Highly Efficient Multi-Step Oxidation Bioanode Using Microfluidic Channels
Abstract
:Highlights
- Development of microfluidic electrode system.
- Efficient electron transfer in a multi-step cascade reaction.
- Tetra-enzyme immobilized electrode.
- Eight-fold increase in current density.
1. Introduction
2. Results and Discussion
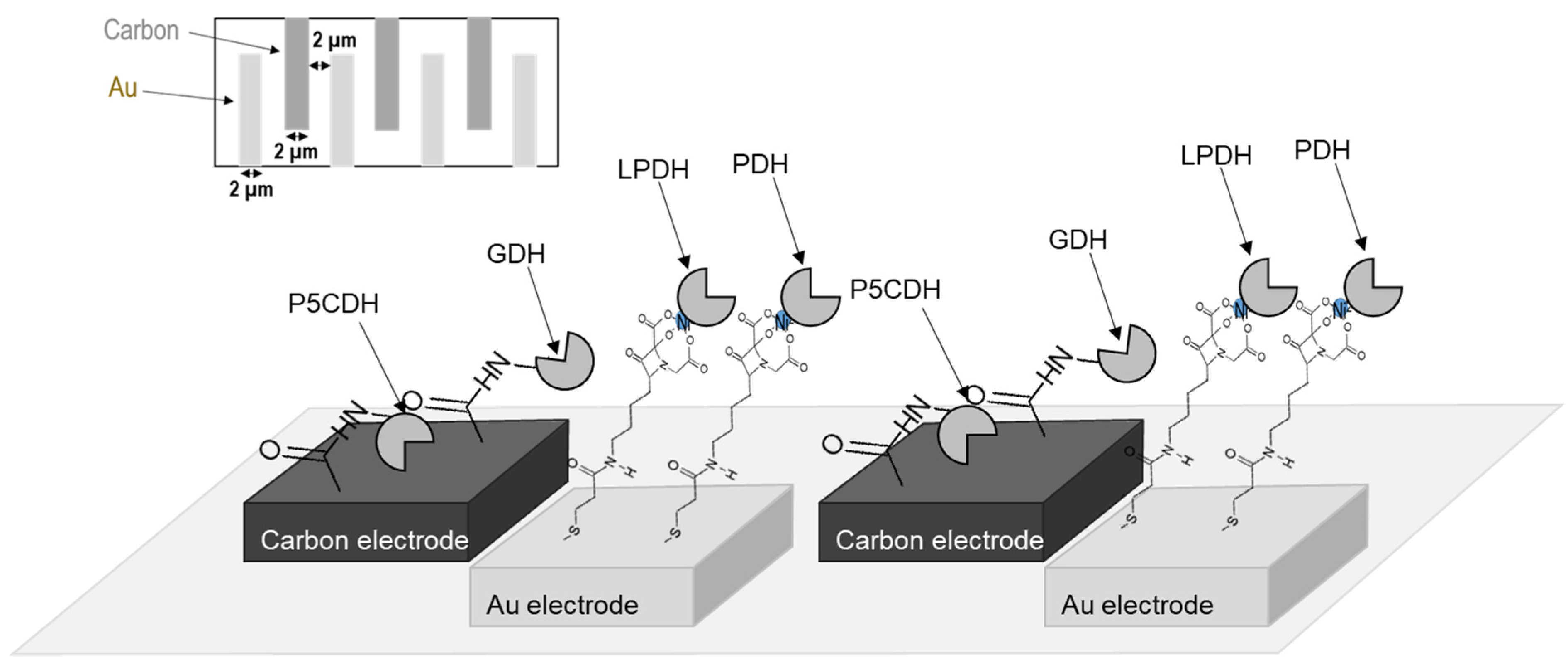
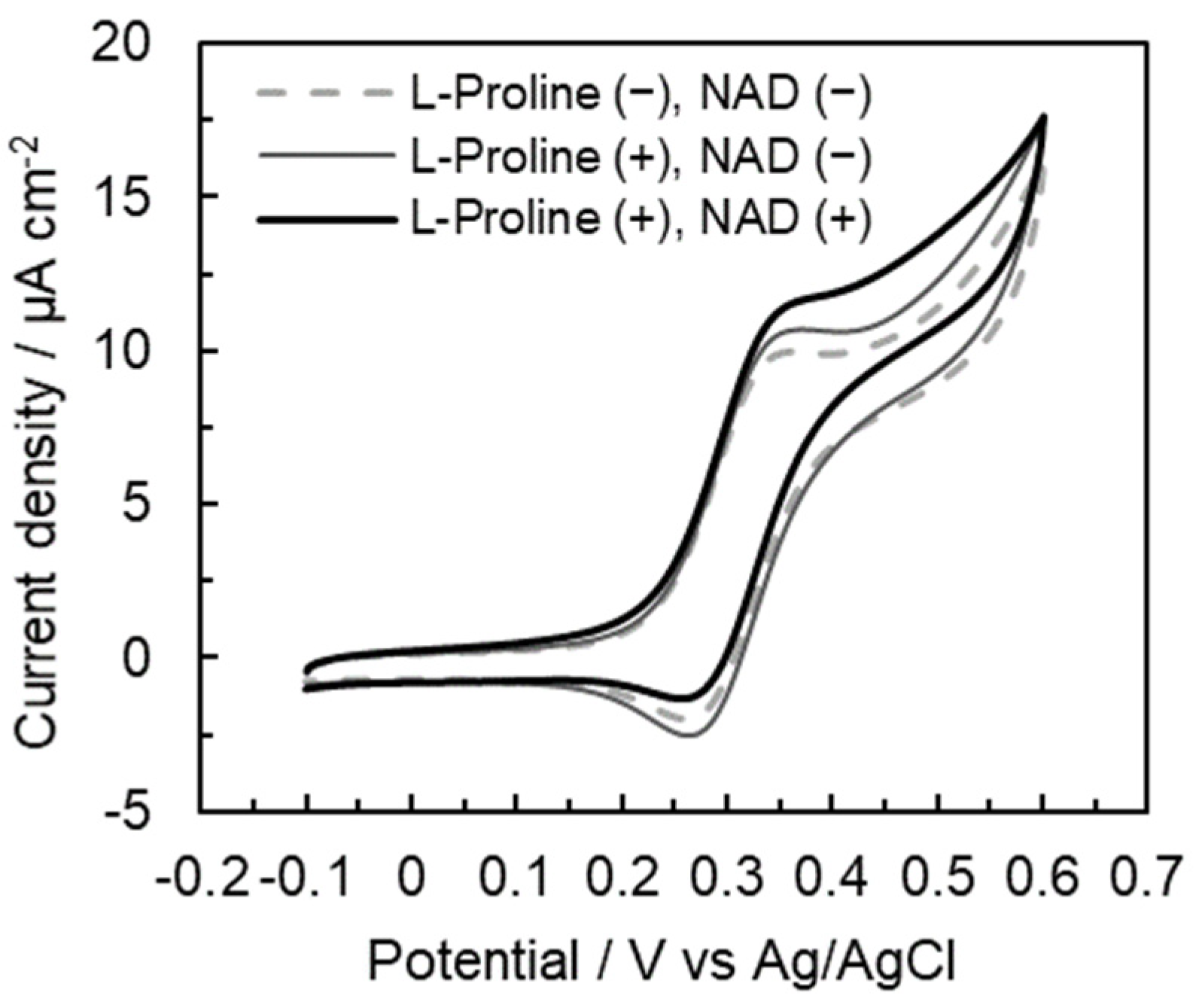
2.1. Confirmation of Electron Transfer during the Tetra-Enzymatic Cascade Reaction on the Gold Disc Electrode
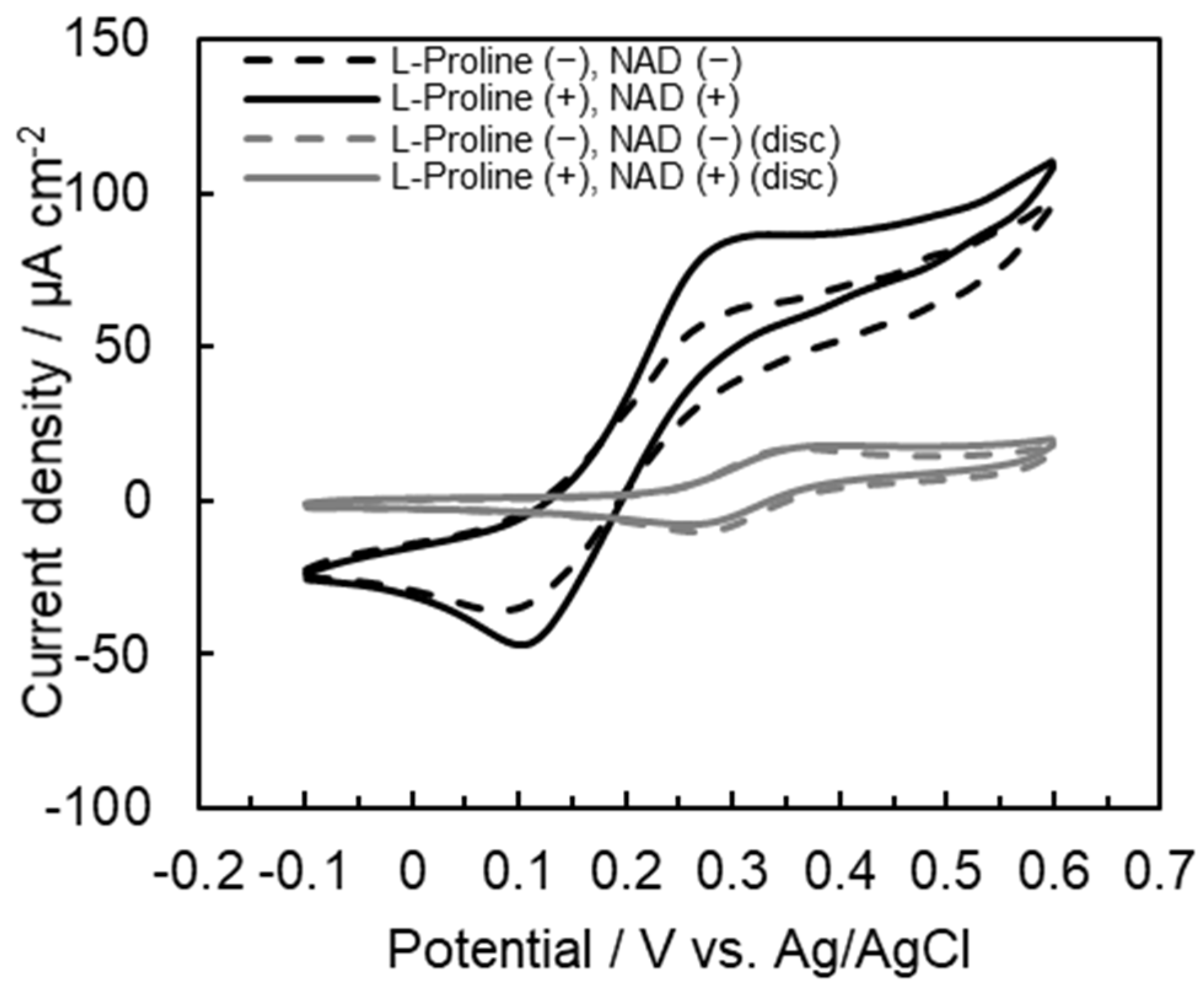
2.2. Comparison of Electrochemical Characterization of the Microfluidic System Electrode with That of Gold Disc Electrode Using Tetra-Enzyme Cascade
2.3. Evaluation of Electrochemical Characteristic Differences between Flow of Substrate and No Flow of Substrate
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Purification of LPDH
3.2.2. Purification of P5CDH
3.2.3. Purification of GDH
3.2.4. Purification of Proline/NADH Dehydrogenase (PDH)
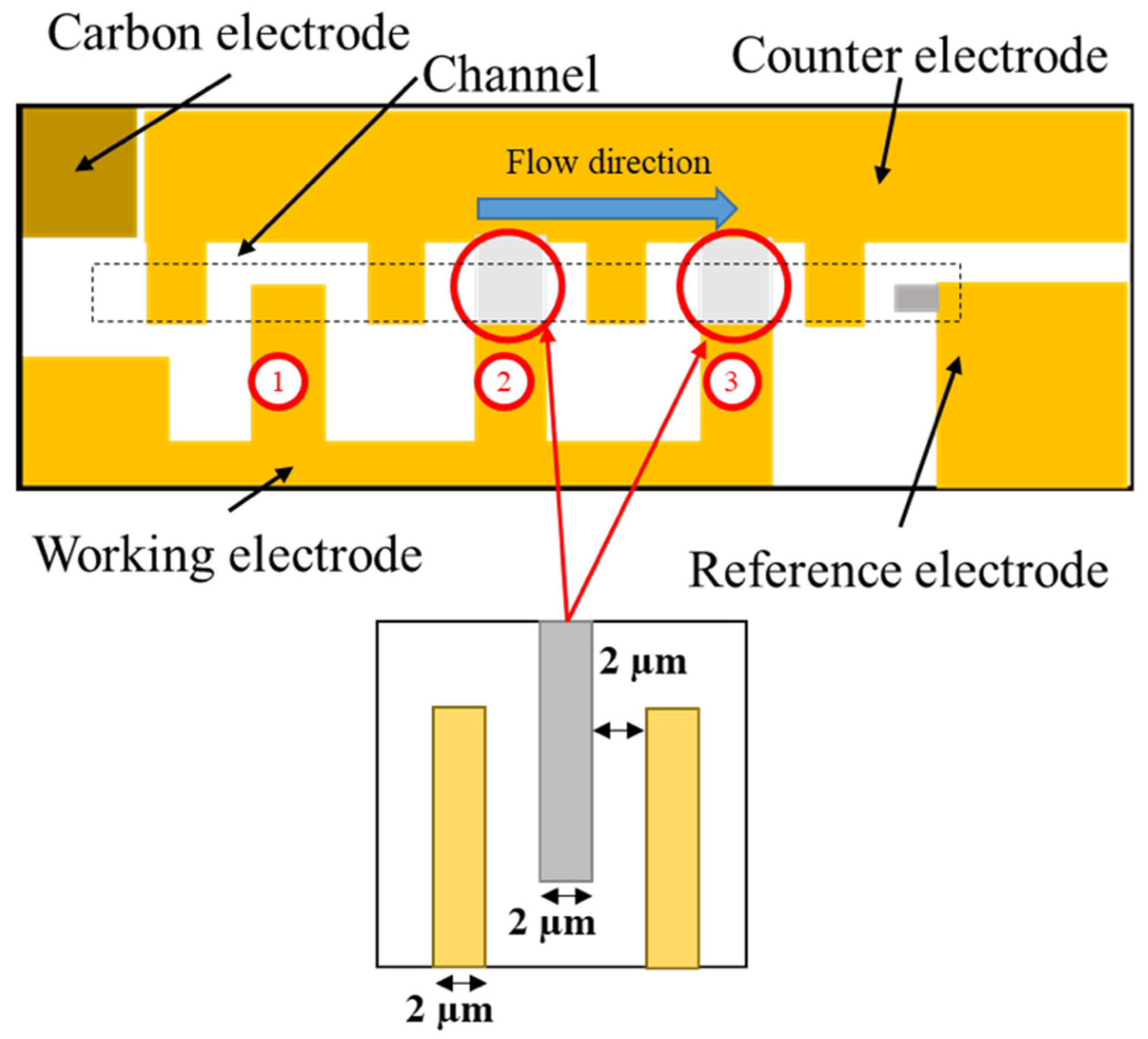
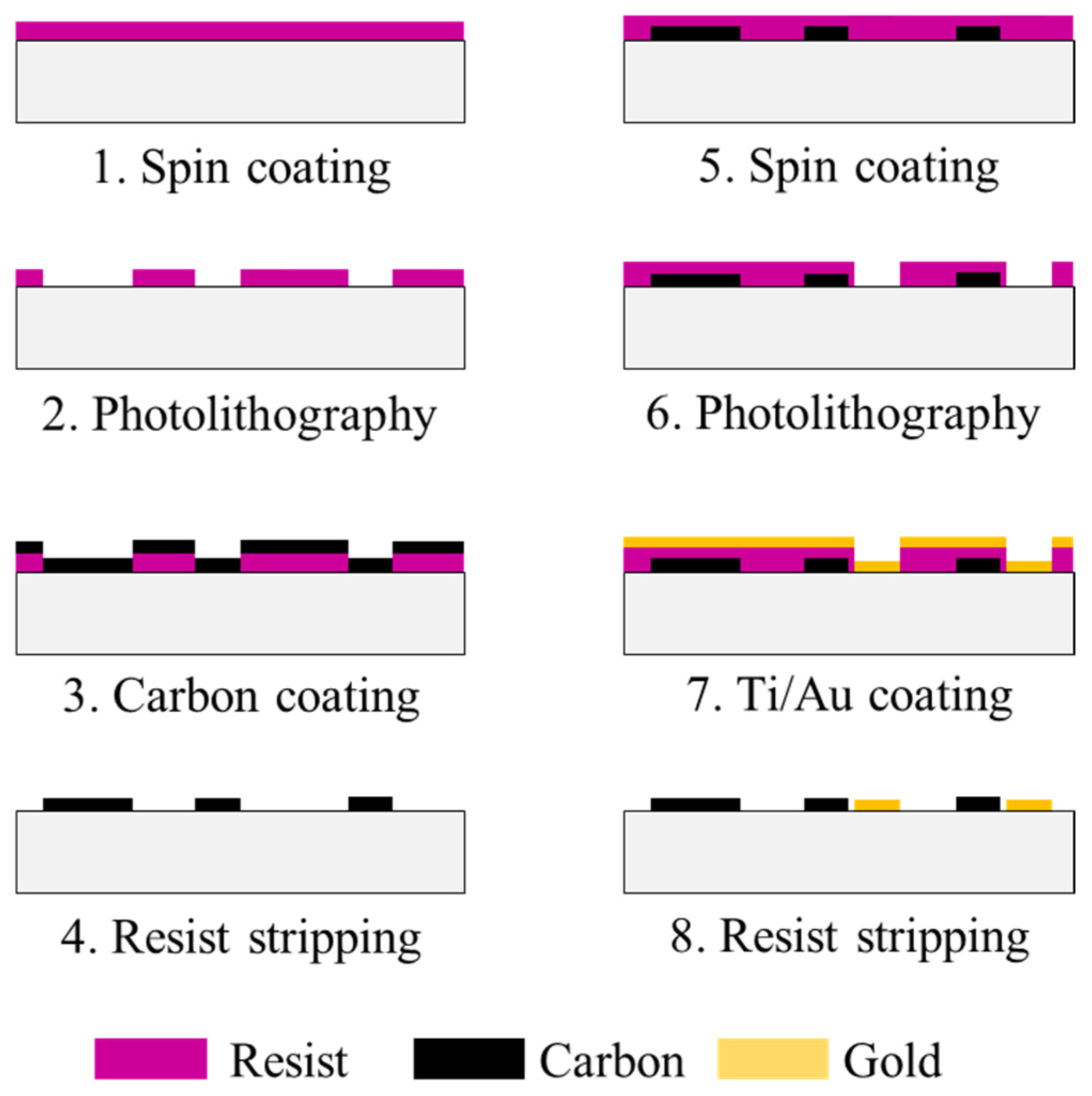
3.2.5. Fabrication of Microfluidic Channel
3.2.6. Preparation of Tetra-Enzyme Immobilized Electrode
3.2.7. Electrochemical Characterization of the Tetra-Enzyme Immobilized Electrode
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Fan, S.; Liang, B.; Xiao, X.; Bai, L.; Tang, X.; Lojou, E.; Cosnier, S.; Liu, A. Controllable display of sequential enzymes on yeast surface with enhanced biocatalytic activity toward efficient enzymatic biofuel cells. J. Am. Chem. Soc. 2020, 142, 3222–3230. [Google Scholar] [CrossRef] [PubMed]
- Sarbon, N.M.; Badii, F.; Howell, N.K. Preparation and characterisation of chicken skin gelatin as an alternative to mammalian gelatin. Food Hydrocollois 2013, 30, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Hickey, D.P.; Giroud, F.; Schmidtke, D.W.; Glatzhofer, D.T.; Minteer, S.D. Enzyme cascade for catalyzing sucrose oxidation in a biofuel cell. ACS Catal. 2013, 3, 2729–2737. [Google Scholar] [CrossRef]
- Xu, S.; Minteer, S.D. Enzymatic biofuel cell for oxidation of glucose to CO2. ACS Catal. 2012, 2, 91–94. [Google Scholar] [CrossRef]
- Chansaenpak, K.; Kamkaew, A.; Lisnund, S.; Prachai, P.; Ratwirunkit, P.; Jingpho, T.; Blah, V.; Pinyou, P. Development of a Sensitive Self-Powered Glucose Biosensor Based on an Enzymatic Biofuel Cell. Biosensors 2021, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Shitanda, I.; Tsunaga, M.; Watanabe, H.; Itagaki, M.; Tsujimura, S.; Mikawa, T. High Capacity Lactate Biofuel Cell Using Enzyme Cascade without NAD. Chem. Lett. 2021, 50, 1160–1163. [Google Scholar] [CrossRef]
- Matsumoto, T.; Shimada, S.; Yamamoto, K.; Tanaka, T.; Kondo, A. Two-Stage Oxidation of Glucose by an Enzymatic Bioanode. Fuel Cells 2013, 13, 960–964. [Google Scholar] [CrossRef]
- Kim, J.; Jia, H.; Wang, P. Challenges in biocatalysis for enzyme-based biofuel cells. Biotechnol. Adv. 2006, 24, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Chand, S.; Zhang, Y.; Nussbaum, S.; Rajeshwar, K.; Perera, R. Implications of active site orientation in myoglobin for direct electron transfer and electrocatalysis based on monolayer and multilayer covalent immobilization on gold electrodes. Electr. Act. 2013, 99, 85–93. [Google Scholar] [CrossRef]
- Yewle, J.N.; Wei, Y.; Puleo, D.A.; Daunert, S.; Bachas, L.G. Oriented immobilization of proteins on hydroxyapatite surface using bifunctional bisphosphonates as linkers. Biomacromolecules 2012, 13, 1742–1749. [Google Scholar] [CrossRef] [PubMed]
- Krzemiński, L.; Cronin, S.; Ndamba, L.; Canters, G.W.; Aartsma, T.J.; Evans, S.D.; Jeuken, L.J. Orientational control over nitrite reductase on modified gold electrode and its effects on the interfacial electron transfer. J. Phys. Chem. 2011, 115, 12607–12614. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotech. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, J.; Kamiya, N.; Doi, S.; Ichinose, H.; Maruyama, T.; Goto, M. Design of a specific peptide tag that affords covalent and site-specific enzyme immobilization catalyzed by microbial transglutaminase. Biomacromolecules 2005, 6, 2299–2304. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Kyratzis, I. Covalent immobilization of proteins on carbon nanotubes using the cross-linker 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide—A critical assessment. Bioconj. Chem. 2008, 19, 1945–1950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balakrishnan, B.; Joshi, N.; Jayakrishnan, A.; Banerjee, R. Self-crosslinked oxidized alginate/gelatin hydrogel as injectable, adhesive biomimetic scaffolds for cartilage regeneration. Acta Biomat. 2014, 10, 3650–3663. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.P.; Park, S.J.; Lee, D.; Kim, H.S. Electrochemical glucose biosensor by electrostatic binding of PQQ-glucose dehydrogenase onto self-assembled monolayers on gold. J. Appl. Electrochem. 2012, 42, 383–390. [Google Scholar] [CrossRef]
- Neto, S.A.; Minteer, S.D.; de Andrade, A.R. Developing ethanol bioanodes using a hydrophobically modified linear polyethylenimine hydrogel for immobilizing an enzyme cascade. J. Electroanal. Chem. 2018, 812, 153–158. [Google Scholar] [CrossRef]
- Wu, Z.Q.; Li, Z.Q.; Li, J.Y.; Gu, J.; Xia, X.H. Contribution of convection and diffusion to the cascade reaction kinetics of β-galactosidase/glucose oxidase confined in a microchannel. Phys. Chem. Chem. Phys. 2016, 18, 14460–14465. [Google Scholar] [CrossRef] [PubMed]
- Kanso, H.; García, M.B.G.; Llano, L.F.; Ma, S.; Ludwig, R.; Bolado, P.F.; Santos, D.H. Novel thin layer flow-cell screen-printed graphene electrode for enzymatic sensors. Biosens. Bioelectron. 2017, 93, 298–304. [Google Scholar] [CrossRef] [PubMed]

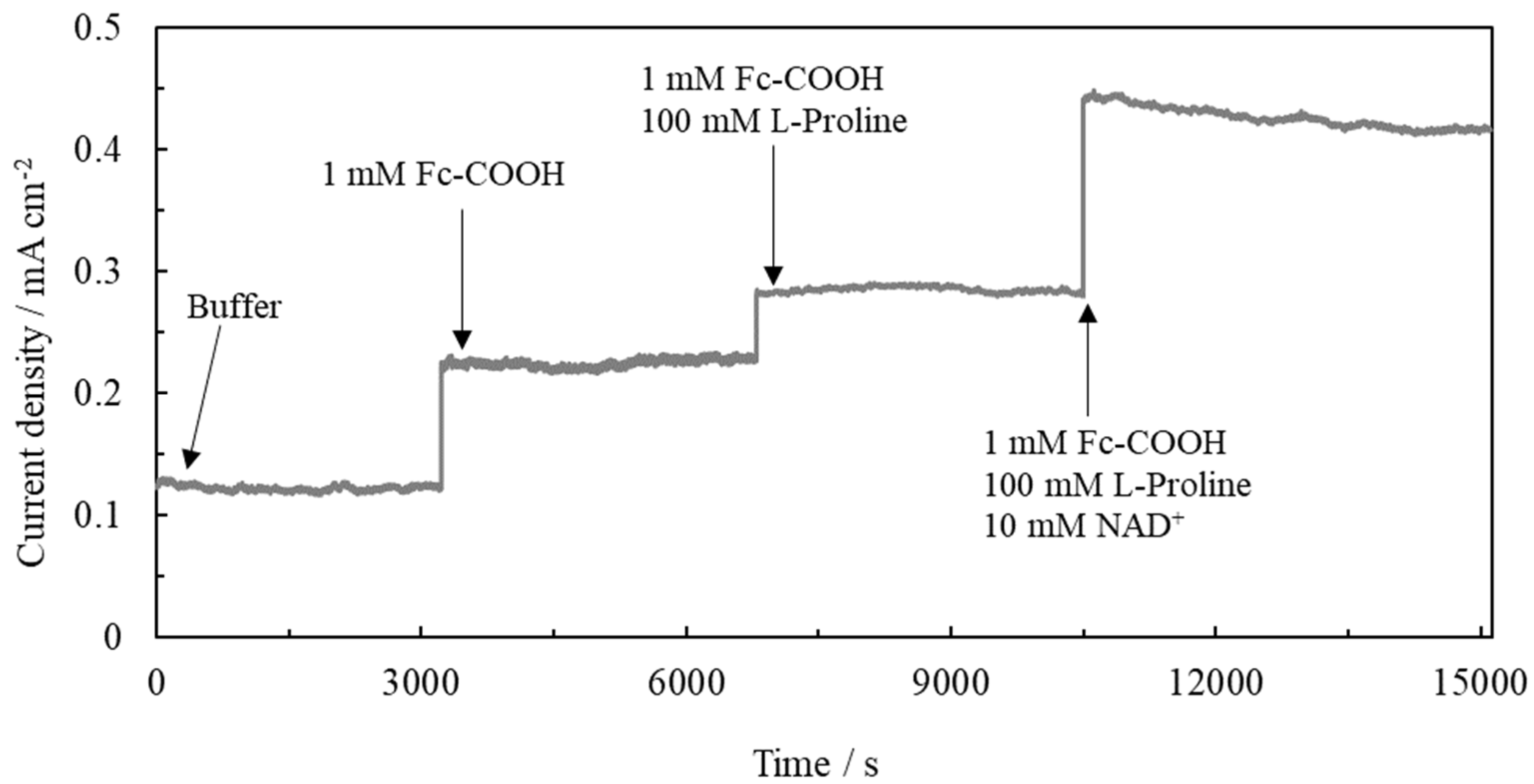
| Electrode | Δ Current Density (Ipa) µA cm−2 |
|---|---|
| Disc electrode | 1.1 |
| Microfluidic electrode | 23.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komatsu, T.; Hishii, K.; Kimura, M.; Amaya, S.; Sakamoto, H.; Takamura, E.; Satomura, T.; Suye, S.-i. Highly Efficient Multi-Step Oxidation Bioanode Using Microfluidic Channels. Int. J. Mol. Sci. 2021, 22, 13503. https://doi.org/10.3390/ijms222413503
Komatsu T, Hishii K, Kimura M, Amaya S, Sakamoto H, Takamura E, Satomura T, Suye S-i. Highly Efficient Multi-Step Oxidation Bioanode Using Microfluidic Channels. International Journal of Molecular Sciences. 2021; 22(24):13503. https://doi.org/10.3390/ijms222413503
Chicago/Turabian StyleKomatsu, Tomohiro, Kazuki Hishii, Michiko Kimura, Satoshi Amaya, Hiroaki Sakamoto, Eiichiro Takamura, Takenori Satomura, and Shin-ichiro Suye. 2021. "Highly Efficient Multi-Step Oxidation Bioanode Using Microfluidic Channels" International Journal of Molecular Sciences 22, no. 24: 13503. https://doi.org/10.3390/ijms222413503
APA StyleKomatsu, T., Hishii, K., Kimura, M., Amaya, S., Sakamoto, H., Takamura, E., Satomura, T., & Suye, S.-i. (2021). Highly Efficient Multi-Step Oxidation Bioanode Using Microfluidic Channels. International Journal of Molecular Sciences, 22(24), 13503. https://doi.org/10.3390/ijms222413503






