Transient Activation of Hedgehog Signaling Inhibits Cellular Senescence and Inflammation in Radiated Swine Salivary Glands through Preserving Resident Macrophages
Abstract
:1. Introduction
2. Results
2.1. Hedgehog Signaling in Pig Parotids Is Inhibited by Radiation and Restored by Shh Gene Transfer with the Preservation of Salivary Gland Function
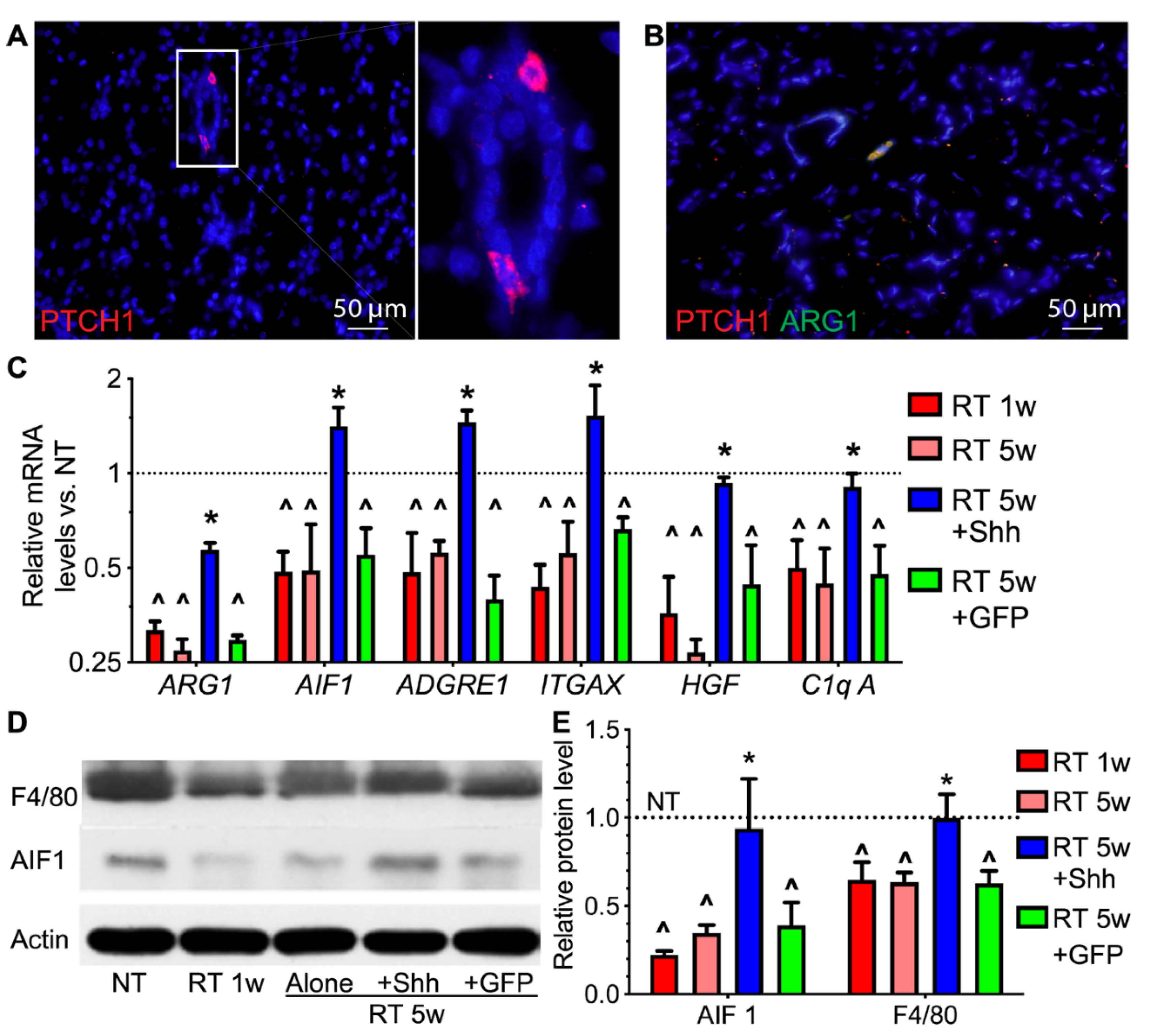
2.2. Hedgehog Receptor Ptch1 Is Mainly Expressed in Parotid Macrophages That Are Decreased by Radiation and Recovered by Transient Hedgehog Activation
2.3. Hedgehog Activation Repressed the Persistent Inflammation in Radiated Swine Parotids
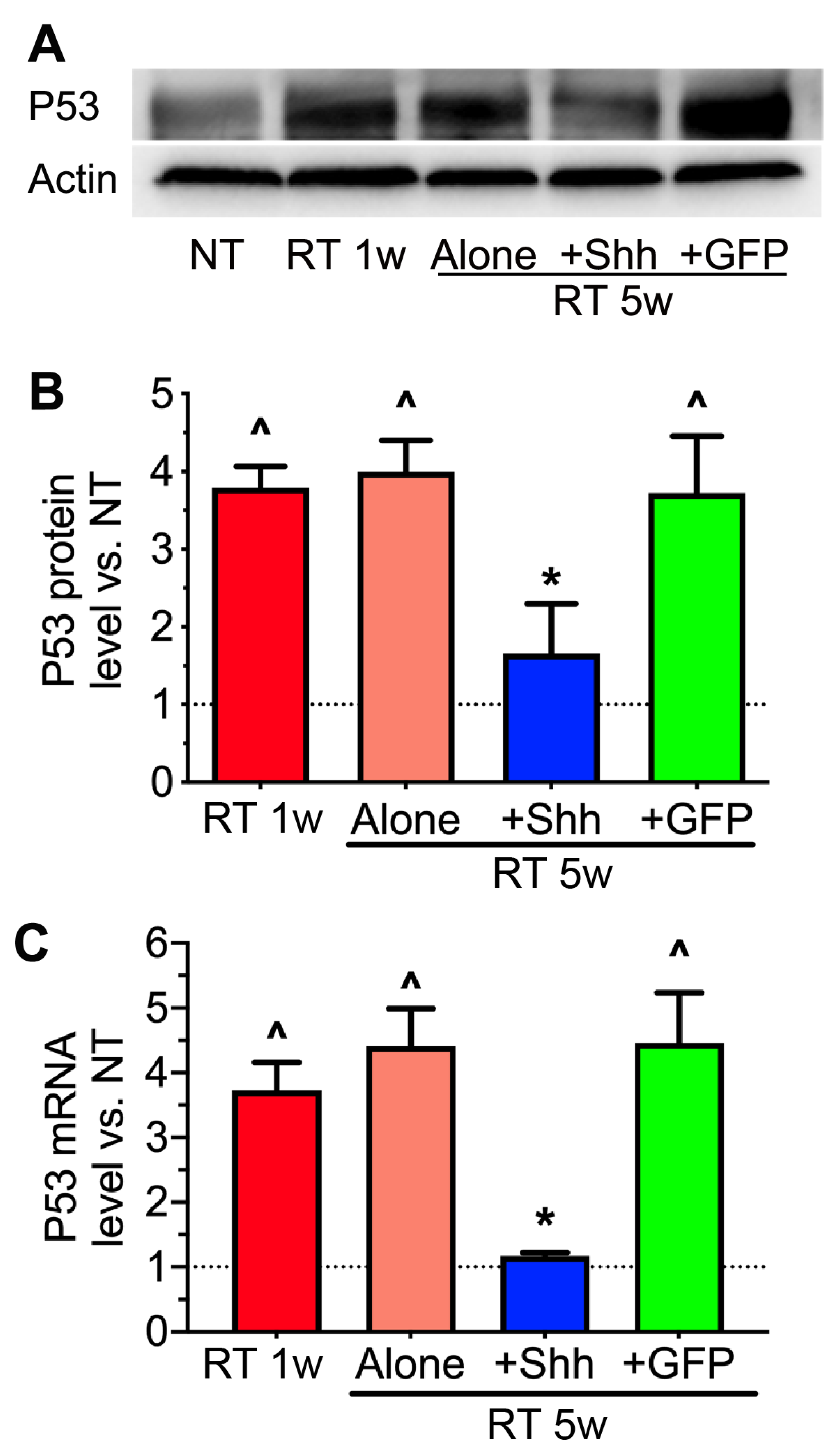
2.4. The Elevation of P53 Levels by Radiation Is Reversed by Hedgehog Activation
2.5. VEGF Level Is Decreased by Radiation but Restored by Hedgehog Activation
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Irradiation
4.3. Gene Transfer and Saliva Collection
4.4. Histological and Immunohistochemical Analyses
4.5. Quantitative RT-PCR and Western Blotting
4.6. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [Green Version]
- Jensen, S.B.; Pedersen, A.M.; Vissink, A.; Andersen, E.; Brown, C.G.; Davies, A.N.; Dutilh, J.; Fulton, J.S.; Jankovic, L.; Lopes, N.N.; et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: Management strategies and economic impact. Support. Care Cancer 2010, 18, 1061–1079. [Google Scholar] [CrossRef]
- Ghosh-Laskar, S.; Yathiraj, P.H.; Dutta, D.; Rangarajan, V.; Purandare, N.; Gupta, T.; Budrukkar, A.; Murthy, V.; Kannan, S.; Agarwal, J.P. Prospective randomized controlled trial to compare 3-dimensional conformal radiotherapy to intensity-modulated radiotherapy in head and neck squamous cell carcinoma: Long-term results. Head Neck 2015, 38 (Suppl. S1), E1481–E1487. [Google Scholar] [CrossRef]
- Jensen, S.B.; Vissink, A.; Limesand, K.H.; Reyland, M.E. Salivary Gland Hypofunction and Xerostomia in Head and Neck Radiation Patients. J. Natl. Cancer Inst. Monogr. 2019, 2019, lgz016. [Google Scholar] [CrossRef]
- Mercadante, V.; Jensen, S.B.; Smith, D.K.; Bohlke, K.; Bauman, J.; Brennan, M.T.; Coppes, R.P.; Jessen, N.; Malhotra, N.K.; Murphy, B.; et al. Salivary Gland Hypofunction and/or Xerostomia Induced by Nonsurgical Cancer Therapies: ISOO/MASCC/ASCO Guideline. J. Clin. Oncol. 2021, 39, 2825–2843. [Google Scholar] [CrossRef]
- Marmary, Y.; Adar, R.; Gaska, S.; Wygoda, A.; Maly, A.; Cohen, J.; Eliashar, R.; Mizrachi, L.; Orfaig-Geva, C.; Baum, B.J.; et al. Radiation-Induced Loss of Salivary Gland Function Is Driven by Cellular Senescence and Prevented by IL6 Modulation. Cancer Res. 2016, 76, 1170–1180. [Google Scholar] [CrossRef] [Green Version]
- Grundmann, O.; Mitchell, G.C.; Limesand, K.H. Sensitivity of salivary glands to radiation: From animal models to therapies. J. Dent. Res. 2009, 88, 894–903. [Google Scholar] [CrossRef]
- Hai, B.; Qin, L.; Yang, Z.; Zhao, Q.; Shangguan, L.; Ti, X.; Zhao, Y.; Kim, S.; Rangaraj, D.; Liu, F. Transient activation of hedgehog pathway rescued irradiation-induced hyposalivation by preserving salivary stem/progenitor cells and parasympathetic innervation. Clin. Cancer Res. 2014, 20, 140–150. [Google Scholar] [CrossRef] [Green Version]
- Hai, B.; Zhao, Q.; Qin, L.; Rangaraj, D.; Gutti, V.R.; Liu, F. Rescue Effects and Underlying Mechanisms of Intragland Shh Gene Delivery on Irradiation-Induced Hyposalivation. Hum. Gene Ther. 2016, 27, 390–399. [Google Scholar] [CrossRef] [Green Version]
- Hai, B.; Zhao, Q.; Deveau, M.A.; Liu, F. Delivery of Sonic Hedgehog Gene Repressed Irradiation-induced Cellular Senescence in Salivary Glands by Promoting DNA Repair and Reducing Oxidative Stress. Theranostics 2018, 8, 1159–1167. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Zhang, L.; Hai, B.; Wang, J.; Baetge, C.L.; Deveau, M.A.; Kapler, G.M.; Feng, J.Q.; Liu, F. Transient activation of the Hedgehog-Gli pathway rescues radiotherapy-induced dry mouth via recovering salivary gland resident macrophages. Cancer Res. 2020. [Google Scholar] [CrossRef]
- Pappo, J.; Ebersole, J.L.; Taubman, M.A. Resident salivary gland macrophages function as accessory cells in antigen-dependent T-cell proliferation. Immunology 1988, 63, 99–104. [Google Scholar]
- Cohen, R.E.; Noble, B.; Neiders, M.E.; Comeau, R.L. Mononuclear cells in salivary glands of normal and isoproterenol-treated rats. Arch. Oral Biol. 1995, 40, 1015–1021. [Google Scholar] [CrossRef]
- Thom, J.T.; Walton, S.M.; Torti, N.; Oxenius, A. Salivary gland resident APCs are Flt3L- and CCR2-independent macrophage-like cells incapable of cross-presentation. Eur. J. Immunol. 2014, 44, 706–714. [Google Scholar] [CrossRef]
- Stolp, B.; Thelen, F.; Ficht, X.; Altenburger, L.M.; Ruef, N.; Inavalli, V.; Germann, P.; Page, N.; Moalli, F.; Raimondi, A.; et al. Salivary gland macrophages and tissue-resident CD8+ T cells cooperate for homeostatic organ surveillance. Sci. Immunol. 2020, 5. [Google Scholar] [CrossRef]
- Takahashi, S.; Nakamura, S.; Suzuki, R.; Islam, N.; Domon, T.; Yamamoto, T.; Wakita, M. Apoptosis and mitosis of parenchymal cells in the duct-ligated rat submandibular gland. Tissue Cell 2000, 32, 457–463. [Google Scholar] [CrossRef]
- Amano, O.; Mizobe, K.; Bando, Y.; Sakiyama, K. Anatomy and histology of rodent and human major salivary glands: -overview of the Japan salivary gland society-sponsored workshop. Acta Histochem. Cytochem. 2012, 45, 241–250. [Google Scholar] [CrossRef] [Green Version]
- Gluck, C.; Min, S.; Oyelakin, A.; Smalley, K.; Sinha, S.; Romano, R.A. RNA-seq based transcriptomic map reveals new insights into mouse salivary gland development and maturation. BMC genomics 2016, 17, 923. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Liu, Y.; Fang, D.; Shi, S. The miniature pig: A useful large animal model for dental and orofacial research. Oral Dis. 2007, 13, 530–537. [Google Scholar] [CrossRef]
- Shan, Z.; Li, J.; Zheng, C.; Liu, X.; Fan, Z.; Zhang, C.; Goldsmith, C.M.; Wellner, R.B.; Baum, B.J.; Wang, S. Increased fluid secretion after adenoviral-mediated transfer of the human aquaporin-1 cDNA to irradiated miniature pig parotid glands. Mol. Ther. 2005, 11, 444–451. [Google Scholar] [CrossRef]
- Xu, J.; Yan, X.; Gao, R.; Mao, L.; Cotrim, A.P.; Zheng, C.; Zhang, C.; Baum, B.J.; Wang, S. Effect of irradiation on microvascular endothelial cells of parotid glands in the miniature pig. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 897–903. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Zhu, Z.; Hai, B.; Chang, S.; Ma, L.; Xu, Y.; Li, X.; Feng, X.; Wu, X.; Zhao, Q.; et al. Intragland Shh gene delivery mitigated irradiation-induced hyposalivation in a miniature pig model. Theranostics 2018, 8, 4321–4331. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, K.A.; Song, C.J.; Li, Z.; Lever, J.M.; Crossman, D.K.; Rains, A.; Aloria, E.J.; Gonzalez, N.M.; Bassler, J.R.; Zhou, J.; et al. Tissue-Resident Macrophages Promote Renal Cystic Disease. J. Am. Soc. Nephrol. 2019, 30, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Avila, J.L.; Grundmann, O.; Burd, R.; Limesand, K.H. Radiation-induced salivary gland dysfunction results from p53-dependent apoptosis. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 523–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, Y.; Oda-Sato, E.; Tobiume, K.; Kawauchi, K.; Taya, Y.; Okamoto, K.; Oren, M.; Tanaka, N. Hedgehog signaling overrides p53-mediated tumor suppression by activating Mdm2. Proc. Natl. Acad. Sci. USA 2008, 105, 4838–4843. [Google Scholar] [CrossRef] [Green Version]
- Fan, S.; Ma, Y.X.; Wang, J.A.; Yuan, R.Q.; Meng, Q.; Cao, Y.; Laterra, J.J.; Goldberg, I.D.; Rosen, E.M. The cytokine hepatocyte growth factor/scatter factor inhibits apoptosis and enhances DNA repair by a common mechanism involving signaling through phosphatidyl inositol 3′ kinase. Oncogene 2000, 19, 2212–2223. [Google Scholar] [CrossRef] [Green Version]
- Verneret, M.; Tacnet-Delorme, P.; Osman, R.; Awad, R.; Grichine, A.; Kleman, J.P.; Frachet, P. Relative contribution of c1q and apoptotic cell-surface calreticulin to macrophage phagocytosis. J. Innate Immunity 2014, 6, 426–434. [Google Scholar] [CrossRef]
- Son, M.; Porat, A.; He, M.; Suurmond, J.; Santiago-Schwarz, F.; Andersson, U.; Coleman, T.R.; Volpe, B.T.; Tracey, K.J.; Al-Abed, Y.; et al. C1q and HMGB1 reciprocally regulate human macrophage polarization. Blood 2016, 128, 2218–2228. [Google Scholar] [CrossRef] [Green Version]
- Benoit, M.E.; Clarke, E.V.; Morgado, P.; Fraser, D.A.; Tenner, A.J. Complement protein C1q directs macrophage polarization and limits inflammasome activity during the uptake of apoptotic cells. J. Immunol. 2012, 188, 5682–5693. [Google Scholar] [CrossRef] [Green Version]
- Lasry, A.; Ben-Neriah, Y. Senescence-associated inflammatory responses: Aging and cancer perspectives. Trends Immunol. 2015, 36, 217–228. [Google Scholar] [CrossRef]
- Cotrim, A.P.; Sowers, A.; Mitchell, J.B.; Baum, B.J. Prevention of irradiation-induced salivary hypofunction by microvessel protection in mouse salivary glands. Mol. Ther. 2007, 15, 2101–2106. [Google Scholar] [CrossRef]
- Watanabe, Y.; Lee, S.W.; Detmar, M.; Ajioka, I.; Dvorak, H.F. Vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) delays and induces escape from senescence in human dermal microvascular endothelial cells. Oncogene 1997, 14, 2025–2032. [Google Scholar] [CrossRef] [Green Version]
- Foersch, S.; Sperka, T.; Lindner, C.; Taut, A.; Rudolph, K.L.; Breier, G.; Boxberger, F.; Rau, T.T.; Hartmann, A.; Sturzl, M.; et al. VEGFR2 Signaling Prevents Colorectal Cancer Cell Senescence to Promote Tumorigenesis in Mice With Colitis. Gastroenterology 2015, 149, 177–189.e10. [Google Scholar] [CrossRef] [Green Version]
- Moran, C.M.; Myers, C.T.; Lewis, C.M.; Krieg, P.A. Hedgehog regulates angiogenesis of intersegmental vessels through the VEGF signaling pathway. Dev. Dyn. 2012, 241, 1034–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumura, A.; Kubota, T.; Taiyoh, H.; Fujiwara, H.; Okamoto, K.; Ichikawa, D.; Shiozaki, A.; Komatsu, S.; Nakanishi, M.; Kuriu, Y.; et al. HGF regulates VEGF expression via the c-Met receptor downstream pathways, PI3K/Akt, MAPK and STAT3, in CT26 murine cells. Int. J. Oncol. 2013, 42, 535–542. [Google Scholar] [CrossRef] [Green Version]
- Fan, G.; Li, Q.; Qian, J. C1q contributes to post-stroke angiogenesis via LAIR1-HIF1alpha-VEGF pathway. Front. Biosci. 2019, 24, 1050–1059. [Google Scholar] [CrossRef]
- Jasmer, K.J.; Gilman, K.E.; Munoz Forti, K.; Weisman, G.A.; Limesand, K.H. Radiation-Induced Salivary Gland Dysfunction: Mechanisms, Therapeutics and Future Directions. J. Clin. Med. 2020, 9, 4095. [Google Scholar] [CrossRef]
- Yu, W.L.; Hua, Z.C. Evaluation of effectiveness of granulocyte-macrophage colony-stimulating factor therapy to cancer patients after chemotherapy: A meta-analysis. Oncotarget 2018, 9, 28226–28239. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Ghosh, S.; Yang, H. Nanomedicines for dysfunctional macrophage-associated diseases. J. Control. Release 2017, 247, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keysar, S.B.; Le, P.N.; Anderson, R.T.; Morton, J.J.; Bowles, D.W.; Paylor, J.J.; Vogler, B.W.; Thorburn, J.; Fernandez, P.; Glogowska, M.J.; et al. Hedgehog signaling alters reliance on EGF receptor signaling and mediates anti-EGFR therapeutic resistance in head and neck cancer. Cancer Res. 2013, 73, 3381–3392. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, D.; Chow, A.; Noizat, C.; Teo, P.; Beasley, M.B.; Leboeuf, M.; Becker, C.D.; See, P.; Price, J.; Lucas, D.; et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 2013, 38, 792–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Ruysscher, D.; Niedermann, G.; Burnet, N.G.; Siva, S.; Lee, A.W.M.; Hegi-Johnson, F. Radiotherapy toxicity. Nat. Rev. Dis. Primers 2019, 5, 13. [Google Scholar] [CrossRef]
- Hai, B.; Yang, Z.; Millar, S.E.; Choi, Y.S.; Taketo, M.M.; Nagy, A.; Liu, F. Wnt/beta-catenin signaling regulates postnatal development and regeneration of the salivary gland. Stem Cells Dev. 2010, 19, 1793–1801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hai, B.; Yang, Z.; Shangguan, L.; Zhao, Y.; Boyer, A.; Liu, F. Concurrent transient activation of Wnt/beta-catenin pathway prevents radiation damage to salivary glands. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, e109–e116. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, L.; Du, C.; Yang, Z.; Yang, Y.; Zhu, Z.; Shan, Z.; Zhang, C.; Wang, S.; Liu, F. Transient Activation of Hedgehog Signaling Inhibits Cellular Senescence and Inflammation in Radiated Swine Salivary Glands through Preserving Resident Macrophages. Int. J. Mol. Sci. 2021, 22, 13493. https://doi.org/10.3390/ijms222413493
Hu L, Du C, Yang Z, Yang Y, Zhu Z, Shan Z, Zhang C, Wang S, Liu F. Transient Activation of Hedgehog Signaling Inhibits Cellular Senescence and Inflammation in Radiated Swine Salivary Glands through Preserving Resident Macrophages. International Journal of Molecular Sciences. 2021; 22(24):13493. https://doi.org/10.3390/ijms222413493
Chicago/Turabian StyleHu, Liang, Conglin Du, Zi Yang, Yang Yang, Zhao Zhu, Zhaochen Shan, Chunmei Zhang, Songlin Wang, and Fei Liu. 2021. "Transient Activation of Hedgehog Signaling Inhibits Cellular Senescence and Inflammation in Radiated Swine Salivary Glands through Preserving Resident Macrophages" International Journal of Molecular Sciences 22, no. 24: 13493. https://doi.org/10.3390/ijms222413493
APA StyleHu, L., Du, C., Yang, Z., Yang, Y., Zhu, Z., Shan, Z., Zhang, C., Wang, S., & Liu, F. (2021). Transient Activation of Hedgehog Signaling Inhibits Cellular Senescence and Inflammation in Radiated Swine Salivary Glands through Preserving Resident Macrophages. International Journal of Molecular Sciences, 22(24), 13493. https://doi.org/10.3390/ijms222413493




