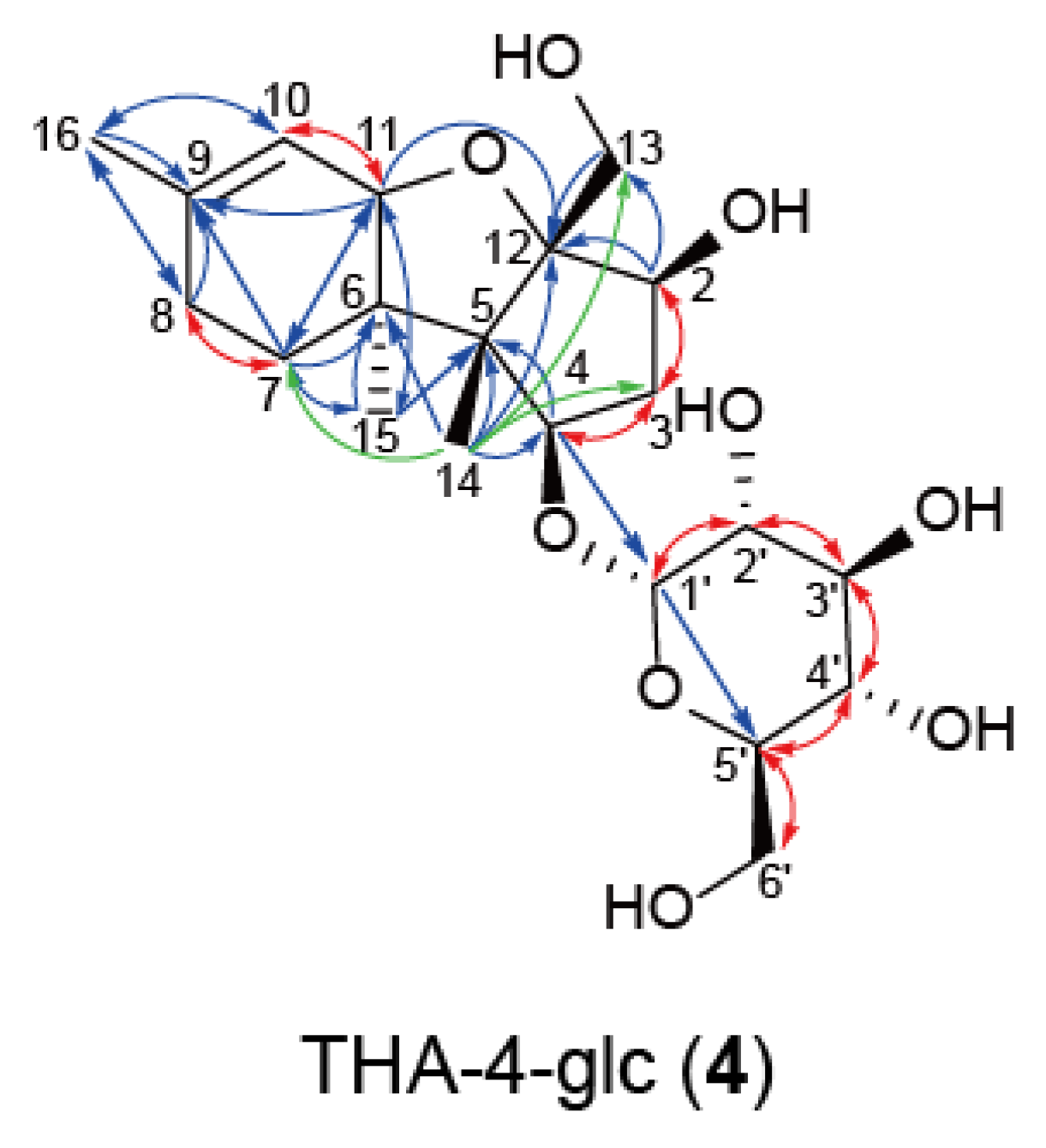
2.2. Purification and Structure Elucidation of Hexose Conjugate of d-Type Trichothecenes
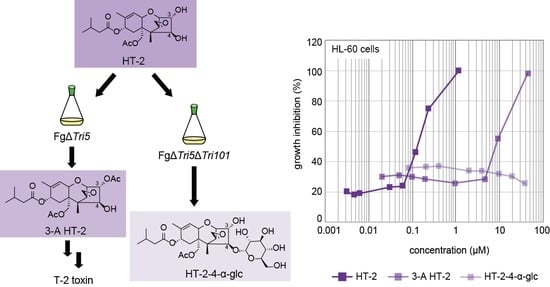
2.3. Glucosylation of a t-Type Type A Trichothecene, HT-2, by a Tri101-Null Mutant of F. graminearum
2.4. Glucosylation of t-Type Type B Trichothecenes by a Tri101-Null Mutant of F. graminearum
2.5. Stability of the Glucoside Conjugates of Trichothecenes
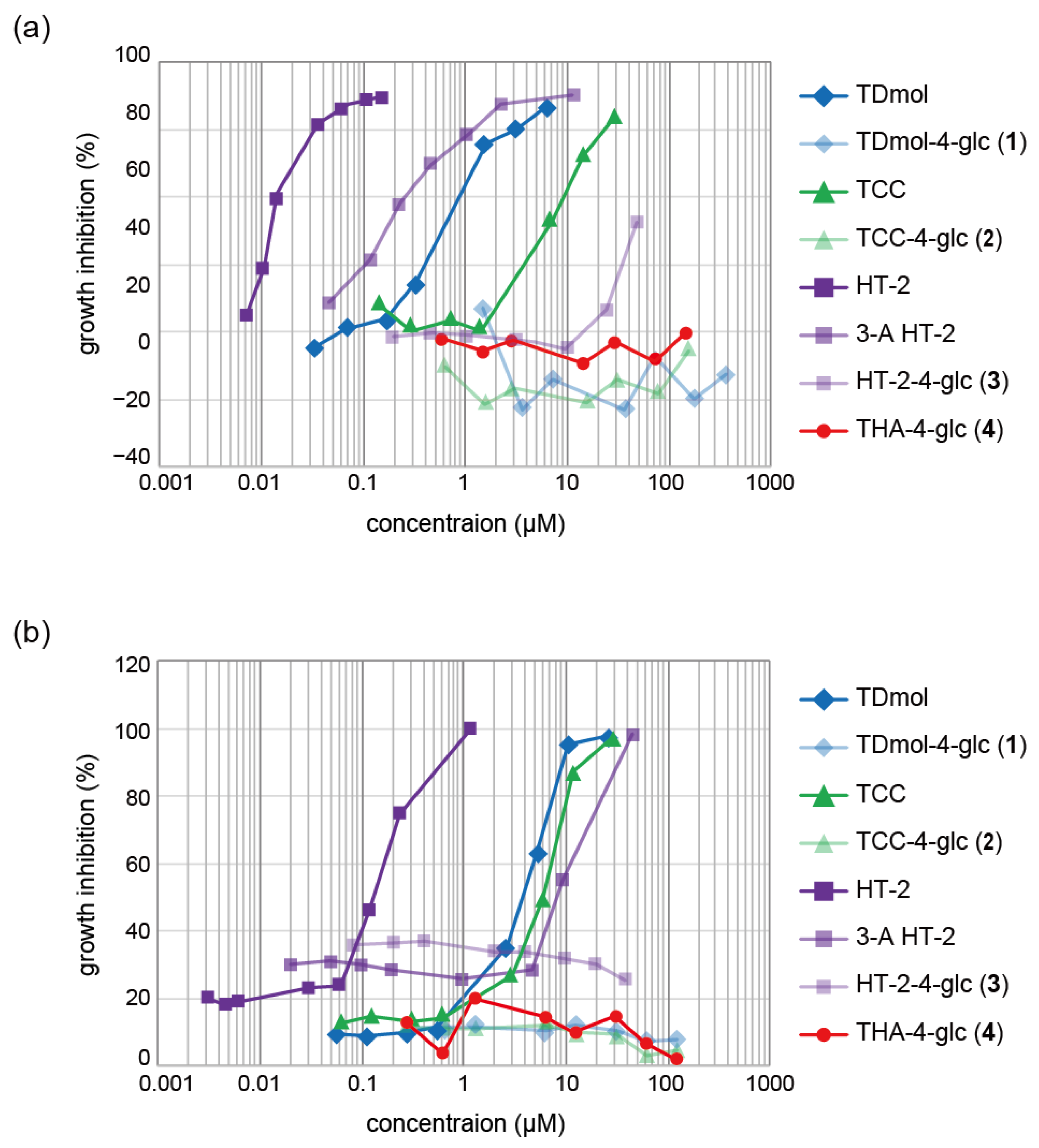
2.6. Cytotoxicity of the Glucoside Conjugates of Trichothecenes
2.7. Possible Role of Trichothecene 4-O-Glucosylation by Fusarium
3. Materials and Methods
3.1. Strains
3.2. Reagents
3.3. Medium and Culture Conditions
3.4. Preparation of Trichothecene Substrates for the Feeding Experiment
3.5. Extraction and Purification of Trichothecenes
3.6. HPLC and LC-MS/MS Analysis
3.7. Feeding Experiments and Purification of Trichothecene Metabolites
3.8. In Vitro Trichothecene 3-O-Acetyltransferase (Tri101p) Activity Assay
3.9. Stability of the Glucoside Conjugates of Trichothecenes and Trichothecene Aglycons
3.10. NMR
3.11. Cytotoxicity Assay
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 15-AS-4-glc | 15-Acetoxyscirpenol-4-O-α-glucopyranoside |
| 3-A HT-2 | 3-Acetyl HT-2 toxin |
| 3-ANIV | 3-Acetylnivalenol |
| 4-ANIV | 4-Acetylnivalenol |
| 15-ANIV | 15-Acetylnivalenol |
| NBRC | Biological Resource Center, NITE |
| COSY | Correlation spectroscopy |
| DON | Deoxynivalenol |
| DON-3-glc | DON-3-O-β-glucopyranoside |
| 8-deTCN | 8-Deoxytrichothecin |
| 3,15-diANIV | 3,15-Diacetylnivalenol |
| 4,15-diANIV | 4,15-Diacetylnivalenol |
| DAS | 4,15-Diacetoxyscirpenol |
| 1D-NOE | 1D-nuclear overhauser effect |
| EPT | 12,13-Epoxy-trichothec-9-ene |
| XIC | Extracted ion chromatogram |
| FBS | Fetal bovine serum |
| glc | Glucopyranoside |
| HMBC | Heteronuclear multiple bond coherence |
| HMQC | Heteronuclear multiple quantum coherence |
| HPLC | High-performance liquid chromatography |
| HT-2 | HT-2 toxin |
| HT-2-4-glc | HT-2 toxin-4-O-α-glucopyranoside |
| IDA | Information dependent acquisition |
| ITD | Isotrichodermin |
| ITDmol | Isotrichodermol |
| LC-MS/MS | Liquid chromatography-tandem mass spectrometry |
| NEO | Neosolaniol |
| NBP | 4-(p-Nitrobenzyl)pyridine |
| NIV | Nivalenol |
| NIV-3-glc | NIV-3-O-β-glucopyranoside |
| NMR | Nuclear magnetic resonance |
| PTFE | Polytetrafluoroethylene |
| RF | Rice flour |
| SDS | Sodium dodecyl sulfate |
| TEPA | Tetraethylenepentamine |
| TLC | Thin-layer chromatography |
| TOF | Time of flight |
| TOCSY | Total correlation spectroscopy |
| 3,4,15-triANIV | 3,4,15-Triacetylnivalenol |
| TDmol | Trichodermol |
| TDmol-4-glc | TDmol-4-O-α-glucopyranoside |
| TCN | Trichothecin |
| TCC | Trichothecolone |
| TCC-4-glc | TCC-4-O-α-glucopyranoside |
| THA | 2,4,13-Trihydroxyapotrichothecene |
| UDP | Uridine diphosphate |
| UGT | UDP-glucosyltransferase |
References
- Ueno, Y.; Nakajima, M.; Sakai, K.; Ishii, K.; Sato, N. Comparative toxicology of trichothec mycotoxins: Inhibition of protein synthesis in animal cells. J. Biochem. 1973, 74, 285–296. [Google Scholar]
- Kimura, M.; Tokai, T.; Takahashi-Ando, N.; Ohsato, S.; Fujimura, M. Molecular and genetic studies of Fusarium trichothecene biosynthesis: Pathways, genes, and evolution. Biosci. Biotechnol. Biochem. 2007, 71, 2105–2123. [Google Scholar] [CrossRef]
- Kimura, M.; Kaneko, I.; Komiyama, M.; Takatsuki, A.; Koshino, H.; Yoneyama, K.; Yamaguchi, I. Trichothecene 3-O-acetyltransferase protects both the producing organism and transformed yeast from related mycotoxins. Cloning and characterization of Tri101. J. Biol. Chem. 1998, 273, 1654–1661. [Google Scholar] [CrossRef]
- McCormick, S.P.; Stanley, A.M.; Stover, N.A.; Alexander, N.J. Trichothecenes: From simple to complex mycotoxins. Toxins 2011, 3, 802–814. [Google Scholar] [CrossRef]
- Zamir, L.O.; Devor, K.A.; Nikolakakis, A.; Sauriol, F. Biosynthesis of Fusarium culmorum trichothecenes. The roles of isotrichodermin and 12,13-epoxytrichothec-9-ene. J. Biol. Chem. 1990, 265, 6713–6725. [Google Scholar] [CrossRef]
- Tokai, T.; Koshino, H.; Takahashi-Ando, N.; Sato, M.; Fujimura, M.; Kimura, M. Fusarium Tri4 encodes a key multifunctional cytochrome P450 monooxygenase for four consecutive oxygenation steps in trichothecene biosynthesis. Biochem. Biophys. Res. Commun. 2007, 353, 412–417. [Google Scholar] [CrossRef]
- McCormick, S.P.; Alexander, N.J.; Trapp, S.E.; Hohn, T.M. Disruption of TRI101, the gene encoding trichothecene 3-O-acetyltransferase, from Fusarium sporotrichioides. Appl. Environ. Microbiol. 1999, 65, 5252–5256. [Google Scholar] [CrossRef]
- McCormick, S.P.; Taylor, S.L.; Plattner, R.D.; Beremand, M.N. Bioconversion of possible T-2 toxin precursors by a mutant strain of Fusarium sporotrichioides NRRL 3299. Appl. Environ. Microbiol. 1990, 56, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Zamir, L.O.; Nikolakakis, A.; Devor, K.A.; Sauriol, F. Biosynthesis of the trichothecene 3-acetyldeoxynivalenol. Is isotrichodermin a biosynthetic precursor? J. Biol. Chem. 1996, 271, 27353–27359. [Google Scholar] [CrossRef]
- Thompson, W.L.; Wannemacher, R.W., Jr. Structure-function relationships of 12,13-epoxytrichothecene mycotoxins in cell culture: Comparison to whole animal lethality. Toxicon 1986, 24, 985–994. [Google Scholar] [CrossRef]
- Wu, Q.; Dohnal, V.; Kuca, K.; Yuan, Z. Trichothecenes: Structure-toxic activity relationships. Curr. Drug Metab. 2013, 14, 641–660. [Google Scholar] [CrossRef]
- Kimura, M.; Matsumoto, G.; Shingu, Y.; Yoneyama, K.; Yamaguchi, I. The mystery of the trichothecene 3-O-acetyltransferase gene. Analysis of the region around Tri101 and characterization of its homologue from Fusarium sporotrichioides. FEBS Lett. 1998, 435, 163–168. [Google Scholar] [CrossRef]
- Muhitch, M.J.; McCormick, S.P.; Alexander, N.J.; Hohn, T.M. Transgenic expression of the TRI101 or PDR5 gene increases resistance of tobacco to the phytotoxic effects of the trichothecene 4,15-diacetoxyscirpenol. Plant Sci. 2000, 157, 201–207. [Google Scholar] [CrossRef]
- Ohsato, S.; Ochiai-Fukuda, T.; Nishiuchi, T.; Takahashi-Ando, N.; Koizumi, S.; Hamamoto, H.; Kudo, T.; Yamaguchi, I.; Kimura, M. Transgenic rice plants expressing trichothecene 3-O-acetyltransferase show resistance to the Fusarium phytotoxin deoxynivalenol. Plant Cell Rep. 2007, 26, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Takushima, R.; Tanaka, A.; Okada, A.; Matsui, K.; Maeda, K.; Aikawa, S.; Kimura, M.; Takahashi-Ando, N. Reduced toxicity of trichothecenes, isotrichodermol, and deoxynivalenol, by transgenic expression of the Tri101 3-O-acetyltransferase gene in cultured mammalian FM3A Cells. Toxins 2019, 11, 654. [Google Scholar] [CrossRef]
- Shima, J.; Takase, S.; Takahashi, Y.; Iwai, Y.; Fujimoto, H.; Yamazaki, M.; Ochi, K. Novel detoxification of the trichothecene mycotoxin deoxynivalenol by a soil bacterium isolated by enrichment culture. Appl. Environ. Microbiol. 1997, 63, 3825–3830. [Google Scholar] [CrossRef] [PubMed]
- He, J.W.; Hassan, Y.I.; Perilla, N.; Li, X.-Z.; Boland, G.J.; Zhou, T. Bacterial epimerization as a route for deoxynivalenol detoxification: The influence of growth and environmental conditions. Front. Microbiol. 2016, 7, 572. [Google Scholar] [CrossRef] [PubMed]
- He, J.W.; Bondy, G.S.; Zhou, T.; Caldwell, D.; Boland, G.J.; Scott, P.M. Toxicology of 3-epi-deoxynivalenol, a deoxynivalenol-transformation product by Devosia mutans 17-2-E-8. Food Chem. Toxicol. 2015, 84, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Carere, J.; Hassan, Y.I.; Lepp, D.; Zhou, T. The enzymatic detoxification of the mycotoxin deoxynivalenol: Identification of DepA from the DON epimerization pathway. Microb. Biotechnol. 2018, 11, 1106–1111. [Google Scholar] [CrossRef]
- Carere, J.; Hassan, Y.I.; Lepp, D.; Zhou, T. The Identification of DepB: An enzyme responsible for the final detoxification step in the deoxynivalenol epimerization pathway in Devosia mutans 17-2-E-8. Front. Microbiol. 2018, 9, 1573. [Google Scholar] [CrossRef]
- Hassan, Y.I.; He, J.W.; Perilla, N.; Tang, K.; Karlovsky, P.; Zhou, T. The enzymatic epimerization of deoxynivalenol by Devosia mutans proceeds through the formation of 3-keto-DON intermediate. Sci. Rep. 2017, 7, 6929. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.D.; Arnison, P.G. Degradation of deoxynivalenol by suspension cultures of the Fusarium head blight resistant wheat cultivar Frontana. Can. J. Plant Pathol. 1986, 8, 147–150. [Google Scholar] [CrossRef]
- Sewald, N.; von Gleissenthall, J.L.; Schuster, M.; Müller, G.; Aplin, R.T. Structure elucidation of a plant metabolite of 4-desoxynivalenol. Tetrahedron Asymmetry 1992, 3, 953–960. [Google Scholar] [CrossRef]
- Poppenberger, B.; Berthiller, F.; Lucyshyn, D.; Sieberer, T.; Schuhmacher, R.; Krska, R.; Kuchler, K.; Glössl, J.; Luschnig, C.; Adam, G. Detoxification of the Fusarium mycotoxin deoxynivalenol by a UDP-glucosyltransferase from Arabidopsis thaliana. J. Biol. Chem. 2003, 278, 47905–47914. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, W.; Boddu, J.; Shin, S.; Poppenberger, B.; Berthiller, F.; Lemmens, M.; Muehlbauer, G.J.; Adam, G. Validation of a candidate deoxynivalenol-inactivating UDP-glucosyltransferase from barley by heterologous expression in yeast. Mol. Plant-Microbe Interact. 2010, 23, 977–986. [Google Scholar] [CrossRef]
- Gatti, M.; Choulet, F.; Macadré, C.; Guérard, F.; Seng, J.-M.; Langin, T.; Dufresne, M. Identification, molecular cloning, and functional characterization of a wheat UDP-glucosyltransferase involved in resistance to Fusarium head blight and to mycotoxin accumulation. Front. Plant Sci. 2018, 9, 1853. [Google Scholar] [CrossRef] [PubMed]
- Michlmayr, H.; Varga, E.; Malachová, A.; Fruhmann, P.; Piątkowska, M.; Hametner, C.; Šofrová, J.; Jaunecker, G.; Häubl, G.; Lemmens, M.; et al. UDP-gulcosyltransferases from rice, Brachypodium, and barley: Substrate specificities and synthesis of type A and B trichothecene-3-O-β-D-glucosides. Toxins 2018, 10, 111. [Google Scholar] [CrossRef]
- Alizadeh, A.; Braber, S.; Akbari, P.; Kraneveld, A.; Garssen, J.; Fink-Gremmels, J. Deoxynivalenol and its modified forms: Are there major differences? Toxins 2016, 8, 334. [Google Scholar] [CrossRef]
- Dall’Erta, A.; Cirlini, M.; Dall’Asta, M.; Del Rio, D.; Galaverna, G.; Dall’Asta, C. Masked mycotoxins are efficiently hydrolyzed by human colonic microbiota releasing their aglycones. Chem. Res. Toxicol. 2013, 26, 305–312. [Google Scholar] [CrossRef]
- Gratz, S.W. Do plant-bound masked mycotoxins contribute to toxicity? Toxins 2017, 9, 85. [Google Scholar] [CrossRef]
- Bryła, M.; Waśkiewicz, A.; Ksieniewicz-Woźniak, E.; Szymczyk, K.; Jędrzejczak, R. Modified Fusarium mycotoxins in cereals and their products—Metabolism, occurrence, and toxicity: An updated review. Molecules 2018, 23, 963. [Google Scholar] [CrossRef] [PubMed]
- Proctor, R.H.; McCormick, S.P.; Kim, H.S.; Cardoza, R.E.; Stanley, A.M.; Lindo, L.; Kelly, A.; Brown, D.W.; Lee, T.; Vaughan, M.M.; et al. Evolution of structural diversity of trichothecenes, a family of toxins produced by plant pathogenic and entomopathogenic fungi. PLoS Pathog. 2018, 14, e1006946. [Google Scholar] [CrossRef]
- Matsui, K.; Shinkai, K.; Adachi, K.; Takahashi-Ando, N. Possibility of novel hexose conjugation of a d-type trichothecene by trichodiene synthase gene-disrupted mutants of Fusarium species. JSM Mycotoxins 2018, 68, 27–29. [Google Scholar] [CrossRef][Green Version]
- Maeda, K.; Tanaka, A.; Sugiura, R.; Koshino, H.; Tokai, T.; Sato, M.; Nakajima, Y.; Tanahashi, Y.; Kanamaru, K.; Kobayashi, T.; et al. Hydroxylations of trichothecene rings in the biosynthesis of Fusarium trichothecenes: Evolution of alternative pathways in the nivalenol chemotype. Environ. Microbiol. 2016, 18, 3798–3811. [Google Scholar] [CrossRef] [PubMed]
- Kamata, K.; Sato, H.; Maeda, K.; Furihata, K.; Aikawa, S.; Adachi, K.; Tanaka, A.; Tokai, T.; Nakajima, Y.; Yoshida, Y.; et al. Exploring an artificial metabolic route in Fusarium sporotrichioides: Production and characterization of 7-hydroxy T-2 toxin. J. Nat. Prod. 2018, 81, 1041–1044. [Google Scholar] [CrossRef]
- Maeda, K.; Tanaka, Y.; Matsuyama, M.; Sato, M.; Sadamatsu, K.; Suzuki, T.; Matsui, K.; Nakajima, Y.; Tokai, T.; Kanamaru, K.; et al. Substrate specificities of Fusarium biosynthetic enzymes explain the genetic basis of a mixed chemotype producing both deoxynivalenol and nivalenol-type trichothecenes. Int. J. Food Microbiol. 2020, 320, 108532. [Google Scholar] [CrossRef] [PubMed]
- Gorst-Allman, C.P.; Steyn, P.S.; Vleggaar, R.; Rabie, C.J. Structure elucidation of a novel trichothecene glycoside using 1H and 13C nuclear magnetic resonance spectroscopy. J. Chem. Soc. Perkin Trans. 1 1985, 1553–1555. [Google Scholar] [CrossRef]
- Steyn, P.S.; Vleggaar, R.; Rabie, C.J.; Kriek, N.P.J.; Harington, J.S. Trichothecene mycotoxins from Fusarium sulphureum. Phytochemistry 1978, 17, 949–951. [Google Scholar] [CrossRef]
- Lee, T.; Han, Y.K.; Kim, K.H.; Yun, S.H.; Lee, Y.W. Tri13 and Tri7 determine deoxynivalenol- and nivalenol-producing chemotypes of Gibberella zeae. Appl. Environ. Microbiol. 2002, 68, 2148–2154. [Google Scholar] [CrossRef]
- Yoshinari, T.; Sakuda, S.; Furihata, K.; Furusawa, H.; Ohnishi, T.; Sugita-Konishi, Y.; Ishizaki, N.; Terajima, J. Structural determination of a nivalenol glucoside and development of an analytical method for the simultaneous determination of nivalenol and deoxynivalenol, and their glucosides, in wheat. J. Agric. Food Chem. 2014, 62, 1174–1180. [Google Scholar] [CrossRef]
- Malachová, A.; Štočková, L.; Wakker, A.; Varga, E.; Krska, R.; Michlmayr, H.; Adam, G.; Berthiller, F. Critical evaluation of indirect methods for the determination of deoxynivalenol and its conjugated forms in cereals. Anal. Bioanal. Chem. 2015, 407, 6009–6020. [Google Scholar] [CrossRef]
- Michlmayr, H.; Varga, E.; Malachova, A.; Nguyen, N.T.; Lorenz, C.; Haltrich, D.; Berthiller, F.; Adam, G.; Cullen, D. A versatile family 3 glycoside hydrolase from Bifidobacterium adolescentis hydrolyzes β-glucosides of the Fusarium mycotoxins deoxynivalenol, nivalenol, and HT-2 toxin in cereal matrices. Appl. Environ. Microbiol. 2015, 81, 4885–4893. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, D.; Cheng, Z.; Proksch, P.; Lin, W. Cytotoxic trichothecene-type sesquiterpenes from the sponge-derived fungus Stachybotrys chartarum with tyrosine kinase inhibition. RSC Adv. 2017, 7, 7259–7267. [Google Scholar] [CrossRef]
- Pierron, A.; Mimoun, S.; Murate, L.S.; Loiseau, N.; Lippi, Y.; Bracarense, A.P.; Liaubet, L.; Schatzmayr, G.; Berthiller, F.; Moll, W.D.; et al. Intestinal toxicity of the masked mycotoxin deoxynivalenol-3-β-D-glucoside. Arch. Toxicol. 2016, 90, 2037–2046. [Google Scholar] [CrossRef]
- Takahashi-Ando, N.; Tanaka, A.; Sekimoto, Y.; Yamauchi, K.; Echigo, A.; Usami, R.; Abe, F.; Minegishi, H. Functional screening for resistance genes against trichothecenes in the library of Saccharomyces cerevisiae deletion mutants. JSM Mycotoxins 2013, 63, 9–15. [Google Scholar] [CrossRef][Green Version]
- Gessner, T.; Jacknowitz, A.; Vollmer, C.A. Studies of mammalian glucoside conjugation. Biochem. J. 1973, 132, 249–258. [Google Scholar] [CrossRef]
- Shipkova, M.; Strassburg, C.P.; Braun, F.; Streit, F.; Gröne, H.J.; Armstrong, V.W.; Tukey, R.H.; Oellerich, M.; Wieland, E. Glucuronide and glucoside conjugation of mycophenolic acid by human liver, kidney and intestinal microsomes. Br. J. Pharmacol. 2001, 132, 1027–1034. [Google Scholar] [CrossRef]
- Cerniglia, C.E. Fungal metabolism of polycyclic aromatic hydrocarbons: Past, present and future applications in bioremediation. J. Ind. Microbiol. Biotechnol. 1997, 19, 324–333. [Google Scholar] [CrossRef]
- Kamimura, H. Conversion of zearalenone to zearalenone glycoside by Rhizopus sp. Appl. Environ. Microbiol. 1986, 52, 515–519. [Google Scholar] [CrossRef]
- Sutherland, J.B.; Selby, A.L.; Freeman, J.P.; Evans, F.E.; Cerniglia, C.E. Metabolism of phenanthrene by Phanerochaete chrysosporium. Appl. Environ. Microbiol. 1991, 57, 3310–3316. [Google Scholar] [CrossRef]
- Bezalel, L.; Hadar, Y.; Cerniglia, C.E. Enzymatic mechanisms involved in phenanthrene degradation by the white rot fungus Pleurotus ostreatus. Appl. Environ. Microbiol. 1997, 63, 2495–2501. [Google Scholar] [CrossRef] [PubMed]
- Hammer, E.; Schoefer, L.; Schäfer, A.; Hundt, K.; Schauer, F. Formation of glucoside conjugates during biotransformation of dibenzofuran by Penicillium canescens SBUG-M 1139. Appl. Microbiol. Biotechnol. 2001, 57, 390–394. [Google Scholar] [PubMed]
- Huang, H.H.; Ma, G.L.; Sun, Y.M.; Li, Q.; Zhong, D.F. Phase II metabolites of etofesalamide in filamentous fungi. Acta Pharmacol. Sin. 2005, 26, 893–896. [Google Scholar] [CrossRef]
- Campoy, S.; Álvarez-Rodríguez, M.L.; Recio, E.; Rumbero, A.; Coque, J.J.R. Biodegradation of 2,4,6-TCA by the white-rot fungus Phlebia radiata is initiated by a phase I (O-demethylation)–phase II (O-conjugation) reactions system: Implications for the chlorine cycle. Environ. Microbiol. 2009, 11, 99–110. [Google Scholar] [CrossRef]
- Carvalho, M.B.; Tavares, S.; Medeiros, J.; Núñez, O.; Gallart-Ayala, H.; Leitão, M.C.; Galceran, M.T.; Hursthouse, A.; Pereira, C.S. Degradation pathway of pentachlorophenol by Mucor plumbeus involves phase II conjugation and oxidation–reduction reactions. J. Hazard. Mater. 2011, 198, 133–142. [Google Scholar] [CrossRef]
- Lemmens, M.; Scholz, U.; Berthiller, F.; Dall’Asta, C.; Koutnik, A.; Schuhmacher, R.; Adam, G.; Buerstmayr, H.; Mesterházy, A.; Krska, R.; et al. The ability to detoxify the mycotoxin deoxynivalenol colocalizes with a major quantitative trait locus for Fusarium head blight resistance in wheat. Mol. Plant-Microbe Interact. 2005, 18, 1318–13124. [Google Scholar] [CrossRef]
- Schmidt, H.S.; Schulz, M.; Focke, C.; Becker, S.; Cramer, B.; Humpf, H.U. Glucosylation of T-2 and HT-2 toxins using biotransformation and chemical synthesis: Preparation, stereochemistry, and stability. Mycotoxin Res. 2018, 34, 159–172. [Google Scholar] [CrossRef]
- Tanaka, A.; Saikawa, S.; Suzuki, T.; Echigo, A.; Maeda, K.; Sato, M.; Fujimura, M.; Tokai, T.; Usami, R.; Yoshida, Y.; et al. Acetyltransferase activity in Pseudomonas sp. capable of acetylating the C-4 hydroxyl group of nivalenol-type trichothecenes. J. Gen. Appl. Microbiol. 2016, 62, 326–329. [Google Scholar] [CrossRef]
- Nakajima, Y.; Koseki, N.; Sugiura, R.; Tominaga, N.; Maeda, K.; Tokai, T.; Izawa, M.; Kanamaru, K.; Kamakura, T.; Kobayashi, T.; et al. Effect of disrupting the trichothecene efflux pump encoded by FgTri12 in the nivalenol chemotype of Fusarium graminearum. J. Gen. Appl. Microbiol. 2015, 61, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Sakai, K.; Ueno, Y.; Tsunoda, H.; Enomoto, M. Solaniol, a toxic metabolite of Fusarium solani. Appl. Microbiol. 1971, 22, 718–720. [Google Scholar] [CrossRef]
- Tanaka, K.; Plattner, R.D.; Yamagishi, R.; Minamisawa, M.; Manabe, M.; Kawasugi, S.; Gareis, M.; Okada, G. 8-Deoxy-trichothecin production by Spicellum roseum isolated from a cultivated mushroom in Japan. Mycotoxins 2001, 51, 71–77. [Google Scholar] [CrossRef]
- Nakajima, Y.; Kawamura, T.; Maeda, K.; Ichikawa, H.; Motoyama, T.; Kondoh, Y.; Saito, T.; Kobayashi, T.; Yoshida, M.; Osada, H.; et al. Identification and characterization of an inhibitor of trichothecene 3-O-acetyltransferase, TRI101, by the chemical array approach. Biosci. Biotechnol. Biochem. 2013, 77, 1958–1960. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, N.; Tokai, T.; Takahashi-Ando, N.; Fujimura, M.; Kimura, M. Genetically engineered Fusarium as a tool to evaluate the effects of environmental factors on initiation of trichothecene biosynthesis. FEMS Microbiol. Lett. 2007, 275, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Takahashi-Ando, N.; Tokai, T.; Yoshida, M.; Fujimura, M.; Kimura, M. An easy method to identify 8-keto-15-hydroxytrichothecenes by thin-layer chromatography. Mycotoxins 2008, 58, 115–117. [Google Scholar] [CrossRef]
- Takitani, S.; Asabe, Y.; Kato, T.; Suzuki, M.; Ueno, Y. Spectrodensitometric determination of trichothecene mycotoxins with 4-(p-nitrobenzyl)pyridine on silica gel thin-layer chromatograms. J. Chromatogr. 1979, 172, 335–342. [Google Scholar] [CrossRef]
- Kamata, K.; Tanaka, A.; Maeda, K.; Takushima, R.; Sato, H.; Aikawa, S.; Yoshida, Y.; Kimura, M.; Takahashi-Ando, N. Evaluation of toxicities of 7-hydroxyisotrichodermin and 8-hydroxyisotrichodermin, shunt intermediates in the biosynthetic grid of deoxynivalenol, by using a sensitive yeast assay. JSM Mycotoxins 2015, 65, 7–9. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsui, K.; Takeda, H.; Shinkai, K.; Kakinuma, T.; Koizumi, Y.; Kase, M.; Yoshinari, T.; Minegishi, H.; Nakajima, Y.; Aikawa, S.; et al. 4-O-Glucosylation of Trichothecenes by Fusarium Species: A Phase II Xenobiotic Metabolism for t-Type Trichothecene Producers. Int. J. Mol. Sci. 2021, 22, 13542. https://doi.org/10.3390/ijms222413542
Matsui K, Takeda H, Shinkai K, Kakinuma T, Koizumi Y, Kase M, Yoshinari T, Minegishi H, Nakajima Y, Aikawa S, et al. 4-O-Glucosylation of Trichothecenes by Fusarium Species: A Phase II Xenobiotic Metabolism for t-Type Trichothecene Producers. International Journal of Molecular Sciences. 2021; 22(24):13542. https://doi.org/10.3390/ijms222413542
Chicago/Turabian StyleMatsui, Kosuke, Hirone Takeda, Koki Shinkai, Takao Kakinuma, Yoshiaki Koizumi, Masahiro Kase, Tomoya Yoshinari, Hiroaki Minegishi, Yuichi Nakajima, Shunichi Aikawa, and et al. 2021. "4-O-Glucosylation of Trichothecenes by Fusarium Species: A Phase II Xenobiotic Metabolism for t-Type Trichothecene Producers" International Journal of Molecular Sciences 22, no. 24: 13542. https://doi.org/10.3390/ijms222413542
APA StyleMatsui, K., Takeda, H., Shinkai, K., Kakinuma, T., Koizumi, Y., Kase, M., Yoshinari, T., Minegishi, H., Nakajima, Y., Aikawa, S., Takahashi-Ando, N., & Kimura, M. (2021). 4-O-Glucosylation of Trichothecenes by Fusarium Species: A Phase II Xenobiotic Metabolism for t-Type Trichothecene Producers. International Journal of Molecular Sciences, 22(24), 13542. https://doi.org/10.3390/ijms222413542