Main and Minor Types of Collagens in the Articular Cartilage: The Role of Collagens in Repair Tissue Evaluation in Chondral Defects
Abstract
1. Introduction
2. Articular Cartilage
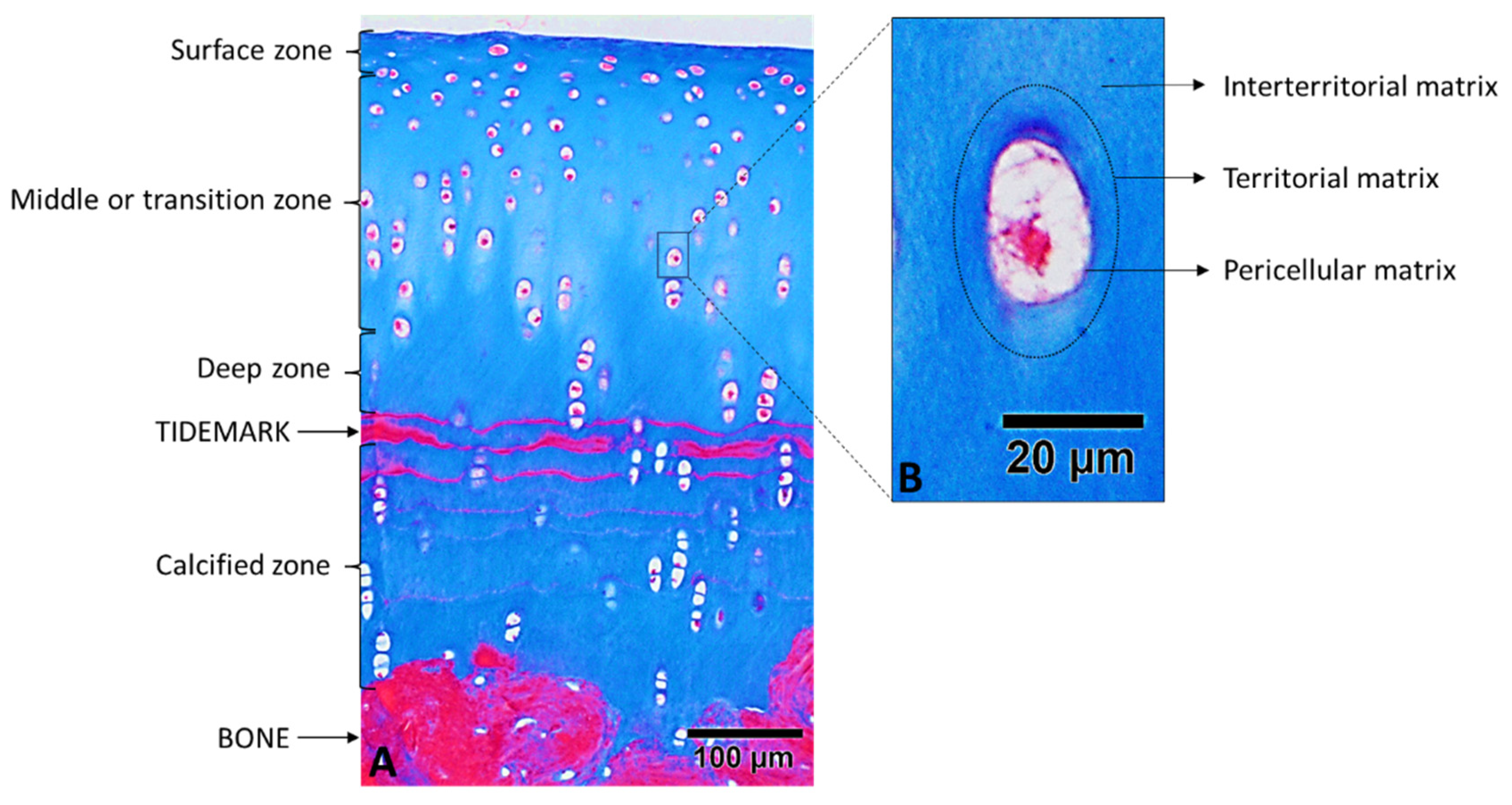
2.1. Cartilage Zones
2.2. Regions of the Extracellular Cartilage Matrix
3. Collagens
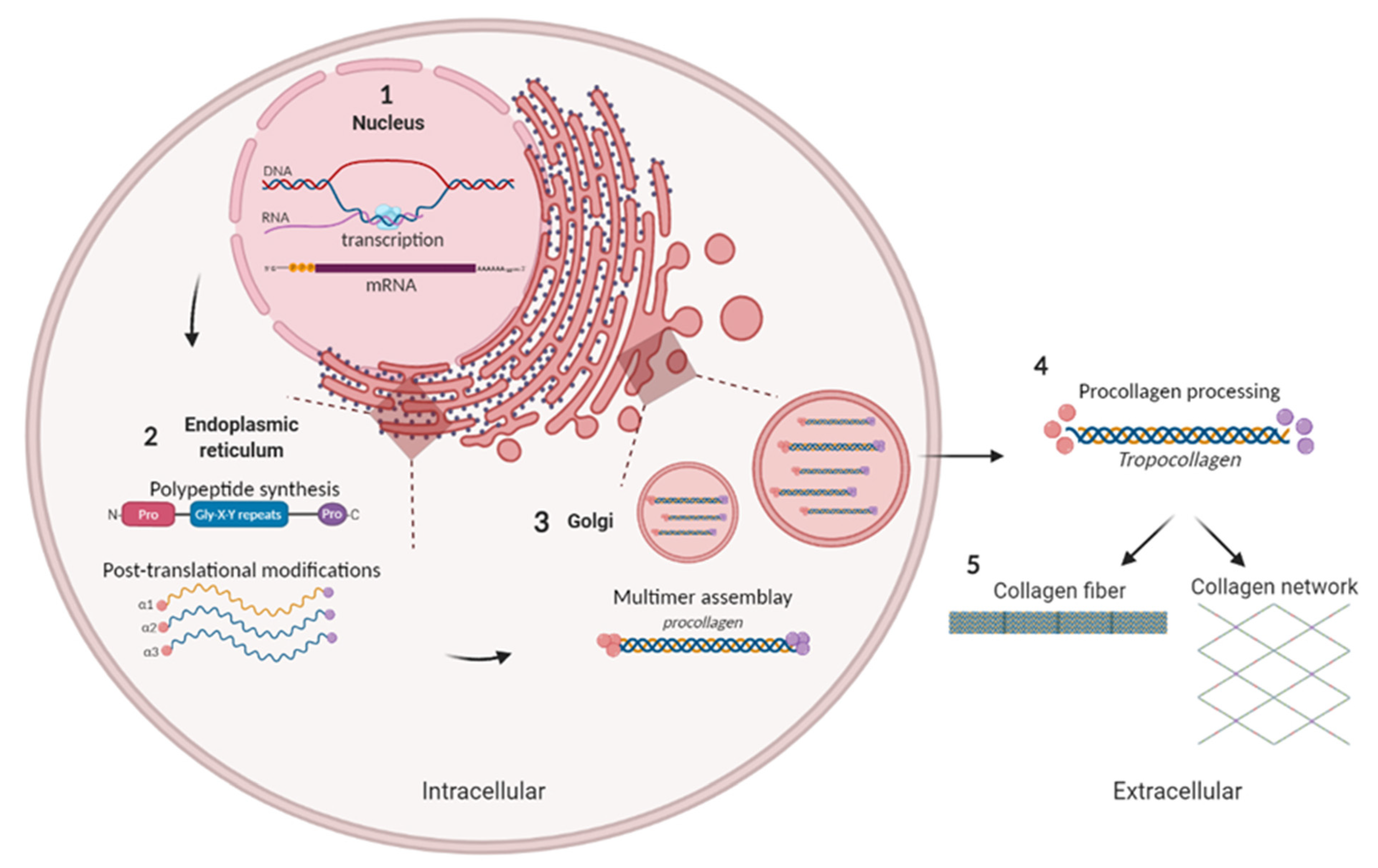
3.1. Biosynthesis of Collagens Fibers
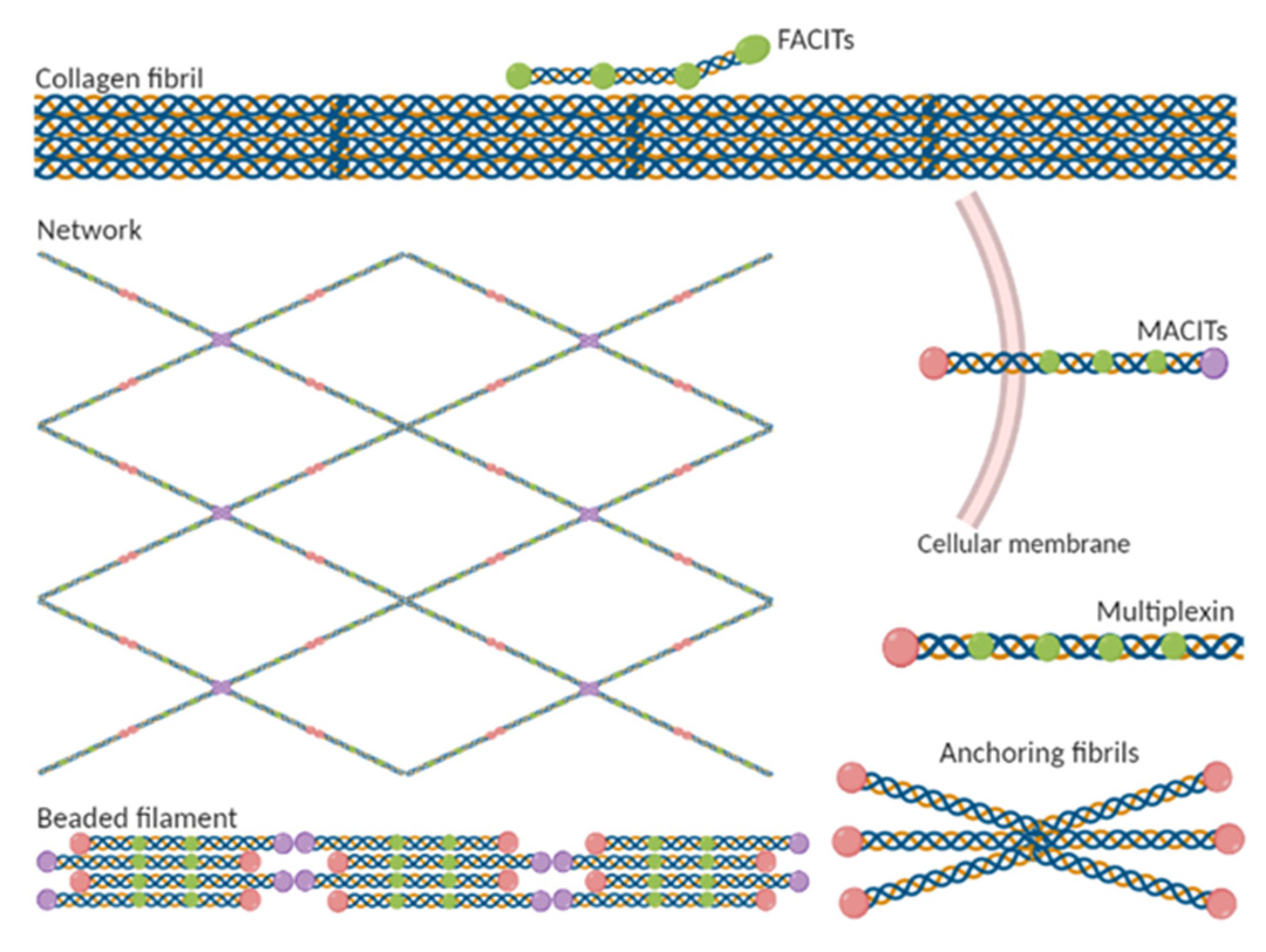
3.2. Classification of Collagen Types
4. Types of Collagens in Articular Cartilage
4.1. Main Collagens of Healthy Articular Cartilage
4.1.1. Type II Collagen
4.1.2. Type IX Collagen
4.1.3. Type XI Collagen
4.2. Minor Collagens of Healthy Articular Cartilage
4.2.1. Type III Collagen
4.2.2. Type IV Collagen
4.2.3. Type V Collagen
4.2.4. Type VI Collagen
4.2.5. Type X Collagen
4.2.6. Type XII, XIV, XVI, XXII, and XXVII Collagens
4.3. Articular Collagen Types Synthetized in Pathological Processes
Type I Collagen
4.4. Collagens in Pathological Situations
5. Role of Collagen Fibrils in the Quality of Repaired Tissue in Chondral Defects
5.1. Methods of Cartilage Tissue Reparation
5.2. Methods for Evaluating Morphological and Structural Characteristics of a Repaired Cartilage
5.3. Methods Allowing Evaluating Collagen of a Repaired Cartilage
- Main collagens:
- -
- 90–95% type II collagen distributed throughout all areas of the ECM.
- -
- 1–5% type IX collagen distributed throughout all areas of the ECM.
- -
- 1–5% type XI collagen distributed throughout all areas of the cartilage and around the chondrocytes.
- Minor collagens:
- -
- 1–2% type VI collagen distributed around the chondrocytes.
- -
- 1% type X collagen present only in the calcified area.
- -
- Presence of type IV and V collagen around the chondrocytes.
- -
- Possible presence of type III, XII, XIV, XVI, XXII, and XXVII collagens.
- Absence of type I collagen.
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ECM | Extracellular matrix |
| FACITs | Fibril-associated collagens with interrupted tripled helices |
| MACITs | membrane-associated collagens with interrupted tripled helices |
| MMP13 | Matrix metalloproteinase 13 |
| OA | Osteoarthritis |
| PCM | Pericellular matrix |
| PG | Proteoglycan |
| RER | Rough endoplasmic reticulum |
| SMD | Spondylometaphyseal dysplasia |
References
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef]
- Baumann, S.; Hennet, T. Collagen accumulation in osteosarcoma cells lacking GLT25D1 collagen galactosyltransferase. J. Biol. Chem. 2016, 291, 18514–18524. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Tashiro, T.; Katsuragawa, Y.; Sawabe, M.; Furukawa, H.; Fukui, N. Expression of minor cartilage collagens and small leucine rich proteoglycans may be relatively reduced in osteoarthritic cartilage. BMC Musculoskelet. Disord. 2019, 20, 232. [Google Scholar] [CrossRef]
- Cohen, N.P.; Foster, R.J.; Mow, V.C. Composition and dynamics of articular cartilage: Structure, function, and maintaining healthy state. J. Orthop. Sports Phys. Ther. 1998, 28, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Cumming, M.H.; Hall, B.; Hofman, K. Isolation and characterisation of major and minor collagens from hyaline cartilage of hoki (Macruronus novaezelandiae). Mar. Drugs 2019, 17, 223. [Google Scholar] [CrossRef]
- Miller, E.J.; Matukas, V.J. Chick cartilage collagen: A new type of alpha 1 chain not present in bone or skin of the species. Proc. Natl. Acad. Sci. USA 1969, 64, 1264–1268. [Google Scholar] [CrossRef]
- Miller, E.J.; Gay, S. Collagen: An Overview. Methods Enzymol. 1982, 82, 3–32. [Google Scholar]
- Ricard-Blum, S.; Ruggiero, F. The collagen superfamily: From the extracellular matrix to the cell membrane. Pathol. Biol. 2005, 53, 430–442. [Google Scholar] [CrossRef]
- Kadler, K.E.; Baldock, C.; Bella, J.; Boot-Handford, R.P. Collagens at a glance. J. Cell Sci. 2007, 120, 1955–1958. [Google Scholar] [CrossRef]
- Gordon, M.K.; Hahn, R.A. Collagens. Cell Tissue Res. 2010, 339, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, A.M.; Richardson, J.B. Articular cartilage: Structure, injuries and review of management. Br. Med. Bull. 2008, 87, 77–95. [Google Scholar] [CrossRef]
- Eyre, D. Collagen of articular cartilage. Arthritis Res. Ther. 2002, 4, 30–35. [Google Scholar] [CrossRef][Green Version]
- Luo, Y.; Sinkeviciute, D.; He, Y.; Karsdal, M.; Henrotin, Y.; Mobasheri, A.; Önnerfjord, P.; Bay-Jensen, A. The minor collagens in articular cartilage. Protein Cell 2017, 8, 560–572. [Google Scholar] [CrossRef]
- Wu, L.; Petrigliano, F.A.; Ba, K.; Lee, S.; Bogdanov, J.; McAllister, D.R.; Adams, J.S.; Rosenthal, A.K.; Van Handel, B.; Crooks, G.M.; et al. Lysophosphatidic acid mediates fibrosis in injured joints by regulating collagen type I biosynthesis. Osteoarthr. Cartil. 2015, 23, 308–318. [Google Scholar] [CrossRef]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef]
- Luria, A.; Chu, C.R. Articular Cartilage Changes in Maturing Athletes: New Targets for Joint Rejuvenation. Sports Health 2014, 6, 18–30. [Google Scholar] [CrossRef]
- Thorp, H.; Kim, K.; Kondo, M.; Maak, T.; Grainger, D.W.; Okano, T. Trends in Articular Cartilage Tissue Engineering: 3D Mesenchymal Stem Cell Sheets as Candidates for Engineered Hyaline-Like Cartilage. Cells 2011, 10, 643. [Google Scholar] [CrossRef]
- Sun, Y.; Yan, L.; Chen, S.; Pei, M. Functionality of decellularized matrix in cartilage regeneration: A comparison of tissue versus cell sources. Acta Biomater. 2018, 74, 56–73. [Google Scholar] [CrossRef] [PubMed]
- Decker, R.S. Articular cartilage and joint development from embryogenesis to adulthood. Semin. Cell Dev. Biol. 2017, 62, 50–56. [Google Scholar] [CrossRef]
- Medvedeva, E.V.; Grebenik, E.A.; Gornostaeva, A.N.; Telpuhov, V.I.; Lychagin, A.V.; Timashev, P.S.; Chagin, A.S. Repair of damaged articular cartilage: Current approaches and future directions. Int. J. Mol. Sci. 2017, 19, 2366. [Google Scholar] [CrossRef]
- Vinod, E.; Boopalan, P.R.J.V.C.; Sathishkumar, S. Reserve or Resident Progenitors in Cartilage? Comparative Analysis of Chondrocytes versus Chondroprogenitors and Their Role in Cartilage Repair. Cartilage 2018, 9, 171–182. [Google Scholar] [CrossRef]
- Patel, J.M.; Saleh, K.S.; Burdick, J.A.; Mauck, R.L. Bioactive factors for cartilage repair and regeneration: Improving delivery, retention, and activity. Acta Biomater. 2019, 93, 222–238. [Google Scholar] [CrossRef]
- Phull, A.R.; Eo, S.H.; Abbas, Q.; Ahmed, M.; Kim, S.J. Applications of Chondrocyte-Based Cartilage Engineering: An Overview. BioMed Res. Int. 2016, 2016, 1879837. [Google Scholar] [CrossRef]
- Wilusz, R.E.; Sanchez-Adams, J.; Guilak, F. The structure and function of the pericellular matrix of articular cartilage. Matrix Biol. 2014, 39, 25–32. [Google Scholar] [CrossRef]
- Tiku, M.L.; Sabaawy, H.E. Cartilage regeneration for treatment of osteoarthritis: A paradigm for nonsurgical intervention. Ther. Adv. Musculoskelet. Dis. 2015, 7, 76–87. [Google Scholar] [CrossRef]
- Chau, M.; Lui, J.C.; Landman, E.B.M.; Späth, S.S.; Vortkamp, A.; Baron, J.; Nilsson, O. Gene expression profiling reveals similarities between the spatial architectures of postnatal articular and growth plate cartilage. PLoS ONE 2014, 9, 103061. [Google Scholar]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, 1801651. [Google Scholar] [CrossRef]
- Fidler, A.L.; Boudko, S.P.; Rokas, A.; Hudson, B.G. The triple helix of collagens—An ancient protein structure that enabled animal multicellularity and tissue evolution. J. Cell Sci. 2018, 131, 209350. [Google Scholar] [CrossRef]
- Regoli, M.; Tosi, G.M.; Neri, G.; Altera, A.; Orazioli, D.; Bertelli, E. The Peculiar Pattern of Type IV Collagen Deposition in Epiretinal Membranes. J. Histochem. Cytochem. 2020, 68, 149–162. [Google Scholar] [CrossRef]
- Calvo, A.C.; Moreno, L.; Moreno, L.; Toivonen, J.M.; Manzano, R.; Molina, N.; de la Torre, M.; López, T.; Miana-Mena, F.J.; Muñoz, M.J.; et al. Type XIX collagen: A promising biomarker from the basement membranes. Neural Regen. Res. 2020, 15, 988–995. [Google Scholar]
- Gelse, K.; Pöschl, E.; Aigner, T. Collagens—Structure, function, and biosynthesis. Adv. Drug Del. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef]
- Arseni, L.; Lombardi, A.; Orioli, D. From structure to phenotype: Impact of collagen alterations on human health. Int. J. Mol. Sci. 2018, 19, 1407. [Google Scholar] [CrossRef]
- Albaugh, V.L.; Mukherjee, K.; Barbul, A. Proline precursors and collagen synthesis: Biochemical challenges of nutrient supplementation and wound healing. J. Nutr. 2017, 147, 2011–2017. [Google Scholar] [CrossRef]
- Taye, N.; Karoulias, S.Z.; Hubmacher, D. The “other” 15–40%: The Role of Non-Collagenous Extracellular Matrix Proteins and Minor Collagens in Tendon. J. Orthop. Res. 2020, 38, 23–35. [Google Scholar] [CrossRef]
- Senadheera, T.R.L.; Dave, D.; Shahidi, F. Sea cucumber derived type I collagen: A comprehensive review. Mar. Drugs 2020, 18, 471. [Google Scholar] [CrossRef]
- Bourgot, I.; Primac, I.; Louis, T.; Noël, A.; Maquoi, E. Reciprocal Interplay between Fibrillar Collagens and Collagen-Binding Integrins: Implications in Cancer Progression and Metastasis. Front. Oncol. 2020, 10, 1488. [Google Scholar] [CrossRef]
- León-López, A.; Morales-Peñaloza, A.; Martínez-Juárez, V.M.; Vargas-Torres, A.; Zeugolis, A.I.; Aguirre-Álvarez, G. Hydrolyzed collagen-sources and applications. Molecules 2019, 24, 4031. [Google Scholar] [CrossRef]
- Oudart, J.B.; Monboisse, J.C.; Maquart, F.X.; Brassart, B.; Brassart-Pasco, S.; Ramont, L. Type XIX collagen: A new partner in the interactions between tumor cells and their microenvironment. Matrix Biol. 2017, 57–58, 169–177. [Google Scholar] [CrossRef]
- Knupp, C.; Squire, J.M. Molecular packing in network-forming collagens. Sci. World J. 2003, 3, 558–577. [Google Scholar] [CrossRef]
- Nyström, A.; Kiritsi, D. Transmembrane collagens—Unexplored mediators of epidermal-dermal communication and tissue homeostasis. Exp. Dermatol. 2021, 30, 10–16. [Google Scholar] [CrossRef]
- Clementz, A.G.; Harris, A. Collagen XV: Exploring its structure and role within the tumor microenvironment. Mol. Cancer Res. 2013, 11, 1481–1486. [Google Scholar] [CrossRef]
- Csorba, K.; Chiriac, M.C.; Florea, F.; Ghinia, M.G.; Licarete, E.; Rados, A.; Sas, A.; Vuta, V.; Sitaru, C. Blister-inducing antibodies target multiple epitopes on collagen VII in mice. J. Cell Mol. Med. 2014, 18, 1727–1739. [Google Scholar] [CrossRef]
- Wullink, B.; Pas, H.H.; Van Der Worp, R.J.; Kuijer, R.; Los, L.I. Type VII collagen expression in the human vitreoretinal interface, corpora amylacea and inner retinal layers. PLoS ONE 2015, 10, 0145502. [Google Scholar] [CrossRef]
- Godwin, A.R.F.; Starborg, T.; Sherratt, M.J.; Roseman, A.M.; Baldock, C. Defining the hierarchical organisation of collagen VI microfibrils at nanometre to micrometre length scales. Acta Biomater. 2017, 52, 21–32. [Google Scholar] [CrossRef]
- Izu, Y.; Ezura, Y.; Koch, M.; Birk, D.E.; Noda, M. Collagens VI and XII form complexes mediating osteoblast interactions during osteogenesis. Cell Tissue Res. 2016, 364, 623–635. [Google Scholar] [CrossRef]
- McAlinden, A.; Traeger, G.; Hansen, U.; Weis, M.A.; Ravindran, S.; Wirthlin, L.; Eyre, D.R.; Fernandes, R.J. Molecular properties and fibril ultrastructure of types II and XI collagens in cartilage of mice expressing exclusively the α1(IIA) collagen isoform. Matrix Biol. 2014, 34, 105–113. [Google Scholar] [CrossRef]
- Lian, H.; Gao, B.; Wu, Z.; Qiu, X.; Peng, Y.; Liang, A.; Xu, C.; Su, P.; Huang, D. Collagen type II is downregulated in the degenerative nucleus pulposus and contributes to the degeneration and apoptosis of human nucleus pulposus cells. Mol. Med. Rep. 2017, 16, 4730–4736. [Google Scholar] [CrossRef]
- Zhu, Y.; Oganesian, A.; Keene, D.R.; Sandell, L.J. Type IIA procollagen containing the cysteine-rich amino propeptide is deposited in the extracellular matrix of prechondrogenic tissue and binds to TGF-β1 and BMP-2. J. Cell Biol. 1999, 144, 1069–1080. [Google Scholar] [CrossRef]
- Wu, J.J.; Weis, M.A.; Kim, L.S.; Eyre, D.R. Type III collagen, a fibril network modifier in articular cartilage. J. Biol. Chem. 2010, 285, 18537–18544. [Google Scholar] [CrossRef]
- Eyre, D.R.; Wu, J.J.; Fernandes, R.J.; Pietka, T.A.; Weis, M.A. Recent developments in cartilage research: Matrix biology of the collagen II/IX/XI heterofibril network. Biochem. Soc. Trans. 2002, 30, 893–899. [Google Scholar] [CrossRef]
- Lai, C.S.; Tu, C.W.; Kuo, H.C.; Sun, P.P.; Tsai, M.L. Type II Collagen from Cartilage of Acipenser baerii Promotes Wound Healing in Human Dermal Fibroblasts and in Mouse Skin. Mar. Drugs 2020, 18, 511. [Google Scholar] [CrossRef]
- Lian, C.; Wang, X.; Qiu, X.; Gao, B.; Liu, L.; Liang, G.; Zhou, H.; Yang, X.; Peng, Y.; Liang, A.; et al. Collagen type II suppresses articular chondrocyte hypertrophy and osteoarthritis progression by promoting integrin β1−SMAD1 interaction. Bone Res. 2019, 7, 1–15. [Google Scholar] [CrossRef]
- Carlsen, S.; Nandakumar, K.S.; Holmdahl, R. Type IX collagen deficiency enhances the binding of cartilage-specific antibodies and arthritis severity. Arthritis Res. Ther. 2006, 8, 1–8. [Google Scholar] [CrossRef]
- Bönnemann, C.G.; Cox, G.F.; Shapiro, F.; Wu, J.J.; Feener, C.A.; Thompson, T.G.; Anthony, D.C.; Eyre, D.R.; Darras, T.B.; Kunkel, L.M. A mutation in the alpha 3 chain of type IX collagen causes autosomal dominant multiple epiphyseal dysplasia with mild myopathy. Proc. Natl. Acad. Sci. USA 2000, 97, 1212–1217. [Google Scholar] [CrossRef]
- Eyre, D.R.; Pietka, T.; Weis, M.A.; Wu, J.J. Covalent Cross-linking of the NC1 Domain of Collagen Type IX to Collagen Type II in Cartilage. J. Biol. Chem. 2004, 279, 2568–2574. [Google Scholar] [CrossRef]
- Imagawa, K.; de Andrés, M.C.; Hashimoto, K.; Itoi, E.; Otero, M.; Roach, H.I.; Golgring, M.B.; Oreffo, R.O.C. Association of reduced type IX collagen gene expression in human osteoarthritic chondrocytes with epigenetic silencing by DNA hypermethylation. Arthritis Rheumatol. 2014, 66, 3040–3051. [Google Scholar] [CrossRef]
- Lohiniva, J.; Paassilta, P.; Seppänen, U.; Vierimaa, O.; Kivirikko, S.; Ala-Kokko, L. Splicing mutations in the COL3 domain of collagen IX cause multiple epiphyseal dysplasia. Am. J. Med. Genet. 2000, 90, 216–222. [Google Scholar] [CrossRef]
- Czarny-Ratajczak, M.; Lohiniva, J.; Rogala, P.; Kozlowski, K.; Perälä, M.; Carter, L.; Spector, T.D.; Kolodziej, L.; Seppänen, U.; Glazar, R.; et al. A mutation in COL9A1 causes multiple epiphyseal dysplasia: Further evidence for locus heterogeneity. Am. J. Hum. Genet. 2001, 69, 969–980. [Google Scholar] [CrossRef]
- Parsons, P.; Gilbert, S.J.; Vaughan-Thomas, A.; Sorrell, D.A.; Notman, R.; Bishop, M.; Hayes, A.J.; Mason, D.J.; Duance, V.C. Type IX collagen interacts with fibronectin providing an important molecular bridge in articular cartilage. J. Biol. Chem. 2011, 286, 34986–34997. [Google Scholar] [CrossRef] [PubMed]
- Mio, F.; Chiba, K.; Hirose, Y.; Kawaguchi, Y.; Mikami, Y.; Oya, T.; Mori, M.; Kamata, M.; Matsumoto, M.; Ozaki, K.; et al. A functional polymorphism in COL11A1, which encodes the α1 chain of type XI collagen, is associated with susceptibility to lumbar disc herniation. Am. J. Hum. Genet. 2007, 81, 1271–1277. [Google Scholar] [CrossRef]
- Warner, L.R.; Blasick, C.M.; Brown, R.J.; Oxford, J.T. Expression, purification, and refolding of recombinant collagen α1(XI) amino terminal domain splice variants. Protein Expr. Purif. 2007, 52, 403–409. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luo, Y.Y.; Karsdal, M.A. Type XI Collagen. In Biochemistry of Collagens, Laminins and Elastin: Structure, Function and Biomarkers, 1st ed.; Elsevier: London, UK, 2016; pp. 77–80. [Google Scholar]
- Nallanthighal, S.; Rada, M.; Heiserman, J.P.; Cha, J.; Sage, J.; Zhou, B.; Yang, W.; Hu, Y.; Korgaonkar, C.; Hanos, C.T.; et al. Inhibition of collagen XI alpha 1-induced fatty acid oxidation triggers apoptotic cell death in cisplatin-resistant ovarian cancer. Cell Death Dis. 2020, 11, 258. [Google Scholar] [CrossRef]
- Fernandes, R.J.; Weis, M.A.; Scott, M.A.; Seegmiller, R.E.; Eyre, D.R. Collagen XI chain misassembly in cartilage of the chondrodysplasia (cho) mouse. Matrix Biol. 2007, 26, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Tong, D.; Lönnblom, E.; Yau, A.C.Y.; Nandakumar, K.S.; Liang, B.; Ge, C.; Viljanen, J.; Li, L.; Bãlan, M.; Klareskog, L.; et al. A shared epitope of collagen type XI and type II is recognized by pathogenic antibodies in mice and humans with arthritis. Front. Immunol. 2018, 9, 12. [Google Scholar] [CrossRef]
- Xu, J.; Wang, W.; Ludeman, M.; Cheng, K.; Hayami, T.; Lotz, J.C.; Kapila, S. Chondrogenic differentiation of human mesenchymal stem cells in three-dimensional alginate gels. Tissue Eng. Part A 2008, 14, 667–680. [Google Scholar] [CrossRef]
- Shanmugasundaram, S.; Logan-Mauney, S.; Burgos, K.; Nurminskaya, M. Tissue transglutaminase regulates chondrogenesis in mesenchymal stem cells on collagen type XI matrices. Amino Acids 2012, 42, 1045–1053. [Google Scholar] [CrossRef]
- Wang, C.; Brisson, B.K.; Terajima, M.; Li, Q.; Hoxha, K.; Han, B.; Goldberg, A.M.; Liu, X.S.; Marcolongo, M.S.; Enomoto-Iwamoto, M.; et al. Type III collagen is a key regulator of the collagen fibrillar structure and biomechanics of articular cartilage and meniscus. Matrix Biol. 2020, 85–86, 47–67. [Google Scholar] [CrossRef]
- Prockop, D.J.; Kivirikko, K.I. Collagens: Molecular Biology, Diseases, and Potentials for Therapy. Annu. Rev. Biochem. 1995, 64, 403–434. [Google Scholar] [CrossRef]
- Kuivaniemi, H.; Tromp, G. Type III collagen (COL3A1): Gene and protein structure, tissue distribution, and associated diseases. Gene 2019, 707, 151–171. [Google Scholar] [CrossRef]
- Balleisen, L.; Gay, S.; Marx, R.; Kühn, K. Comparative investigation on the influence of human bovine collagen types I, II and III on the aggregation of human platelets. Klin. Wochenschr. 1975, 53, 903–905. [Google Scholar] [CrossRef]
- Chiang, T.M.; Seyer, J.M.; Kang, A.H. Collagen-platelet interaction: Separate receptor sites for types I and III collagen. Thromb. Res. 1993, 71, 443–456. [Google Scholar] [CrossRef]
- Monnet, E.; Fauvel-Lafève, F. A new platelet receptor specific to type III collagen. Type III collagen-binding protein. J. Biol. Chem. 2000, 275, 10912–10917. [Google Scholar] [CrossRef] [PubMed]
- Eyre, D.R.; Weis, M.A.; Wu, J.J. Articular cartilage collagen: An irreplaceable framework? Eur. Cell Mater. 2006, 12, 57–63. [Google Scholar] [CrossRef]
- Young, R.D.; Lawrence, P.A.; Duance, V.C.; Aigner, T.; Monaghan, P. Immunolocalization of collagen Types II and III in single fibrils of human articular cartilage. J. Histochem. Cytochem. 2000, 48, 423–432. [Google Scholar] [CrossRef]
- Hosseininia, S.; Wei, M.A.; Rai, J.; Funk, S.; Dahlberg, L.E.; Eyre, D.R. Evidence for enhanced collagen type III deposition focally in the territorial matrix of osteoarthritic hip articular cartilage. Osteoarthr. Cartil. 2016, 24, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Borza, D.B.; Bondar, O.; Ninomiya, Y.; Sado, Y.; Naito, I.; Todd, P.; Hudson, B.G. The NC1 Domain of Collagen IV Encodes a Novel Network Composed of the α1, α2, α5, and α6 Chains in Smooth Muscle Basement Membranes. J. Biol. Chem. 2001, 276, 28532–28540. [Google Scholar] [CrossRef]
- Maziers, N.; Dahan, K.; Pirson, Y. From Alport syndrome to benign familial hematuria: Clinical and genetic aspect. Nephrol. Ther. 2005, 1, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Foldager, C.B.; Toh, W.S.; Christensen, B.B.; Lind, M.; Gomoll, A.H.; Spector, M. Collagen Type IV and Laminin Expressions during Cartilage Repair and in Late Clinically Failed Repair Tissues from Human Subjects. Cartilage 2016, 7, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Komori, T.; Ono, M.; Hara, E.S.; Ueda, J.; Nguyen, H.T.T.; Nguyen, H.T.; Yonezawa, T.; Maeba, T.; Kimura-Ono, A.; Takarada, T.; et al. Type IV collagen α6 chain is a regulator of keratin 10 in keratinization of oral mucosal epithelium. Sci. Rep. 2018, 8, 2612. [Google Scholar] [CrossRef]
- Nakano, K.; Naito, I.; Momota, R.; Sado, Y.; Hasegawa, H.; Ninomiya, Y.; Ohtsuka, A. The distribution of type IV collagen α chains in the mouse ovary and its correlation with follicular development. Arch. Histol. Cytol. 2007, 70, 243–253. [Google Scholar] [CrossRef][Green Version]
- Jeng, L.; Hsu, H.P.; Spector, M. Tissue-engineered cartilaginous constructs for the treatment of caprine cartilage defects, including distribution of laminin and type IV collagen. Tissue Eng. Part A. 2013, 19, 2267–2274. [Google Scholar] [CrossRef] [PubMed]
- Birk, D.E. Type V collagen: Heterotypic type I/V collagen interactions in the regulation of fibril assembly. Micron 2001, 32, 223–237. [Google Scholar] [CrossRef]
- Huang, G.; Ge, G.; Izzi, V.; Greenspan, D.S. α3 Chains of type v collagen regulate breast tumour growth via glypican-1. Nat. Commun. 2017, 8, 14351. [Google Scholar] [CrossRef]
- Huang, G.; Ge, G.; Wang, D.; Gopalakrishnan, B.; Butz, D.H.; Colman, R.J.; Nagy, A.; Greenspan, D.S. α3(V) Collagen is critical for glucose homeostasis in mice due to effects in pancreatic islets and peripheral tissues. J. Clin. Investig. 2011, 121, 769–783. [Google Scholar] [CrossRef] [PubMed]
- Bobadilla, J.L.; Jankowska-Gan, E.; Xu, Q.; Haynes, L.D.; del Rio, A.M.; Meyer, K.; Greenspan, D.S.; De Oliveira, N.; Burlingham, W.J.; Maloney, J.D. Reflux-induced collagen type V sensitization: Potential mediator of bronchiolitis obliterans syndrome. Chest 2010, 138, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Weis, M.A.; Kim, L.S.; Carter, B.G.; Eyre, D.R. Differences in chain usage and cross-linking specificities of cartilage type V/XI collagen isoforms with age and tissue. J. Biol. Chem. 2009, 284, 5539–5545. [Google Scholar] [CrossRef]
- Braun, R.K.; Molitor-Dart, M.; Wigfield, C.; Xiang, Z.; Fain, S.B.; Jankowska-Gan, E.; Seroogy, C.M.; Burlingham, W.J.; Wilkes, D.S.; Brand, D.D.; et al. Transfer of tolerance to collagen type v suppresses T-helper-cell-17 lymphocyte-mediated acute lung transplant rejection. Transplantation 2009, 88, 1341–1348. [Google Scholar] [CrossRef]
- Parra, E.R.; Teodoro, W.R.; Velosa, A.P.P.; de Oliveira, C.C.; Yoshinari, N.H.; Capelozzi, V.L. Interstitial and Vascular Type V Collagen Morphologic Disorganization in Usual Interstitial Pneumonia. J. Histochem. Cytochem. 2006, 54, 1315–1325. [Google Scholar] [CrossRef]
- Domínguez-Pérez, J.M.; Fernández-Sarmiento, J.A.; García, D.; Granados-Machuca, M.M.; Morgaz-Rodríguez, J.; Navarrete-Calvo, R.; Pérez-Arévalo, J.; Carrillo-Poveda, J.M.; Alentorn-Geli, E.; Laiz-Boada, P.; et al. Cartilage regeneration using a novel autologous growth factors-based matrix for full-thickness defects in sheep. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 950–961. [Google Scholar] [CrossRef] [PubMed]
- Ritelli, M.; Dordoni, C.; Venturini, M.; Chiarelli, N.; Quinzani, S.; Traversa, M.; Zoppi, N.; Vascellaro, A.; Wischmeijer, A.; Manfredini, E.; et al. Clinical and molecular characterization of 40 patients with classic Ehlers-Danlos syndrome: Identification of 18 COL5A1 and 2 COL5A2 novel mutations. Orphanet J. Rare Dis. 2013, 8, 58. [Google Scholar] [CrossRef]
- Wang, J.; Pan, W. The biological role of the collagen alpha-3 (Vi) chain and its cleaved c5 domain fragment endotrophin in cancer. OncoTargets Ther. 2020, 13, 5779–5793. [Google Scholar] [CrossRef]
- Koudouna, E.; Young, R.D.; Ueno, M.; Kinoshita, S.; Quantock, A.J.; Knupp, C. Three-dimensional architecture of collagen type VI in the human trabecular meshwork. Mol. Vis. 2014, 20, 638–648. [Google Scholar] [PubMed]
- Lettmann, S.; Bloch, W.; MaaB, T.; Niehoff, A.; Schulz, J.N.; Eckes, B.; Eming, S.A.; Bonaldo, P.; Paulsson, M.; Wagener, R. Col6a1 null mice as a model to study skin phenotypes in patients with collagen VI related myopathies: Expression of classical and novel collagen VI variants during wound healing. PLoS ONE 2014, 9, 105686. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alexopoulos, L.G.; Youn, I.; Bonaldo, P.; Guilak, F. Developmental and osteoarthritic changes in Col6a1 -knockout mice: Biomechanics of type VI collagen in the cartilage pericellular matrix. Arthritis Rheum. 2009, 60, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Veidal, S.S.; Karsdal, M.A.; Vassiliadis, E.; Nawrocki, A.; Larsen, M.R.; Nguyen, Q.H.T.; Hägglund, P.; Luo, Y.; Zheng, Q.; Vainer, B.; et al. MMP mediated degradation of type VI collagen is highly associated with liver Fibrosis—Identification and validation of a novel biochemical marker assay. PLoS ONE 2011, 6, 24753. [Google Scholar] [CrossRef] [PubMed]
- Stickel, F.; Urbaschek, R.; Schuppan, D.; Poeschl, G.; Oesterling, C.; Conradt, C.; McCuskey, R.S.; Simanowski, U.A.; Seitz, H.K. Serum collagen type VI and XIV and hyaluronic acid as early indicators for altered connective tissue turnover in alcoholic liver disease. Dig. Dis. Sci. 2001, 46, 2025–2032. [Google Scholar] [CrossRef]
- Gerling, B.; Becker, M.; Staab, D.; Schuppan, D. Prediction of Liver Fibrosis According to Serum Collagen VI Level in Children with Cystic Fibrosis. N. Engl. J. Med. 1997, 336, 1611–1612. [Google Scholar] [CrossRef] [PubMed]
- Endicott, J.; Holden, P.; Fitzgerald, J. Authentication of collagen VI antibodies. BMC Res. Notes 2017, 10, 358. [Google Scholar] [CrossRef]
- Pan, T.C.; Zhang, R.Z.; Arita, M.; Bogdanovich, S.; Adams, S.M.; Gara, S.K.; Wagener, R.; Khurana, T.; Birk, D.E.; Chu, M.L. A mouse model for dominant collagen VI disorders Heterozygous Deletion of Col6a3 EXON 16. J. Biol. Chem. 2014, 289, 10293–10307. [Google Scholar] [CrossRef]
- Allamand, V.; Briñas, L.; Richard, P.; Stojkovic, T.; Quijano-Roy, S.; Bonne, G. ColVI myopathies: Where do we stand, where do we go? Skelet. Muscle 2011, 1, 30. [Google Scholar] [CrossRef] [PubMed]
- Jansen, I.D.C.; Hollander, A.P.; Buttle, D.J.; Everts, V. Type II and VI collagen in nasal and articular cartilage and the effect of IL-1α on the distribution of these collagens. J. Mol. Histol. 2010, 41, 9–17. [Google Scholar] [CrossRef][Green Version]
- Zelenski, N.A.; Leddy, H.A.; Sanchez-Adams, J.; Zhang, J.; Bonaldo, P.; Liedtke, W.; Guilak, F. Type VI collagen regulates pericellular matrix properties, chondrocyte swelling, and mechanotransduction in mouse articular cartilage. Arthritis Rheumatol. 2015, 67, 1286–1294. [Google Scholar] [CrossRef] [PubMed]
- Shen, G. The role of type X collagen in facilitating and regulating endochondral ossification of articular cartilage. Orthod. Craniofacial Res. 2015, 8, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Lu, Y.; Qiao, L.; Wang, Q.; Li, N.; Borgia, J.A.; Deng, Y.; Lei, G.; Zheng, Q. Identification and characterization of the novel Col10a1 regulatory mechanism during chondrocyte hypertrophic differentiation. Cell Death Dis. 2014, 5, 1469. [Google Scholar] [CrossRef]
- He, Y.; Siebuhr, A.S.; Brandt-Hanses, N.U.; Wang, J.; Su, D.; Zheng, Q.; Simonsen, O.; Petersen, K.K.; Arendt-Nielsen, L.; Eskehave, T.; et al. Type X collagen levels are elevated in serum from human osteoarthritis patients and associated with biomarkers of cartilage degradation and inflammation. BMC Musculoskelet. Disord. 2014, 15, 309. [Google Scholar] [CrossRef]
- Brew, C.J.; Clegg, P.D.; Boot-Handford, R.P.; Andrew, J.G.; Hardingham, T. Gene expression in human chondrocytes in late osteoarthritis is changed in both fibrillated and intact cartilage without evidence of generalised chondrocyte hypertrophy. Ann. Rheum. Dis. 2010, 69, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, J.; Balbín, M.; Santos, F.; Fernández, M.; Ferrando, S.; López, J.M. Different bone growth rates are associated with changes in the expression pattern of types II and X collagens and collagenase 3 in proximal growth plates of the rat tibia. J. Bone Miner. Res. 2000, 15, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Van der Kraan, P.M.; Van den Berg, W.B. Chondrocyte hypertrophy and osteoarthritis: Role in initiation and progression of cartilage degeneration? Osteoarthr. Cartil. 2012, 20, 223–232. [Google Scholar] [CrossRef] [PubMed]
- De Andrea, C.E.; Wiweger, M.I.; Bovée, J.V.M.G.; Romeo, S.; Hogendoorn, P.C.W. Peripheral chondrosarcoma progression is associated with increased type X collagen and vascularisation. Virchows Arch. 2012, 460, 95–102. [Google Scholar] [CrossRef][Green Version]
- Luckman, S.P.; Rees, E.; Kwan, A.P.L. Partial characterization of cell-type X collagen interactions. Biochem. J. 2003, 372, 485–493. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, H.; Liu, W.; Li, X.; Zhang, W.; Shang, X. A novel sequence variant in COL10A1 causing spondylometaphyseal dysplasia accompanied with coxa valga: A case report. Medicine 2019, 98, 16485. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.W.; Ahmed, N.; Parreno, J.; Lunstrum, G.P.; Gross, A.E.; Diamandis, E.P.; Kandel, R.A. Collagen type XII and versican are present in the early stages of cartilage tissue formation by both redifferentating passaged and primary chondrocytes. Tissue Eng. Part A 2015, 21, 683–693. [Google Scholar] [CrossRef]
- Polacek, M.; Bruun, J.A.; Elvenes, J.; Figenschau, Y.; Martinez, I. The secretory profiles of cultured human articular chondrocytes and mesenchymal stem cells: Implications for autologous cell transplantation strategies. Cell Transplant. 2011, 20, 1381–1393. [Google Scholar] [CrossRef] [PubMed]
- Hemmavanh, C.; Koch, M.; Birk, D.E.; Espana, E.M. Abnormal corneal endothelial maturation in collagen XII and XIV Null mice. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3297–3308. [Google Scholar] [CrossRef]
- Kassner, A.; Hansen, U.; Miosge, N.; Reinhardt, D.P.; Aigner, T.; Bruckner-Tuderman, L.; Bruckner, P.; Grässel, S. Discrete integration of collagen XVI into tissue-specific collagen fibrils or beaded microfibrils. Matrix Biol. 2003, 22, 131–143. [Google Scholar] [CrossRef]
- Koch, M.; Schulze, J.; Hansen, U.; Ashwodt, T.; Keene, D.R.; Brunken, W.J.; Burgeson, R.E.; Bruckner, P.; Bruckner-Tuderman, L. A novel marker of tissue junctions, collagen XXII. J. Biol. Chem. 2004, 279, 22514–22521. [Google Scholar] [CrossRef]
- Boot-Handford, R.P.; Tuckwell, D.S.; Plumb, D.A.; Farrington Rock, C.; Poulsom, R. A novel and highly conserved collagen (proα1(XXVII)) with a unique expression pattern and unusual molecular characteristics establishes a new clade within the vertebrate fibrillar collagen family. J. Biol. Chem. 2003, 278, 31067–31077. [Google Scholar] [CrossRef]
- Plumb, D.A.; Ferrara, L.; Torbica, T.; Knowles, L.; Mironov, A.; Kadler, K.E.; Briggs, M.D.; Boot-Handford, R.P. Collagen XXVII organises the pericellular matrix in the growth plate. PLoS ONE 2011, 6, 29422. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Chen, Z.; Huang, R.; Yao, Y.; Ma, G. Transforming Growth Factor β1 Induces the Expression of Collagen Type I by DNA Methylation in Cardiac Fibroblasts. PLoS ONE 2013, 8, 60335. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, W.R. Connective Tissue and Its Heritable Disorders: Molecular, Genetic, and Medical Aspects. Am. J. Hum. Genet. 2003, 72, 503. [Google Scholar] [CrossRef]
- Kratochwil, K.; Ghaffari-Tabrizi, N.; Holzinger, I.; Harbers, K. Restricted expression of Mov13 mutant α1(I) collagen gene in osteoblasts and its consequences for bone development. Dev. Dyn. 1993, 198, 273–283. [Google Scholar] [CrossRef]
- Fleischmajer, R.; Douglas MacDonald, E.; Perlish, J.S.; Burgeson, R.E.; Fisher, L.W. Dermal collagen fibrils are hybrids of type I and type III collagen molecules. J. Struct. Biol. 1990, 105, 162–169. [Google Scholar] [CrossRef]
- Birk, D.E.; Fitch, J.M.; Babiarz, J.P.; Linsenmayer, T.F. Collagen type I and type V are present in the same fibril in the avian corneal stroma. J. Cell Biol. 1998, 106, 999–1008. [Google Scholar] [CrossRef]
- Von Der Mark, K. Localization of collagen types in tissues. Int. Rev. Connect. Tissue Res. 1981, 9, 265–324. [Google Scholar]
- Shibata, S.; Sakamoto, Y.; Baba, O.; Qin, C.; Murakami, G.; Cho, B.H. An immunohistochemical study of matrix proteins in the craniofacial cartilage in midterm human fetuses. Eur. J. Histochem. 2013, 57, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Liang, C.; Huang, J.; He, J.; Wang, Z.; Cao, X.; Peng, C.; Wu, S. Pericellular collagen I coating for enhanced homing and chondrogenic differentiation of mesenchymal stem cells in direct intra-articular injection. Stem Cell Res. Ther. 2018, 9, 174. [Google Scholar] [CrossRef] [PubMed]
- Bielajew, B.J.; Hu, J.C.; Athanasiou, K.A. Collagen: Quantification, biomechanics, and role of minor subtypes in cartilage. Nat. Rev. Mater. 2020, 5, 730–747. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.S.; Kim, C.W.; Jung, D.W. Management of Focal Chondral Lesion in the Knee Joint. Knee Surg Relat Res. 2011, 23, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Gelber, A.C.; Hochberg, M.C.; Mead, L.A.; Wang, N.Y.; Wigley, F.M.; Klag, M.J. Joint injury in young adults and risk for subsequent knee and hip osteoarthritis. Ann. Intern. Med. 2000, 133, 321–328. [Google Scholar] [CrossRef]
- Mainil-Varlet, P.; Aigner, T.; Brittberg, M.; Bullough, P.; Hollander, A.; Hunziker, E.; Kandel, R.; Nehrer, S.; Pritzker, K.; Roberts, S.; et al. Histological assessment of cartilage repair: A report by the Histology Endpoint Committee of the International Cartilage Repair Society (ICRS). J. Bone Jt. Surg 2003, 85, 45–57. [Google Scholar] [CrossRef]
- Aigner, T.; Stöve, J. Collagens—major component of the physiological cartilage matrix, major target of cartilage degeneration, major tool in cartilage repair. Adv. Drug Deliv. Rev. 2003, 55, 1569–1593. [Google Scholar] [CrossRef]
- Hoemann, C.; Kandel, R.; Roberts, S.; Saris, D.B.F.; Creemers, L.; Mainil-Varlet, P.; Méthot, S.; Hollander, A.P.; Buschmann, M.D. International cartilage repair society (ICRS) recommended guidelines for histological endpoints for cartilage repair studies in animal models and clinical trials. Cartilage 2011, 2, 153–172. [Google Scholar] [CrossRef]
- Lindert, U.; Gnoli, M.; Maioli, M.; Bedeschi, M.F.; Sangiorgi, L.; Rohrbach, M.; Giunta, C. Insight into the Pathology of a COL1A1 Signal Peptide Heterozygous Mutation Leading to Severe Osteogenesis Imperfecta. Calcif. Tissue Int. 2018, 102, 373–379. [Google Scholar] [CrossRef]
- Tabeta, K.; Du, X.; Arimatsu, K.; Yokoji, M.; Takahashi, N.; Amizuka, N.; Hasegawa, T.; Crozat, K.; Maekawa, T.; Miyauchi, S.; et al. An ENU-induced splice site mutation of mouse Col1a1 causing recessive osteogenesis imperfecta and revealing a novel splicing rescue. Sci. Rep. 2017, 7, 11717. [Google Scholar] [CrossRef]
- Sen, R.; Hurley, J.A. Osteoarthritis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Hosseininia, S.; Önnerfjord, P.; Dahlberg, L.E. Targeted proteomics of hip articular cartilage in OA and fracture patients. J. Orthop. Res. 2019, 37, 131–135. [Google Scholar] [CrossRef]
- Hosseininia, S.; Lindberg, L.R.; Dahlberg, L.E. Cartilage collagen damage in hip osteoarthritis similar to that seen in knee osteoarthritis; a case-control study of relationship between collagen, glycosaminoglycan and cartilage swelling. BMC Musculoskelet. Disord. 2013, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Xu, X.; Ye, Y.; Song, K.; Cheng, Y.; Di, J.; Hu, Q.; Li, J.; Ju, H.; Jiang, Q.; et al. Photo-cross-linked scaffold with kartogenin-encapsulated nanoparticles for cartilage regeneration. ACS Nano 2016, 10, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Peck, Y.; He, P.; Chilla, G.S.V.N.; Poh, C.L.; Wang, D.-A. A preclinical evaluation of an autologous living hyaline-like cartilaginous graft for articular cartilage repair: A pilot study. Sci. Rep. 2015, 5, 16225. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Kondo, H.; Kawaguchi, Y.; Kitamura, N.; Nagai, N.; Lida, H.; Yasuda, K. Development of a salmon-derived crosslinked atelocollagen sponge disc containing osteogenic protein-1 for articular cartilage regeneration: In vivo evaluations with rabbits. BMC Musculoskelet. Disord. 2013, 14, 174. [Google Scholar] [CrossRef] [PubMed]
- Sobol, E.; Baum, O.; Shekhter, A.; Wachsmann-Hogiu, S.; Shnirelman, A.; Alexandrovskaya, Y.; Sadovskky, I.; Vinokur, V. Laser induced micropore formation and modification of cartilage structure in osteoarthritis healing. J. Biomed. Opt. 2017, 22, 091515. [Google Scholar] [CrossRef] [PubMed]
- Richter, D.L.; Schenck, R.C.; Wascher, D.C.; Treme, G. Knee Articular Cartilage Repair and Restoration Techniques: A Review of the Literature. Sports Health 2016, 8, 153–160. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, B.; Liu, W.; Wang, P.; Lv, X.; Chen, S.; Liu, H.; Shao, Z. Articular cartilage regeneration: The role of endogenous mesenchymal stem/progenitor cell recruitment and migration. Semin. Arthritis Rheum. 2020, 50, 198–208. [Google Scholar] [CrossRef]
- Musumeci, G.; Castrogiovanni, P.; Leonardi, R.; Trovato, F.M.; Szychlinska, M.A.; Di Giunta, A.; Loreto, C.; Castorin, S. New perspectives for articular cartilage repair treatment through tissue engineering: A contemporary review. World J. Orthop. 2014, 5, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.B.; Pineda, M.; Harris, J.D.; Flanigan, D.C. Return to Sport after Articular Cartilage Repair in Athletes’ Knees: A Systematic Review. Arthroscopy 2016, 32, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Orozco, R.; Olague-Franco, J.K.; Miranda-Ramírez, E. Implantación de condrocitos autólogos versus microfracturas para el tratamiento de lesiones del cartílago en la rodilla. Acta Ortopédica Mex. 2018, 32, 322–328. [Google Scholar] [CrossRef]
- Jackson, R.W.; Dieterichs, C. The results of arthroscopic lavage and debridement of osteoarthritic knees based on the severity of degeneration: A 4- to 6-year symptomatic follow-up. Arthroscopy 2003, 19, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Stanish, W.D.; McCormack, R.; Forriol, F.; Mohtadi, N.; Pelet, S.; Desnoyers, J.; Restrepo, A.; Shive, M.S. Novel scaffold-based bst-cargel treatment results in superior cartilage repair compared with microfracture in a randomized controlled trial. J. Bone Jt. Surg. 2013, 95, 1640–1650. [Google Scholar] [CrossRef]
- Matsiko, A.; Levingstone, T.J.; O’Brien, F.J. Advanced Strategies for Articular Cartilage Defect Repair. Materials 2013, 6, 637–668. [Google Scholar] [CrossRef] [PubMed]
- Horbert, V.; Xin, L.; Foehr, P.; Brinkmann, O.; Bungartz, M.; Burgkart, R.H.; Graeve, T.; Kinne, R.W. In Vitro Analysis of Cartilage Regeneration Using a Collagen Type I Hydrogel (CaReS) in the Bovine Cartilage Punch Model. Cartilage 2019, 10, 346–363. [Google Scholar] [CrossRef]
- Vázquez-Portalatĺn, N.; Kilmer, C.E.; Panitch, A.; Liu, J.C. Characterization of Collagen Type i and II Blended Hydrogels for Articular Cartilage Tissue Engineering. Biomacromolecules 2021, 17, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Mainil-Varlet, P.; Damme, B.V.; Nesic, D.; Knutsen, G.; Kandel, R.; Roberts, S. A New Histology Scoring System for the Assessment of the Quality of Human Cartilage Repair: ICRS II. Am. J. Sports Med. 2010, 38, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Rutgers, M.; van Pelt, M.J.P.; Dhert, W.J.A.; Creemers, L.B.; Saris, D.B.F. Evaluation of histological scoring systems for tissue-engineered, repaired and osteoarthritic cartilage. Osteoarthr. Cartil. 2010, 18, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Thorp, H.; Kim, K.; Kondo, M.; Grainger, D.W.; Okano, T. Fabrication of hyaline-like cartilage constructs using mesenchymal stem cell sheets. Sci. Rep. 2020, 10, 20869. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, R.O.; Dreiman, R.W.; Montenegro-Rizzardini, M.A. Evaluation of Collagen and Elastic Fibers in Human TMJ Tissue. Int. J. Odontostomatol. 2010, 4, 277–284. [Google Scholar]
- Wegner, K.A.; Keikhosravi, A.; Eliceiri, K.W.; Vezina, C.M. Fluorescence of Picrosirius Red Multiplexed With Immunohistochemistry for the Quantitative Assessment of Collagen in Tissue Sections. J. Histochem. Cytochem. 2017, 65, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Stolz, M.; Gottardi, R.; Raiteri, R.; Miot, S.; Martin, I.; Imer, R.; Staufer, U.; Raducanu, A.; Düggelin, M.; Baschong, W.; et al. Early detection of aging cartilage and osteoarthritis in mice and patient samples using atomic force microscopy. Nat. Nanotechnol. 2009, 4, 186–192. [Google Scholar] [CrossRef] [PubMed]
| Collagen | Chains | Genes | Clasification | % * | Distribution in Articular Cartilage | Distribution in the Human Body |
|---|---|---|---|---|---|---|
| Type I | [α1(I)]2α2(I) | COL1A1 COL1A2 | Fibrill-forming collagen | 0% | Fibrocartilage | Bone, skin, cornea, and many interstitial connective tissues with the exception of hyaline cartilage, brain and vitreous body. |
| Type II | [α1(II)]3 | COL2A1 | Fibrill-forming collagen | 90–95% | ECM of all zones | Cartilage, vitreous, and intervertebral disc. |
| Type III | [α1(III)]3 | COL3A1 | Fibrill-forming collagen | n/a | n/a | Bloods vessels, uterus, bowel, skin, tendon, ligament, cartilage, periodontal ligament, and synovial membranes. |
| Type IV | [α1(IV)]2α2(IV) α3(IV)α4(IV)α5(IV) [α5(IV)]2α6(IV) | COL4A1 COL4A2 COL4A3 COL4A4 COL4A5 COL4A6 | Network-forming collagen | n/a | PCM | Skin, basement membranes, lung, kidney, cochlea eye, smooth muscle, oesophagus, and cartilage. |
| Type V | α1(V)2α2(V) | COL5A1 COL5A2 | Fibrill-forming collagen | n/a | PCM | Adipose tissue, skeletal muscle, cartilage, pancreatic islets, skin, placenta, and lung. |
| Type VI | α1(VI)α2(V)α3(V) α1(VI)α2(V)α4(V) α1(VI)α2(V)α5(V) α1(VI)α2(V)α6(V) | COL6A1 COL6A2 COL6A3 COL6A4 COL6A5 COL6A6 | Beaded filament collagen | 1–2% | PCM | Skin, cornea, blood vessels, heart, lung, adipose tissue, nervous, pancreas, bone, cartilage, and muscle. |
| Type IX | α1(IX)α2(IX)α3(IX) | COL9A1 COL9A2 COL9A3 | FACIT | 1–5% | ECM of all zones and growth plate in adults | Cartilages, eye vitreum, avian cornea, ear, and intervertebral disc. |
| Type X | [α1(X)]3 | COL10A1 | Network-forming collagen | 1% | Calcified zone and hypertrophic cartilage | Hypertrophic cartilage and the calcified zone. |
| Type XI | α1(XI)α2(XI)α3(XI) | COL11A1 COL11A2 COL2A1 | Fibrill-forming collagen | 1–5% | ECM of all zones and PCM | Cartilage, tendons, trabecular bone, testis, trachea, skeletal muscle, placenta, ovarian, lung, and brain. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcaide-Ruggiero, L.; Molina-Hernández, V.; Granados, M.M.; Domínguez, J.M. Main and Minor Types of Collagens in the Articular Cartilage: The Role of Collagens in Repair Tissue Evaluation in Chondral Defects. Int. J. Mol. Sci. 2021, 22, 13329. https://doi.org/10.3390/ijms222413329
Alcaide-Ruggiero L, Molina-Hernández V, Granados MM, Domínguez JM. Main and Minor Types of Collagens in the Articular Cartilage: The Role of Collagens in Repair Tissue Evaluation in Chondral Defects. International Journal of Molecular Sciences. 2021; 22(24):13329. https://doi.org/10.3390/ijms222413329
Chicago/Turabian StyleAlcaide-Ruggiero, Lourdes, Verónica Molina-Hernández, María M. Granados, and Juan M. Domínguez. 2021. "Main and Minor Types of Collagens in the Articular Cartilage: The Role of Collagens in Repair Tissue Evaluation in Chondral Defects" International Journal of Molecular Sciences 22, no. 24: 13329. https://doi.org/10.3390/ijms222413329
APA StyleAlcaide-Ruggiero, L., Molina-Hernández, V., Granados, M. M., & Domínguez, J. M. (2021). Main and Minor Types of Collagens in the Articular Cartilage: The Role of Collagens in Repair Tissue Evaluation in Chondral Defects. International Journal of Molecular Sciences, 22(24), 13329. https://doi.org/10.3390/ijms222413329







