Infection by High-Risk Human Papillomaviruses, Epithelial-to-Mesenchymal Transition and Squamous Pre-Malignant or Malignant Lesions of the Uterine Cervix: A Series of Chained Events?
Abstract
1. Introduction
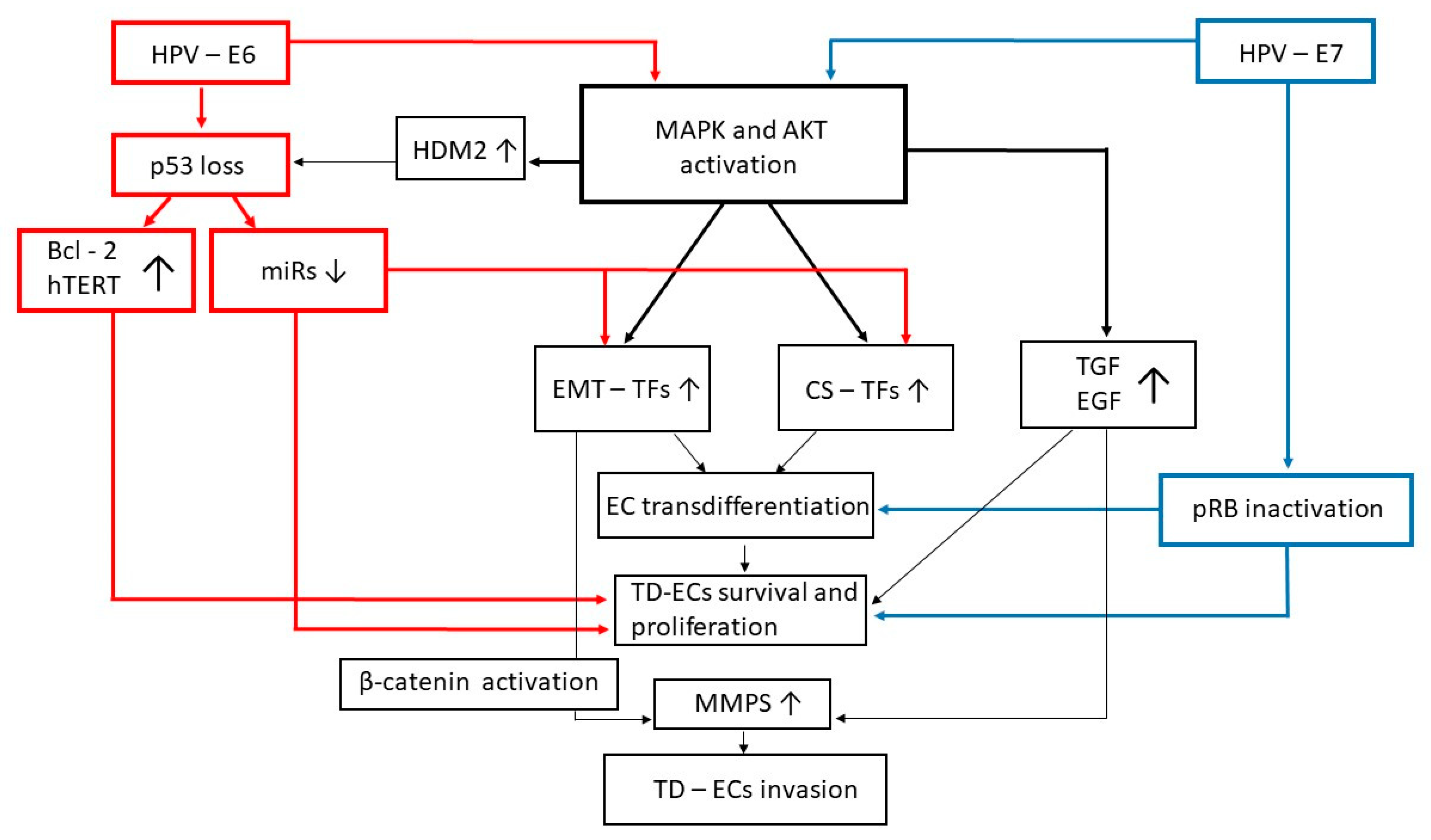
2. The E5, E6, and E7 Proteins of HR-HPVs Trigger EMT in Cervical Epithelial Cells
3. Inflammation Cooperates with the E6 and E7 Proteins of HR-HPVs at Promoting EMT in Normal or Neoplastic Uterine Cervical Epithelial Cells
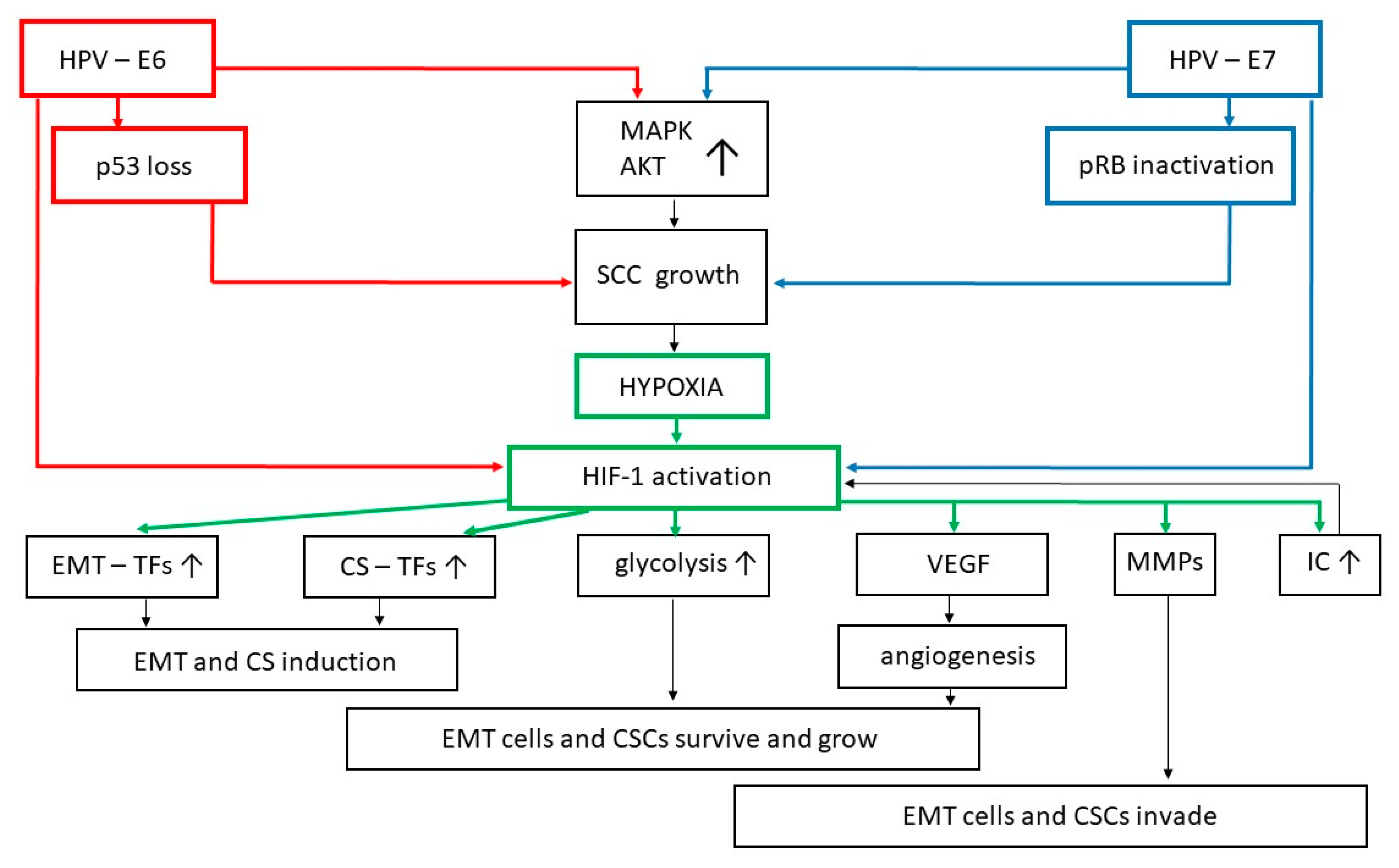
4. Cyclic Hypoxia Exacerbates EMT and Favors the Appearance of Stem-Like Cells in Cervical Squamous Lesions
5. EMT and Cellular Stemness Not Only Facilitate SCC Cells Invasion and Spreading, but also Increase SCC Cells Resistance to Anticancer Chemo- or Radiotherapy
6. Concluding Remarks and Future Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AKT | protein kinase B |
| CSC | cancer stem cell |
| E-cadherin | epithelial-cadherin |
| EGF | epidermal growth factor |
| EGFR | epidermal growth factor receptor |
| EMT | epithelial-to-mesenchymal transition |
| ERK | extracellular-regulated kinase |
| FGFR | fibroblast growth factor receptor |
| HIF | hypoxia-inducible factor |
| HLA | human leukocyte antigen |
| H/MDM2 | human/murine double minute 2 |
| HR-HPV | high-risk human papillomavirus |
| H-SIL | high-grade squamous intraepithelial lesion |
| IFN | interferon |
| IL | interleukin |
| L-SIL | low-grade squamous intraepithelial lesion |
| MAPK | mitogen-activated protein kinase |
| MET | mesenchymal-to-epithelial transition |
| MHC | major histocompatibility complex |
| miR | microRNA |
| MMP | matrix metalloproteinase |
| mTOR | mammalian target of rapamycin |
| N-cadherin | neuronal-cadherin |
| NF-kB | nuclear factor-kappa B |
| PI3K | phosphoinositide-3-kinase |
| pRb | retinoblastoma protein |
| SCC | squamous cell carcinoma |
| SIL | squamous intraepithelial lesion |
| TGF | transforming growth factor |
| TNF | tumor necrosis factor |
| VEGF | vascular endothelial growth factor |
| Wnt | wingless-type mouse mammary tumor virus integration site |
| Zeb | zinc finger E-box-binding homeobox |
References
- Haensel, D.; Dai, X. Epithelial-to-mesenchymal transition in cutaneous wound healing: Where we are and where we are heading. Dev. Dyn. 2018, 247, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.H.; Huang, B.S.; Horng, H.C.; Yeh, C.C.; Chen, Y.J. Wound healing. J. Chin. Med. Assoc. 2018, 81, 94–101. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Sha, Y.; Haensel, D.; Gutierrez, G.; Du, H.; Dai, X.; Nie, Q. Intermediate cell states in epithelial-to-mesenchymal transition. Phys. Biol. 2019, 16, 021001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Weinberg, R.A. Epithelial-to-mesenchymal transition in cancer: Complexity and opportunities. Front. Med. 2018, 12, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Liarte, S.; Bernabé-García, Á.; Nicolás, F.J. Human Skin Keratinocytes on Sustained TGF-β Stimulation Reveal Partial EMT Features and Weaken Growth Arrest Responses. Cells 2020, 9, 255. [Google Scholar] [CrossRef] [PubMed]
- Elsum, I.A.; Martin, C.; Humbert, P.O. Scribble regulates an EMT polarity pathway through modulation of MAPK-ERK signaling to mediate junction formation. J. Cell Sci. 2013, 126, 3990–3999. [Google Scholar] [CrossRef] [PubMed]
- Tanahashi, T.; Osada, S.; Yamada, A.; Kato, J.; Yawata, K.; Mori, R.; Imai, H.; Sasaki, Y.; Saito, S.; Tanaka, Y.; et al. Extracellular signal-regulated kinase and Akt activation play a critical role in the process of hepatocyte growth factor-induced epithelial-mesenchymal transition. Int. J. Oncol. 2013, 42, 556–564. [Google Scholar] [CrossRef]
- Carpenter, R.L.; Paw, I.; Dewhirst, M.W.; Lo, H.W. Akt phosphorylates and activates HSF-1 independent of heat shock, leading to Slug overexpression and epithelial-mesenchymal transition (EMT) of HER2-overexpressing breast cancer cells. Oncogene 2015, 34, 546–557. [Google Scholar] [CrossRef]
- Tang, H.; Massi, D.; Hemmings, B.A.; Mandalà, M.; Hu, Z.; Wicki, A.; Xue, G. AKT-ions with a TWIST between EMT and MET. Oncotarget 2016, 7, 62767–62777. [Google Scholar] [CrossRef]
- Liu, J.Y.; Jiang, L.; He, T.; Liu, J.J.; Fan, J.Y.; Xu, X.H.; Tang, B.; Shi, Y.; Zhao, Y.L.; Qian, F.; et al. NETO2 promotes invasion and metastasis of gastric cancer cells via activation of PI3K/Akt/NF-κB/Snail axis and predicts outcome of the patients. Cell Death Dis. 2019, 10, 162. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, T.; Bozanovic, T.; Hooper, S.; Ljubic, A.; Slettenaar, V.I.; Wilson, J.L.; Singh, N.; Gayther, S.A.; Shepherd, J.H.; van Trappen, P.O. Molecular profiling of cervical cancer progression. Br. J. Cancer 2007, 96, 321–328. [Google Scholar] [CrossRef]
- Hatta, M.; Miyake, Y.; Uchida, K.; Yamazaki, J. Keratin 13 gene is epigenetically suppressed during transforming growth factor-β1-induced epithelial-mesenchymal transition in a human keratinocyte cell line. Biochem. Biophys. Res. Commun. 2018, 496, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Markopoulos, G.S.; Roupakia, E.; Marcu, K.B.; Kolettas, E. Epigenetic Regulation of Inflammatory Cytokine-Induced Epithelial-To-Mesenchymal Cell Transition and Cancer Stem Cell Generation. Cells 2019, 8, 1143. [Google Scholar] [CrossRef]
- Lin, Y.T.; Wu, K.J. Epigenetic regulation of epithelial-mesenchymal transition: Focusing on hypoxia and TGF-β signaling. J. Biomed. Sci. 2020, 27, 39. [Google Scholar] [CrossRef] [PubMed]
- Georgakopoulos-Soares, I.; Chartoumpekis, D.V.; Kyriazopoulou, V.; Zaravinos, A. EMT Factors and Metabolic Pathways in Cancer. Front. Oncol. 2020, 10, 499. [Google Scholar] [CrossRef]
- Zhang, W.N.; Li, W.; Wang, X.L.; Hu, Z.; Zhu, D.; Ding, W.C.; Liu, D.; Li, K.Z.; Ma, D.; Wang, H. CLDN1 expression in cervical cancer cells is related to tumor invasion and metastasis. Oncotarget 2016, 7, 87449–87461. [Google Scholar] [CrossRef]
- Lv, J.; Sun, B.; Mai, Z.; Jiang, M.; Du, J. CLDN-1 promoted the epithelial to migration and mesenchymal transition (EMT) in human bronchial epithelial cells via Notch pathway. Mol. Cell. Biochem. 2017, 432, 91–98. [Google Scholar] [CrossRef]
- Nishimoto, Y.; Murakami, A.; Sato, S.; Kajimura, T.; Nakashima, K.; Yakabe, K.; Sueoka, K.; Sugino, N. Decreased carbonyl reductase 1 expression promotes tumor growth via epithelial mesenchymal transition in uterine cervical squamous cell carcinomas. Reprod. Med. Biol. 2018, 17, 173–181. [Google Scholar] [CrossRef]
- Kang, S.U.; Choi, J.W.; Chang, J.W.; Kim, K.I.; Kim, Y.S.; Park, J.K.; Kim, Y.E.; Lee, Y.S.; Yang, S.S.; Kim, C.H. N2 non-thermal atmospheric pressure plasma promotes wound healing in vitro and in vivo: Potential modulation of adhesion molecules and matrix metalloproteinase-9. Exp. Dermatol. 2017, 26, 163–170. [Google Scholar] [CrossRef]
- Banyard, J.; Bielenberg, D.R. The role of EMT and MET in cancer dissemination. Connect. Tissue Res. 2015, 56, 403–413. [Google Scholar] [CrossRef]
- Bracken, C.P.; Gregory, P.A.; Kolesnikoff, N.; Bert, A.G.; Wang, J.; Shannon, M.F.; Goodall, G.J. A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Res. 2008, 68, 7846–7854. [Google Scholar] [CrossRef]
- Burk, U.; Schubert, J.; Wellner, U.; Schmalhofer, O.; Vincan, E.; Spaderna, S.; Brabletz, T. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008, 9, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Siemens, H.; Jackstadt, R.; Hünten, S.; Kaller, M.; Menssen, A.; Götz, U.; Hermeking, H. miR-34 and SNAIL form a double-negative feedback loop to regulate epithelial-mesenchymal transitions. Cell Cycle 2011, 10, 4256–4271. [Google Scholar] [CrossRef]
- Chakrabarti, R.; Hwang, J.; Andres Blanco, M.; Wei, Y.; Lukačišin, M.; Romano, R.A.; Smalley, K.; Liu, S.; Yang, Q.; Ibrahim, T.; et al. Elf5 inhibits the epithelial-mesenchymal transition in mammary gland development and breast cancer metastasis by transcriptionally repressing Snail2. Nat. Cell Biol. 2012, 14, 1212–1222. [Google Scholar] [CrossRef] [PubMed]
- Cieply, B.; Farris, J.; Denvir, J.; Ford, H.L.; Frisch, S.M. Epithelial-mesenchymal transition and tumor suppression are controlled by a reciprocal feedback loop between ZEB1 and Grainyhead-like-2. Cancer Res. 2013, 73, 6299–6309. [Google Scholar] [CrossRef] [PubMed]
- Roca, H.; Hernandez, J.; Weidner, S.; McEachin, R.C.; Fuller, D.; Sud, S.; Schumann, T.; Wilkinson, J.E.; Zaslavsky, A.; Li, H.; et al. Transcription factors OVOL1 and OVOL2 induce the mesenchymal to epithelial transition in human cancer. PLoS ONE 2013, 8, e76773. [Google Scholar] [CrossRef] [PubMed]
- Jolly, M.K.; Jia, D.; Boareto, M.; Mani, S.A.; Pienta, K.J.; Ben-Jacob, E.; Levine, H. Coupling the modules of EMT and stemness: A tunable ‘stemness window’ model. Oncotarget 2015, 6, 25161–25174. [Google Scholar] [CrossRef]
- Jolly, M.K.; Tripathi, S.C.; Jia, D.; Mooney, S.M.; Celiktas, M.; Hanash, S.M.; Mani, S.A.; Pienta, K.J.; Ben-Jacob, E.; Levine, H. Stability of the hybrid epithelial/mesenchymal phenotype. Oncotarget 2016, 7, 27067–27084. [Google Scholar] [CrossRef]
- Chung, V.Y.; Tan, T.Z.; Tan, M.; Wong, M.K.; Kuay, K.T.; Yang, Z.; Ye, J.; Muller, J.; Koh, C.M.; Guccione, E.; et al. GRHL2-miR-200-ZEB1 maintains the epithelial status of ovarian cancer through transcriptional regulation and histone modification. Sci. Rep. 2016, 6, 19943. [Google Scholar] [CrossRef]
- Mooney, S.M.; Talebian, V.; Jolly, M.K.; Jia, D.; Gromala, M.; Levine, H.; McConkey, B.J. The GRHL2/ZEB Feedback Loop-A Key Axis in the Regulation of EMT in Breast Cancer. J. Cell Biochem. 2017, 118, 2559–2570. [Google Scholar] [CrossRef]
- Liu, D.; Skomorovska, Y.; Song, J.; Bowler, E.; Harris, R.; Ravasz, M.; Bai, S.; Ayati, M.; Tamai, K.; Koyuturk, M.; et al. ELF3 is an antagonist of oncogenic-signalling-induced expression of EMT-TF ZEB1. Cancer Biol. Ther. 2019, 20, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Morel, A.P.; Hinkal, G.W.; Thomas, C.; Fauvet, F.; Courtois-Cox, S.; Wierinckx, A.; Devouassoux-Shisheboran, M.; Treilleux, I.; Tissier, A.; Gras, B.; et al. EMT inducers catalyze malignant transformation of mammary epithelial cells and drive tumorigenesis towards claudin-low tumors in transgenic mice. PLoS Genet. 2012, 8, e1002723. [Google Scholar] [CrossRef] [PubMed]
- Parfenyev, S.; Singh, A.; Fedorova, O.; Daks, A.; Kulshreshtha, R.; Barlev, N.A. Interplay between p53 and non-coding RNAs in the regulation of EMT in breast cancer. Cell Death Dis. 2021, 12, 17. [Google Scholar] [CrossRef]
- Ma, L.; Young, J.; Prabhala, H.; Pan, E.; Mestdagh, P.; Muth, D.; Teruya-Feldstein, J.; Reinhardt, F.; Onder, T.T.; Valastyan, S.; et al. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat. Cell Biol. 2010, 12, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.T.; Yang, M.H. Revisiting epithelial-mesenchymal transition in cancer metastasis: The connection between epithelial plasticity and stemness. Mol. Oncol. 2017, 11, 792–804. [Google Scholar] [CrossRef]
- Yokoyama, K.; Kamata, N.; Fujimoto, R.; Tsutsumi, S.; Tomonari, M.; Taki, M.; Hosokawa, H.; Nagayama, M. Increased invasion and matrix metalloproteinase-2 expression by Snail-induced mesenchymal transition in squamous cell carcinomas. Int. J. Oncol. 2003, 22, 891–898. [Google Scholar] [CrossRef]
- Jordà, M.; Olmeda, D.; Vinyals, A.; Valero, E.; Cubillo, E.; Llorens, A.; Cano, A.; Fabra, A. Upregulation of MMP-9 in MDCK epithelial cell line in response to expression of the Snail transcription factor. J. Cell Sci. 2005, 118, 3371–3385. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, T.; Che, N.; Sun, D.; Zhao, N.; Dong, X.; Gu, Q.; Yao, Z.; Sun, B. Promotion of hepatocellular carcinoma metastasis through matrix metalloproteinase activation by epithelial-mesenchymal transition regulator Twist1. Cell. Mol. Med. 2011, 15, 691–700. [Google Scholar] [CrossRef]
- Zhao, M.; Hu, H.G.; Huang, J.; Zou, Q.; Wang, J.; Liu, M.Q.; Zhao, Y.; Li, G.Z.; Xue, S.; Wu, Z.S. Expression and correlation of Twist and gelatinases in breast cancer. Exp. Ther. Med. 2013, 6, 97–100. [Google Scholar] [CrossRef]
- García-Cuellar, C.M.; Santibáñez-Andrade, M.; Chirino, Y.I.; Quintana-Belmares, R.; Morales-Bárcenas, R.; Quezada-Maldonado, E.M.; Sánchez-Pérez, Y. Particulate Matter (PM10) Promotes Cell Invasion through Epithelial-Mesenchymal Transition (EMT) by TGF-β Activation in A549 Lung Cells. Int. J. Mol. Sci. 2021, 22, 12632. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; Fan, C.C.; Chen, Y.A.; Cheng, C.W.; Sung, Y.J.; Hsu, C.P.; Kao, T.Y. Curcumin Inhibits Invasiveness and Epithelial-Mesenchymal Transition in Oral Squamous Cell Carcinoma Through Reducing Matrix Metalloproteinase 2, 9 and Modulating p53-E-Cadherin Pathway. Integr. Cancer Ther. 2015, 14, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zha, Z.; Zhang, P.; Li, D.; Liu, G. NSE, positively regulated by LINC00657-miR-93-5p axis, promotes small cell lung cancer (SCLC) invasion and epithelial-mesenchymal transition (EMT) process. Int. J. Med. Sci. 2021, 18, 3768–3779. [Google Scholar] [CrossRef]
- Wu, W.S.; You, R.I.; Cheng, C.C.; Lee, M.C.; Lin, T.Y.; Hu, C.T. Snail collaborates with EGR-1 and SP-1 to directly activate transcription of MMP 9 and ZEB1. Sci. Rep. 2018, 8, 14226. [Google Scholar] [CrossRef] [PubMed]
- Hseu, Y.C.; Lin, Y.C.; Rajendran, P.; Thigarajan, V.; Mathew, D.C.; Lin, K.Y.; Way, T.D.; Liao, J.W.; Yang, H.L. Antrodia salmonea suppresses invasion and metastasis in triple-negative breast cancer cells by reversing EMT through the NF-κB and Wnt/β-catenin signaling pathway. Food Chem. Toxicol. 2019, 124, 219–230. [Google Scholar] [CrossRef]
- Yu, L.; Lu, S.; Tian, J.; Ma, J.; Li, J.; Wang, H.; Xu, W. TWIST expression in hypopharyngeal cancer and the mechanism of TWIST-induced promotion of metastasis. Oncol. Rep. 2012, 27, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Li, X.; Hua, Z.; Ma, J.; Wu, X.; Liu, Z.; Chen, H.; Cui, Z. S100A7 promotes the migration, invasion and metastasis of human cervical cancer cells through epithelial-mesenchymal transition. Oncotarget 2017, 8, 24964–24977. [Google Scholar] [CrossRef]
- Zheng, H.; Fu, Y.; Yang, T. Propofol inhibits proliferation, migration, and invasion of hepatocellular carcinoma cells by downregulating Twist. J. Cell Biochem. 2019, 120, 12803–12809. [Google Scholar] [CrossRef]
- Sonongbua, J.; Siritungyong, S.; Thongchot, S.; Kamolhan, T.; Utispan, K.; Thuwajit, P.; Pongpaibul, A.; Wongkham, S.; Thuwajit, C. Periostin induces epithelial to mesenchymal transition via the integrin α5β1/TWIST 2 axis in cholangiocarcinoma. Oncol. Rep. 2020, 43, 1147–1158. [Google Scholar] [CrossRef]
- Yao, Y.; Bellon, M.; Shelton, S.N.; Nicot, C. Tumor suppressors p53, p63TAα, p63TAy, p73α, and p73β use distinct pathways to repress telomerase expression. J. Biol. Chem. 2012, 287, 20737–20747. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, K.N. HPV-p53-miR-34a axis in HPV-associated cancers. Ann. Transl. Med. 2015, 3, 331–337. [Google Scholar] [CrossRef]
- Popper, H. Primary tumor and metastasis-sectioning the different steps of the metastatic cascade. Transl. Lung Cancer Res. 2020, 9, 2277–2300. [Google Scholar] [CrossRef]
- Lin, C.C.; Cheng, T.L.; Tsai, W.H.; Tsai, H.J.; Hu, K.H.; Chang, H.C.; Yeh, C.W.; Chen, Y.C.; Liao, C.C.; Chang, W.T. Loss of the respiratory enzyme citrate synthase directly links the Warburg effect to tumor malignancy. Sci. Rep. 2012, 2, 785. [Google Scholar] [CrossRef] [PubMed]
- Courtnay, R.; Ngo, D.C.; Malik, N.; Ververis, K.; Tortorella, S.M.; Karagiannis, T.C. Cancer metabolism and the Warburg effect: The role of HIF-1 and PI3K. Mol. Biol. Rep. 2015, 42, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.; Kulkarni, S. Butein induces intrinsic pathway of apoptosis, vimentin proteolysis, and inhibition of cancer stem cell population in a human papillary thyroid cancer cell line. Toxicol. In Vitro 2021, 77, 105244. [Google Scholar] [CrossRef]
- Huang, R.; Zong, X. Aberrant cancer metabolism in epithelial-mesenchymal transition and cancer metastasis: Mechanisms in cancer progression. Crit. Rev. Oncol. Hematol. 2017, 115, 13–22. [Google Scholar] [CrossRef]
- Li, H.; Zhang, W.; Yan, M.; Qiu, J.; Chen, J.; Sun, X.; Chen, X.; Song, L.; Zhang, Y. Nucleolar and spindle associated protein 1 promotes metastasis of cervical carcinoma cells by activating Wnt/β-catenin signaling. J. Exp. Clin. Cancer Res. 2019, 38, 33. [Google Scholar] [CrossRef]
- Stumbar, S.E.; Stevens, M.; Feld, Z. Cervical Cancer and Its Precursors: A Preventative Approach to Screening, Diagnosis, and Management. Prim. Care 2019, 46, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Fidan, U.; Keskin, U.; Ulubay, M.; Öztürk, M.; Bodur, S. Value of vaginal cervical position in estimating uterine anatomy. Clin. Anat. 2017, 30, 404–408. [Google Scholar] [CrossRef]
- Reich, O.; Fritsch, H. The developmental origin of cervical and vaginal epithelium and their clinical consequences: A systematic review. J. Low. Genit. Tract Dis. 2014, 18, 358–360. [Google Scholar] [CrossRef]
- Yang, E.J.; Quick, M.C.; Hanamornroongruang, S.; Lai, K.; Doyle, L.A.; McKeon, F.D.; Xian, W.; Crum, C.P.; Herfs, M. Microanatomy of the cervical and anorectal squamocolumnar junctions: A proposed model for anatomical differences in HPV-related cancer risk. Mod. Pathol. 2015, 28, 994–1000. [Google Scholar] [CrossRef]
- Doorbar, J.; Griffin, H. Refining our understanding of cervical neoplasia and its cellular origins. Papillomavirus Res. 2019, 7, 176–179. [Google Scholar] [CrossRef]
- Bodily, J.; Laimins, L.A. Persistence of human papillomavirus infection: Keys to malignant progression. Trends Microbiol. 2011, 19, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Latsuzbaia, A.; Wienecke-Baldacchino, A.; Tapp, J.; Arbyn, M.; Karabegović, I.; Chen, Z.; Fischer, M.; Mühlschlegel, F.; Weyers, S.; Pesch, P.; et al. Characterization and Diversity of 243 Complete Human Papillomavirus Genomes in Cervical Swabs Using Next Generation Sequencing. Viruses 2020, 12, 1437. [Google Scholar] [CrossRef] [PubMed]
- Vande Pol, S.B.; Klingelhutz, A.J. Papillomavirus E6 oncoproteins. Virology 2013, 445, 115–137. [Google Scholar] [CrossRef] [PubMed]
- Hoppe-Seyler, K.; Bossler, F.; Braun, J.A.; Herrmann, A.L.; Hoppe-Seyler, F. The HPV E6/E7 Oncogenes: Key Factors for Viral Carcinogenesis and Therapeutic Targets. Trends Microbiol. 2018, 26, 158–168. [Google Scholar] [CrossRef]
- Jiang, Z.; Albanese, J.; Kesterson, J.; Warrick, J.; Karabakhtsian, R.; Dadachova, E.; Phaëton, R. Monoclonal Antibodies Against Human Papillomavirus E6 and E7 Oncoproteins Inhibit Tumor Growth in Experimental Cervical Cancer. Transl. Oncol. 2019, 12, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Venuti, A.; Paolini, F.; Nasir, L.; Corteggio, A.; Roperto, S.; Campo, M.S.; Borzacchiello, G. Papillomavirus E5: The smallest oncoprotein with many functions. Mol. Cancer 2011, 10, 140. [Google Scholar] [CrossRef]
- Basukala, O.; Banks, L. The Not-So-Good, the Bad and the Ugly: HPV E5, E6 and E7 Oncoproteins in the Orchestration of Carcinogenesis. Viruses 2021, 13, 1892. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, D.; French, D.; Raffa, S.; Guttieri, L.; Torrisi, M.R.; Belleudi, F. Expression of the E5 Oncoprotein of HPV16 Impacts on the Molecular Profiles of EMT-Related and Differentiation Genes in Ectocervical Low-Grade Lesions. Int. J. Mol. Sci. 2021, 22, 6534. [Google Scholar] [CrossRef]
- Jenkins, D. Histopathology and cytopathology of cervical cancer. Dis. Markers 2007, 23, 199–212. [Google Scholar] [CrossRef]
- Petignat, P.; Roy, M. Diagnosis and management of cervical cancer. BMJ 2007, 335, 765–768. [Google Scholar] [CrossRef]
- Horn, L.C.; Klostermann, K. Precancerous lesions of the uterine cervix: Morphology and molecular pathology. Pathologe 2011, 32, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, A.K.; Riemer, A.B. The invisible enemy—How human papillomaviruses avoid recognition and clearance by the host immune system. Open Virol. J. 2012, 6, 249–256. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, A.C.; de Oliveira, T.H.A.; Barros, M.R., Jr.; Venuti, A. hrHPV E5 oncoprotein: Immune evasion and related immunotherapies. J. Exp. Clin. Cancer Res. 2017, 36, 71–86. [Google Scholar] [CrossRef]
- Mittal, S.; Basu, P.; Muwonge, R.; Banerjee, D.; Ghosh, I.; Sengupta, M.M.; Das, P.; Dey, P.; Mandal, R.; Panda, C. Risk of high-grade precancerous lesions and invasive cancers in high-risk HPV-positive women with normal cervix or CIN 1 at baseline-A population-based cohort study. Int. J. Cancer 2017, 140, 1850–1859. [Google Scholar] [CrossRef]
- McBride, A.A.; Warburton, A. The role of integration in oncogenic progression of HPV-associated cancers. PLoS Pathog. 2017, 13, e1006211. [Google Scholar] [CrossRef]
- Day, E.; Duffy, S.; Bryson, G.; Syed, S.; Shanbhag, S.; Burton, K.; Lindsay, R.; Siddiqui, N.; Millan, D. Multifocal FIGO Stage IA1 Squamous Carcinoma of the Cervix: Criteria for Identification, Staging, and its Good Clinical Outcome. Int. J. Gynecol. Pathol. 2016, 35, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, W.; Wang, W.; Yang, R.; Wang, T.; Su, T.; Weng, D.; Tao, T.; Li, W.; Ma, D.; et al. Correlation of TWIST2 up-regulation and epithelial-mesenchymal transition during tumorigenesis and progression of cervical carcinoma. Gynecol. Oncol. 2012, 124, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, S.; Huang, K.; Zhang, Q.; Wang, J.; Li, X.; Hu, T.; Wang, S.; Yang, R.; Jia, Y.; et al. The nuclear protein expression levels of SNAI1 and ZEB1 are involved in the progression and lymph node metastasis of cervical cancer via the epithelial-mesenchymal transition pathway. Hum. Pathol. 2013, 44, 2097–2105. [Google Scholar] [CrossRef]
- Zhao, W.; Zhou, Y.; Xu, H.; Cheng, Y.; Kong, B. Snail family proteins in cervical squamous carcinoma: Expression and significance. Clin. Investig. Med. 2013, 36, E223–E233. [Google Scholar] [CrossRef]
- Wang, T.; Li, Y.; Wang, W.; Tuerhanjiang, A.; Wu, Z.; Yang, R.; Yuan, M.; Ma, D.; Wang, W.; Wang, S. Twist2, the key Twist isoform related to prognosis, promotes invasion of cervical cancer by inducing epithelial-mesenchymal transition and blocking senescence. Hum. Pathol. 2014, 45, 1839–1846. [Google Scholar] [CrossRef]
- Liu, Y.; Qian, W.; Zhang, J.; Dong, Y.; Shi, C.; Liu, Z.; Wu, S. The indicative function of Twist2 and E-cadherin in HPV oncogene-induced epithelial-mesenchymal transition of cervical cancer cells. Oncol. Rep. 2015, 33, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zheng, X.; Zhou, J.; Zhang, Y.; Chen, K. ZEB1 promotes the progression and metastasis of cervical squamous cell carcinoma via the promotion of epithelial-mesenchymal transition. Int. J. Clin. Exp. Pathol. 2015, 8, 11258–11267. [Google Scholar] [PubMed]
- Ran, J.; Lin, D.L.; Wu, R.F.; Chen, Q.H.; Huang, H.P.; Qiu, N.X.; Quan, S. ZEB1 promotes epithelial-mesenchymal transition in cervical cancer metastasis. Fertil. Steril. 2015, 103, 1606–1614.e2. [Google Scholar] [CrossRef]
- Yang, H.; Hu, H.; Gou, Y.; Hu, Y.; Li, H.; Zhao, H.; Wang, B.; Li, P.; Zhang, Z. Combined detection of Twist1, Snail1 and squamous cell carcinoma antigen for the prognostic evaluation of invasion and metastasis in cervical squamous cell carcinoma. Int. J. Clin. Oncol. 2018, 23, 321–328. [Google Scholar] [CrossRef]
- Yu, J.Q.; Zhou, Q.; Zheng, Y.F.; Bao, Y. Expression of Vimentin and Ki-67 Proteins in Cervical Squamous Cell Carcinoma and their Relationships with Clinicopathological Features. Asian Pac. J. Cancer Prev. 2015, 16, 4271–4275. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Feng, D.; Peng, C.; Li, C.; Zhou, Y.; Li, M.; Ling, B.; Wei, H.; Tian, Z. Identification and characterization of cancer stem-like cells from primary carcinoma of the cervix uteri. Oncol. Rep. 2009, 22, 1129–1134. [Google Scholar] [CrossRef][Green Version]
- Yao, T.; Chen, Q.; Zhang, B.; Zhou, H.; Lin, Z. The expression of ALDH1 in cervical carcinoma. Med. Sci. Monit. 2011, 17, HY21–HY26. [Google Scholar] [CrossRef]
- Bae, H.S.; Chung, Y.W.; Lee, J.K.; Lee, N.W.; Yeom, B.W.; Lee, K.W.; Song, J.Y. Nestin expression as an indicator of cervical cancer initiation. Eur. J. Gynaecol. Oncol. 2013, 34, 238–242. [Google Scholar]
- Gao, Q.; Liu, W.; Cai, J.; Li, M.; Gao, Y.; Lin, W.; Li, Z. EphB2 promotes cervical cancer progression by inducing epithelial-mesenchymal transition. Hum. Pathol. 2014, 45, 372–381. [Google Scholar] [CrossRef]
- Liu, W.; Gao, Q.; Chen, K.; Xue, X.; Li, M.; Chen, Q.; Zhu, G.; Gao, Y. Hiwi facilitates chemoresistance as a cancer stem cell marker in cervical cancer. Oncol. Rep. 2014, 32, 1853–1860. [Google Scholar] [CrossRef]
- Liu, X.F.; Yang, W.T.; Xu, R.; Liu, J.T.; Zheng, P.S. Cervical cancer cells with positive Sox2 expression exhibit the properties of cancer stem cells. PLoS ONE 2014, 9, e87092. [Google Scholar] [CrossRef]
- Lin, J.; Liu, X.; Ding, D. Evidence for epithelial-mesenchymal transition in cancer stem-like cells derived from carcinoma cell lines of the cervix uteri. Int. J. Clin. Exp. Pathol. 2015, 8, 847–855. [Google Scholar] [PubMed]
- Javed, S.; Sharma, B.K.; Sood, S.; Sharma, S.; Bagga, R.; Bhattacharyya, S.; Rayat, C.S.; Dhaliwal, L.; Srinivasan, R. Significance of CD133 positive cells in four novel HPV-16 positive cervical cancer-derived cell lines and biopsies of invasive cervical cancer. BMC Cancer 2018, 18, 357. [Google Scholar] [CrossRef]
- Zhang, Y.; An, J.; Liu, M.; Li, N.; Wang, W.; Yao, H.; Li, N.; Yang, X.; Sun, Y.; Xu, N.; et al. Efficient isolation, culture, purification, and stem cell expression profiles of primary tumor cells derived from uterine cervical squamous cell carcinoma. Am. J. Reprod. Immunol. 2020, 84, e13251. [Google Scholar] [CrossRef]
- Zamulaeva, I.; Selivanova, E.; Matchuk, O.; Kiseleva, V.; Mkrtchyan, L.; Krikunova, L. Radiation Response of Cervical Cancer Stem Cells Is Associated with Pretreatment Proportion of These Cells and Physical Status of HPV DNA. Int. J. Mol. Sci. 2021, 22, 1445. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Chen, C.; Zhang, J.; Qian, W.; Dong, Y.; Liu, Z.; Zhang, X.; Wang, X.; Zhang, Z.; et al. LSD1 binds to HPV16 E7 and promotes the epithelial-mesenchymal transition in cervical cancer by demethylating histones at the Vimentin promoter. Oncotarget 2017, 8, 11329–11342. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carrillo, D.; Muñoz, J.P.; Huerta, H.; Leal, G.; Corvalán, A.; León, O.; Calaf, G.M.; Urzúa, U.; Boccardo, E.; Tapia, J.C.; et al. Upregulation of PIR gene expression induced by human papillomavirus E6 and E7 in epithelial oral and cervical cells. Open Biol. 2017, 7, 170111. [Google Scholar] [CrossRef]
- Morgan, E.L.; Scarth, J.A.; Patterson, M.R.; Wasson, C.W.; Hemingway, G.C.; Barba-Moreno, D.; Macdonald, A. E6-mediated activation of JNK drives EGFR signalling to promote proliferation and viral oncoprotein expression in cervical cancer. Cell Death Differ. 2021, 28, 1669–1687. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, Q.; Nishitani, J.; Brown, J.; Shi, S.; Le, A.D. Overexpression of human papillomavirus type 16 oncoproteins enhances hypoxia-inducible factor 1 alpha protein accumulation and vascular endothelial growth factor expression in human cervical carcinoma cells. Clin. Cancer Res. 2007, 13, 2568–2576. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Feng, X.; Liu, F.; Zhang, P.; Liang, J.; Tang, X. Roles of PI3K/Akt and c-Jun signaling pathways in human papillomavirus type 16 oncoprotein-induced HIF-1α, VEGF, and IL-8 expression and in vitro angiogenesis in non-small cell lung cancer cells. PLoS ONE 2014, 9, e103440. [Google Scholar] [CrossRef]
- Liu, F.; Lin, B.; Liu, X.; Zhang, W.; Zhang, E.; Hu, L.; Ma, Y.; Li, X.; Tang, X. ERK Signaling Pathway Is Involved in HPV-16 E6 but not E7 Oncoprotein-Induced HIF-1α Protein Accumulation in NSCLC Cells. Oncol. Res. 2016, 23, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Rho, S.B.; Lee, S.H.; Byun, H.J.; Kim, B.R.; Lee, C.H. IRF-1 Inhibits Angiogenic Activity of HPV16 E6 Oncoprotein in Cervical Cancer. Int. J. Mol. Sci. 2020, 21, 7622. [Google Scholar] [CrossRef] [PubMed]
- Peralta-Zaragoza, O.; Bermúdez-Morales, V.; Gutiérrez-Xicotencatl, L.; Alcocer-González, J.; Recillas-Targa, F.; Madrid-Marina, V. E6 and E7 oncoproteins from human papillomavirus type 16 induce activation of human transforming growth factor beta1 promoter throughout Sp1 recognition sequence. Viral Immunol. 2006, 19, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Cui, H.; Xu, X.; Lin, Z.; Zhang, X.; Kang, L.; Han, B.; Meng, J.; Yan, Z.; Yan, X.; et al. MiR-125a suppresses tumor growth, invasion and metastasis in cervical cancer by targeting STAT3. Oncotarget 2015, 6, 25266–25280. [Google Scholar] [CrossRef]
- Olivos, D.J.; Mayo, L.D. Emerging Non-Canonical Functions and Regulation by p53: p53 and Stemness. Int. J. Mol. Sci. 2016, 17, 1982. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Xiong, Y.; Hanley, S.J.B.; Yue, J.; Watari, H. Musashi-2, a novel oncoprotein promoting cervical cancer cell growth and invasion, is negatively regulated by p53-induced miR-143 and miR-107 activation. J. Exp. Clin. Cancer Res. 2017, 36, 150. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, T.; Otsuka, Y.; Sabe, H. p53-Dependent and -Independent Epithelial Integrity: Beyond miRNAs and Metabolic Fluctuations. Cancers 2018, 10, 162. [Google Scholar] [CrossRef]
- Wan, H.Y.; Li, Q.Q.; Zhang, Y.; Tian, W.; Li, Y.N.; Liu, M.; Li, X.; Tang, H. MiR-124 represses vasculogenic mimicry and cell motility by targeting amotL1 in cervical cancer cells. Cancer Lett. 2014, 355, 148–158. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, D.; Meng, L.; Wang, B. MicroRNA-124 inhibits proliferation, invasion, migration and epithelial-mesenchymal transition of cervical carcinoma cells by targeting astrocyte-elevated gene-1. Oncol. Rep. 2016, 36, 2321–2328. [Google Scholar] [CrossRef] [PubMed]
- Li, G.C.; Xin, L.; Wang, Y.S.; Chen, Y. Long Intervening Noncoding 00467 RNA Contributes to Tumorigenesis by Acting as a Competing Endogenous RNA against miR-107 in Cervical Cancer Cells. Am. J. Pathol. 2019, 189, 2293–2310. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Xu, F.; Wang, L.; Sun, G.; Wang, J.; Yang, Y. By downregulating PBX3, miR-526b suppresses the epithelial-mesenchymal transition process in cervical cancer cells. Future Oncol. 2019, 15, 1577–1591. [Google Scholar] [CrossRef] [PubMed]
- Arima, Y.; Inoue, Y.; Shibata, T.; Hayashi, H.; Nagano, O.; Saya, H.; Taya, Y. Rb depletion results in deregulation of E-cadherin and induction of cellular phenotypic changes that are characteristic of the epithelial-to-mesenchymal transition. Cancer Res. 2008, 68, 5104–5112. [Google Scholar] [CrossRef]
- Rezaei, M.; Mostafaei, S.; Aghaei, A.; Hosseini, N.; Darabi, H.; Nouri, M.; Etemadi, A.; Neill, A.O.; Nahand, J.S.; Mirzaei, H.; et al. The association between HPV gene expression, inflammatory agents and cellular genes involved in EMT in lung cancer tissue. BMC Cancer 2020, 20, 916. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, E.S.; McClendon, A.K.; Franco, J.; Ertel, A.; Fortina, P.; Witkiewicz, A.K. RB loss contributes to aggressive tumor phenotypes in MYC-driven triple negative breast cancer. Cell Cycle 2015, 14, 109–122. [Google Scholar] [CrossRef]
- Dominguez, C.; David, J.M.; Palena, C. Epithelial-mesenchymal transition and inflammation at the site of the primary tumor. Semin. Cancer Biol. 2017, 47, 177–184. [Google Scholar] [CrossRef]
- Hammes, L.S.; Tekmal, R.R.; Naud, P.; Edelweiss, M.I.; Kirma, N.; Valente, P.T.; Syrjänen, K.J.; Cunha-Filho, J.S. Macrophages, inflammation and risk of cervical intraepithelial neoplasia (CIN) progression—Clinicopathological correlation. Gynecol. Oncol. 2007, 105, 157–165. [Google Scholar] [CrossRef]
- Hiraku, Y.; Tabata, T.; Ma, N.; Murata, M.; Ding, X.; Kawanishi, S. Nitrative and oxidative DNA damage in cervical intraepithelial neoplasia associated with human papilloma virus infection. Cancer Sci. 2007, 98, 964–972. [Google Scholar] [CrossRef]
- Mhatre, M.; McAndrew, T.; Carpenter, C.; Burk, R.D.; Einstein, M.H.; Herold, B.C. Cervical intraepithelial neoplasia is associated with genital tract mucosal inflammation. Sex. Transm Dis. 2012, 39, 591–597. [Google Scholar] [CrossRef]
- Saldivar, J.S.; Lopez, D.; Feldman, R.A.; Tharappel-Jacob, R.; de la Rosa, A.; Terreros, D.; Baldwin, W.S. COX-2 overexpression as a biomarker of early cervical carcinogenesis: A pilot study. Gynecol. Oncol. 2007, 107, S155–S162. [Google Scholar] [CrossRef]
- de Castro-Sobrinho, J.M.; Rabelo-Santos, S.H.; Fugueiredo-Alves, R.R.; Derchain, S.; Sarian, L.O.; Pitta, D.R.; Campos, E.A.; Zeferino, L.C. Bacterial vaginosis and inflammatory response showed association with severity of cervical neoplasia in HPV-positive women. Diagn. Cytopathol. 2016, 44, 80–86. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, J.; Ling, M.T.; Zhao, L.; Zhao, K.N. The role of the PI3K/Akt/mTOR signalling pathway in human cancers induced by infection with human papillomaviruses. Mol. Cancer 2015, 14, 87. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.W.; Liu, L.J.; Huang, J. Interleukin-6-induced epithelial-mesenchymal transition through signal transducer and activator of transcription 3 in human cervical carcinoma. Int. J. Oncol. 2014, 45, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Carrero, Y.; Mosquera, J.; Callejas, D.; Alvarez-Mon, M. In situ increased chemokine expression in human cervical intraepithelial neoplasia. Pathol. Res. Pract. 2015, 211, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tian, X.; Mumtahana, F.; Jiao, J.; Zhang, T.; Croce, K.D.; Ma, D.; Kong, B.; Cui, B. The existence of Th22, pure Th17 and Th1 cells in CIN and Cervical Cancer along with their frequency variation in different stages of cervical cancer. BMC Cancer 2015, 15, 717. [Google Scholar] [CrossRef]
- de Matos, L.G.; Cândido, E.B.; Vidigal, P.V.; Bordoni, P.H.; Lamaita, R.M.; Carneiro, M.M.; da Silva-Filho, A.L. Association between Toll-like receptor and tumor necrosis factor immunological pathways in uterine cervical neoplasms. Tumori 2017, 103, 81–86. [Google Scholar] [CrossRef]
- Takano, H.; Harigaya, K.; Ishii, G.; Sugaya, Y.; Soeta, S.; Nunoyama, T.; Shirasawa, H.; Shimizu, K.; Tokita, H.; Simizu, B.; et al. Interleukin-6 (IL-6) production in carcinoma of the cervix. Arch. Gynecol. Obstet. 1996, 258, 25–33. [Google Scholar] [CrossRef]
- Woods, K.V.; Adler-Storthz, K.; Clayman, G.L.; Francis, G.M.; Grimm, E.A. Interleukin-1 regulates interleukin-6 secretion in human oral squamous cell carcinoma in vitro: Possible influence of p53 but not human papillomavirus E6/E7. Cancer Res. 1998, 58, 3142–3149. [Google Scholar]
- Ho, Y.; Wu, C.Y.; Chin, Y.T.; Li, Z.L.; Pan, Y.S.; Huang, T.Y.; Su, P.Y.; Lee, S.Y.; Crawford, D.R.; Su, K.W.; et al. NDAT suppresses pro-inflammatory gene expression to enhance resveratrol-induced anti-proliferation in oral cancer cells. Food Chem. Toxicol. 2020, 136, 111092. [Google Scholar] [CrossRef]
- Gaiotti, D.; Chung, J.; Iglesias, M.; Nees, M.; Baker, P.D.; Evans, C.H.; Woodworth, C.D. Tumor necrosis factor-alpha promotes human papillomavirus (HPV) E6/E7 RNA expression and cyclin-dependent kinase activity in HPV-immortalized keratinocytes by a ras-dependent pathway. Mol. Carcinog. 2000, 27, 97–109. [Google Scholar] [CrossRef]
- Arany, I.; Muldrow, M.; Tyring, S.K. Correlation between mRNA levels of IL-6 and TNF alpha and progression rate in anal squamous epithelial lesions from HIV-positive men. Anticancer Res. 2001, 21, 425–428. [Google Scholar]
- Iwata, T.; Fujii, T.; Morii, K.; Saito, M.; Sugiyama, J.; Nishio, H.; Morisada, T.; Tanaka, K.; Yaguchi, T.; Kawakami, Y.; et al. Cytokine profile in cervical mucosa of Japanese patients with cervical intraepithelial neoplasia. Int. J. Clin. Oncol. 2015, 20, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.E.; Ma, Y.; Kuzmich, L.; Moscicki, A.B. Diminished IFN-gamma and IL-10 and elevated Foxp3 mRNA expression in the cervix are associated with CIN 2 or 3. Int. J. Cancer 2009, 124, 1379–1383. [Google Scholar] [CrossRef]
- Chen, Z.; Ding, J.; Pang, N.; Du, R.; Meng, W.; Zhu, Y.; Zhang, Y.; Ma, C.; Ding, Y. The Th17/Treg balance and the expression of related cytokines in Uygur cervical cancer patients. Diagn. Pathol. 2013, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Jiang, C.; Hu, X.; Liu, H.; Li, Q.; Li, T.; Yang, Y.; Li, O. Methylation patterns of the IFN-γ gene in cervical cancer tissues. Sci. Rep. 2014, 4, 6331. [Google Scholar] [CrossRef]
- Sikorski, M.; Bobek, M.; Zrubek, H.; Marcinkiewicz, J. Dynamics of selected MHC class I and II molecule expression in the course of HPV positive CIN treatment with the use of human recombinant IFN-gamma. Acta Obstet. Gynecol. Scand. 2004, 83, 299–307. [Google Scholar]
- Valyi-Nagy, I.; Jensen, P.J.; Albelda, S.M.; Rodeck, U. Cytokine-induced expression of transforming growth factor-alpha and the epidermal growth factor receptor in neonatal skin explants. J. Investig. Dermatol. 1992, 99, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Liu, X. Inflammatory cytokines augments TGF-beta1-induced epithelial-mesenchymal transition in A549 cells by up-regulating TbetaR-I. Cell Motil. Cytoskelet. 2008, 65, 935–944. [Google Scholar] [CrossRef]
- Hamburger, A.W.; Pinnamaneni, G.D. Increased epidermal growth factor receptor gene expression by gamma-interferon in a human breast carcinoma cell line. Br. J. Cancer 1991, 64, 64–68. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boente, M.P.; Berchuck, A.; Rodriguez, G.C.; Davidoff, A.; Whitaker, R.; Xu, F.J.; Marks, J.; Clarke-Pearson, D.L.; Bast, R.C., Jr. The effect of interferon gamma on epidermal growth factor receptor expression in normal and malignant ovarian epithelial cells. Am. J. Obstet. Gynecol. 1992, 167, 1877–1882. [Google Scholar] [CrossRef]
- Schmiegel, W.; Roeder, C.; Schmielau, J.; Rodeck, U.; Kalthoff, H. Tumor necrosis factor alpha induces the expression of transforming growth factor alpha and the epidermal growth factor receptor in human pancreatic cancer cells. Proc. Natl. Acad. Sci. USA 1993, 90, 863–867. [Google Scholar] [CrossRef]
- Lizard, G.; Chignol, M.C.; Chardonnet, Y.; Schmitt, D. Differences of reactivity to interferon gamma in HeLa and CaSki cells: A combined immunocytochemical and flow-cytometric study. J. Cancer Res. Clin. Oncol. 1996, 122, 223–230. [Google Scholar] [CrossRef]
- Wan, Y.; Belt, A.; Wang, Z.; Voorhees, J.; Fisher, G. Transmodulation of epidermal growth factor receptor mediates IL-1 beta-induced MMP-1 expression in cultured human keratinocytes. Int. J. Mol. Med. 2001, 7, 329–334. [Google Scholar]
- Segawa, R.; Shigeeda, K.; Hatayama, T.; Dong, J.; Mizuno, N.; Moriya, T.; Hiratsuka, M.; Hirasawa, N. EGFR transactivation is involved in TNF-α-induced expression of thymic stromal lymphopoietin in human keratinocyte cell line. J. Dermatol. Sci. 2018, 89, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, I.; Higa-Nakamine, S.; Uehara, A.; Sugahara, K.; Kakinohana, M.; Yamamoto, H. Regulation of epidermal growth factor receptor expression and morphology of lung epithelial cells by interleukin-1β. J. Biochem. 2020, 168, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.S.M.R.; Moriyama, M.; Kubota, K.; Ishiguro, N.; Sakamoto, M.; Chinju, A.; Mochizuki, K.; Sakamoto, T.; Kaneko, N.; Munemura, R.; et al. CD206+ tumor-associated macrophages promote proliferation and invasion in oral squamous cell carcinoma via EGF production. Sci. Rep. 2019, 9, 14611. [Google Scholar] [CrossRef]
- Lee, M.Y.; Chou, C.Y.; Tang, M.J.; Shen, M.R. Epithelial-mesenchymal transition in cervical cancer: Correlation with tumor progression, epidermal growth factor receptor overexpression, and snail up-regulation. Clin. Cancer Res. 2008, 14, 4743–4750. [Google Scholar] [CrossRef]
- Zhen, L.; Fan, D.; Yi, X.; Cao, X.; Chen, D.; Wang, L. Curcumin inhibits oral squamous cell carcinoma proliferation and invasion via EGFR signaling pathways. Int. J. Clin. Exp. Pathol. 2014, 7, 6438–6446. [Google Scholar]
- Kim, J.W.; Kim, Y.T.; Kim, D.K.; Song, C.H.; Lee, J.W. Expression of epidermal growth factor receptor in carcinoma of the cervix. Gynecol. Oncol. 1996, 60, 283–287. [Google Scholar] [CrossRef]
- Boiko, I.V.; Mitchell, M.F.; Hu, W.; Pandey, D.K.; Mathevet, P.; Malpica, A.; Hittelman, W.N. Epidermal growth factor receptor expression in cervical intraepithelial neoplasia and its modulation during an alpha-difluoromethylornithine chemoprevention trial. Clin. Cancer Res. 1998, 4, 1383–1391. [Google Scholar] [PubMed]
- Lesur, O.; Brisebois, M.; Thibodeau, A.; Chagnon, F.; Lane, D.; Füllöp, T. Role of IFN-gamma and IL-2 in rat lung epithelial cell migration and apoptosis after oxidant injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2004, 286, L4–L14. [Google Scholar] [CrossRef][Green Version]
- Jiang, G.X.; Zhong, X.Y.; Cui, Y.F.; Liu, W.; Tai, S.; Wang, Z.D.; Shi, Y.G.; Zhao, S.Y.; Li, C.L. IL-6/STAT3/TFF3 signaling regulates human biliary epithelial cell migration and wound healing in vitro. Mol. Biol. Rep. 2010, 37, 3813–3818. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Cao, G.F.; Jiang, Q.; Yao, J. TNF-α promotes human retinal pigment epithelial (RPE) cell migration by inducing matrix metallopeptidase 9 (MMP-9) expression through activation of Akt/mTORC1 signaling. Biochem. Biophys. Res. Commun. 2012, 425, 33–38. [Google Scholar] [CrossRef]
- Tseng, H.C.; Lee, I.T.; Lin, C.C.; Chi, P.L.; Cheng, S.E.; Shih, R.H.; Hsiao, L.D.; Yang, C.M. IL-1β promotes corneal epithelial cell migration by increasing MMP-9 expression through NF-κB- and AP-1-dependent pathways. PLoS ONE 2013, 8, e57955. [Google Scholar] [CrossRef]
- Taipale, J.; Matikainen, S.; Hurme, M.; Keski-Oja, J. Induction of transforming growth factor beta 1 and its receptor expression during myeloid leukemia cell differentiation. Cell Growth Differ. 1994, 5, 1309–1319. [Google Scholar] [PubMed]
- Yamauchi, Y.; Kohyama, T.; Takizawa, H.; Kamitani, S.; Desaki, M.; Takami, K.; Kawasaki, S.; Kato, J.; Nagase, T. Tumor necrosis factor-alpha enhances both epithelial-mesenchymal transition and cell contraction induced in A549 human alveolar epithelial cells by transforming growth factor-beta1. Exp. Lung Res. 2010, 36, 12–24. [Google Scholar] [CrossRef]
- Kamitani, S.; Yamauchi, Y.; Kawasaki, S.; Takami, K.; Takizawa, H.; Nagase, T.; Kohyama, T. Simultaneous stimulation with TGF-β1 and TNF-α induces epithelial mesenchymal transition in bronchial epithelial cells. Int. Arch. Allergy Immunol. 2011, 155, 119–128. [Google Scholar] [CrossRef]
- Voloshenyuk, T.G.; Hart, A.D.; Khoutorova, E.; Gardner, J.D. TNF-α increases cardiac fibroblast lysyl oxidase expression through TGF-β and PI3Kinase signaling pathways. Biochem. Biophys. Res. Commun. 2011, 413, 370–375. [Google Scholar] [CrossRef]
- Cheng, K.; Hao, M. Metformin Inhibits TGF-β1-Induced Epithelial-to-Mesenchymal Transition via PKM2 Relative-mTOR/p70s6k Signaling Pathway in Cervical Carcinoma Cells. Int. J. Mol. Sci. 2016, 17, 2000. [Google Scholar] [CrossRef]
- Cheng, K.Y.; Hao, M. Mammalian Target of Rapamycin (mTOR) Regulates Transforming Growth Factor-β1 (TGF-β1)-Induced Epithelial-Mesenchymal Transition via Decreased Pyruvate Kinase M2 (PKM2) Expression in Cervical Cancer Cells. Med. Sci. Monit. 2017, 23, 2017–2028. [Google Scholar] [CrossRef]
- Kim, K.K.; Sheppard, D.; Chapman, H.A. TGF-β1 Signaling and Tissue Fibrosis. Cold Spring Harb. Perspect. Biol. 2018, 10, a022293. [Google Scholar] [CrossRef] [PubMed]
- Sitole, B.N.; Mavri-Damelin, D. Peroxidasin is regulated by the epithelial-mesenchymal transition master transcription factor Snai1. Gene 2018, 646, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Tyszka-Czochara, M.; Lasota, M.; Majka, M. Caffeic Acid and Metformin Inhibit Invasive Phenotype Induced by TGF-β1 in C-4I and HTB-35/SiHa human Cervical Squamous Carcinoma Cells by Acting on Different Molecular Targets. Int. J. Mol. Sci. 2018, 19, 266. [Google Scholar] [CrossRef]
- Ji, H.; Liu, G.; Han, J.; Zhu, F.; Dong, X.; Li, B. C-phycocyanin inhibits epithelial-to-mesenchymal transition in Caski cells. Cancer Cell Int. 2020, 20, 292. [Google Scholar] [CrossRef]
- Stolfi, C.; Troncone, E.; Marafini, I.; Monteleone, G. Role of TGF-Beta and Smad7 in Gut Inflammation, Fibrosis and Cancer. Biomolecules 2020, 11, 17. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, F.; van Dinther, M.; Ten Dijke, P. Determining TGF-β Receptor Levels in the Cell Membrane. Methods Mol. Biol. 2016, 1344, 35–47. [Google Scholar] [CrossRef]
- Baritaki, S.; Sifakis, S.; Huerta-Yepez, S.; Neonakis, I.K.; Soufla, G.; Bonavida, B.; Spandidos, D.A. Overexpression of VEGF and TGF-beta1 mRNA in Pap smears correlates with progression of cervical intraepithelial neoplasia to cancer: Implication of YY1 in cervical tumorigenesis and HPV infection. Int. J. Oncol. 2007, 31, 69–79. [Google Scholar] [PubMed]
- Viloria, M.E.; Bravo, J.; Carrero, Y.; Mosquera, J.A. In situ expressions of protein 16 (p16CDKN2A) and transforming growth factor beta-1 in patients with cervical intraepithelial neoplasia and cervical cancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 228, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Wang, S.; Xi, L.; Wu, S.; Chen, G.; Zhao, Y.; Wu, Y.; Ma, D. Effects of human papillomavirus type 16 E7 protein on the growth of cervical carcinoma cells and immuno-escape through the TGF-beta1 signaling pathway. Gynecol. Oncol. 2006, 101, 132–139. [Google Scholar] [CrossRef]
- Mo, N.; Li, Z.Q.; Li, J.; Cao, Y.D. Curcumin inhibits TGF-β1-induced MMP-9 and invasion through ERK and Smad signaling in breast cancer MDA- MB-231 cells. Asian Pac. J. Cancer Prev. 2012, 13, 5709–5714. [Google Scholar] [CrossRef]
- Huang, Y.; Yu, T.; Fu, X.; Chen, J.; Liu, Y.; Li, C.; Xia, Y.; Zhang, Z.; Li, L. EGFR inhibition prevents in vitro tumor growth of salivary adenoid cystic carcinoma. BMC Cell Biol. 2013, 14, 13. [Google Scholar] [CrossRef]
- Huang, W.C.; Wu, S.J.; Tu, R.S.; Lai, Y.R.; Liou, C.J. Phloretin inhibits interleukin-1β-induced COX-2 and ICAM-1 expression through inhibition of MAPK, Akt, and NF-κB signaling in human lung epithelial cells. Food Funct. 2015, 6, 1960–1967. [Google Scholar] [CrossRef]
- Jiménez-Garduño, A.M.; Mendoza-Rodríguez, M.G.; Urrutia-Cabrera, D.; Domínguez-Robles, M.C.; Pérez-Yépez, E.A.; Ayala-Sumuano, J.T.; Meza, I. IL-1β induced methylation of the estrogen receptor ERα gene correlates with EMT and chemoresistance in breast cancer cells. Biochem Biophys Res Commun 2017, 490, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Nishikai-Yan Shen, T.; Kanazawa, S.; Kado, M.; Okada, K.; Luo, L.; Hayashi, A.; Mizuno, H.; Tanaka, R. Interleukin-6 stimulates Akt and p38 MAPK phosphorylation and fibroblast migration in non-diabetic but not diabetic mice. PLoS ONE 2017, 12, e0178232. [Google Scholar] [CrossRef]
- Yang, D.; Xiao, C.X.; Su, Z.H.; Huang, M.W.; Qin, M.; Wu, W.J.; Jia, W.W.; Zhu, Y.Z.; Hu, J.F.; Liu, X.H. (-)-7(S)-hydroxymatairesinol protects against tumor necrosis factor-α-mediated inflammation response in endothelial cells by blocking the MAPK/NF-κB and activating Nrf2/HO-1. Phytomedicine 2017, 32, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, A.; Song, J.; Thakur, N.; Itoh, S.; Marcusson, A.; Bergh, A.; Heldin, C.H.; Landström, M. TGF-β promotes PI3K-AKT signaling and prostate cancer cell migration through the TRAF6-mediated ubiquitylation of p85α. Sci. Signal. 2017, 10, eaal4186. [Google Scholar] [CrossRef]
- Rodríguez-García, A.; Samsó, P.; Fontova, P.; Simon-Molas, H.; Manzano, A.; Castaño, E.; Rosa, J.L.; Martinez-Outshoorn, U.; Ventura, F.; Navarro-Sabaté, À.; et al. TGF-β1 targets Smad, p38 MAPK, and PI3K/Akt signaling pathways to induce PFKFB3 gene expression and glycolysis in glioblastoma cells. FEBS J. 2017, 284, 3437–3454. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wan, D.; Zhou, R.; Zhong, W.; Lu, S.; Chai, Y. Geraniin inhibits migration and invasion of human osteosarcoma cancer cells through regulation of PI3K/Akt and ERK1/2 signaling pathways. Anticancer Drugs 2017, 28, 959–966. [Google Scholar] [CrossRef]
- Bai, T.; Liu, F.; Zou, F.; Zhao, G.; Jiang, Y.; Liu, L.; Shi, J.; Hao, D.; Zhang, Q.; Zheng, T.; et al. Epidermal Growth Factor Induces Proliferation of Hair Follicle-Derived Mesenchymal Stem Cells Through Epidermal Growth Factor Receptor-Mediated Activation of ERK and AKT Signaling Pathways Associated with Upregulation of Cyclin D1 and Downregulation of p16. Stem Cells Dev. 2017, 26, 113–122. [Google Scholar] [CrossRef]
- Zibara, K.; Zeidan, A.; Bjeije, H.; Kassem, N.; Badran, B.; El-Zein, N. ROS mediates interferon gamma induced phosphorylation of Src, through the Raf/ERK pathway, in MCF-7 human breast cancer cell line. J. Cell Commun. Signal. 2017, 11, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.P.; Harishankar, M.K.; Pillai, A.A.; Devi, A. Hypoxia induced EMT: A review on the mechanism of tumor progression and metastasis in OSCC. Oral Oncol. 2018, 80, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, J.; Tripathy, A.; Thangaraju, M.; Suar, M.; Elangovan, S. α-Lipoic acid inhibits the migration and invasion of breast cancer cells through inhibition of TGFβ signaling. Life Sci. 2018, 207, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Morales-Garcia, V.; Contreras-Paredes, A.; Martinez-Abundis, E.; Gomez-Crisostomo, N.P.; Lizano, M.; Hernandez-Landero, F.; de la Cruz-Hernandez, E. The high-risk HPV E6 proteins modify the activity of the eIF4E protein via the MEK/ERK and AKT/PKB pathways. FEBS Open Bio 2020, 10, 2541–2552. [Google Scholar] [CrossRef]
- Jin, J.; Zhang, Z.; Zhang, S.; Chen, X.; Chen, Z.; Hu, P.; Wang, J.; Xie, C. Fatty acid binding protein 4 promotes epithelial-mesenchymal transition in cervical squamous cell carcinoma through AKT/GSK3β/Snail signaling pathway. Mol. Cell. Endocrinol. 2018, 461, 155–164. [Google Scholar] [CrossRef]
- Hernández-Padilla, L.; Reyes de la Cruz, H.; Campos-García, J. Antiproliferative effect of bacterial cyclodipeptides in the HeLa line of human cervical cancer reveals multiple protein kinase targeting, including mTORC1/C2 complex inhibition in a TSC1/2-dependent manner. Apoptosis 2020, 25, 632–647. [Google Scholar] [CrossRef]
- Wang, D.; Li, Q.; Li, K.; Xiao, P.; Yin, R. Twist-related protein 1-mediated regulation of mesenchymal change contributes to the migration and invasion of cervical cancer cells. Oncol. Lett. 2015, 10, 3107–3112. [Google Scholar] [CrossRef]
- Srinivas, K.P.; Viji, R.; Dan, V.M.; Sajitha, I.S.; Prakash, R.; Rahul, P.V.; Santhoshkumar, T.R.; Lakshmi, S.; Pillai, M.R. DEPTOR promotes survival of cervical squamous cell carcinoma cells and its silencing induces apoptosis through downregulating PI3K/AKT and by up-regulating p38 MAP kinase. Oncotarget 2016, 7, 24154–24171. [Google Scholar] [CrossRef]
- Liu, C.; Ding, L.; Bai, L.; Chen, X.; Kang, H.; Hou, L.; Wang, J. Folate receptor alpha is associated with cervical carcinogenesis and regulates cervical cancer cells growth by activating ERK1/2/c-Fos/c-Jun. Biochem. Biophys. Res. Commun. 2017, 491, 1083–1091. [Google Scholar] [CrossRef]
- Saxena, K.; Jolly, M.K.; Balamurugan, K. Hypoxia, partial EMT and collective migration: Emerging culprits in metastasis. Transl. Oncol. 2020, 13, 100845. [Google Scholar] [CrossRef]
- Ghatak, D.; Das Ghosh, D.; Roychoudhury, S. Cancer Stemness: p53 at the Wheel. Front. Oncol. 2021, 10, 604124. [Google Scholar] [CrossRef]
- Viallard, C.; Larrivée, B. Tumor angiogenesis and vascular normalization: Alternative thera-peutic targets. Angiogenesis 2017, 20, 409–426. [Google Scholar] [CrossRef]
- Pezzuto, A.; Carico, E. Role of HIF-1 in Cancer Progression: Novel Insights. A Review. Curr. Mol. Med. 2018, 18, 343–351. [Google Scholar] [CrossRef]
- Acs, G.; Zhang, P.J.; McGrath, C.M.; Acs, P.; McBroom, J.; Mohyeldin, A.; Liu, S.; Lu, H.; Verma, A. Hypoxia-inducible erythropoietin signaling in squamous dysplasia and squamous cell carcinoma of the uterine cervix and its potential role in cervical carcinogenesis and tumor progression. Am. J. Pathol. 2003, 162, 1789–1806. [Google Scholar] [CrossRef]
- Sartori-Cintra, A.R.; Mara, C.S.; Argolo, D.L.; Coimbra, I.B. Regulation of hypoxia-inducible factor-1α (HIF-1α) expression by interleukin-1β (IL-1 β), insulin-like growth factors I (IGF-I) and II (IGF-II) in human osteoarthritic chondrocytes. Clinics 2012, 67, 35–40. [Google Scholar] [CrossRef]
- Zhang, H.X.; Yang, J.J.; Zhang, S.A.; Zhang, S.M.; Wang, J.X.; Xu, Z.Y.; Lin, R.Y. HIF-1α promotes inflammatory response of chronic obstructive pulmonary disease by activating EGFR/PI3K/AKT pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 6077–6084. [Google Scholar] [CrossRef]
- Gao, X.; Li, Y.; Wang, H.; Li, C.; Ding, J. Inhibition of HIF-1α decreases expression of pro-inflammatory IL-6 and TNF-α in diabetic retinopathy. Acta Ophthalmol. 2017, 95, e746–e750. [Google Scholar] [CrossRef] [PubMed]
- Ogryzko, N.V.; Lewis, A.; Wilson, H.L.; Meijer, A.H.; Renshaw, S.A.; Elks, P.M. Hif-1α-Induced Expression of Il-1β Protects against Mycobacterial Infection in Zebrafish. J. Immunol. 2019, 202, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Warbrick, I.; Rabkin, S.W. Hypoxia-inducible factor 1-alpha (HIF-1α) as a factor mediating the relationship between obesity and heart failure with preserved ejection fraction. Obes. Rev. 2019, 20, 701–712. [Google Scholar] [CrossRef]
- Kim, K.W.; Lee, S.J.; Kim, J.C. TNF-α upregulates HIF-1α expression in pterygium fibroblasts and enhances their susceptibility to VEGF independent of hypoxia. Exp. Eye Res. 2017, 164, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Yu, C.; Ma, X.; Li, Y.; Shen, Y.; Chen, Y.; Huang, S.; Zhang, T.; Deng, W.; Wang, Y. IL-6 promotes nuclear translocation of HIF-1α to aggravate chemoresistance of ovarian cancer cells. Eur. J. Pharmacol. 2021, 894, 173817. [Google Scholar] [CrossRef]
- Nakamura, M.; Bodily, J.M.; Beglin, M.; Kyo, S.; Inoue, M.; Laimins, L.A. Hypoxia-specific stabilization of HIF-1alpha by human papillomaviruses. Virology 2009, 387, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Rodolico, V.; Arancio, W.; Amato, M.C.; Aragona, F.; Cappello, F.; Di Fede, O.; Pannone, G.; Campisi, G. Hypoxia inducible factor-1 alpha expression is increased in infected positive HPV16 DNA oral squamous cell carcinoma and positively associated with HPV16 E7 oncoprotein. Infect. Agent Cancer 2011, 6, 18. [Google Scholar] [CrossRef]
- Fan, R.; Hou, W.J.; Zhao, Y.J.; Liu, S.L.; Qiu, X.S.; Wang, E.H.; Wu, G.P. Overexpression of HPV16 E6/E7 mediated HIF-1α upregulation of GLUT1 expression in lung cancer cells. Tumour Biol. 2016, 37, 4655–4663. [Google Scholar] [CrossRef] [PubMed]
- Fujiwaki, R.; Hata, K.; Iida, K.; Maede, Y.; Miyazaki, K. Vascular endothelial growth factor expression in progression of cervical cancer: Correlation with thymidine phosphorylase expression, angiogenesis, tumor cell proliferation, and apoptosis. Anticancer Res. 2000, 20, 1317–1322. [Google Scholar]
- Triratanachat, S.; Niruthisard, S.; Trivijitsilp, P.; Tresukosol, D.; Jarurak, N. Angiogenesis in cervical intraepithelial neoplasia and early-staged uterine cervical squamous cell carcinoma: Clinical significance. Int. J. Gynecol. Cancer 2006, 16, 575–580. [Google Scholar] [CrossRef]
- Kim, N.S.; Kang, Y.J.; Jo, J.O.; Kim, H.Y.; Oh, Y.R.; Kim, Y.O.; Jung, M.H.; Ock, M.S.; Cha, H.J. Elevated expression of thymosin β4, vascular endothelial growth factor (VEGF), and hypoxia inducible factor (HIF)-1α in early-stage cervical cancers. Pathol. Oncol. Res. 2011, 17, 493–502. [Google Scholar] [CrossRef]
- Durand, R.E.; Aquino-Parsons, C. The fate of hypoxic (pimonidazole-labelled) cells in human cervix tumours undergoing chemo-radiotherapy. Radiother. Oncol. 2006, 80, 138–142. [Google Scholar] [CrossRef]
- Sundfør, K.; Lyng, H.; Rofstad, E.K. Tumour hypoxia and vascular density as predictors of metastasis in squamous cell carcinoma of the uterine cervix. Br. J. Cancer 1998, 78, 822–827. [Google Scholar] [CrossRef]
- Höckel, M.; Schlenger, K.; Höckel, S.; Vaupel, P. Hypoxic cervical cancers with low apoptotic index are highly aggressive. Cancer Res. 1999, 59, 4525–4528. [Google Scholar] [PubMed]
- Chen, H.; Chan, D.C. Mitochondrial Dynamics in Regulating the Unique Phenotypes of Cancer and Stem Cells. Cell Metab. 2017, 26, 39–48. [Google Scholar] [CrossRef]
- Sachdeva, M.; Zhu, S.; Wu, F.; Wu, H.; Walia, V.; Kumar, S.; Elble, R.; Watabe, K.; Mo, Y.Y. p53 represses c-Myc through induction of the tumor suppressor miR-145. Proc. Natl. Acad. Sci. USA 2009, 106, 3207–3212. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Allton, K.; Iacovino, M.; Mahen, E.; Milczarek, R.J.; Zwaka, T.P.; Kyba, M.; Barton, M.C. p53 regulates cell cycle and microRNAs to promote differentiation of human embryonic stem cells. PLoS Biol. 2012, 10, e1001268. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, K.; Li, C.; Yao, Y.; Tao, D.; Liu, Y.; Zhang, S.; Ma, Y. Piwil2 suppresses p53 by inducing phosphorylation of signal transducer and activator of transcription 3 in tumor cells. PLoS ONE 2012, 7, e30999. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Rokavec, M.; Jiang, L.; Horst, D.; Hermeking, H. Antagonistic Effects of p53 and HIF1A on microRNA-34a Regulation of PPP1R11 and STAT3 and Hypoxia-induced Epithelial to Mesenchymal Transition in Colorectal Cancer Cells. Gastroenterology 2017, 153, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fan, D. The epithelial-mesenchymal transition and cancer stem cells: Functional and mechanistic links. Curr. Pharm. Des. 2015, 21, 1279–1291. [Google Scholar] [CrossRef]
- Xia, P.; Xu, X.Y. Epithelial-mesenchymal transition and gastric cancer stem cell. Tumour Biol. 2017, 39, 1010428317698373. [Google Scholar] [CrossRef]
- Du, B.; Shim, J.S. Targeting Epithelial-Mesenchymal Transition (EMT) to Overcome Drug Resistance in Cancer. Molecules 2016, 21, 965. [Google Scholar] [CrossRef]
- Shibue, T.; Weinberg, R.A. EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 2017, 14, 611–629. [Google Scholar] [CrossRef]
- Li, S.W.; Wu, X.L.; Dong, C.L.; Xie, X.Y.; Wu, J.F.; Zhang, X. The differential expression of OCT4 isoforms in cervical carcinoma. PLoS ONE 2015, 10, e0118033. [Google Scholar] [CrossRef][Green Version]
- Su, P.H.; Hsu, Y.W.; Huang, R.L.; Chen, L.Y.; Chao, T.K.; Liao, C.C.; Chen, C.W.; Wu, T.I.; Mao, S.P.; Balch, C.; et al. TET1 promotes 5hmC-dependent stemness, and inhibits a 5hmC-independent epithelial-mesenchymal transition, in cervical precancerous lesions. Cancer Lett. 2019, 450, 53–62. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, X.; Bian, L.; Wang, Y.; Liu, H. CD44+/CD24+-Expressing Cervical Cancer Cells and Radioresistant Cervical Cancer Cells Exhibit Cancer Stem Cell Characteristics. Gynecol. Obstet. Investig. 2019, 84, 174–182. [Google Scholar] [CrossRef]
- De Brux, J. Natural history of the cervical precancerous lesions and their evolutions. Eur. J. Gynaecol. Oncol. 1982, 3, 106–112. [Google Scholar]
- Organista-Nava, J.; Gómez-Gómez, Y.; Gariglio, P. Embryonic stem cell-specific signature in cervical cancer. Tumour Biol. 2014, 35, 1727–1738. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, H.; Wang, H.; Chen, Q.; Zhang, L.; Song, C.; Zhou, Q.; Hong, Y. The inhibition of miR-126 in cell migration and invasion of cervical cancer through regulating ZEB1. Hereditas 2019, 156, 11. [Google Scholar] [CrossRef]
- Tian, W.; Zhang, W.; Zhang, Y.; Zhu, T.; Hua, Y.; Li, H.; Zhang, Q.; Xia, M. FABP4 promotes invasion and metastasis of colon cancer by regulating fatty acid transport. Cancer Cell Int. 2020, 20, 512. [Google Scholar] [CrossRef] [PubMed]
- Mun, J.G.; Han, Y.H.; Jeon, H.D.; Yoon, D.H.; Lee, Y.G.; Hong, S.H.; Kee, J.Y. Inhibitory Effect of Gallotannin on Lung Metastasis of Metastatic Colorectal Cancer Cells by Inducing Apoptosis, Cell Cycle Arrest and Autophagy. Am. J. Chin. Med. 2021, 49, 1535–1555. [Google Scholar] [CrossRef]
- Zheng, L.; Li, N.; Guo, F.; Jian, X.C.; Jiang, C.H.; Yin, P.; Min, A.J.; Huang, L. Twist-related protein 1 enhances oral tongue squamous cell carcinoma cell invasion through β-catenin signaling. Mol. Med. Rep. 2015, 11, 2255–2261. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kashyap, T.; Nath, N.; Mishra, P.; Jha, A.; Nagini, S.; Mishra, R. Pluripotency transcription factor Nanog and its association with overall oral squamous cell carcinoma progression, cisplatin-resistance, invasion and stemness acquisition. Head Neck 2020, 42, 3282–3294. [Google Scholar] [CrossRef]
- Zheng, J.H.; Jiao, S.J.; Na, L.; Zheng, S.Q.; Ma, Z.H.; Wang, S.W.; Aili, A.; Hasim, A. Defective expression of polarity protein Par3 promotes cervical tumorigenesis and metastasis. Eur. J. Gynaecol. Oncol. 2017, 38, 199–206. [Google Scholar] [PubMed]
- Lu, K.; Dong, J.L.; Fan, W.J. Twist1/2 activates MMP2 expression via binding to its promoter in colorectal cancer. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8210–8219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X. Targeting the Wnt/β-catenin signaling pathway in cancer. J. Hematol. Oncol. 2020, 13, 165. [Google Scholar] [CrossRef]
- Chang, B.; Kim, J.; Jeong, D.; Jeong, Y.; Jeon, S.; Jung, S.I.; Yang, Y.; Kim, K.I.; Lim, J.S.; Kim, C.; et al. Klotho inhibits the capacity of cell migration and invasion in cervical cancer. Oncol. Rep. 2012, 28, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Huang, Y.; Cao, X.; Xu, J.; Zhang, L.; Wang, J.; Huang, L.; Huang, S.; Yuan, L.; Jia, W.; et al. WNT2 Promotes Cervical Carcinoma Metastasis and Induction of Epithelial-Mesenchymal Transition. PLoS ONE 2016, 11, e0160414. [Google Scholar] [CrossRef]
- Müller, T.; Bain, G.; Wang, X.; Papkoff, J. Regulation of epithelial cell migration and tumor formation by beta-catenin signaling. Exp. Cell Res. 2002, 280, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Choi, K.Y. EGF receptor is involved in WNT3a-mediated proliferation and motility of NIH3T3 cells via ERK pathway activation. Cell Signal. 2007, 19, 1554–1564. [Google Scholar] [CrossRef]
- Shi, I.; Hashemi Sadraei, N.; Duan, Z.H.; Shi, T. Aberrant signaling pathways in squamous cell lung carcinoma. Cancer Inform. 2011, 10, 273–285. [Google Scholar] [CrossRef]
- Zhou, S. TGF-β regulates β-catenin signaling and osteoblast differentiation in human mesenchymal stem cells. J. Cell Biochem. 2011, 112, 1651–1660. [Google Scholar] [CrossRef]
- Landman, E.B.; Miclea, R.L.; van Blitterswijk, C.A.; Karperien, M. Small molecule inhibitors of WNT/β-catenin signaling block IL-1β- and TNFα-induced cartilage degradation. Arthritis Res. Ther. 2013, 15, R93. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, H.; Wang, N.; Zhao, C.; Zhang, H.; Deng, F.; Wu, N.; He, Y.; Chen, X.; Zhang, J.; et al. Modulation of β-catenin signaling by the inhibitors of MAP kinase, tyrosine kinase, and PI3-kinase pathways. Int. J. Med. Sci. 2013, 10, 1888–1898. [Google Scholar] [CrossRef]
- Zhang, H.; Nan, W.; Wang, S.; Zhang, T.; Si, H.; Yang, F.; Li, G. Epidermal Growth Factor Promotes Proliferation and Migration of Follicular Outer Root Sheath Cells via Wnt/β-Catenin Signaling. Cell Physiol. Biochem. 2016, 39, 360–370. [Google Scholar] [CrossRef]
- Ratz, L.; Laible, M.; Kacprzyk, L.A.; Wittig-Blaich, S.M.; Tolstov, Y.; Duensing, S.; Altevogt, P.; Klauck, S.M.; Sültmann, H. TMPRSS2/ERG gene fusion variants induce TGF-β signaling and epithelial to mesenchymal transition in human prostate cancer cells. Oncotarget 2017, 8, 25115–25130. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Hu, J.; Wu, X.; Liang, Z. PMA treated THP-1-derived-IL-6 promotes EMT of SW48 through STAT3/ERK-dependent activation of Wnt/β-catenin signaling pathway. Biomed. Pharm. 2018, 108, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, W.; Wu, L.; Jiang, L.; Liang, N.; Tan, L.; Liang, M.; Tang, N. TGF-β1 Restores Hippocampal Synaptic Plasticity and Memory in Alzheimer Model via the PI3K/Akt/Wnt/β-Catenin Signaling Pathway. J. Mol. Neurosci. 2019, 67, 142–149. [Google Scholar] [CrossRef]
- Lee, M.S.; Lee, J.; Kim, Y.M.; Lee, H. The metastasis suppressor CD82/KAI1 represses the TGF-β 1 and Wnt signalings inducing epithelial-to-mesenchymal transition linked to invasiveness of prostate cancer cells. Prostate 2019, 79, 1400–1411. [Google Scholar] [CrossRef]
- Bas, E.; Anwar, M.R.; Van De Water, T.R. TGF β-1 and WNT Signaling Pathways Collaboration Associated with Cochlear Implantation Trauma-Induced Fibrosis. Anat. Rec. 2020, 303, 608–618. [Google Scholar] [CrossRef]
- Du, L.; Lee, J.H.; Jiang, H.; Wang, C.; Wang, S.; Zheng, Z.; Shao, F.; Xu, D.; Xia, Y.; Li, J.; et al. β-Catenin induces transcriptional expression of PD-L1 to promote glioblastoma immune evasion. J. Exp. Med. 2020, 217, e20191115. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Shen, X.; Zhang, Y.; Wang, S.; Zhou, L. Astragaloside IV suppresses transforming growth factor-β1-induced epithelial-mesenchymal transition through inhibition of Wnt/β-catenin pathway in glioma U251 cells. Biosci. Biotechnol. Biochem. 2020, 84, 1345–1352. [Google Scholar] [CrossRef]
- Seomun, Y.; Kim, J.T.; Joo, C.K. MMP-14 mediated MMP-9 expression is involved in TGF-beta1-induced keratinocyte migration. J. Cell Biochem. 2008, 104, 934–941. [Google Scholar] [CrossRef]
- Sinpitaksakul, S.N.; Pimkhaokham, A.; Sanchavanakit, N.; Pavasant, P. TGF-beta1 induced MMP-9 expression in HNSCC cell lines via Smad/MLCK pathway. Biochem. Biophys. Res. Commun. 2008, 371, 713–718. [Google Scholar] [CrossRef]
- Chang, C.C.; Ling, X.H.; Hsu, H.F.; Wu, J.M.; Wang, C.P.; Yang, J.F.; Fang, L.W.; Houng, J.Y. Siegesbeckia orientalis Extract Inhibits TGFβ1-Induced Migration and Invasion of Endometrial Cancer Cells. Molecules 2016, 21, 1021. [Google Scholar] [CrossRef]
- Huang, Z.; Li, S.; Fan, W.; Ma, Q. Transforming growth factor β1 promotes invasion of human JEG-3 trophoblast cells via TGF-β/Smad3 signaling pathway. Oncotarget 2017, 8, 33560–33570. [Google Scholar] [CrossRef]
- Xie, F.; Jin, K.; Shao, L.; Fan, Y.; Tu, Y.; Li, Y.; Yang, B.; van Dam, H.; Ten Dijke, P.; Weng, H.; et al. FAF1 phosphorylation by AKT accumulates TGF-β type II receptor and drives breast cancer metastasis. Nat. Commun. 2017, 8, 15021. [Google Scholar] [CrossRef]
- Pramanik, K.K.; Nagini, S.; Singh, A.K.; Mishra, P.; Kashyap, T.; Nath, N.; Alam, M.; Rana, A.; Mishra, R. Glycogen synthase kinase-3β mediated regulation of matrix metalloproteinase-9 and its involvement in oral squamous cell carcinoma progression and invasion. Cell. Oncol. 2018, 41, 47–60. [Google Scholar] [CrossRef]
- Drews, C.M.; Case, S.; Vande Pol, S.B. E6 proteins from high-risk HPV, low-risk HPV, and animal papillomaviruses activate the Wnt/β-catenin pathway through E6AP-dependent degradation of NHERF1. PLoS Pathog. 2019, 15, e1007575. [Google Scholar] [CrossRef]
- Li, B.; Guo, X.; Li, N.; Chen, Q.; Shen, J.; Huang, X.; Huang, G.; Wang, F. WNT1, a target of miR-34a, promotes cervical squamous cell carcinoma proliferation and invasion by induction of an E-P cadherin switch via the WNT/β-catenin pathway. Cell. Oncol. 2020, 43, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Jang, Y.S.; Min, S.Y.; Song, J.Y. Overexpression of MMP-9 and HIF-1α in Breast Cancer Cells under Hypoxic Conditions. J. Breast Cancer 2011, 14, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Tinganelli, W.; Durante, M. Tumor Hypoxia and Circulating Tumor Cells. Int. J. Mol. Sci. 2020, 21, 9592. [Google Scholar] [CrossRef]
- Jiang, J.; Li, X.; Yin, X.; Zhang, J.; Shi, B. Association of low expression of E-cadherin and β-catenin with the progression of early stage human squamous cervical cancer. Oncol. Lett. 2019, 17, 5729–5739. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Yang, J.; Wang, M.; Zheng, D.; Liu, Y. Endoplasmic reticulum stress regulates epithelial mesenchymal transition in human lens epithelial cells. Mol. Med. Rep. 2020, 21, 173–180. [Google Scholar] [CrossRef]
- Eddy, R.J.; Weidmann, M.D.; Sharma, V.P.; Condeelis, J.S. Tumor Cell Invadopodia: Invasive Protrusions that Orchestrate Metastasis. Trends Cell Biol. 2017, 27, 595–607. [Google Scholar] [CrossRef]
- Stenman, J.; Lintula, S.; Hotakainen, K.; Vartiainen, J.; Lehväslaiho, H.; Stenman, U.H. Detection of squamous-cell carcinoma antigen-expressing tumour cells in blood by reverse transcriptase-polymerase chain reaction in cancer of the uterine cervix. Int. J. Cancer 1997, 74, 75–80. [Google Scholar] [CrossRef]
- Wei, X.Q.; Ma, Y.; Chen, Y.; Liu, X.; Zhao, M.; Zhou, L.W. Laparoscopic surgery for early cervical squamous cell carcinoma and its effect on the micrometastasis of cancer cells. Medicine 2018, 97, e11921. [Google Scholar] [CrossRef]
- Wen, Y.F.; Cheng, T.T.; Chen, X.L.; Huang, W.J.; Peng, H.H.; Zhou, T.C.; Lin, X.D.; Zeng, L.S. Elevated circulating tumor cells and squamous cell carcinoma antigen levels predict poor survival for patients with locally advanced cervical cancer treated with radiotherapy. PLoS ONE 2018, 13, e0204334. [Google Scholar] [CrossRef] [PubMed]
- Braunholz, D.; Saki, M.; Niehr, F.; Öztürk, M.; Borràs Puértolas, B.; Konschak, R.; Budach, V.; Tinhofer, I. Spheroid Culture of Head and Neck Cancer Cells Reveals an Important Role of EGFR Signalling in Anchorage Independent Survival. PLoS ONE 2016, 11, e0163149. [Google Scholar] [CrossRef] [PubMed]
- Micalizzi, D.S.; Haber, D.A.; Maheswaran, S. Cancer metastasis through the prism of epithelial-to-mesenchymal transition in circulating tumor cells. Mol. Oncol. 2017, 11, 770–780. [Google Scholar] [CrossRef]
- Rudzinski, J.K.; Govindasamy, N.P.; Asgari, A.; Saito, M.S.; Lewis, J.D.; Jurasz, P. Preferential interaction of platelets with prostate cancer cells with stem cell markers. Thromb. Res. 2021, 206, 42–51. [Google Scholar] [CrossRef]
- St Hill, C.A. Interactions between endothelial selectins and cancer cells regulate metastasis. Front. Biosci. 2011, 16, 3233–3251. [Google Scholar] [CrossRef]
- Fischer, V.; Wong, M.; Li, F.Q.; Takemaru, K.I. Chibby1 knockdown promotes mesenchymal-to-epithelial transition-like changes. Cell Cycle 2017, 16, 448–456. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dellas, K.; Bache, M.; Pigorsch, S.U.; Taubert, H.; Kappler, M.; Holzapfel, D.; Zorn, E.; Holzhausen, H.J.; Haensgen, G. Prognostic impact of HIF-1alpha expression in patients with definitive radiotherapy for cervical cancer. Strahlenther. Onkol. 2008, 184, 169–174. [Google Scholar] [CrossRef]
- Hou, T.; Zhang, W.; Tong, C.; Kazobinka, G.; Huang, X.; Huang, Y.; Zhang, Y. Putative stem cell markers in cervical squamous cell carcinoma are correlated with poor clinical outcome. BMC Cancer 2015, 15, 785. [Google Scholar] [CrossRef]
- Shen, L.; Huang, X.; Xie, X.; Su, J.; Yuan, J.; Chen, X. High Expression of SOX2 and OCT4 Indicates Radiation Resistance and an Independent Negative Prognosis in Cervical Squamous Cell Carcinoma. J. Histochem. Cytochem. 2014, 62, 499–509. [Google Scholar] [CrossRef]
- Zamulaeva, I.A.; Selivanova, E.I.; Kiseleva, V.I.; Matchuk, O.N.; Krikunova, L.I.; Mkrtchyan, L.S.; Kaprin, A.D. Correlation of Radiation Response of Cervical Cancer Stem Cells with Their Initial Number before Treatment and Molecular Genetic Features of Papillomavirus Infection. Bull. Exp. Biol. Med. 2020, 170, 241–245. [Google Scholar] [CrossRef]
- Jarosz-Biej, M.; Smolarczyk, R.; Cichoń, T.; Kułach, N. Tumor Microenvironment as A “Game Changer” in Cancer Radiotherapy. Int. J. Mol. Sci. 2019, 20, 3212. [Google Scholar] [CrossRef]
- Joura, E.A.; Ulied, A.; Vandermeulen, C.; Rua Figueroa, M.; Seppä, I.; Hernandez Aguado, J.J.; Ahonen, A.; Reich, O.; Virta, M.; Perino, A.; et al. Immunogenicity and safety of a nine-valent human papillomavirus vaccine in women 27–45 years of age compared to women 16–26 years of age: An open-label phase 3 study. Vaccine 2021, 39, 2800–2809. [Google Scholar] [CrossRef]
- Ge, Y.; Liu, Y.; Cheng, Y.; Liu, Y. Predictors of recurrence in patients with high-grade cervical intraepithelial neoplasia after cervical conization. Medicine 2021, 100, e26359. [Google Scholar] [CrossRef]
- Hong, K.O.; Kim, J.H.; Hong, J.S.; Yoon, H.J.; Lee, J.I.; Hong, S.P.; Hong, S.D. Inhibition of Akt activity induces the mesenchymal-to-epithelial reverting transition with restoring E-cadherin expression in KB and KOSCC-25B oral squamous cell carcinoma cells. J. Exp. Clin. Cancer Res. 2009, 28, 28. [Google Scholar] [CrossRef]
- De Amicis, F.; Perri, A.; Vizza, D.; Russo, A.; Panno, M.L.; Bonofiglio, D.; Giordano, C.; Mauro, L.; Aquila, S.; Tramontano, D.; et al. Epigallocatechin gallate inhibits growth and epithelial-to-mesenchymal transition in human thyroid carcinoma cell lines. J. Cell. Physiol. 2013, 228, 2054–2062. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Zhang, S.; Miao, Y.; Zhang, Y.; Wen, F.; Guo, K. Down-regulation of Frizzled-7 expression inhibits migration, invasion, and epithelial-mesenchymal transition of cervical cancer cell lines. Med. Oncol. 2015, 32, 102. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Tan, R.; Dai, C.; Li, Y.; Wang, D.; Hao, S.; Kahn, M.; Liu, Y. Plasminogen activator inhibitor-1 is a transcriptional target of the canonical pathway of Wnt/beta-catenin signaling. J. Biol. Chem. 2010, 285, 24665–24675. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, S.; Li, W.; Chen, J.; Xiao, X.; Wang, Y.; Yan, G.; Chen, L. Inactivation of lysyl oxidase by β-aminopropionitrile inhibits hypoxia-induced invasion and migration of cervical cancer cells. Oncol. Rep. 2013, 29, 541–548. [Google Scholar] [CrossRef] [PubMed]

| E5 Activity | Effect on HPV-Infected EC |
|---|---|
| Inhibition of EGFR degradation, enhancement of ET-1 growth effect, p21 and p27 downregulation, cx43 counteraction | Proliferation |
| Downregulation of the expression of epithelial FGFR2b | Lack of differentiation |
| Reduction in CD1d and MHC levels on the plasma membrane | Impaired clearance by immune cells |
| Fas downregulation and Bax degradation | Survival |
| Induction of the expression of mesenchymal FGFR2c, activation of AKT and MAPK | EMT and tumorigenic behavior |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barillari, G.; Bei, R.; Manzari, V.; Modesti, A. Infection by High-Risk Human Papillomaviruses, Epithelial-to-Mesenchymal Transition and Squamous Pre-Malignant or Malignant Lesions of the Uterine Cervix: A Series of Chained Events? Int. J. Mol. Sci. 2021, 22, 13543. https://doi.org/10.3390/ijms222413543
Barillari G, Bei R, Manzari V, Modesti A. Infection by High-Risk Human Papillomaviruses, Epithelial-to-Mesenchymal Transition and Squamous Pre-Malignant or Malignant Lesions of the Uterine Cervix: A Series of Chained Events? International Journal of Molecular Sciences. 2021; 22(24):13543. https://doi.org/10.3390/ijms222413543
Chicago/Turabian StyleBarillari, Giovanni, Roberto Bei, Vittorio Manzari, and Andrea Modesti. 2021. "Infection by High-Risk Human Papillomaviruses, Epithelial-to-Mesenchymal Transition and Squamous Pre-Malignant or Malignant Lesions of the Uterine Cervix: A Series of Chained Events?" International Journal of Molecular Sciences 22, no. 24: 13543. https://doi.org/10.3390/ijms222413543
APA StyleBarillari, G., Bei, R., Manzari, V., & Modesti, A. (2021). Infection by High-Risk Human Papillomaviruses, Epithelial-to-Mesenchymal Transition and Squamous Pre-Malignant or Malignant Lesions of the Uterine Cervix: A Series of Chained Events? International Journal of Molecular Sciences, 22(24), 13543. https://doi.org/10.3390/ijms222413543







