The Chemokine Systems at the Crossroads of Inflammation and Energy Metabolism in the Development of Obesity
Abstract
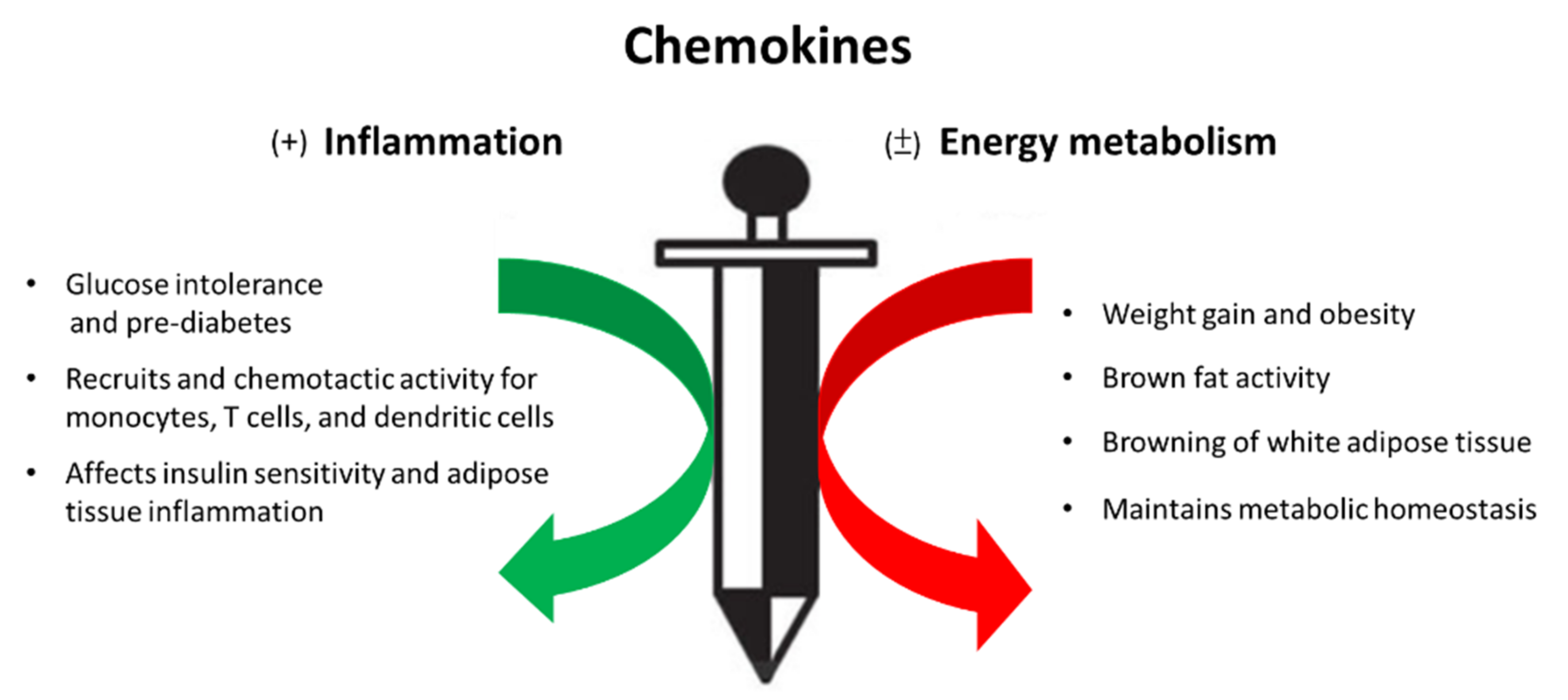
1. Introduction
2. Chemokines in Obesity and Metabolic Syndrome
3. The Involvement of Chemokines and Chemokine Receptors in the Development of Inflammation and Energy Metabolism
3.1. CCL5
3.2. CX3CL1-CX3CR1 Signaling
3.3. CXCL12-CXCR4 Signaling
3.4. CXCL14
4. The Cellular and Molecular Mechanisms of Chemokines and Chemokine Receptors in the Regulation of Inflammation and Energy Metabolism
4.1. The Cellular and Molecular Mechanisms of Chemokine-Mediated Tissue Inflammation
4.2. The Cellular and Molecular Mechanisms of Chemokine and Chemokine Effects on Energy Metabolism
4.3. The Potential Effects of Post-Translational Modification of Chemokines on Inflammation and Energy Dysregulation in the Development of Obesity
5. Modulation of the Chemokine/Chemokine Receptor Axis as a Novel Approach for Treatment of Morbid Obesity
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Surmi, B.K.; Hasty, A.H. The role of chemokines in recruitment of immune cells to the artery wall and adipose tissue. Vasc. Pharmacol. 2010, 52, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Painter, J.D.; Akbari, O. Type 2 Innate lymphoid cells: Protectors in type 2 diabetes. Front. Immunol. 2021, 12, 727008. [Google Scholar] [CrossRef]
- Mahlakõiv, T.; Flamar, A.-L.; Johnston, L.K.; Moriyama, S.; Putzel, G.G.; Bryce, P.J.; Artis, D. Stromal cells maintain immune cell homeostasis in adipose tissue via production of interleukin-33. Sci. Immunol. 2019, 4, 416. [Google Scholar] [CrossRef] [PubMed]
- Bolus, W.; Hasty, A.H. Contributions of innate type 2 inflammation to adipose function. J. Lipid Res. 2019, 60, 1698–1709. [Google Scholar] [CrossRef]
- Rao, R.R.; White, J.P.; Svensson, K.J.; Lou, J.; Lokurkar, I.; Jedrychowski, M.P.; Ruas, J.L.; Wrann, C.D.; Lo, J.C.; Camera, D.M.; et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 2014, 5, 1279–1291. [Google Scholar]
- Boulenouar, S.; Michelet, X.; Duquette, D.; Alvarez, D.; Hogan, A.E.; Dold, C.; O’Connor, D.; Stutte, S.; Tavakkoli, A.; Winters, D.; et al. Adipose type one innate lymphoid cells regulate macrophage homeostasis through targeted cytotoxicity. Immunity 2017, 46, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Bachelerie, F.; Ben-Baruch, A.; Burkhardt, A.M.; Combadiere, C.; Farber, J.M.; Graham, G.J.; Horuk, R.; Sparre-Ulrich, A.H.; Locati, M.; Luster, A.D.; et al. International union of basic and clinical pharmacology. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol. Rev. 2013, 11, 1–79. [Google Scholar] [CrossRef]
- Ikeda, K.; Yamada, T. UCP1 dependent and independent thermogenesis in brown and beige adipocytes. Front. Endocrinol. 2020, 11, 498. [Google Scholar] [CrossRef]
- Rakotoarivelo, V.; Variya, B.; Ilangumaran, S.; Langlois, M.-F.; Ramanathan, S. Inflammation in human adipose tissues-Shades of gray, rather than white and brown. Cytokine Growth Factor Rev. 2018, 44, 28–37. [Google Scholar]
- Rakotoarivelo, V.; Lacraz, G.; Mayhue, M.; Brown, C.; Rottembourg, D.; Fradette, J.; Ilangumaran, S.; Menendez, A.; Langlois, M.-F.; Ramanathan, S. Inflammatory cytokine profiles in visceral and subcutaneous adipose tissues of obese patients undergoing bariatric surgery reveal lack of correlation with obesity or diabetes. EBioMedicine 2018, 30, 237–247. [Google Scholar]
- Pessentheiner, A.R.; Ducasa, G.M.; Gordts, P.L.S.M. Proteoglycans in obesity-associated metabolic dysfunction and meta-inflammation. Front. Immunol. 2020, 11, 769. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Herlea-Pana, O.; Heuser-Baker, J.; Chen, Y.; Barlic-Dicen, J. Roles of the chemokine system in development of obesity, insulin resistance, and cardiovascular disease. J. Immunol. Res. 2014, 2014, 181450. [Google Scholar] [CrossRef] [PubMed]
- Kanda, H.; Tateya, S.; Tamori, Y.; Kotani, K.; Hiasa, K.-I.; Kitazawa, R.; Kitazawa, S.; Miyachi, H.; Maeda, S.; Egashira, K.; et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Investig. 2006, 116, 1494–1505. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, D.; Das, S.; Guria, S.; Basu, S.; Mukherjee, S. Fetuin-A regulates adipose tissue macrophage content and activation in insulin resistant mice through MCP-1 and iNOS: Involvement of IFNγ-JAK2-STAT1 pathway. Biochem. J. 2021, 478, 4027–4043. [Google Scholar] [CrossRef]
- Dommel, S.; Blüher, M. Does C-C motif chemokine ligand 2 (CCL2) link obesity to a pro-inflammatory state? Int. J. Mol. Sci. 2021, 22, 1500. [Google Scholar] [CrossRef]
- Kamei, N.; Tobe, K.; Suzuki, R.; Ohsugi, M.; Watanabe, T.; Kubota, N.; Ohtsuka-Kowatari, N.; Kumagai, K.; Sakamoto, K.; Kobayashi, M.; et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J. Biol. Chem. 2006, 281, 26602–26614. [Google Scholar]
- Inouye, K.E.; Shi, H.; Howard, J.K.; Daly, C.H.; Lord, G.M.; Rollins, B.J.; Flier, J.S. Absence of CC chemokine ligand 2 does not limit obesity-associated infiltration of macrophages Into Adipose Tissue. Diabetes 2007, 56, 2242–2250. [Google Scholar] [CrossRef]
- Laurent, V.; Guérard, A.; Mazerolles, C.; Le Gonidec, S.; Toulet, A.; Nieto, L.; Zaidi, F.; Majed, B.; Garandeau, D.; Socrier, Y.; et al. Periprostatic adipocytes act as a driving force for prostate cancer progression in obesity. Nat. Commun. 2016, 12, 10230. [Google Scholar]
- Kochumon, S.; Al-Rashed, F.; Abu-Farha, M.; Devarajan, S.; Tuomilehto, J.; Ahmad, R. Adipose tissue expression of CCL19 chemokine is positively associated with insulin resistance. Diabetes Metab. Res. Rev. 2019, 35, e3087. [Google Scholar]
- Keophiphath, M.; Rouault, C.; Divoux, A.; Clément, K.; Lacasa, D. CCL5 promotes macrophage recruitment and survival in human adipose tissue. Arter. Thromb. Vasc. Biol. 2010, 30, 39–45. [Google Scholar] [CrossRef]
- Cereijo, R.; Gavaldà-Navarro, A.; Montserrat, C.; Quesada-López, T.; Villarroya, J.; Morón-Ros, S.; Sànchez-Infantes, D.; Peyrou, M.; Iglesias, R.; Mampel, T.; et al. CXCL14, a brown adipokine that mediates brown-fat-to-macrophage communication in thermogenic adaptation. Cell Metab. 2018, 28, 750–763. [Google Scholar]
- Wang, S.-W.; Wu, H.-H.; Liu, S.-C.; Wang, P.-C.; Ou, W.-C.; Chou, W.-Y.; Shen, Y.-S.; Tang, C.-H. CCL5 and CCR5 interaction promotes cell motility in human osteosarcoma. PLoS ONE 2012, 7, e35101. [Google Scholar]
- Long, H.; Xie, R.; Xiang, T.; Zhao, Z.; Lin, S.; Liang, Z.; Chen, Z.; Zhu, B. Autocrine CCL5 signaling promotes invasion and migration of CD133+ ovarian cancer stem-like cells via NF-κB-mediated MMP-9 upregulation. Stem Cells 2012, 30, 2309–2319. [Google Scholar]
- Li, M.; Sun, X.; Zhao, J.; Xia, L.; Li, J.; Xu, M.; Wang, B.; Guo, H.; Yu, C.; Gao, Y.; et al. CCL5 deficiency promotes liver repair by improving inflammation resolution and liver regeneration through M2 macrophage polarization. Cell. Mol. Immunol. 2020, 17, 753–764. [Google Scholar]
- Slominski, B.; Lawrynowicz, U.; Ryba-Stanislawowska, M.; Skrzypkowska, M.; Mysliwska, J.; Mysliwiec, M. CCR5-Δ32 polymorphism is a genetic risk factor associated with dyslipidemia in patients with type 1 diabetes. Cytokine 2019, 114, 81–85. [Google Scholar]
- Inayat, H.; Azim, M.K.; Baloch, A.A. Analysis ofinflammatory gene expression profile of peripheral blood leukocytes in type 2 diabetes. Immunol. Investig. 2019, 48, 618–631. [Google Scholar]
- Ota, T. Chemokine systems link obesity to insulin resistance. Diabetes Metab. J. 2013, 37, 165–172. [Google Scholar] [CrossRef]
- Dworacka, M.; Krzyzagorska, E.; Iskakova, S.; Bekmukhambetov, Y.; Urazayev, O.; Dworacki, G. Increased circulating RANTES in type 2 diabetes. Eur. Cytokine Netw. 2014, 25, 46–51. [Google Scholar]
- Ahnstedt, H.; Roy-O’Reilly, M.; Spychala, M.S.; Mobley, A.S.; Bravo-Alegria, J.; Chauhan, A.; Aronowski, J.; Marrelli, S.P.; McCullough, L.D. Sex differences in adipose tissue CD8 + T Cells and regulatory T cells in middle-aged mice. Front. Immunol. 2018, 4, 659. [Google Scholar]
- Braunersreuther, V.; Pellieux, C.; Pelli, G.; Burger, F.; Steffens, S.; Montessuit, C.; Weber, C.; Proudfoot, A.; Mach, F.; Arnaud, C. Chemokine CCL5/RANTES inhibition reduces myocardial reperfusion injury in atherosclerotic mice. J. Mol. Cell. Cardiol. 2010, 48, 789–798. [Google Scholar]
- Walens, A.; DiMarco, A.V.; Lupo, R.; Kroger, B.R.; Damrauer, J.S.; Alvarez, J.V. CCL5 promotes breast cancer recurrence through macrophage recruitment in residual tumors. eLife 2019, 16, e43653. [Google Scholar]
- Kim, E.; Kim, Y.; Kim, S.; Kim, J.; Tian, Y.; Doh, E.; Lee, D.; Chung, J. Adipochemokines induced by ultraviolet irradiation contribute to impaired fat metabolism in subcutaneous fat cells. Br. J. Dermatol. 2018, 178, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.-Y.; Ajoy, R.; Changou, C.A.; Hsieh, Y.-T.; Wang, Y.-K.; Hoffer, B. CCL5/RANTES contributes to hypothalamic insulin signaling for systemic insulin responsiveness through CCR5. Sci. Rep. 2016, 29, 37659. [Google Scholar] [CrossRef] [PubMed]
- Baturcam, E.; Abubaker, J.; Tiss, A.; Abu-Farha, M.; Khadir, A.; Al-Ghimlas, F.; Al-Khairi, I.; Cherian, P.; Elkum, N.; Hammad, M.; et al. Physical exercise reduces the expression of RANTES and its CCR5 receptor in the adipose tissue of obese humans. Mediat. Inflamm. 2014, 2014, 627150. [Google Scholar] [CrossRef]
- Chan, P.-C.; Hung, L.-M.; Huang, J.-P.; Day, Y.-J.; Yu, C.-L.; Kuo, F.-C.; Lu, C.-H.; Tian, Y.-F.; Hsieh, P.-S. Augmented CCL5/CCR5 signaling in brown adipose tissue inhibits adaptive thermogenesis and worsens insulin resistance in obesity. Clin. Sci. 2021. [Google Scholar] [CrossRef]
- Apostolakis, S.; Spandidos, D. Chemokines and atherosclerosis: Focus on the CX3CL1/CX3CR1 pathway. Acta Pharmacol. Sin. 2013, 34, 1251–1256. [Google Scholar] [CrossRef]
- Shah, R.; Hinkle, C.C.; Ferguson, J.F.; Mehta, N.N.; Li, M.; Qu, L.; Lu, Y.; Putt, M.E.; Ahima, R.S.; Reilly, M.P. Fractalkine is a novel human adipochemokine associated with type 2 diabetes. Diabetes 2011, 60, 1512–1518. [Google Scholar] [CrossRef]
- Lee, Y.S.; Morinaga, H.; Kim, J.J.; Lagakos, W.; Taylor, S.; Keshwani, M.; Perkins, G.; Dong, H.; Kayali, A.G.; Sweet, I.R.; et al. The Fractalkine/CX3CR1 system regulates β cell function and insulin secretion. Cell 2013, 153, 413–425. [Google Scholar] [CrossRef]
- Morari, J.; Anhe, G.F.; Nascimento, L.F.; de Moura, R.F.; Razolli, D.; Solon, C.; Guadagnini, D.; Souza, G.; Mattos, A.H.; Tobar, N.; et al. Fractalkine (CX3CL1) is involved in the early activation of hypothalamic inflammation in experimental obesity. Diabetes 2014, 63, 3770–3784. [Google Scholar] [CrossRef]
- Polyak, A.; Ferenczi, S.; Denes, A.; Winkler, Z.; Kriszt, R.; Pinter-Kubler, B.; Kovacs, K.J. The fractalkine/Cx3CR1 system is implicated in the development of metabolic visceral adipose tissue inflammation in obesity. Brain Behav. Immun. 2014, 38, 25–35. [Google Scholar] [CrossRef][Green Version]
- Polyak, A.; Winkler, Z.; Kuti, D.; Ferenczi, S.; Kovacs, K.J. Brown adipose tissue in obesity: Fractalkine-receptor dependent immune cell recruitment affects metabolic-related gene expression. Biochim. Biophys. Acta 2016, 186, 1614–1622. [Google Scholar] [CrossRef]
- Nagashimada, M.; Sawamoto, K.; Ni, Y.; Kitade, H.; Nagata, N.; Xu, L.; Kobori, M.; Mukaida, N.; Yamashita, T.; Kaneko, S.; et al. CX3CL1-CX3CR1 Signaling Deficiency Exacerbates Obesity-induced Inflammation and Insulin Resistance in Male Mice. Endocrinology 2021, 162, bqab064. [Google Scholar] [CrossRef]
- Hill, W.D.; Hess, D.C.; Martin-Studdard, A.; Carothers, J.J.; Zheng, J.; Hale, D.; Maeda, M.; Fagan, S.C.; Carroll, J.E.; Conway, S.J. SDF-1 (CXCL12) is upregulated in the ischemic penumbra following stroke: Association with bone marrow cell homing to injury. J. Neuropathol. Exp. Neurol. 2004, 63, 84–96. [Google Scholar] [CrossRef]
- Kim, D.; Kim, J.; Yoon, J.H.; Ghim, J.; Yea, K.; Song, P.; Park, S.; Lee, A.; Hong, C.P.; Jang, M.S.; et al. CXCL12 secreted from adipose tissue recruits macrophages and induces insulin resistance in mice. Diabetologia 2014, 57, 1456–1465. [Google Scholar] [CrossRef]
- Burger, J.A.; Kipps, T.J. CXCR4: A key receptor in the crosstalk between tumor cells and their microenvironment. Blood 2006, 107, 1761–1767. [Google Scholar] [CrossRef]
- Yao, L.; Heuser-Baker, J.; Herlea-Pana, O.; Zhang, N.; Szweda, L.I.; Griffin, T.M.; Barlic-Dicen, J. Deficiency in adipocyte chemokine receptor CXCR4 exacerbates obesity and compromises thermoregulatory responses of brown adipose tissue in a mouse model of diet-induced obesity. FASEB J. 2014, 28, 4534–4550. [Google Scholar] [CrossRef]
- Hazan, U.; Romero, I.A.; Cancello, R.; Valente, S.; Perrin, V.; Mariot, V.; Dumonceaux, J.; Gerhardt, C.C.; Strosberg, A.D.; Couraud, P.-O.; et al. Human adipose cells express CD4, CXCR4, and CCR5 receptors: A new target cell type for the immunodeficiency virus-1? FASEB J. 2002, 16, 1254–1256. [Google Scholar] [CrossRef]
- Girousse, A.; Gil-Ortega, M.; Bourlier, V.; Bergeaud, C.; Sastourné-Arrey, Q.; Moro, C.; Barreau, C.; Guissard, C.; Vion, J.; Arnaud, E.; et al. The release of adipose stromal cells from subcutaneous adipose tissue regulates ectopic intramuscular adipocyte deposition. Cell Rep. 2019, 27, 323–333. [Google Scholar] [CrossRef]
- Kurita, K.; Ishikawa, K.; Takeda, K.; Fujimoto, M.; Ono, H.; Kumagai, J.; Inoue, H.; Yokoh, H.; Yokote, K. CXCL12-CXCR4 pathway activates brown adipocytes and induces insulin resistance in CXCR4-deficient mice under high-fat diet. Sci. Rep. 2019, 9, 6165. [Google Scholar] [CrossRef]
- Nara, N.; Nakayama, Y.; Okamoto, S.; Tamura, H.; Kiyono, M.; Muraoka, M.; Tanaka, K.; Taya, C.; Shitara, H.; Ishii, R.; et al. Disruption of CXC motif chemokine ligand-14 in mice ameliorates obesity-induced insulin resistance. J. Biol. Chem. 2007, 282, 30794–30803. [Google Scholar] [CrossRef]
- Tanegashima, K.; Okamoto, S.; Nakayama, Y.; Taya, C.; Shitara, H.; Ishii, R.; Yonekawa, H.; Minokoshi, Y.; Hara, T. CXCL14 deficiency in mice attenuates obesity and inhibits feeding behavior in a novel environment. PLoS ONE 2010, 5, e10321. [Google Scholar] [CrossRef]
- Hara, T.; Nakayama, Y. CXCL14 and insulin action. Vitam. Horm. 2009, 80, 107–123. [Google Scholar] [CrossRef]
- Atanes, P.; Hawkes, R.G.; Olaniru, O.E.; Ruz-Maldonado, I.; Amisten, S.; Persaud, S.J. CXCL14 inhibits insulin secretion Independently of CXCR4 or CXCR7 receptor activation or cAMP inhibition. Cell. Physiol. Biochem. 2019, 52, 879–892. [Google Scholar] [CrossRef]
- Takahashi, M.; Takahashi, Y.; Takahashi, K.; Zolotaryov, F.N.; Hong, K.S.; Iida, K.; Okimura, Y.; Kaji, H.; Chihara, K. CXCL14 enhances insulin-dependent glucose uptake in adipocytes and is related to high-fat diet-induced obesity. Biochem. Biophys. Res. Commun. 2007, 364, 1037–1042. [Google Scholar] [CrossRef]
- Nibbs, R.J.; Graham, G.J. Immune regulation by atypical chemokine receptors. Nat. Rev. Immunol. 2013, 13, 815–829. [Google Scholar] [CrossRef]
- Vaillant, A.A.J.; Vashisht, R.; Zito, P.M. Immediate Hypersensitivity Reactions; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Yao, C.; Narumiya, S. Prostaglandin-cytokine crosstalk in chronic inflammation. Br. J. Pharmacol. 2019, 176, 337–354. [Google Scholar] [CrossRef] [PubMed]
- Tirone, M.; Tran, N.L.; Ceriotti, C.; Gorzanelli, A.; Canepari, M.; Bottinelli, R.; Raucci, A.; Di Maggio, S.; Santiago, C.; Mellado, M.; et al. High mobility group box 1 orchestrates tissue regeneration via CXCR4. J. Exp. Med. 2018, 215, 303–318. [Google Scholar] [CrossRef]
- Castan, L.; Magnan, A.; Bouchaud, G. Chemokine receptors in allergic diseases. Allergy 2016, 72, 682–690. [Google Scholar] [CrossRef]
- Le Thuc, O.; Blondeau, N.; Nahon, J.-L.; Rovère, C. The complex contribution of chemokines to neuroinflammation: Switching from beneficial to detrimental effects. Ann. N. Y. Acad. Sci. 2015, 1351, 127–140. [Google Scholar] [CrossRef]
- Nishimura, M.; Kuboi, Y.; Muramoto, K.; Kawano, T.; Imai, T. Chemokines as novel therapeutic targets for inflammatory bow-el disease. Ann. N. Y. Acad. Sci. 2009, 1173, 350–356. [Google Scholar] [CrossRef]
- van der Vorst, E.P.; Döring, Y.; Weber, C. Chemokines. Arterioscler. Thromb. Vasc. Biol. 2015, 35, e52–e56. [Google Scholar] [CrossRef]
- Nagarsheth, N.; Wicha, M.S.; Zou, W. Chemokines in the cancer microenvironment and their relevance in cancer immuno-therapy. Nat. Rev. Immunol. 2017, 17, 559–572. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Zhou, R.; Tschopp, J. The NLRP3 inflammasome: A sensor for metabolic danger? Science 2010, 327, 296–300. [Google Scholar] [CrossRef]
- Maedler, K.; Sergeev, P.; Ris, F.; Oberholzer, J.; Joller-Jemelka, H.I.; Spinas, G.A.; Kaiser, N.; Halban, P.A.; Donath, M.Y. Glu-cose-induced beta cell production of IL-1β contributes to glucotoxicity in human pancreatic islets. J. Clin. Investig. 2017, 127, 1589. [Google Scholar] [CrossRef]
- Ferrari, S.M.; Fallahi, P.; Ruffilli, I.; Elia, G.; Ragusa, F.; Paparo, S.R.; Patrizio, A.; Mazzi, V.; Colaci, M.; Giuggioli, D.; et al. Immunomodulation of CXCL10 secretion by hepatitis C virus: Could CXCL10 be a prognostic marker of chronic hepatitis C? J. Immunol. Res. 2019, 2019, 5878960. [Google Scholar] [CrossRef]
- Singh, K.P.; Zerbato, J.M.; Zhao, W.; Braat, S.; Deleage, C.; Tennakoon, G.S.; Mason, H.; Dantanarayana, A.; Rhodes, A.; Rhodes, J.W.; et al. Intrahepatic CXCL10 is strongly associated with liver fibrosis in HIV-Hepatitis B co-infection. PLoS Pathog. 2020, 16, e1008744. [Google Scholar] [CrossRef]
- Nishimura, S.; Manabe, I.; Nagasaki, M.; Eto, K.; Yamashita, H.; Ohsugi, M.; Otsu, M.; Hara, K.; Ueki, K.; Sugiura, S.; et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 2009, 15, 914–920. [Google Scholar] [CrossRef]
- Winer, S.; Chan, Y.; Paltser, G.; Truong, D.; Tsui, H.; Bahrami, J.; Dorfman, R.; Wang, Y.; Zielenski, J.; Mastronardi, F.; et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat. Med. 2009, 15, 921–929. [Google Scholar] [CrossRef]
- Shadidi, K.R.; Aarvak, T.; Henriksen, J.E.; Natvig, J.B.; Thompson, K.M. The chemokines CCL5, CCL2 and CXCL12 play sig-nificant roles in the migration of Th1 cells into rheumatoid synovial tissue. Scand. J. Immunol. 2003, 57, 192–198. [Google Scholar] [CrossRef]
- Bahlas, S.; Damiati, L.; Dandachi, N.; Sait, H.; Alsefri, M.; Pushparaj, P.N. Rapid immunoprofiling of cytokines, chemokines and growth factors in patients with active rheumatoid arthritis using luminex multiple analyte profiling technology for precision medicine. Clin. Exp. Rheumatol. 2019, 37, 112–119. [Google Scholar]
- Roca, H.; Varsos, Z.S.; Pienta, K. CCL2 Is a negative regulator of AMP-activated proteinkKinase to sustain mTOR Complex-1 activation, survivin expression, and cell survival in human prostate cancer PC3 cells. Neoplasia 2009, 11, 1309–1317. [Google Scholar] [CrossRef]
- Hayashi, M.; Iwashita, M.; Nishimura, Y.; Shinjo, T.; Sano, T.; Yamashita, A.; Fukuda, T.; Sanui, T.; Asano, T.; Nishimura, F. Adi-pose-specific C-C motif chemokine ligand (CCL) 19 overexpression drives the mice to both insulin resistance and weight gain. BMJ Open Diabetes Res. Care 2021, 9, e001871. [Google Scholar] [CrossRef]
- Joven, J.; Rull, A.; Rodriguez-Gallego, E.; Camps, J.; Riera-Borrull, M.; Hernández-Aguilera, A.; Martin-Paredero, V.; Segura-Carretero, A.; Micol, V.; Alonso-Villaverde, C.; et al. Multifunctional targets of dietary polyphenols in disease: A case for the chemokine net-work and energy metabolism. Food Chem. Toxicol. 2013, 51, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Proudfoot, A.E.I.; Handel, T.M.; Johnson, Z.; Lau, E.K.; LiWang, P.; Clark-Lewis, I.; Borlat, F.; Wells, T.; Kosco-Vilbois, M.H. Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines. Proc. Natl. Acad. Sci. USA 2003, 100, 1885–1890. [Google Scholar] [CrossRef]
- Sharif, O.; Brunner, J.S.; Vogel, A.; Schabbauer, G. Macrophage rewiring by nutrient associated PI3K dependent pPathways. Front. Immunol. 2019, 10, 2002. [Google Scholar] [CrossRef]
- McCormick, B.; Chu, J.Y.; Vermeren, S. Cross-talk between Rho GTPases and PI3K in the neutrophil. Small GTPases 2017, 10, 187–195. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, X.; Miao, W.; Wang, B.; Qiu, Y. CXCL8 promotes the invasion of human osteosarcoma cells by regulation of PI3K/Akt signaling pathway. APMIS 2017, 125, 773–780. [Google Scholar] [CrossRef]
- Shen, X.; Artinyan, A.; Jackson, D.; Thomas, R.M.; Lowy, A.M.; Kim, J. Chemokine Receptor CXCR4 Enhances Proliferation in Pancreatic Cancer Cells Through AKT and ERK Dependent Pathways. Pancreas 2010, 39, 81–87. [Google Scholar] [CrossRef]
- Yang, Z.-Z.; Tschopp, O.; Baudry, A.; Dümmler, B.; Hynx, D.; Hemmings, B. Physiological functions of protein kinase B/Akt. Biochem. Soc. Trans. 2004, 32, 350–354. [Google Scholar] [CrossRef]
- Zhang, Y.; Guan, X.-Y.; Jiang, P. Cytokine and Chemokine Signals of T-Cell Exclusion in Tumors. Front. Immunol. 2020, 11, 594609. [Google Scholar] [CrossRef]
- Chan, O.; Burke, J.D.; Gao, D.F.; Fish, E.N. The chemokine CCL5 regulates glucose uptake and AMP kinase signaling in activated T cells to facilitate chemotaxis. J. Biol. Chem. 2012, 287, 29406–29416. [Google Scholar] [CrossRef]
- Chien, H.-C.; Chan, P.-C.; Tu, C.-C.; Day, Y.-J.; Hung, L.-M.; Juan, C.-C.; Tian, Y.-F.; Hsieh, P.-S. Importance of PLC-Dependent PI3K/AKT and AMPK Signaling in RANTES/CCR5 Mediated Macrophage Chemotaxis. Chin. J. Physiol. 2018, 61, 266–279. [Google Scholar] [CrossRef]
- Loos, T.; Mortier, A.; Gouwy, M.; Ronsse, I.; Put, W.; Lenaerts, J.-P.; Van Damme, J.; Proost, P. Citrullination of CXCL10 and CXCL11 by peptidylarginine deiminase: A naturally occurring posttranslational modification of chemokines and new dimen-sion of immunoregulation. Blood 2008, 112, 2648–2656. [Google Scholar] [CrossRef]
- Struyf, S.; Noppen, S.; Loos, T.; Mortier, A.; Gouwy, M.; Verbeke, H.; Huskens, D.; Luangsay, S.; Parmentier, M.; Geboes, K. Citrullination of CXCL12 differentially reduces CXCR4 and CXCR7 binding with loss of inflammatory and anti-HIV-1 activity via CXCR4. J. Immunol. 2009, 182, 666–674. [Google Scholar] [CrossRef]
- Proost, P.; Loos, T.; Mortier, A.; Schutyser, E.; Gouwy, M.; Noppen, S.; Dillen, C.; Ronsse, I.; Conings, R.; Struyf, S. Citrullina-tion of CXCL8 by peptidylarginine deiminase alters receptor usage, prevents proteolysis, and dampens tissue inflammation. J. Exp. Med. 2008, 205, 2085–2097. [Google Scholar] [CrossRef]
- Barker, C.E.; Thompson, S.; O’Boyle, G.; Lortat-Jacob, H.; Sheerin, N.S.; Ali, S.; Kirby, J.A. CCL2 nitration is a negative regula-tor of chemokine-mediated inflammation. Sci. Rep. 2017, 7, 44384. [Google Scholar] [CrossRef]
- Molon, B.; Ugel, S.; Del Pozzo, F.; Soldani, C.; Zilio, S.; Avella, D.; De Palma, A.; Mauri, P.; Monegal, A.; Rescigno, M.; et al. Chemo-kine nitration prevents intratumoral infiltration of antigen-specific T cells. J. Exp. Med. 2011, 208, 1949–1962. [Google Scholar] [CrossRef]
- Janssens, R.; Mortier, A.; Boff, D.; Vanheule, V.; Gouwy, M.; Franck, C.; Larsen, O.; Rosenkilde, M.M.; Van Damme, J.; Amaral, F.A.; et al. Natural nitration of CXCL12 reduces its signaling capacity and chemotactic activity in vitro and abrogates intra-articular lymphocyte recruitment in vivo. Oncotarget 2016, 7, 62439–62459. [Google Scholar] [CrossRef]
- Metzemaekers, M.; Van Damme, J.; Mortier, A.; Proost, P. Regulation of Chemokine Activity—A Focus on the Role of Dipeptidyl Peptidase IV/CD26. Front. Immunol. 2016, 7, 483. [Google Scholar] [CrossRef]
- Proost, P.; Struyf, S.; Van Damme, J.; Fiten, P.; Ugarte-Berzal, E.; Opdenakker, G. Chemokine isoforms and processing in in-flammation and immunity. J. Autoimmun. 2017, 85, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Van Lint, P.; Libert, C. Chemokine and cytokine processing by matrix metalloproteinases and its effect on leukocyte migra-tion and inflammation. J. Leukoc. Biol. 2007, 82, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Monneau, Y.; Arenzana-Seisdedos, F.; Lortat-Jacob, H. The sweet spot: How GAGs help chemokines guide migrating cells. J. Leukoc. Biol. 2015, 99, 935–953. [Google Scholar] [CrossRef] [PubMed]
- Salanga, C.; Handel, T. Chemokine oligomerization and interactions with receptors and glycosaminoglycans: The role of structural dynamics in function. Exp. Cell Res. 2011, 317, 590–601. [Google Scholar] [CrossRef]
- Dyer, D.P.; Thomson, J.M.; Hermant, A.; Jowitt, T.A.; Handel, T.M.; Proudfoot, A.E.I.; Day, A.; Milner, C.M. TSG-6 inhibits neutrophil migration via direct interaction with the chemokine CXCL8. J. Immunol. 2014, 192, 2177–2185. [Google Scholar] [CrossRef]
- Dyer, D.P.; Salanga, C.L.; Johns, S.C.; Valdambrini, E.; Fuster, M.M.; Milner, C.M.; Day, A.J.; Handel, T.M. The an-ti-inflammatory protein TSG-6 regulates chemokine function by inhibiting chemokine/glycosaminoglycan interactions. J. Biol. Chem. 2016, 291, 12627–12640. [Google Scholar] [CrossRef]
- Dyer, D.P.; Salanga, C.L.; Volkman, B.F.; Kawamura, T.; Handel, T.M. The dependence of chemokine–glycosaminoglycan interactions on chemokine oligomerization. Glycobiology 2015, 26, 312–326. [Google Scholar] [CrossRef]
- Liang, W.G.; Triandafillou, C.G.; Huang, T.-Y.; Zulueta, M.M.L.; Banerjee, S.; Dinner, A.R.; Hung, S.-C.; Tang, W.-J. Structural basis for oligomerization and glycosaminoglycan binding of CCL5 and CCL3. Proc. Natl. Acad. Sci. USA 2016, 113, 5000–5005. [Google Scholar] [CrossRef]
- Von Hundelshausen, P.; Koenen, R.R.; Sack, M.; Mause, S.F.; Adriaens, W.; Proudfoot, A.E.I.; Hackeng, T.M.; Weber, C. Het-erophilic interactions of platelet factor 4 and RANTES promote monocyte arrest on endothelium. Blood 2005, 105, 924–930. [Google Scholar] [CrossRef]
- Koenen, R.R.; von Hundelshausen, P.; Nesmelova, I.V.; Zernecke, A.; Liehn, E.A.; Sarabi, A.; Kramp, B.K.; Piccinini, A.M.; Paludan, S.R.; Kowalska, M.A. Disrupting functional interactions between platelet chemokines inhibits atherosclerosis in hy-perlipidemic mice. Nat. Med. 2009, 15, 97–103. [Google Scholar] [CrossRef]
- Von Hundelshausen, P.; Agten, S.M.; Eckardt, V.; Blanchet, X.; Schmitt, M.M.; Ippel, H.; Neideck, C.; Bidzhekov, K.; Leberzammer, J.; Wichapong, K.; et al. Chemokine interactome mapping enables tailored intervention in acute and chronic inflammation. Sci. Transl. Med. 2017, 9, eaah6650. [Google Scholar] [CrossRef]
- Miller, M.C.; Mayo, K.H. Chemokines from a structural perspective. Int. J. Mol. Sci. 2017, 18, 2088. [Google Scholar] [CrossRef]
- Pezhman, L.; Tahrani, A.; Chimen, M. Dysregulation of Leukocyte Trafficking in Type 2 Diabetes: Mechanisms and Po-tential Therapeutic Avenues. Front. Cell Dev. Biol. 2021, 22, 624184. [Google Scholar] [CrossRef]
- Monickaraj, F.; Oruganti, S.R.; McGuire, P.; Das, A. A potential novel therapeutic target in diabetic retinopathy: A chemokine receptor (CCR2/CCR5) inhibitor reduces retinal vascular leakage in an animal model. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 259, 93–100. [Google Scholar] [CrossRef]
- Xue, C.-B.; Wang, A.; Han, Q.; Zhang, Y.; Cao, G.; Feng, H.; Huang, T.; Zheng, C.; Xia, M.; Zhang, K.; et al. Discovery of INCB8761/PF-4136309, a potent, selective, and orally bioavailable CCR2 antagonist. ACS Med. Chem. Lett. 2011, 2, 913–918. [Google Scholar] [CrossRef]
| Chemokines | Chemokine Receptors | Brown/Beige Adipocytes | White Adipocytes | References | ||||
|---|---|---|---|---|---|---|---|---|
| Inflammation | Energy Expenditure | Inflammation | Energy Expenditure | |||||
| Pro | Anti | Pro | Anti | |||||
| CCL5 | CCR5 | ↑ | ↓ | ↓ | ↑ | ↓ | ↓ | [30,31,32,33,34,35] |
| C3XCL1 | CX3CR1 | ↑ | ↓ | ↓ | ↑ | ↓ | ↓ | [37,38,39,40,41,42] |
| CXCL12 | CXCR4 | NA | NA | ↑ | ↑ | ↓ | ↓ | [44,46,47,48,49] |
| CXCL14 | NA | NA | NA | ↑ | ↑ | ↓ | ↓ | [50,51,52,53,54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, P.-C.; Hsieh, P.-S. The Chemokine Systems at the Crossroads of Inflammation and Energy Metabolism in the Development of Obesity. Int. J. Mol. Sci. 2021, 22, 13528. https://doi.org/10.3390/ijms222413528
Chan P-C, Hsieh P-S. The Chemokine Systems at the Crossroads of Inflammation and Energy Metabolism in the Development of Obesity. International Journal of Molecular Sciences. 2021; 22(24):13528. https://doi.org/10.3390/ijms222413528
Chicago/Turabian StyleChan, Pei-Chi, and Po-Shiuan Hsieh. 2021. "The Chemokine Systems at the Crossroads of Inflammation and Energy Metabolism in the Development of Obesity" International Journal of Molecular Sciences 22, no. 24: 13528. https://doi.org/10.3390/ijms222413528
APA StyleChan, P.-C., & Hsieh, P.-S. (2021). The Chemokine Systems at the Crossroads of Inflammation and Energy Metabolism in the Development of Obesity. International Journal of Molecular Sciences, 22(24), 13528. https://doi.org/10.3390/ijms222413528






