Cell Penetrating Peptide-Based Self-Assembly for PD-L1 Targeted Tumor Regression
Abstract
:1. Introduction
2. Results
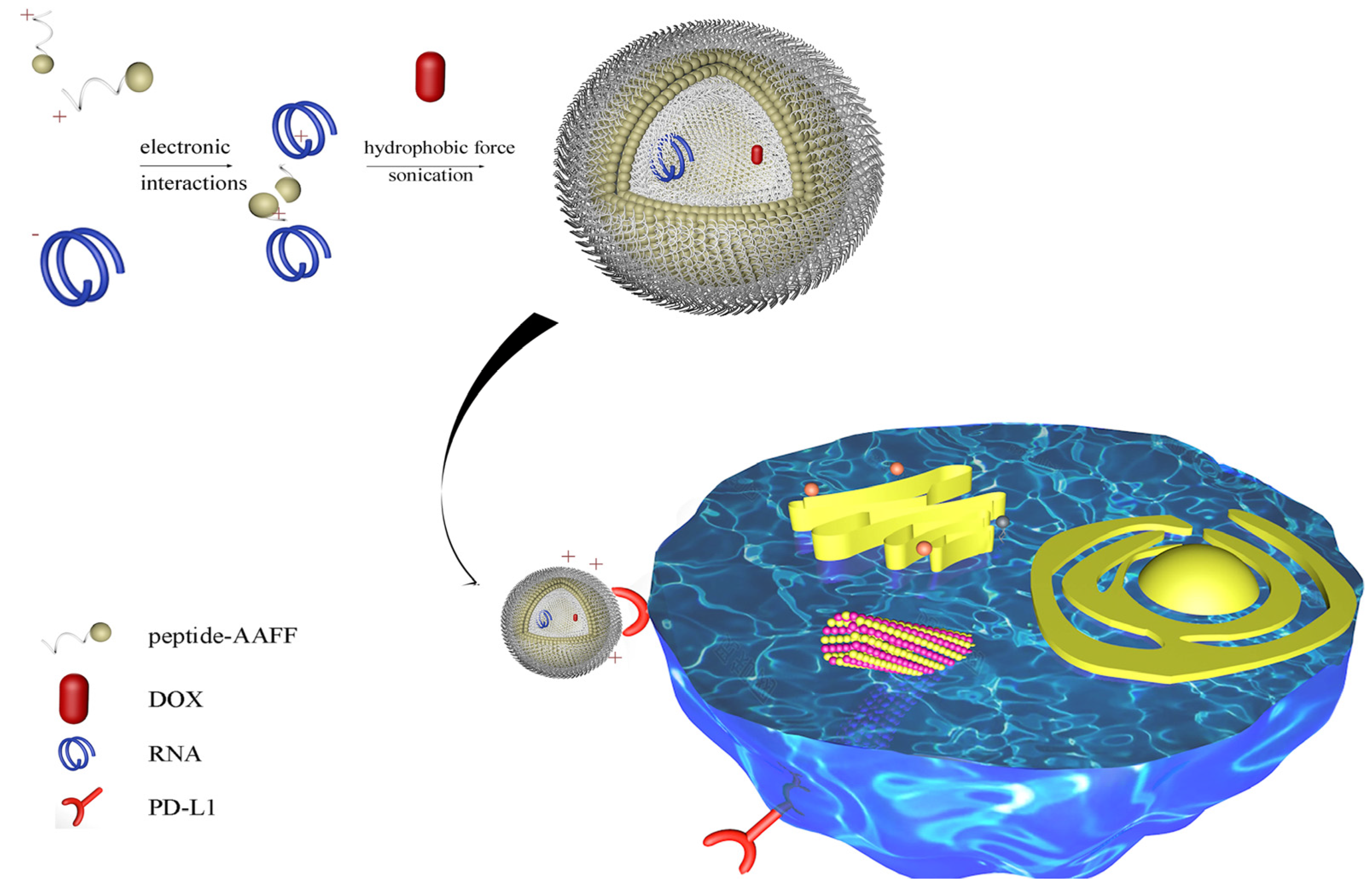
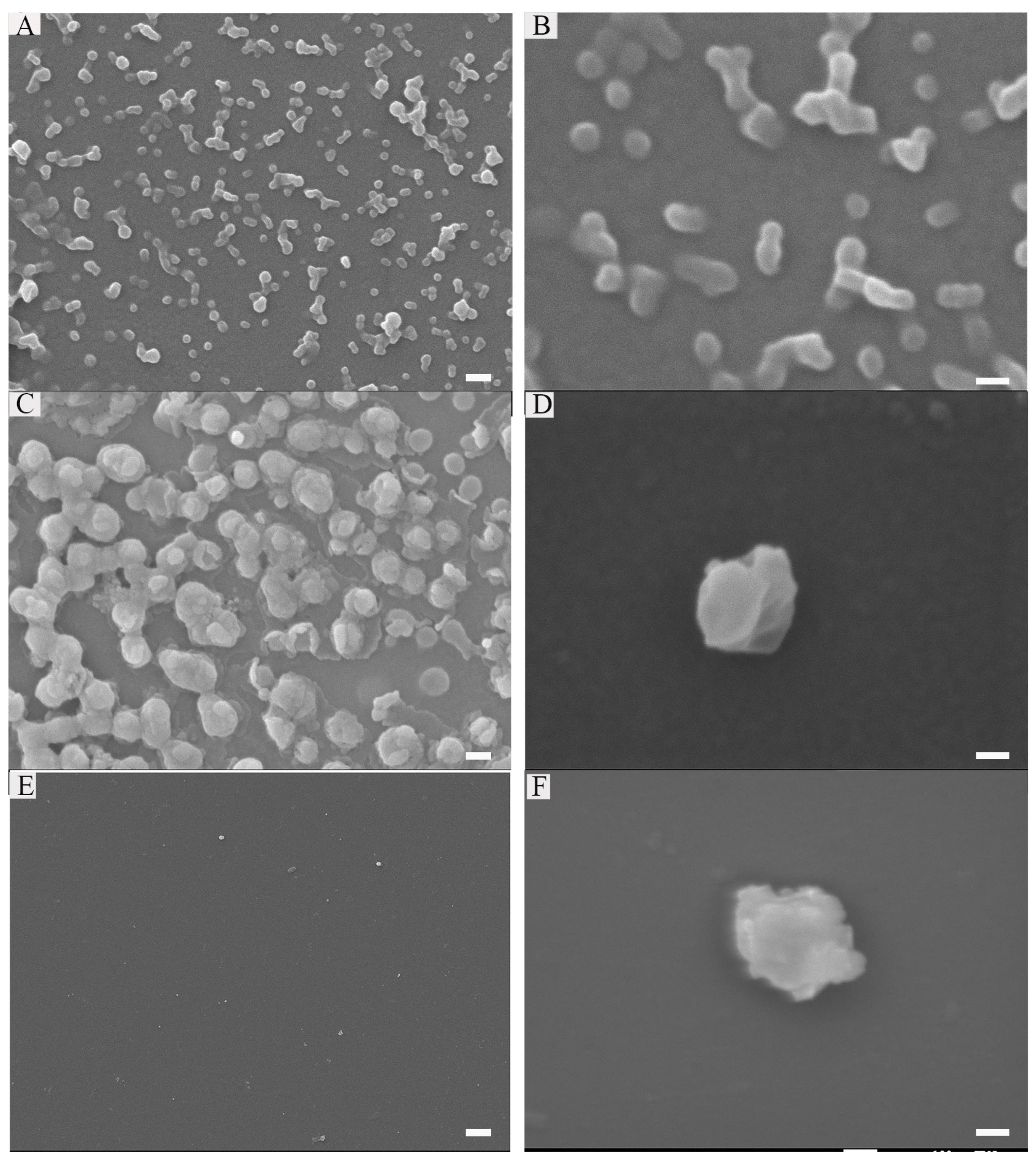
2.1. Characterization of Peptide and Peptide-RNA/DOX
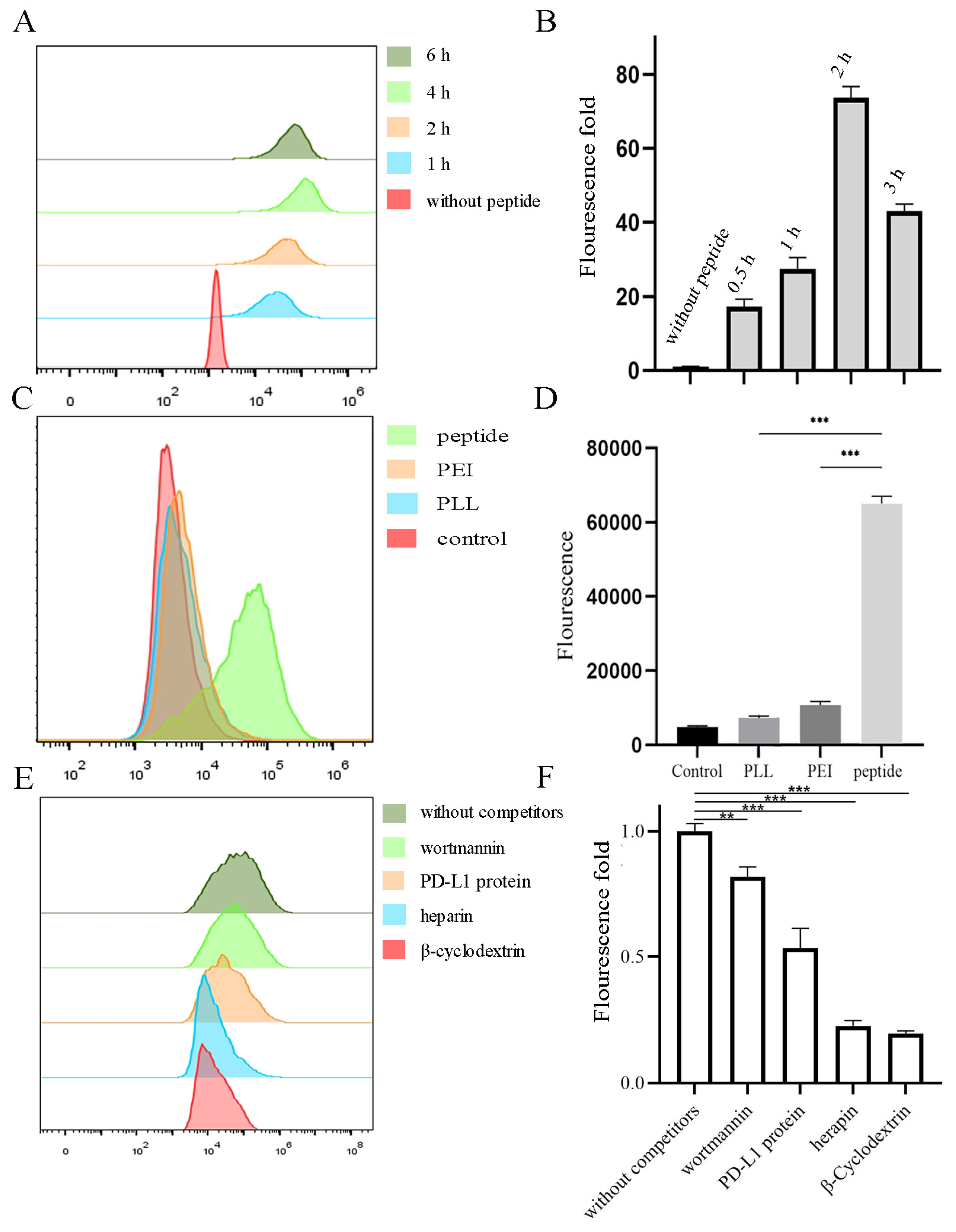
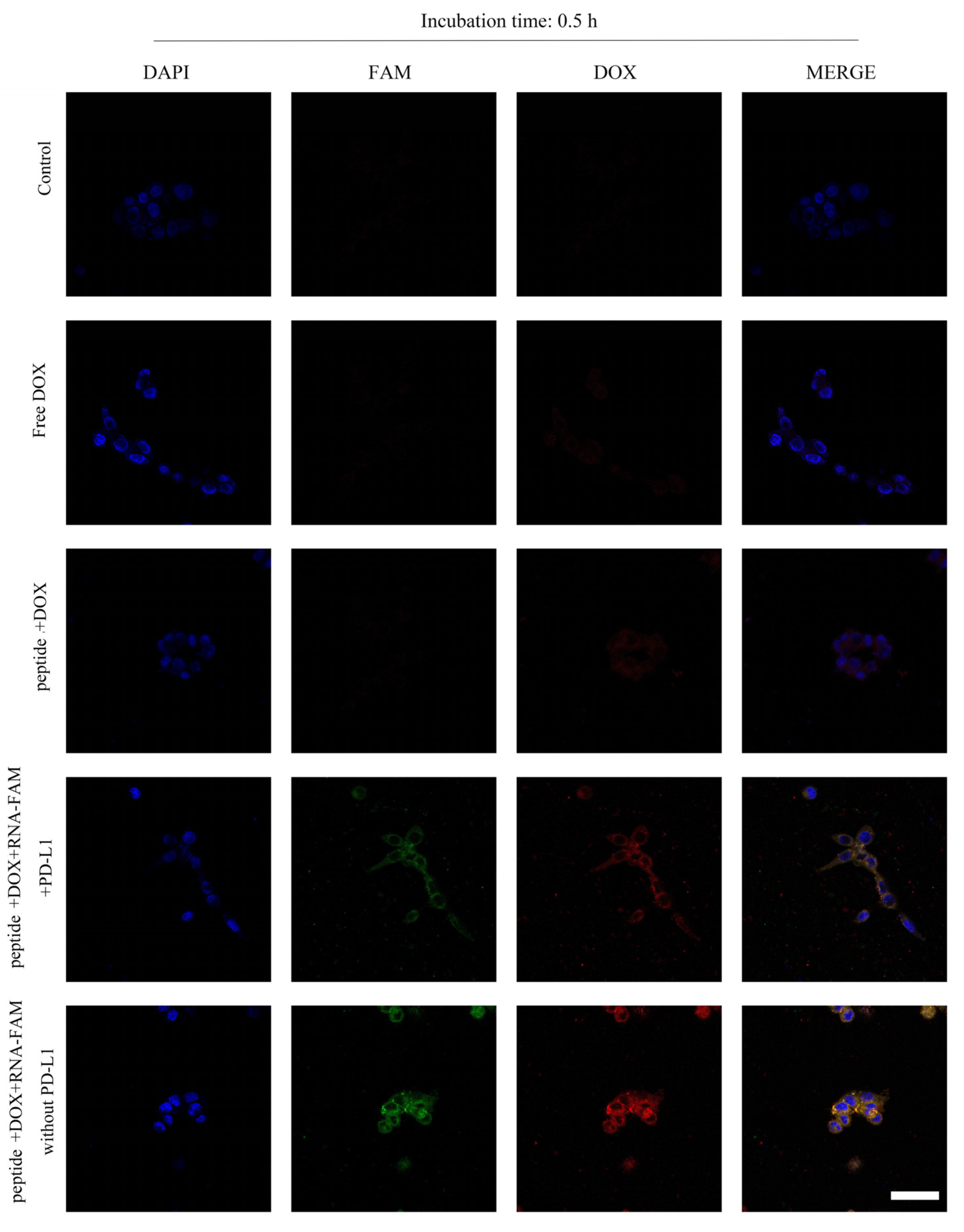
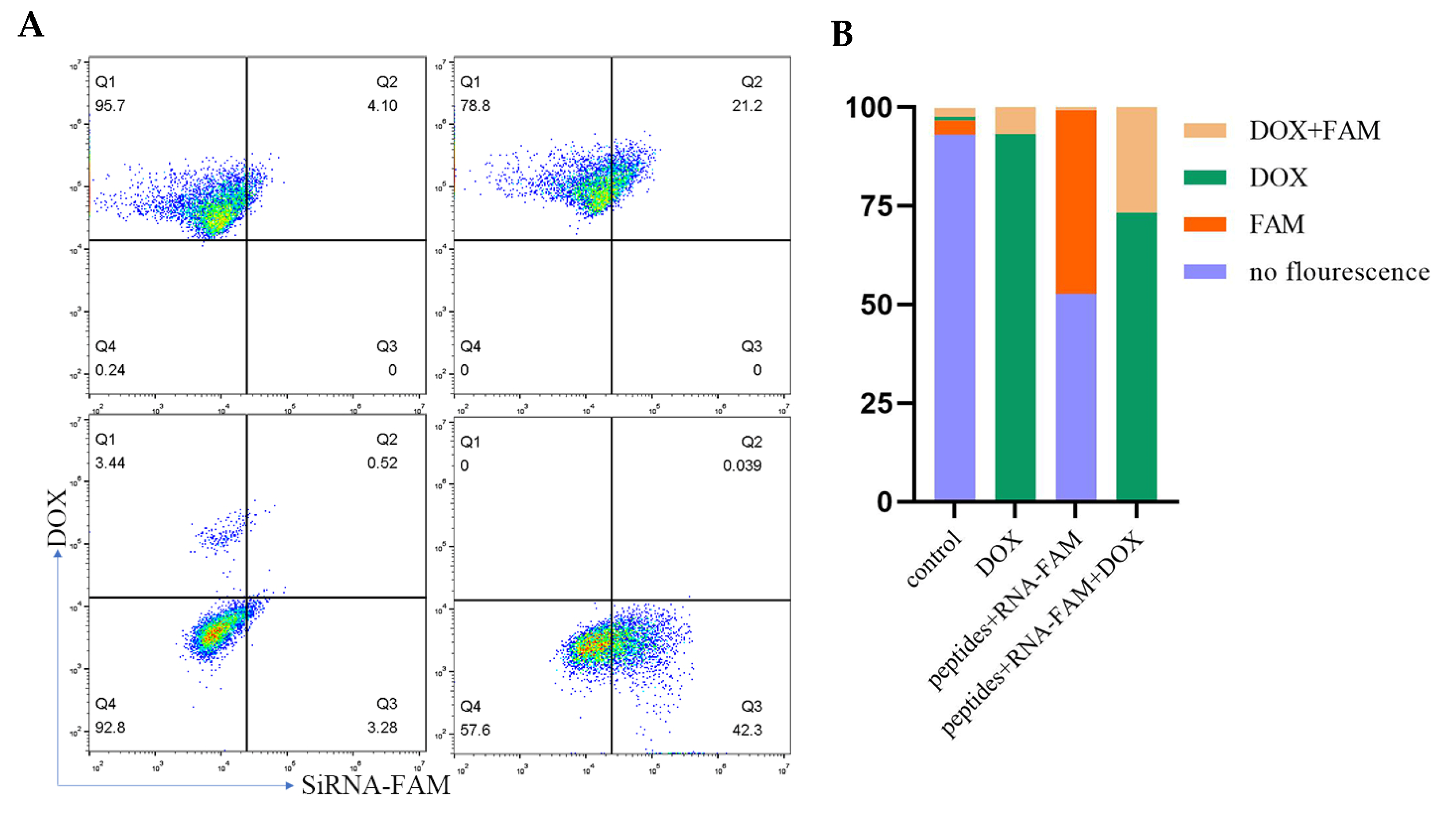
2.2. Internalization of Peptide-RNA/DOX and Its Mechanism
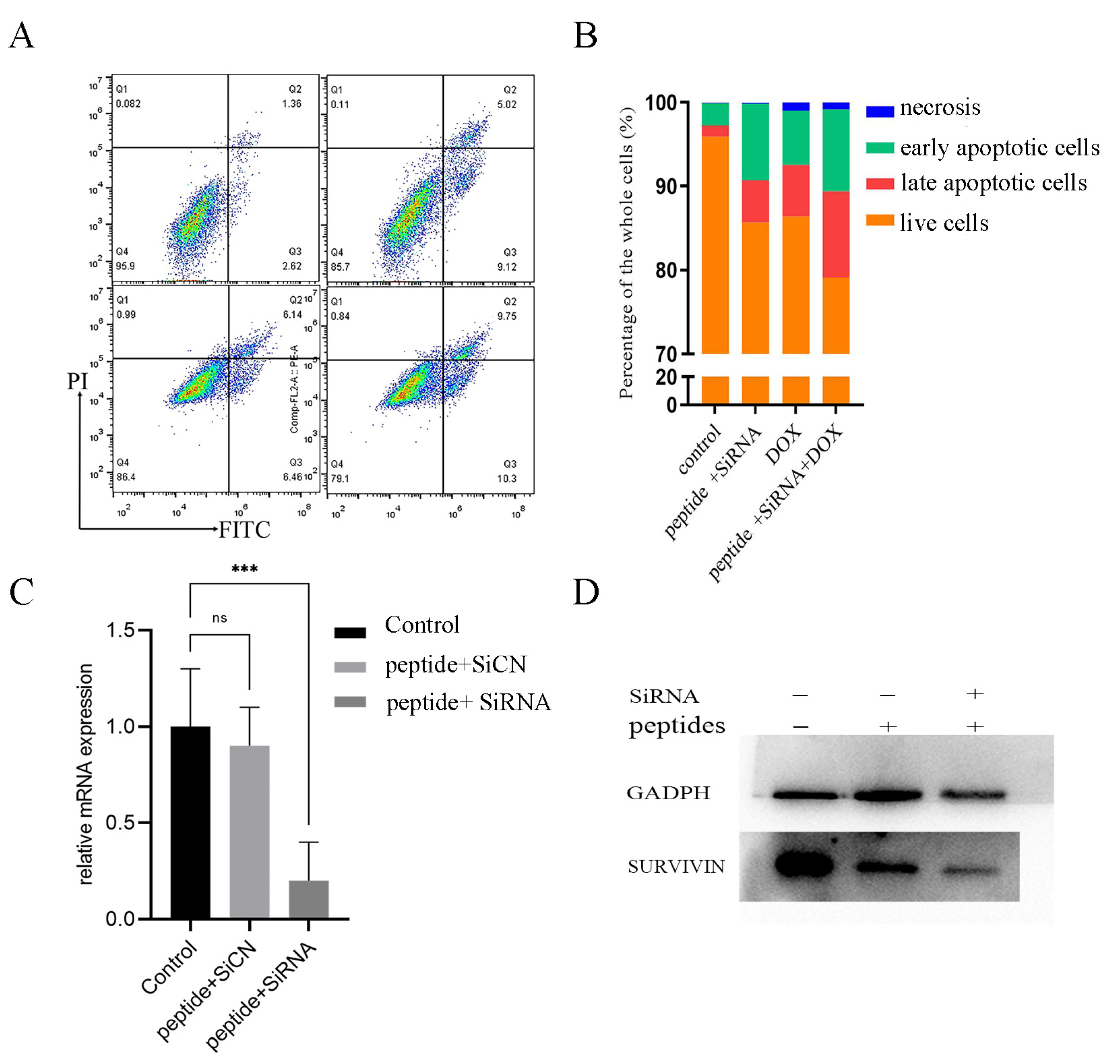
2.3. Anti-Tumor Effects In Vitro
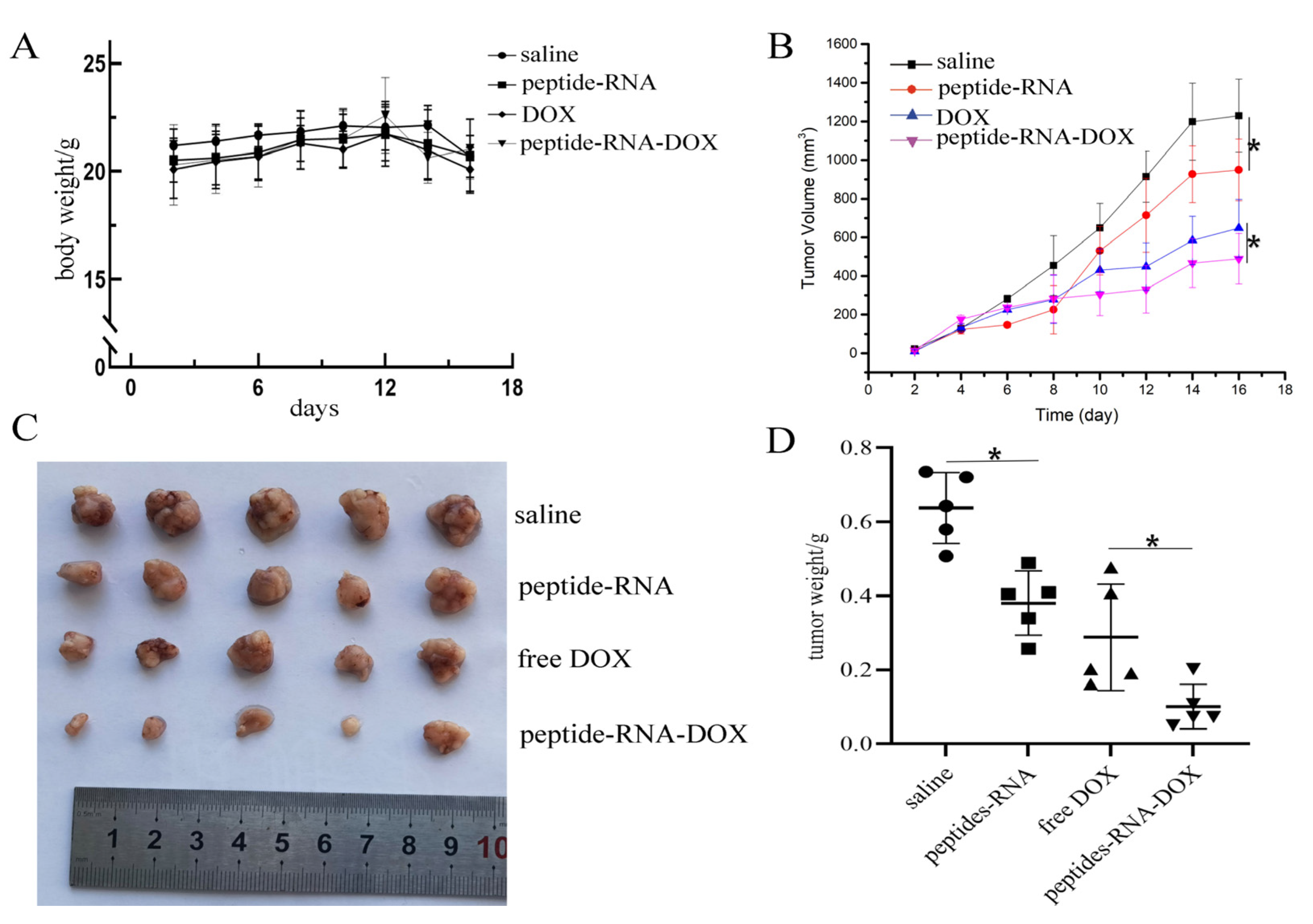
2.4. Anti-Tumor Effects In Vivo
3. Discussion
4. Materials and Methods
4.1. Reagent
4.2. Synthesis of Peptide and Preparation of Nano Vehicles
4.3. Gel Retardation Assay and Drug Loading Efficiency
4.4. Characterization
4.5. Hemolysis Test
4.6. Cell Culture and MTT Assays
4.7. Internalization of Nano Vehicles and Its Mechanism
4.8. Cell Apoptosis
4.9. Quantitative Real-Time-PCR and Western Blot Assays
4.10. In Vivo Administrations of Drug
4.11. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PD-L1 | Programmed cell death 1 ligand 1 |
| FF | Phe-Phe |
| PEI | polyethyleneimine |
| PLL | poly-(l-lysine) |
| PCL | Polycaprolactone |
| IAP | inhibitor of apoptosis protein |
References
- Ganju, A.; Khan, S.; Hafeez, B.B.; Behrman, S.W.; Yallapu, M.M.; Chauhan, S.C.; Jaggi, M. miRNA nanotherapeutics for cancer. Drug Discov. Today 2016, 22, 424–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, F.; Li, Y.; Deng, X.; Wang, X.; Cui, X.; Lu, J.; Pan, Z.; Wu, Y. Self-assembled fluorescent hybrid nanoparticles-mediated collaborative lncRNA CCAT1 silencing and curcumin delivery for synchronous colorectal cancer theranostics. J. Nanobiotechnol. 2021, 19, 238. [Google Scholar] [CrossRef]
- Li, Y.; Chen, G.; He, Y.; Yi, C.; Zhang, X.; Zeng, B.; Huang, Z.; Deng, F.; Yu, D. Selenomethionine-Modified Polyethylenimine-Based Nanoparticles Loaded with miR-132-3p Inhibitor-Biofunctionalized Titanium Implants for Improved Osteointegration. ACS Biomater. Sci. Eng. 2021, 7, 4933–4945. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cao, Y.; Xu, D.; Goh, N.S.; Demirer, G.S.; Cestellos-Blanco, S.; Chen, Y.; Landry, M.P.; Yang, P. Gold-Nanocluster-Mediated Delivery of siRNA to Intact Plant Cells for Efficient Gene Knockdown. Nano Lett. 2021, 21, 5859–5866. [Google Scholar] [CrossRef] [PubMed]
- Delyanee, M.; Akbari, S.; Solouk, A. Amine-terminated dendritic polymers as promising nanoplatform for diagnostic and therapeutic agents’ modification: A review. Eur. J. Med. Chem. 2021, 221, 113572. [Google Scholar] [CrossRef]
- Shi, M.; Wang, Y.; Zhao, X.; Zhang, J.; Hu, H.; Qiao, M.; Zhao, X.; Chen, D. Stimuli-Responsive and Highly Penetrable Nanoparticles as a Multifunctional Nanoplatform for Boosting Nonsmall Cell Lung Cancer siRNA Therapy. ACS Biomater. Sci. Eng. 2021, 7, 3141–3155. [Google Scholar] [CrossRef]
- Regnstrom, K.; Ragnarsson, E.G.; Koping-Hoggard, M.; Torstensson, E.; Nyblom, H.; Artursson, P. PEI—A potent, but not harmless, mucosal immuno-stimulator of mixed T-helper cell response and FasL-mediated cell death in mice. Gene Ther. 2003, 10, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Chollet, P.; Favrot, M.C.; Hurbin, A.; Coll, J.L. Side-effects of a systemic injection of linear polyethylenimine-DNA complexes. J. Gene Med. 2002, 4, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Ferruti, P.; Knobloch, S.; Ranucci, E.; Duncan, R.; Gianasi, E. A novel modification of poly(L-lysine) leading to a soluble cationic polymer with reduced toxicity and with potential as a transfection agent. Macromol. Chem. Phys. 1998, 199, 2565–2575. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Fréchet, J.M.J. Discovery of dendrimers and dendritic polymers: A brief historical perspective. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 2719–2728. [Google Scholar] [CrossRef]
- Sun, Y.; Jiao, Y.; Wang, Y.; Lu, D.; Yang, W. The strategy to improve gene transfection efficiency and biocompatibility of hyperbranched PAMAM with the cooperation of PEGylated hyperbranched PAMAM. Int. J. Pharm. 2014, 465, 112–119. [Google Scholar] [CrossRef]
- Yang, M.; Yuan, C.; Shen, G.; Chang, R.; Xing, R.; Yan, X. Cyclic dipeptide nanoribbons formed by dye-mediated hydrophobic self-assembly for cancer chemotherapy. J. Colloid Interface Sci. 2019, 557, 458–464. [Google Scholar] [CrossRef]
- Yang, J.; Song, J.I.; Song, Q.; Rho, J.Y.; Mansfield, E.D.H.; Hall, S.C.L.; Sambrook, M.; Huang, F.; Perrier, S. Hierarchical Self-Assembled Photo-Responsive Tubisomes from a Cyclic Peptide-Bridged Amphiphilic Block Copolymer. Angew. Chem. Int. Ed. 2020, 59, 8860–8863. [Google Scholar] [CrossRef]
- Schnaider, L.; Brahmachari, S.; Schmidt, N.W.; Mensa, B.; Shaham-Niv, S.; Bychenko, D.; Adler-Abramovich, L.; Shimon, L.J.W.; Kolusheva, S.; DeGrado, W.F.; et al. Self-assembling dipeptide antibacterial nanostructures with membrane disrupting activity. Nat. Commun. 2017, 8, 1365. [Google Scholar] [CrossRef]
- Hamley, I.W. Self-assembly of amphiphilic peptides. Soft Matter. 2011, 7, 4122–4138. [Google Scholar] [CrossRef] [Green Version]
- Reches, M.; Gazit, E. Casting Metal Nanowires within Discrete Self-Assembled Peptide Nanotubes. Science 2003, 300, 625–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Jia, Y.; Dai, L.; Yang, Y.; Li, J. Controlled Rod Nanostructured Assembly of Diphenylalanine and Their Optical Waveguide Properties. ACS Nano 2015, 9, 2689–2695. [Google Scholar] [CrossRef]
- Ariga, K.; Li, J.; Fei, J.; Ji, Q.; Hill, J.P. Nanoarchitectonics for Dynamic Functional Materials from Atomic-/Molecular-Level Manipulation to Macroscopic Action. Adv. Mater. 2016, 28, 1251–1286. [Google Scholar] [CrossRef] [PubMed]
- Reches, M.; Gazit, E. Formation of Closed-Cage Nanostructures by Self-Assembly of Aromatic Dipeptides. Nano Lett. 2004, 4, 581–585. [Google Scholar] [CrossRef]
- Liberato, M.S.; Kogikoski, S.; da Silva, E.R.; de Araujo, D.R.; Guha, S.; Alves, W.A. Polycaprolactone fibers with self-assembled peptide micro/nanotubes: A practical route towards enhanced mechanical strength and drug delivery applications. J. Mater. Chem. B 2016, 4, 1405–1413. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, P.; Bhalla, S.; Usmani, S.S.; Singh, S.; Chaudhary, K.; Raghava, G.P.; Gautam, A. CPPsite 2.0: A repository of experimentally validated cell-penetrating peptides. Nucleic Acids Res. 2016, 44, D1098–D1103. [Google Scholar] [CrossRef] [PubMed]
- Pujals, S.; Fernandez-Carneado, J.; Lopez-Iglesias, C.; Kogan, M.J.; Giralt, E. Mechanistic aspects of CPP-mediated intracellular drug delivery: Relevance of CPP self-assembly. Biochim. Biophys. Acta Biomembr. 2006, 1758, 264–279. [Google Scholar] [CrossRef] [Green Version]
- Taylor, R.E.; Zahid, M. Cell Penetrating Peptides, Novel Vectors for Gene Therapy. Pharmaceutics 2020, 12, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Mei, L.; Xu, C.; Yu, Q.; Shi, K.; Zhang, L.; Wang, Y.; Zhang, Q.; Gao, H.; Zhang, Z.; et al. Dual Receptor Recognizing Cell Penetrating Peptide for Selective Targeting, Efficient Intratumoral Diffusion and Synthesized Anti-Glioma Therapy. Theranostics 2016, 6, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Song, Y.; Su, Y.; Liang, Y.; Wang, L. Effect of the hairpin structure of peptide inhibitors on the blockade of PD-1/PD-L1 axis. Biochem. Biophys. Res. Commun. 2020, 527, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.F.; Araujo, D.R.; Silva, E.R.; Ando, R.A.; Alves, W.A. L-diphenylalanine microtubes as a potential drug-delivery system: Characterization, release kinetics, and cytotoxicity. Langmuir 2013, 29, 10205–10212. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.X.; Wang, Y.; Blake, S.; Yu, M.; Mei, L.; Wang, H.; Shi, J. RNA Nanotechnology-Mediated Cancer Immunotherapy. Theranostics 2020, 10, 281–299. [Google Scholar] [CrossRef]
- Dowdy, S.F. Overcoming cellular barriers for RNA therapeutics. Nat. Biotechnol. 2017, 35, 222–229. [Google Scholar] [CrossRef]
- Abe, K.; Fujiyoshi, Y. Cryo-electron microscopy for structure analyses of membrane proteins in the lipid bilayer. Curr. Opin. Struct. Biol. 2016, 39, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Yonenuma, R.; Ishizuki, A.; Nakabayashi, K.; Mori, H. Synthesis and Hierarchical Self-Assembly of Diphenylalanine-Based Homopolymer and Copolymers by RAFT Polymerization. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 2562–2574. [Google Scholar] [CrossRef]
- Ulijn, R.V.; Smith, A.M. Designing peptide-based nanomaterials. Chem. Soc. Rev. 2008, 37, 664–675. [Google Scholar] [CrossRef]
- Khan, O.; Giles, J.R.; McDonald, S.; Manne, S.; Ngiow, S.F.; Patel, K.P.; Werner, M.T.; Huang, A.C.; Alexander, K.A.; Wu, J.E.; et al. TOX transcriptionally and epigenetically programs CD8+ T cell exhaustion. Nature 2019, 571, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Groner, B.; Weiss, A. Targeting survivin in cancer: Novel drug development approaches. BioDrugs 2014, 28, 27–39. [Google Scholar] [CrossRef] [Green Version]
- Qiu, M.; Ouyang, J.; Wei, Y.; Zhang, J.; Lan, Q.; Deng, C.; Zhong, Z. Selective Cell Penetrating Peptide-Functionalized Envelope-Type Chimeric Lipopepsomes Boost Systemic RNAi Therapy for Lung Tumors. Adv. Healthc. Mater. 2019, 8, e1900500. [Google Scholar] [CrossRef]
- Pan, L.; He, Q.; Liu, J.; Chen, Y.; Ma, M.; Zhang, L.; Shi, J. Nuclear-targeted drug delivery of TAT peptide-conjugated monodisperse mesoporous silica nanoparticles. J. Am. Chem. Soc. 2012, 134, 5722–5725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, Y.; Gan, Y.; Qiu, N.; Gu, Y.; Zhu, H. Conjugates of TAT and folate with DOX-loaded chitosan micelles offer effective intracellular delivery ability. Pharm. Dev. Technol. 2019, 24, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.; Danial, M.; Koh, M.L.; Jolliffe, K.A.; Perrier, S. Design and properties of functional nanotubes from the self-assembly of cyclic peptide templates. Chem. Soc. Rev. 2012, 41, 6023–6041. [Google Scholar] [CrossRef]
- Ni, R.; Chau, Y. Nanoassembly of Oligopeptides and DNA Mimics the Sequential Disassembly of a Spherical Virus. Angew. Chem. Int. Ed. 2020, 59, 3578–3584. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, F.; Ke, J.; Fu, Z.; Han, W.; Wang, L. Cell Penetrating Peptide-Based Self-Assembly for PD-L1 Targeted Tumor Regression. Int. J. Mol. Sci. 2021, 22, 13314. https://doi.org/10.3390/ijms222413314
Guo F, Ke J, Fu Z, Han W, Wang L. Cell Penetrating Peptide-Based Self-Assembly for PD-L1 Targeted Tumor Regression. International Journal of Molecular Sciences. 2021; 22(24):13314. https://doi.org/10.3390/ijms222413314
Chicago/Turabian StyleGuo, Feng, Junfeng Ke, Zhengdong Fu, Wenzhao Han, and Liping Wang. 2021. "Cell Penetrating Peptide-Based Self-Assembly for PD-L1 Targeted Tumor Regression" International Journal of Molecular Sciences 22, no. 24: 13314. https://doi.org/10.3390/ijms222413314
APA StyleGuo, F., Ke, J., Fu, Z., Han, W., & Wang, L. (2021). Cell Penetrating Peptide-Based Self-Assembly for PD-L1 Targeted Tumor Regression. International Journal of Molecular Sciences, 22(24), 13314. https://doi.org/10.3390/ijms222413314




