Electron Holes in G-Quadruplexes: The Role of Adenine Ending Groups
Abstract
1. Introduction
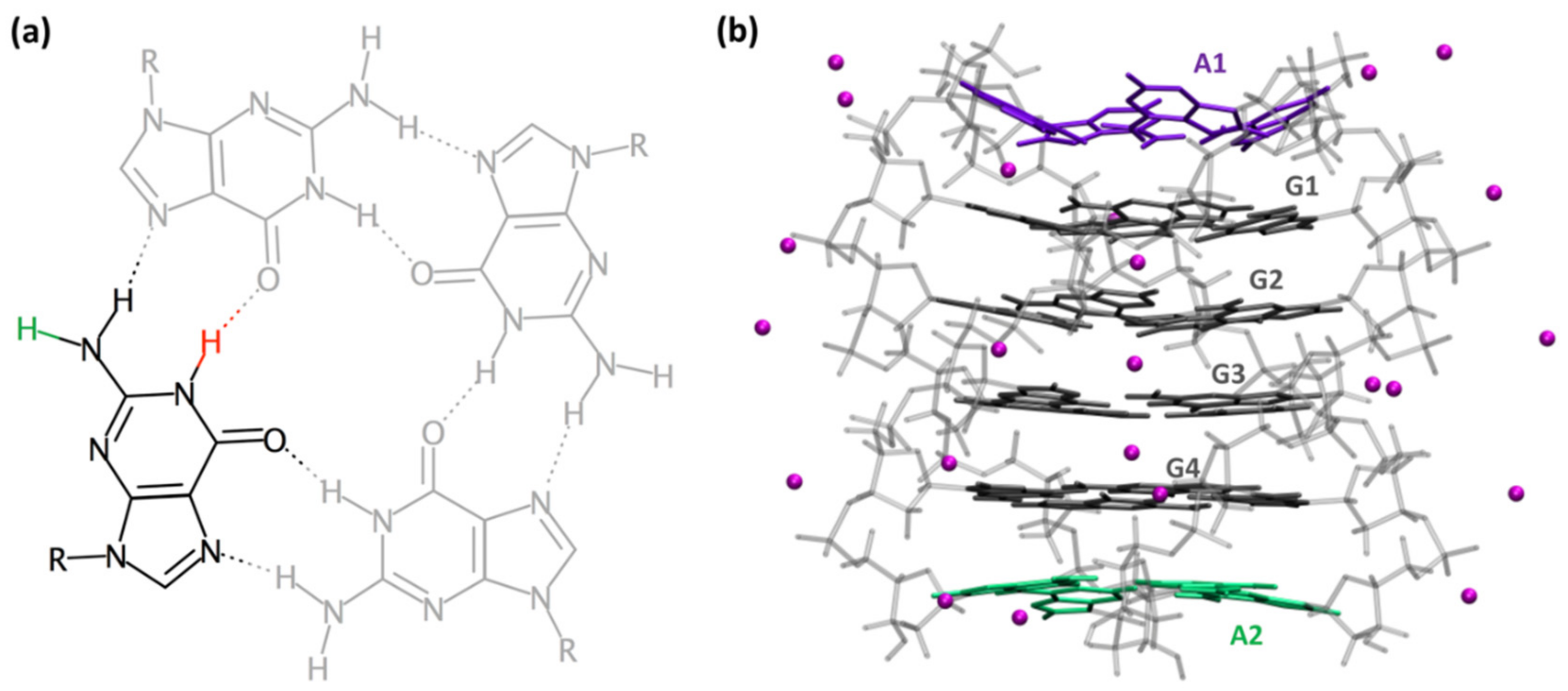
2. Results and Discussion
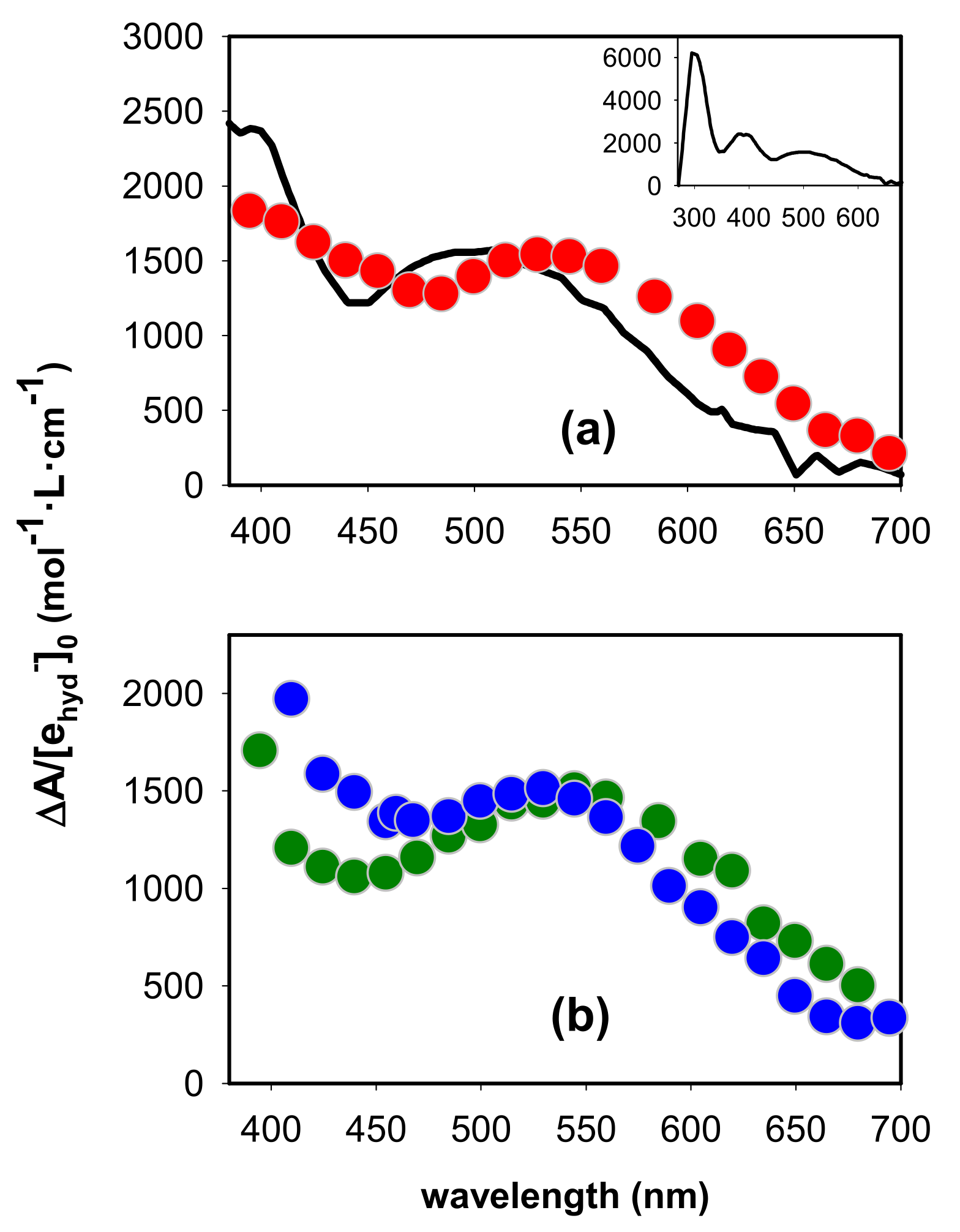
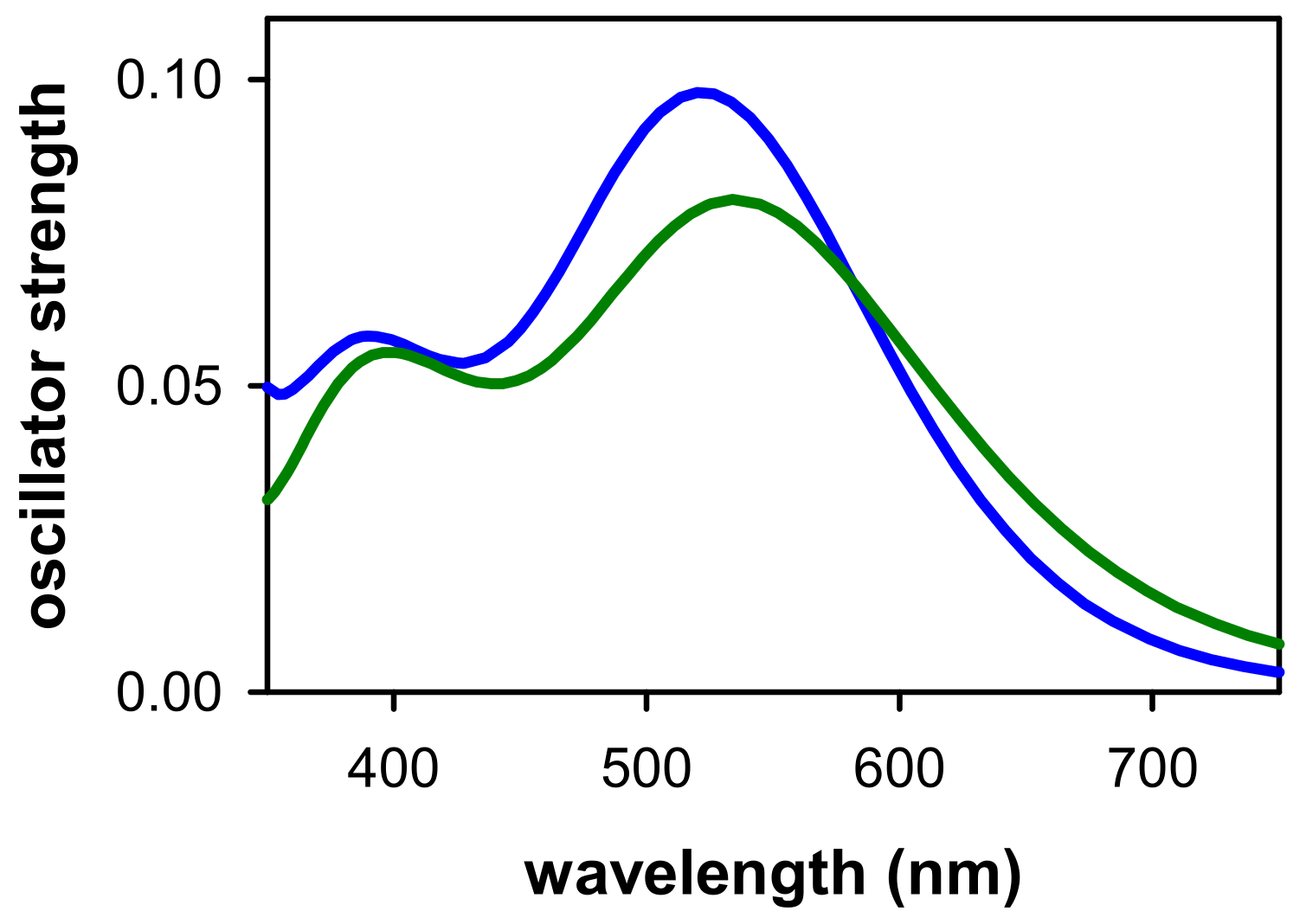
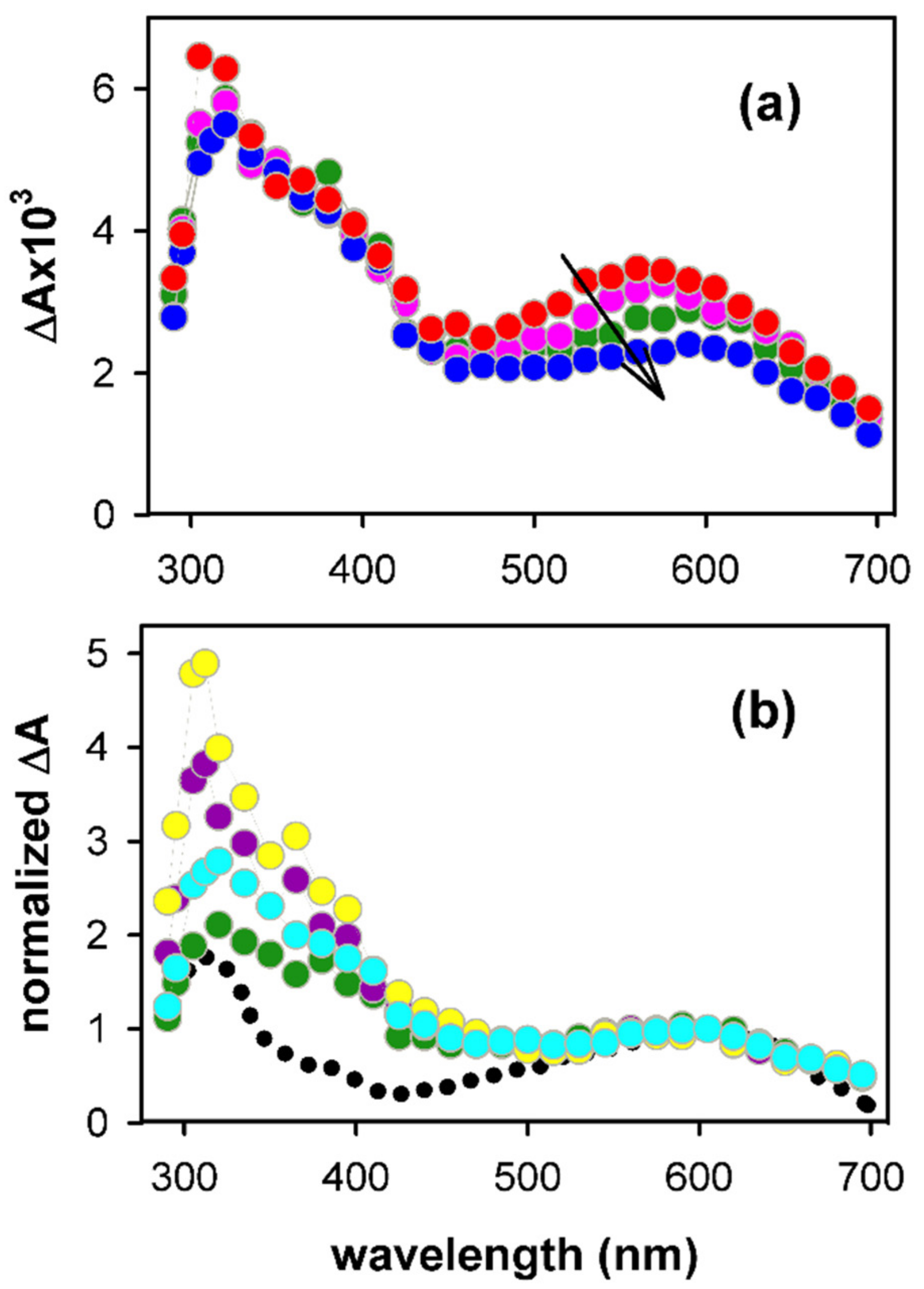
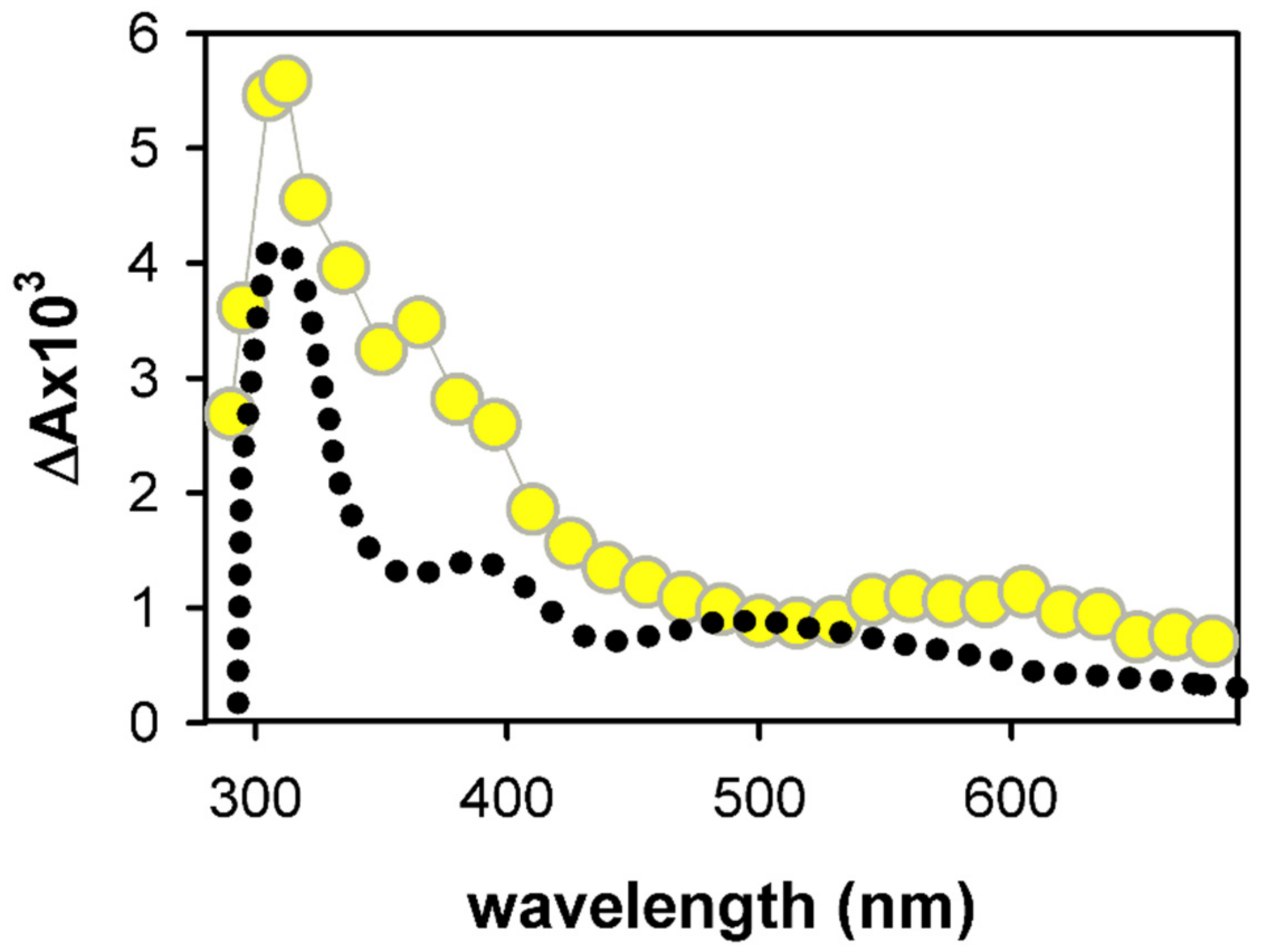
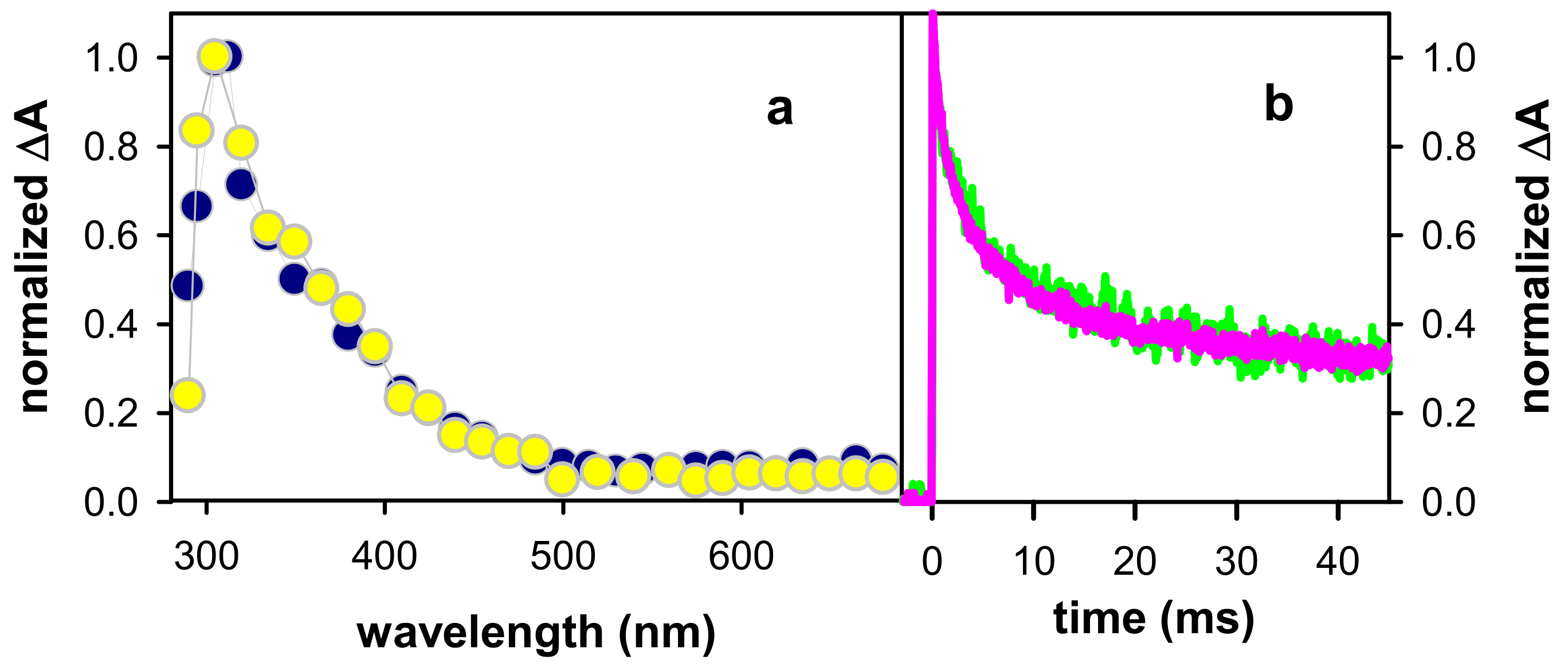
2.1. Spectral Properties
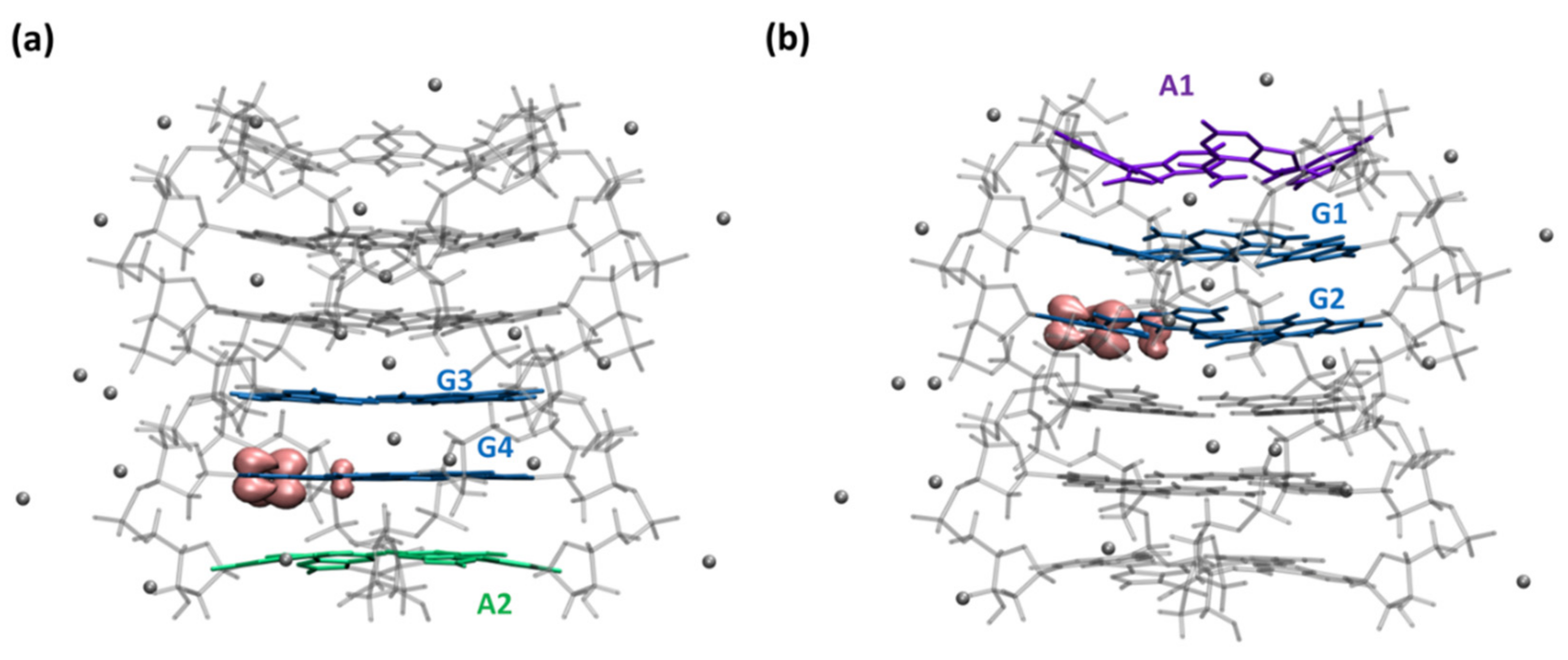
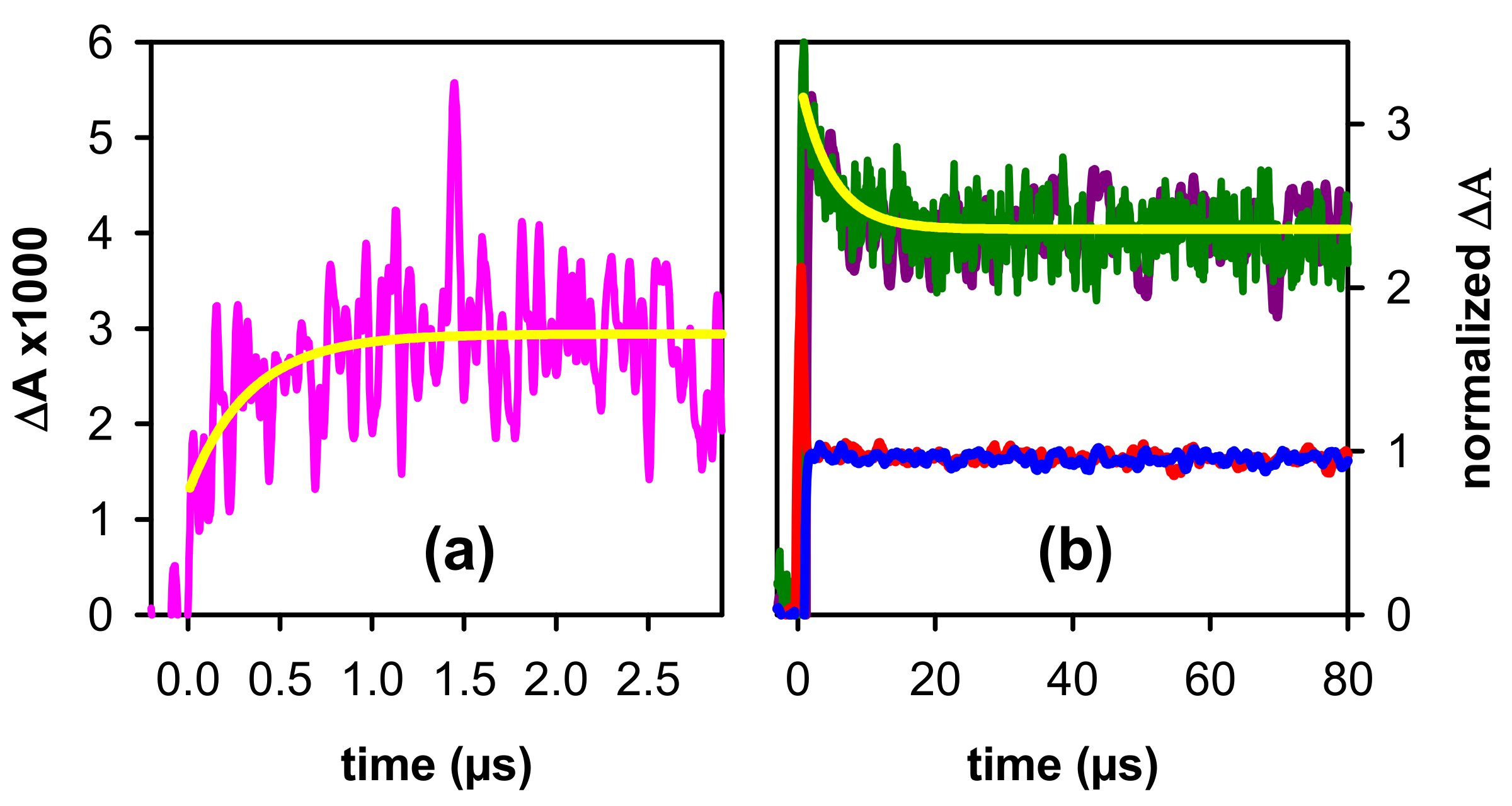
2.2. Dynamical Properties
3. Materials and Methods
3.1. Materials
3.2. Transient Absorption Spectroscopy
3.3. QM/MM Calculations
4. Main Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hall, D.B.; Holmlin, R.E.; Barton, J.K. Oxidative DNA damage through long-range electron transfer. Nature 1996, 382, 731–735. [Google Scholar] [CrossRef]
- Douki, T.; Angelov, D.; Cadet, J. UV laser photolysis of DNA: Effect of duplex stability on charge-transfer efficiency. J. Am. Chem. Soc. 2001, 123, 11360–11366. [Google Scholar] [CrossRef]
- Stemp, E.D.A.; Arkin, M.R.; Barton, J.K. Oxidation of guanine in DNA by Ru(phen)(2)(dppz)(3+) using the flash-quench technique. J. Am. Chem. Soc. 1997, 119, 2921–2925. [Google Scholar] [CrossRef]
- Meggers, E.; Michel-Beyerle, M.E.; Giese, B. Sequence dependent long range hole transport in DNA. J. Am. Chem. Soc. 1998, 120, 12950–12955. [Google Scholar] [CrossRef]
- Saito, I.; Nakamura, T.; Nakatani, K.; Yoshioka, Y.; Yamaguchi, K.; Sugiyama, H. Mapping of the hot spots for DNA damage by one-electron oxidation: Efficacy of GG doublets and GGG triplets as a trap in long-range hole migration. J. Am. Chem. Soc. 1998, 120, 12686–12687. [Google Scholar] [CrossRef]
- Kanvah, S.; Joseph, J.; Schuster, G.B.; Barnett, R.N.; Cleveland, C.L.; Landman, U. Oxidation of DNA: Damage to nucleobases. Acc. Chem. Res. 2010, 43, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Lewis, F.D.; Letsinger, R.L.; Wasielewski, M.R. Dynamics of photoinduced charge transfer and hole transport in synthetic DNA hairpins. Acc. Chem. Res. 2001, 34, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Majima, T. Hole transfer kinetics of DNA. Acc. Chem. Res. 2013, 46, 2616–2625. [Google Scholar] [CrossRef]
- Ma, J.; Marignier, J.L.; Pernot, P.; Houee-Levin, C.; Kumar, A.; Sevilla, M.D.; Adhikary, A.; Mostafavi, M. Direct observation of the oxidation of DNA bases by phosphate radicals formed under radiation: A model of the backbone-to-base hole transfer. Phys. Chem. Chem. Phys. 2018, 20, 14927–14937. [Google Scholar] [CrossRef]
- Palecek, E.; Bartosik, M. Electrochemistry of nucleic acids. Chem. Rev. 2012, 112, 3427–3481. [Google Scholar] [CrossRef] [PubMed]
- Varshney, D.; Spiegel, J.; Zyner, K.; Tannahill, D.; Balasubramanian, S. The regulation and functions of DNA and RNA G-quadruplexes. Nat. Rev. Mol. Cell Biol. 2020, 21, 459–474. [Google Scholar] [CrossRef] [PubMed]
- Kizhuveetil, U.; Omer, S.; Karunagaran, D.; Suraishkumar, G.K. Improved redox anti-cancer treatment efficacy through reactive species rhythm manipulation. Sci. Rep. 2020, 10, 1588. [Google Scholar] [CrossRef]
- Mergny, J.L.; Sen, D. DNA Quadruple helices in nanotechnology. Chem. Rev. 2019, 119, 6290–6325. [Google Scholar] [CrossRef] [PubMed]
- Livshits, G.I.; Stern, A.; Rotem, D.; Borovok, N.; Eidelshtein, G.; Migliore, A.; Penzo, E.; Wind, S.J.; Di Felice, R.; Skourtis, S.S.; et al. Long-range charge transport in single G-quadruplex DNA molecules. Nat. Nanotechnol. 2014, 9, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Delaney, S.; Barton, J.K. Charge transport in DNA duplex/quadruplex conjugates. Biochemistry 2003, 42, 14159–14165. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Park, J.; Tanaka, A.; Park, M.J.; Jang, Y.J.; Fujitsuka, M.; Kim, S.K.; Majima, T. Hole trapping of G-quartets in a G-quadruplex. Angew. Chem. Int. Ed. 2013, 52, 1134–1138. [Google Scholar] [CrossRef]
- Thazhathveetil, A.K.; Harris, M.A.; Young, R.M.; Wasielewski, M.R.; Lewis, F.D. Efficient charge transport via DNA G-quadruplexes. J. Am. Chem. Soc. 2017, 139, 1730–1733. [Google Scholar] [CrossRef]
- Liu, S.P.; Weisbrod, S.H.; Tang, Z.; Marx, A.; Scheer, E.; Erbe, A. Direct measurement of electrical transport through G-quadruplex DNA with mechanically controllable break junction electrodes. Angew. Chem. Int. Ed. 2010, 49, 3313–3316. [Google Scholar] [CrossRef]
- Sha, R.J.; Xiang, L.M.; Liu, C.R.; Balaeff, A.; Zhang, Y.Q.; Zhang, P.; Li, Y.Q.; Beratan, D.N.; Tao, N.J.; Seeman, N.C. Charge splitters and charge transport junctions based on guanine quadruplexes. Nat. Nanotechnol. 2018, 13, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liang, S.D. Topological effects of charge transfer in telomere G-quadruplex: Mechanism on telomerase activation and inhibition. Inter. J. Mod. Phys. B 2013, 27, 1350001. [Google Scholar] [CrossRef]
- Calzolari, A.; Di Felice, R.; Molinari, E.; Garbesi, A. Electron channels in biomolecular nanowires. J. Phys. Chem. B 2004, 108, 2509–2515. [Google Scholar] [CrossRef]
- Lech, C.J.; Anh Tuan, P.; Michel-Beyerle, M.-E.; Voityuk, A.A. Electron-hole transfer in G-quadruplexes with different tetrad stacking geometries: A combined QM and MD study. J. Phys. Chem. B 2013, 117, 9851–9856. [Google Scholar] [CrossRef]
- Kang, D.W.; Sun, M.L.; Zuo, Z.W.; Wang, H.X.; Lv, S.J.; Li, X.Z.; Li, L.B. Charge transport and magnetoresistance of G4-DNA molecular device modulated by counter ions and dephasing effect. Phys. Lett. A 2016, 380, 977–982. [Google Scholar] [CrossRef]
- Sun, W.M.; Varsano, D.; Di Felice, R. Effects of G-quadruplex topology on electronic transfer integrals. Nanomaterials 2016, 6, 6100184. [Google Scholar] [CrossRef]
- Ravindranath, R.; Mondal, P.; Gillet, N. Radical cation transfer in a guanine pair: An insight to the G-quadruplex structure role using constrained DFT/MM. Theor. Chem. Acc. 2021, 140, 89. [Google Scholar] [CrossRef]
- Banyasz, A.; Martinez-Fernandez, L.; Balty, C.; Perron, M.; Douki, T.; Improta, R.; Markovitsi, D. Absorption of low-energy UV Radiation by human telomere g-quadruplexes generates long-lived guanine radical cations. J. Am. Chem. Soc. 2017, 139, 10561–10568. [Google Scholar] [CrossRef] [PubMed]
- Banyasz, A.; Balanikas, E.; Martinez-Fernandez, L.; Baldacchino, G.; Douki, T.; Improta, R.; Markovitsi, D. Radicals generated in tetramolecular guanine quadruplexes by photo-ionization: Spectral and dynamical features. J. Phys. Chem. B 2019, 123, 4950–4957. [Google Scholar] [CrossRef] [PubMed]
- Marguet, S.; Markovitsi, D.; Talbot, F. One and two photon ionization of DNA single and double helices studied by laser flash photolysis at 266 nm. J. Phys. Chem. B 2006, 110, 11037–11039. [Google Scholar] [CrossRef][Green Version]
- Balanikas, E.; Banyasz, A.; Douki, T.; Baldacchino, G.; Markovitsi, D. Guanine radicals induced in DNA by low-energy photoionization. Acc. Chem. Res. 2020, 53, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Balanikas, E.; Markovitsi, D. DNA photoionization: From high to low energies. In DNA Photodamage; Improta, R., Douki, T., Eds.; RSC: Cambridge, UK, 2021; pp. 37–54. [Google Scholar]
- Marguet, S.; Markovitsi, D. Time-resolved study of thymine dimer formation. J. Am. Chem. Soc. 2005, 127, 5780–5781. [Google Scholar] [CrossRef]
- Banyasz, A.; Martinez-Fernandez, L.; Ketola, T.; Muñoz-Losa, A.; Esposito, L.; Markovitsi, D.; Improta, R. Excited state pathways leading to formation of adenine dimers. J. Phys. Chem. Lett. 2016, 7, 2020–2023. [Google Scholar] [CrossRef]
- Candeias, L.P.; Steenken, S. Ionization of purine nucleosides and nucleotides and their components by 193-nm laser photolysis in aqueous solution: Model studies for oxidative damage of DNA. J. Am. Chem. Soc. 1992, 114, 699–704. [Google Scholar] [CrossRef]
- Wu, L.D.; Liu, K.H.; Jie, J.L.; Song, D.; Su, H.M. Direct observation of guanine radical cation deprotonation in G-quadruplex DNA. J. Am. Chem. Soc. 2015, 137, 259–266. [Google Scholar] [CrossRef]
- Balanikas, E.; Banyasz, A.; Baldacchino, G.; Markovitsi, D. Deprotonation dynamics of guanine radical cations. Photochem. Photobiol. 2021, 13, 540. [Google Scholar] [CrossRef]
- Balanikas, E.; Martinez-Fernadez, L.; Improta, R.; Podbevsek, P.; Baldacchino, G.; Markovitsi, D. The structural duality of nucleobases in guanine quadruplexes controls their low-energy photoionization. J. Phys. Chem. Lett. 2021, 12, 8309–8313. [Google Scholar] [CrossRef] [PubMed]
- Pluharova, E.; Slavicek, P.; Jungwirth, P. Modeling photoionization of aqueous DNA and its components. Acc. Chem. Res. 2015, 48, 1209–1217. [Google Scholar] [CrossRef]
- Khanduri, D.; Adhikary, A.; Sevilla, M.D. Highly oxidizing excited states of one-electron-oxidized guanine in DNA: Wavelength and pH dependence. J. Am. Chem. Soc. 2011, 133, 4527–4537. [Google Scholar] [CrossRef] [PubMed]
- Candeias, L.P.; Steenken, S. Stucture and acid-base properties of one-electron-oxidized deoxyguanosine, guanosine, and 1-methylguanosine. J. Am. Chem. Soc. 1989, 111, 1094–1099. [Google Scholar] [CrossRef]
- Sket, P.; Plavec, J. Tetramolecular DNA quadruplexes in solution: Insights into structural diversity and cation movement. J. Am. Chem. Soc. 2010, 132, 12724–12732. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Fernandez, L.; Banyasz, A.; Markovitsi, D.; Improta, I. Topology controls the electronic absorption delocalization of electron hole in guanine quadruplexes. Chem. Eur. J. 2018, 24, 15185–15189. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sevilla, M.D. Density functional theory studies of the extent of hole delocalization in one-electron oxidized adenine and guanine base stacks. J. Phys. Chem. B 2011, 115, 4990–5000. [Google Scholar] [CrossRef]
- Adhikary, A.; Malkhasian, A.Y.S.; Collins, S.; Koppen, J.; Becker, D.; Sevilla, M.D. UVA-visible photo-excitation of guanine radical cations produces sugar radicals in DNA and model structures. Nucl. Ac. Res. 2005, 33, 5553–5564. [Google Scholar] [CrossRef]
- Adhikary, A.; Kumar, A.; Becker, D.; Sevilla, M.D. The guanine cation radical: Investigation of deprotonation states by ESR and DFT. J. Phys. Chem. B 2006, 110, 24171–24180. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Li, Y.; Li, S.; Zhang, M.; Zhou, Z. Collision-induced dissociation (CID) of guanine radical cation in the gas phase: An experimental and computational study. Phys. Chem. Chem. Phys. 2010, 12, 4667–4677. [Google Scholar] [CrossRef] [PubMed]
- Behmand, B.; Balanikas, E.; Martinez-Fernandez, L.; Improta, R.; Banyasz, A.; Baldacchino, G.; Markovitsi, D. Potassium ions enhance guanine radical generation upon absorption of low-energy photons by G-quadruplexes and modify their reactivity. J. Phys. Chem. Lett. 2020, 11, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Fernandez, L.; Esposito, L.; Improta, R. Studying the excited electronic states of guanine rich DNA quadruplexes by quantum mechanical methods: Main achievements and perspectives. Photochem. Photobiol. Sci. 2020, 19, 436–444. [Google Scholar] [CrossRef]
- Santoro, F.; Barone, V.; Lami, A.; Improta, R. The excited electronic states of adenine-guanine stacked dimers in aqueous solution: A PCM/TD-DFT study. Phys. Chem. Chem. Phys. 2010, 12, 4934–4948. [Google Scholar] [CrossRef] [PubMed]
- Aquino, A.J.A.; Nachtigallova, D.; Hobza, P.; Truhlar, D.G.; Hattig, C.; Lischka, H. The charge-transfer states in a stacked nucleobase dimer complex: A benchmark study. J. Comput. Chem. 2011, 32, 1217–1227. [Google Scholar] [CrossRef]
- Martinez-Fernandez, L.; Improta, R. Novel adenine/thymine photodimerization channels mapped by PCM/TD-DFT calculations on dApT and TpdA dinucleotides. Photochem. Photobiol. Sci 2017, 16, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Changenet-Barret, P.; Improta, R.; Vayá, I.; Gustavsson, T.; Kotlyar, A.B.; Zikich, D.; Šket, P.; Plavec, J.; Markovitsi, D. Cation effect on the electronic excited states of guanine nanostructures studied by time-resolved fluorescence spectroscopy. J. Phys. Chem. C 2012, 116, 14682–14689. [Google Scholar] [CrossRef]
- Improta, R. Quantum mechanical calculations unveil the structure and properties of the absorbing and emitting excited electronic states of guanine quadruplex. Chem. Eur. J. 2014, 20, 8106–8115. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Fernandez, L.; Changenet, P.; Banyasz, A.; Gustavsson, T.; Markovitsi, D.; Improta, R. A Comprehensive study of guanine excited state relaxation and photoreactivity in G-quadruplexes. J. Phys. Chem. Lett. 2019, 10, 6873–6877. [Google Scholar] [CrossRef]
- Chatgilialoglu, C. The two faces of the guanyl radical: Molecular context and behavior. Molecules 2021, 26, 3511. [Google Scholar] [CrossRef]
- Von Sonntag, C. Free-Radical-Induced DNA Damage and Its Repair; Springer: Berlin/Heildelberg, Germany, 2006; p. 523. [Google Scholar]
- Balanikas, E.; Banyasz, A.; Baldacchino, G.; Markovitsi, D. Guanine radicals generated in telomeric G-quadruplexes by direct absorption of low-energy UV photons: Effect of potassium ions. Molecules 2020, 25, 2094. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Kumar, A.; Muroya, Y.; Yamashita, S.; Sakurai, T.; Denisov, S.A.; Sevilla, M.D.; Adhikary, A.; Seki, S.; Mostafavi, M. Observation of dissociative quasi-free electron attachment to nucleoside via excited anion radical in solution. Nat. Commun. 2019, 10, 102. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Douki, T.; Ravanat, J.L. Oxidatively generated damage to the guanine moiety of DNA: Mechanistic aspects and formation in cells. Acc. Chem. Res. 2008, 41, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.M.; Burrows, C.J. G-quadruplex folds of the human telomere sequence alter the site reactivity and reaction pathway of guanine oxidation compared to duplex DNA. Chem. Res.Toxicol. 2013, 26, 593–607. [Google Scholar] [CrossRef]
- Bielskute, S.; Plavec, J.; Podbevsek, P. Impact of oxidative lesions on the human telomeric G-quadruplex. J. Am. Chem. Soc. 2019, 141, 2594–2603. [Google Scholar] [CrossRef]
- Dapprich, S.; Komaromi, I.; Byun, K.S.; Morokuma, K.; Frisch, M.J. A new ONIOM implementation in Gaussian98. Part, I. The calculation of energies, gradients, vibrational frequencies and electric field derivatives. J. Mol. Struct.-Theochem 1999, 461, 1–21. [Google Scholar] [CrossRef]
- Zhao, Y.; Schultz, N.E.; Truhlar, D.G. Design of density functionals by combining the method of constraint satisfaction with parametrization for thermochemistry, thermochemical kinetics, and noncovalent interactions. J. Chem. Theory Comput. 2006, 2, 364–382. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Density functionals with broad applicability in chemistry. Acc. Chem. Res. 2008, 41, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Cornell, W.D.; Cieplak, P.; Bayly, C.I.; Gould, I.R.; Merz, K.M.; Ferguson, D.M.; Spellmeyer, D.C.; Fox, T.; Caldwell, J.W.; Kollman, P.A. A 2nd generation force-field for the simulation of proteins, nucleic acids and organic molecules. J. Am. Chem. Soc. 1995, 117, 5179–5197. [Google Scholar] [CrossRef]
- Miertus, S.; Scrocco, E.; Tomasi, J. Electrostatic interaction of a solute with a continuum-A direct utilization of abinitio molecular potentials for the prevision of solvent effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Borrego-Varillas, R.; Cerullo, G.; Markovitsi, D. Exciton trapping dynamics in DNA multimers. J. Phys. Chem. Lett. 2019, 10, 1639–1643. [Google Scholar] [CrossRef]
- Ma, J.; Wang, F.; Denisov, S.A.; Adhikary, A.; Mostafavi, M. Reactivity of prehydrated electrons toward nucleobases and nucleotides in aqueous solution. Sci. Adv. 2017, 3, e1701669. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balanikas, E.; Martinez-Fernandez, L.; Baldacchino, G.; Markovitsi, D. Electron Holes in G-Quadruplexes: The Role of Adenine Ending Groups. Int. J. Mol. Sci. 2021, 22, 13436. https://doi.org/10.3390/ijms222413436
Balanikas E, Martinez-Fernandez L, Baldacchino G, Markovitsi D. Electron Holes in G-Quadruplexes: The Role of Adenine Ending Groups. International Journal of Molecular Sciences. 2021; 22(24):13436. https://doi.org/10.3390/ijms222413436
Chicago/Turabian StyleBalanikas, Evangelos, Lara Martinez-Fernandez, Gérard Baldacchino, and Dimitra Markovitsi. 2021. "Electron Holes in G-Quadruplexes: The Role of Adenine Ending Groups" International Journal of Molecular Sciences 22, no. 24: 13436. https://doi.org/10.3390/ijms222413436
APA StyleBalanikas, E., Martinez-Fernandez, L., Baldacchino, G., & Markovitsi, D. (2021). Electron Holes in G-Quadruplexes: The Role of Adenine Ending Groups. International Journal of Molecular Sciences, 22(24), 13436. https://doi.org/10.3390/ijms222413436






