Regenerative Potential of Mesenchymal Stem Cells’ (MSCs) Secretome for Liver Fibrosis Therapies
Abstract
1. Introduction
2. Liver Fibrosis and Signaling Pathways
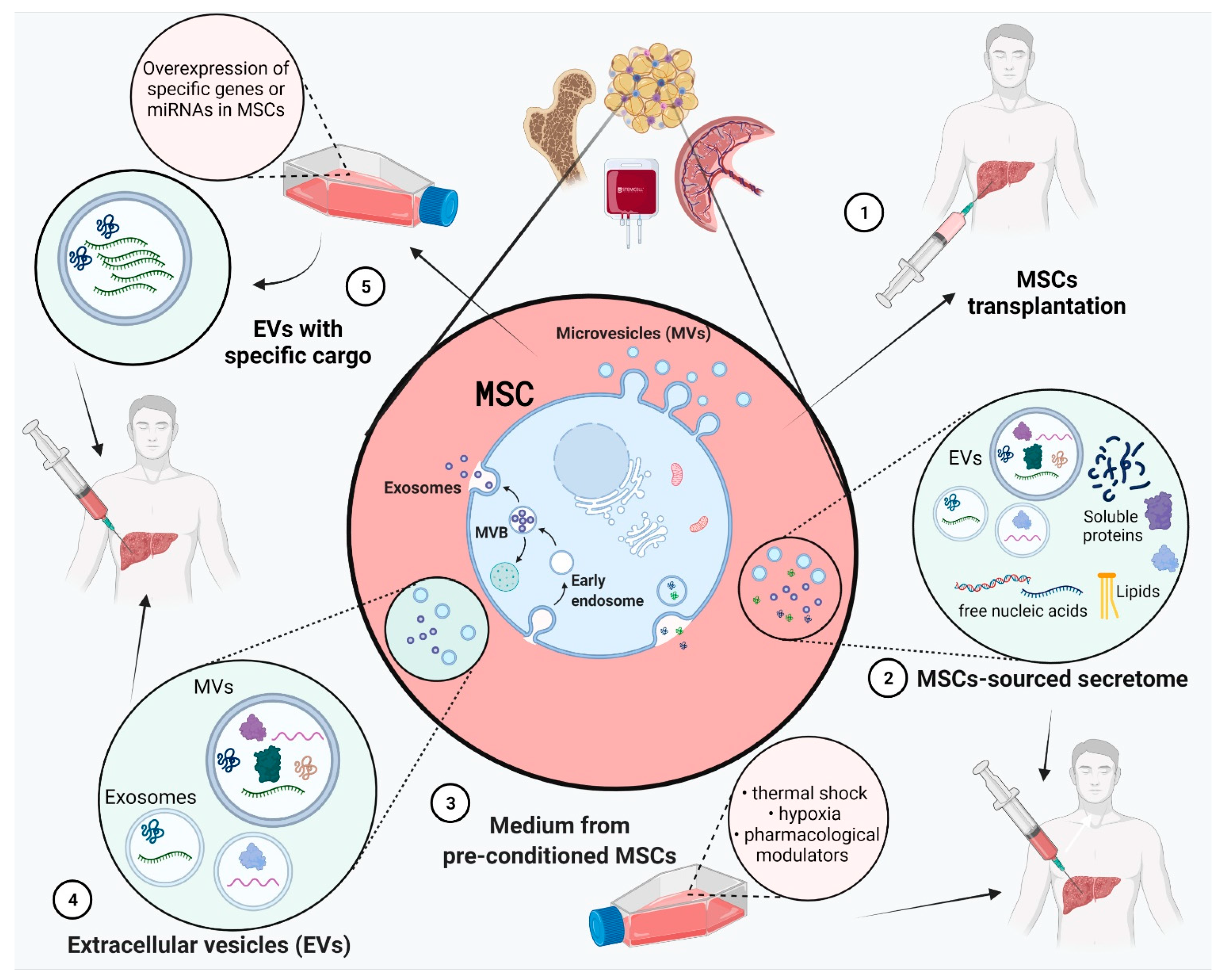
3. Composition of MSCs’ Secretome (Derived Soluble Factors CM/EV)
4. Role of MSCs-Sourced Secretome in Liver Regeneration
4.1. MSCs in Liver Disease Treatment
4.2. Effect of MSC-Derived Conditioned Medium (MSC-CM)
4.3. Pre-Treatment of MSCs for Improved Secretome Content
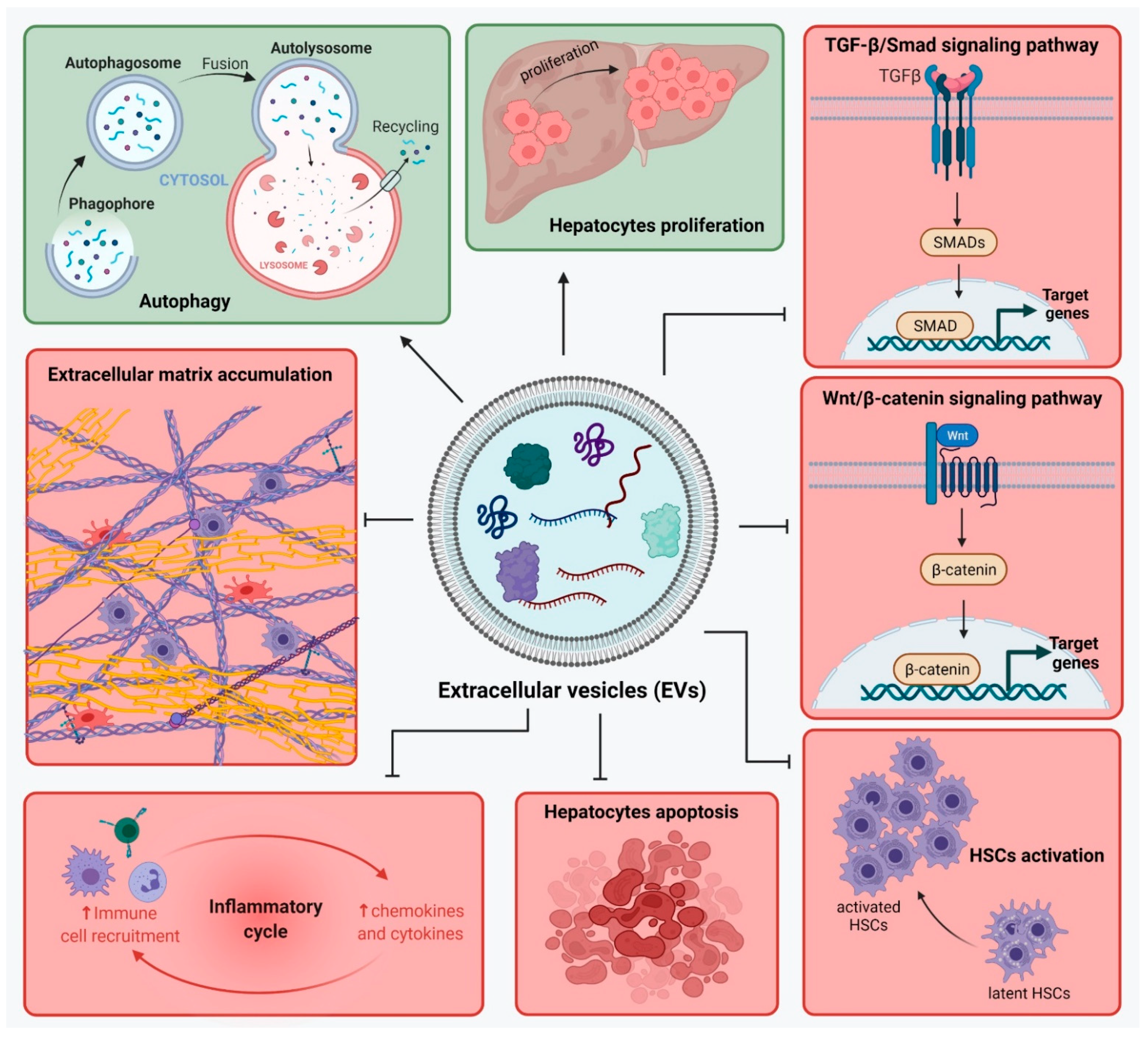
4.4. Effect of EVs
| Source of EV | Type of EV | Liver injury Model | Mechanism of Action | References |
|---|---|---|---|---|
| hAD-MSCs | Exosomes with miR-122 | LX2 cell line | Down-regulated the expression of miR-122 target genes (P4HA1, IGF1R and CCNG1) which are involved in the proliferation and collagen maturation of HSCs | [104] |
| hUC-MSCs | EVs | LX2 cell line | Suppressed HSCs proliferation and activation | [103] |
| Amnion-MSCs | EVs | Rat HSCs and KCs activated with LPS | Inhibits HSCs activation (reduced expression of α-SMA, collagen I, increased expression of mmp-2)Downregulated the expression levels of inflammatory cytokines (Tnf-α, Il-1β, and Mcp-1) in KC | [100] |
| hUC-MSCs | EVs with Insulin Growth Factorlike-I (IGF-I) | Rat CFSC-2G hepatic stellate cell line | Reduced the expression of fibrotic markers (collagen I, α-SMA and TGF-β1, and of pro-inflammatory cytokines IL-6 and TNF-α | [93] |
| hAD-MSCs | Exosomes with miR-181-5p | Mouse HSCs (HST-T6) | Inhibited HSCs activation by downregulating the expression of miR-181-5p target genes (Stat3 and Bcl-2) and activated autophagy (upregulation of Beclin1) | [99] |
| Amnion-MSCs | EVs | Rats with CCl4-induced liver fibrosis | Reduced expression of α-SMA and attenuated formation of fibrotic septa and pseudolobules | [100] |
| Rat BM-MSCs | EVs | Rats with CCl4-induced liver fibrosis | Reduced collagen deposition and attenuated HSC activation | [98] |
| hBM-MSCs | Exosomes | Rats with CCl4-induced liver fibrosis | Inhibited the expression of Wnt/β-catenin pathway components (PPARγ, Wnt3a, Wnt10b, β-catenin, WISP1, Cyclin D1), α-SMA, and collagen I | [94] |
| hUC-MSCs | Exosomes | Mice with CCl4-induced liver fibrosis | Reduced the expression of collagen I and III, inhibited TGF-β1/Smad signaling pathway and epithelial-to-mesenchymal transition (EMT) | [92] |
| hUC-MSCs | Exosomes | Mice with CCl4-induced liver fibrosis | Reduced oxidative stress, decreased TGF-β levels, and inhibited hepatocyte apoptosis and infiltration of inflammatory cells | [97] |
| hUC-MSCs | Exosomes | Rats with CCl4-induced liver fibrosis | Reduced collagen accumulation and reduced α-SMA and collagen I expression, inhibited inflammation, apoptosis, caspase-3 and Bax expression, and increased Bcl-2 expression | [96] |
| hUC-MSCs | EVs with Insulin Growth Factorlike-I (IGF-I) | Mice with TAA-induced liver fibrosis | Reduced the expression of collagen I, α-SMA and the profibrogenic cytokine TGF-β1 | [93] |
| hAD-MSCs | EVs with lncRNA-H19 | D-aminogalactose (GalN)-induced ALF | Downregulated the expression of inflammatory mediators (IL-1ra, IL-1α, IL-1β, IL-6 and IL-17) and chemotactic factors (CCL20, CINC-1, CINC-2α/β, CINC-3, CNTF, CX3CL1, CXCL7, CXCL9, CXCL10 and LECAM-1), inhibited tissue necrosis, promoted hepatocyte proliferation | [101] |
| AD-MSCs | Exosomes with miR-17 | Mice with LPS/GalN-induced ALF | miR-17 from exosomes inhibited NLRP3 inflammasome activation by targeting TXNIIP in hepatic macrophages | [102] |
| hUC-MSCs | Exosomes with upregulated miR-145-5p | Rats with CCl4-induced liver fibrosis | Inhibited the process of liver fibrosis via miR-145-5p-mediated fascin actin-bundling protein 1 (FSCN1) downregulation | [96] |
| AD-MSCs | Exosomes with overexpressed mmu_circ_0000623 | Mice with CCl4-induced liver fibrosis | Regulated autophagy mediated by miR-125/ATG4D, inhibited α-SMA expression | [105] |
| hAD-MSCs | Exosomes with miR-122 | Mice with CCl4-induced liver fibrosis | Reduced the expression of TGF-β1 and α-SMA and suppressed the serum levels of HA, P-III-P, ALT, AST and liver hydroxyproline content | [104] |
| hAD-MSCs | Exosomes with miR-181-5p | Mice with CCl4-induced liver fibrosis | Downregulated expression of fibrotic markers (collagen I, vimentin, α-SMA and fibronectin) and of pro-inflammatory factors (TNFa, IL-6 and IL-17) | [99] |
4.5. Exosomes from MSCs with Specific Overexpressed Cargo Such as miRNAs
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, M.; Kisseleva, T. Reversibility of liver fibrosis. Clin. Res. Hepatol. Gastroenterol. 2015, 39, S60–S63. [Google Scholar] [CrossRef] [PubMed]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Ignat, S.-R.; Dinescu, S.; Hermenean, A.; Costache, M. Cellular Interplay as a Consequence of Inflammatory Signals Leading to Liver Fibrosis Development. Cells 2020, 9, 461. [Google Scholar] [CrossRef] [PubMed]
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Invest. 2005, 115, 209–218. [Google Scholar] [CrossRef]
- Al-Dhamin, Z.; Liu, L.-D.; Li, D.-D.; Zhang, S.-Y.; Dong, S.-M.; Nan, Y.-M. Therapeutic efficiency of bone marrow-derived mesenchymal stem cells for liver fibrosis: A systematic review of in vivo studies. World J. Gastroenterol. 2020, 26, 7444–7469. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.J.; Alison, M.R. Knocking on the door to successful hepatocyte transplantation. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 277–278. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, M.; Liu, W.; Li, Y.; Li, M. Stem Cell-Based Therapies for Liver Diseases: An Overview and Update. Tissue Eng. Regen. Med. 2019, 16, 107–118. [Google Scholar] [CrossRef]
- Tsolaki, E. Stem cell-based regenerative opportunities for the liver: State of the art and beyond. World J. Gastroenterol. 2015, 21, 12334. [Google Scholar] [CrossRef]
- Beer, L.; Mildner, M.; Ankersmit, H.J. Cell secretome based drug substances in regenerative medicine: When regulatory affairs meet basic science. Ann. Transl. Med. 2017, 5, 170–170. [Google Scholar] [CrossRef] [PubMed]
- Fierabracci, A.; Fattore, A.; Muraca, M. The Immunoregulatory Activity of Mesenchymal Stem Cells: ‘State of Art’ and ‘Future Avenues. ’ Curr. Med. Chem. 2016, 23, 3014–3024. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, X.; Zhang, P.; Cai, L.; Yang, X.; Chen, Y.; Jing, Y.; Kong, J.; Yang, X.; Sun, F. Sustained Delivery Growth Factors with Polyethyleneimine-Modified Nanoparticles Promote Embryonic Stem Cells Differentiation and Liver Regeneration. Adv. Sci. 2016, 3, 1500393. [Google Scholar] [CrossRef] [PubMed]
- Assoni, A.; Coatti, G.; Valadares, M.C.; Beccari, M.; Gomes, J.; Pelatti, M.; Mitne-Neto, M.; Carvalho, V.M.; Zatz, M. Different Donors Mesenchymal Stromal Cells Secretomes Reveal Heterogeneous Profile of Relevance for Therapeutic Use. Stem Cells Dev. 2017, 26, 206–214. [Google Scholar] [CrossRef]
- Lu, W.-Y.; Bird, T.G.; Boulter, L.; Tsuchiya, A.; Cole, A.M.; Hay, T.; Guest, R.V.; Wojtacha, D.; Man, T.Y.; Mackinnon, A.; et al. Hepatic progenitor cells of biliary origin with liver repopulation capacity. Nat. Cell Biol. 2015, 17, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Carraro, A.; Flaibani, M.; Cillo, U.; Michelotto, L.; Magrofuoco, E.; Buggio, M.; Abatangelo, G.; Cortivo, R.; Herrera, M.B.; Tetta, C.; et al. A Combining Method to Enhance the In Vitro Differentiation of Hepatic Precursor Cells. Tissue Eng. Part C Methods 2010, 16, 1543–1551. [Google Scholar] [CrossRef]
- Liu, Z.-J.; Zhuge, Y.; Velazquez, O.C. Trafficking and differentiation of mesenchymal stem cells. J. Cell. Biochem. 2009, 106, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Kobolak, J.; Dinnyes, A.; Memic, A.; Khademhosseini, A.; Mobasheri, A. Mesenchymal stem cells: Identification, phenotypic characterization, biological properties and potential for regenerative medicine through biomaterial micro-engineering of their niche. Methods 2016, 99, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Dowidar, M.; El-Belbasi, H.; Ayoub, A.; Rashed, L.; Elged, D. Biochemical and Molecular Studies on Bone Marrow Derived Stromal Stem Cells on Liver Injuries in Rats. Zagazig Vet. J. 2017, 45, 355–365. [Google Scholar] [CrossRef][Green Version]
- Lee, S.K.; Lee, S.C.; Kim, S.-J. A novel cell-free strategy for promoting mouse liver regeneration: Utilization of a conditioned medium from adipose-derived stem cells. Hepatol. Int. 2015, 9, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Zhu, Y.; Yang, J.; Ni, Y.; Zhou, Z.; Chen, Y.; Wen, L. Adipose tissue-derived mesenchymal stem cells differentiated into hepatocyte-like cells in vivo and in vitro. Mol. Med. Rep. 2015, 11, 1722–1732. [Google Scholar] [CrossRef] [PubMed]
- Galateanu, B.; Dinescu, S.; Cimpean, A.; Dinischiotu, A.; Costache, M. Modulation of Adipogenic Conditions for Prospective Use of hADSCs in Adipose Tissue Engineering. Int. J. Mol. Sci. 2012, 13, 15881–15900. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Kim, M.Y.; Eom, Y.W.; Baik, S.K. Mesenchymal Stem Cells for the Treatment of Liver Disease: Present and Perspectives. Gut Liver 2020, 14, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Eggenhofer, E.; Benseler, V.; Kroemer, A.; Popp, F.C.; Geissler, E.K.; Schlitt, H.J.; Baan, C.C.; Dahlke, M.H.; Hoogduijn, M.J. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front. Immunol. 2012, 3, 297. [Google Scholar] [CrossRef]
- Hu, C.; Zhao, L.; Zhang, L.; Bao, Q.; Li, L. Mesenchymal stem cell-based cell-free strategies: Safe and effective treatments for liver injury. Stem Cell Res. Ther. 2020, 11, 377. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Hua, T.; Ouyang, T.; Qian, H.; Yu, B. Applications of Mesenchymal Stem Cells in Liver Fibrosis: Novel Strategies, Mechanisms, and Clinical Practice. Stem Cells Int. 2021, 2021, 6546780. [Google Scholar] [CrossRef]
- Dinescu, S.; Dobranici, A.; Tecucianu, R.; Selaru, A.; Balahura, R.; Ignat, S.; Costache, M. Exosomes as Part of the Human Adipose-Derived Stem Cells Secretome- Opening New Perspectives for Cell-Free Regenerative Applications. 2020, pp. 139–163. Available online: https://link.springer.com/chapter/10.1007/5584_2020_588 (accessed on 5 December 2021).
- Chiabotto, G.; Pasquino, C.; Camussi, G.; Bruno, S. Molecular Pathways Modulated by Mesenchymal Stromal Cells and Their Extracellular Vesicles in Experimental Models of Liver Fibrosis. Front. Cell Dev. Biol. 2020, 8. [Google Scholar] [CrossRef]
- Vizoso, F.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef]
- Higuchi, H.; Gores, G.J. Mechanisms of Liver Injury: An Overview. Curr. Mol. Med. 2003, 3, 483–490. [Google Scholar] [CrossRef]
- Viñas, O. Human hepatic stellate cells show features of antigen-presenting cells and stimulate lymphocyte proliferation. Hepatology 2003, 38, 919–929. [Google Scholar] [CrossRef]
- Krenkel, O.; Tacke, F. Liver macrophages in tissue homeostasis and disease. Nat. Rev. Immunol. 2017, 17, 306–321. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-Y.; Li, X.-F.; Meng, X.-M.; Huang, C.; Zhang, L.; Li, J. Macrophage Phenotype in Liver Injury and Repair. Scand. J. Immunol. 2017, 85, 166–174. [Google Scholar] [CrossRef]
- Rippe, R.A. Liver nbsp fibrosis signals leading to the amplification of the fibrogenic hepatic stellate cell. Front. Biosci. 2003, 8, 887. [Google Scholar] [CrossRef] [PubMed]
- Milani, S.; Herbst, H.; Schuppan, D.; Kim, K.Y.; Riecken, E.O.; Stein, H. Procollagen expression by nonparenchymal rat liver cells in experimental biliary fibrosis. Gastroenterology 1990, 98, 175–184. [Google Scholar] [CrossRef]
- Mederacke, I.; Hsu, C.C.; Troeger, J.S.; Huebener, P.; Mu, X.; Dapito, D.H.; Pradere, J.-P.; Schwabe, R.F. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat. Commun. 2013, 4, 2823. [Google Scholar] [CrossRef] [PubMed]
- Weiskirchen, R.; Tacke, F. Cellular and molecular functions of hepatic stellate cells in inflammatory responses and liver immunology. Hepatobiliary Surg. Nutr. 2014, 3, 344–363. [Google Scholar] [CrossRef] [PubMed]
- Benyon, R.C. Is liver fibrosis reversible? Gut 2000, 46, 443–446. [Google Scholar] [CrossRef]
- Lindquist, J.N.; Marzluff, W.F.; Stefanovic, B. III. Posttranscriptional regulation of type I collagen. Am. J. Physiol. Liver Physiol. 2000, 279, G471–G476. [Google Scholar] [CrossRef] [PubMed]
- Arthur, M.J.P. Fibrogenesis II. Metalloproteinases and their inhibitors in liver fibrosis. Am. J. Physiol. Liver Physiol. 2000, 279, G245–G249. [Google Scholar] [CrossRef]
- Campbell, J.S.; Hughes, S.D.; Gilbertson, D.G.; Palmer, T.E.; Holdren, M.S.; Haran, A.C.; Odell, M.M.; Bauer, R.L.; Ren, H.-P.; Haugen, H.S.; et al. Platelet-derived growth factor C induces liver fibrosis, steatosis, and hepatocellular carcinoma. Proc. Natl. Acad. Sci. USA 2005, 102, 3389–3394. [Google Scholar] [CrossRef] [PubMed]
- Ying, H.-Z.; Chen, Q.; Zhang, W.-Y.; Zhang, H.-H.; Ma, Y.; Zhang, S.-Z.; Fang, J.; Yu, C.-H. PDGF signaling pathway in hepatic fibrosis pathogenesis and therapeutics (Review). Mol. Med. Rep. 2017, 16, 7879–7889. [Google Scholar] [CrossRef]
- Fabregat, I.; Moreno-Càceres, J.; Sánchez, A.; Dooley, S.; Dewidar, B.; Giannelli, G.; ten Dijke, P. TGF-β signalling and liver disease. FEBS J. 2016, 283, 2219–2232. [Google Scholar] [CrossRef]
- Hu, H.-H.; Chen, D.-Q.; Wang, Y.-N.; Feng, Y.-L.; Cao, G.; Vaziri, N.D.; Zhao, Y.-Y. New insights into TGF-β/Smad signaling in tissue fibrosis. Chem. Biol. Interact. 2018, 292, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Tzavlaki, K.; Moustakas, A. TGF-β Signaling. Biomolecules 2020, 10, 487. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, K.; Osawa, Y.; Kimura, K. Wnt/β-Catenin Signaling as a Potential Target for the Treatment of Liver Cirrhosis Using Antifibrotic Drugs. Int. J. Mol. Sci. 2018, 19, 3103. [Google Scholar] [CrossRef] [PubMed]
- Bedford, D.C.; Kasper, L.H.; Fukuyama, T.; Brindle, P.K. Target gene context influences the transcriptional requirement for the KAT3 family of CBP and p300 histone acetyltransferases. Epigenetics 2010, 5, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Berg, T.; DeLanghe, S.; Al Alam, D.; Utley, S.; Estrada, J.; Wang, K.S. β-Catenin Regulates Mesenchymal Progenitor Cell Differentiation During Hepatogenesis. J. Surg. Res. 2010, 164, 276–285. [Google Scholar] [CrossRef]
- Cheng, J.H.; She, H.; Han, Y.-P.; Wang, J.; Xiong, S.; Asahina, K.; Tsukamoto, H. Wnt antagonism inhibits hepatic stellate cell activation and liver fibrosis. Am. J. Physiol. Liver Physiol. 2008, 294, G39–G49. [Google Scholar] [CrossRef]
- Dinescu, S.; Hermenean, A.; Costache, M. Human Adipose-Derived Stem Cells for Tissue Engineering Approaches: Current Challenges and Perspectives. In Stem Cells in Clinical Practice and Tissue Engineering; InTech: London, UK, 2018. [Google Scholar]
- Eleuteri, S.; Fierabracci, A. Insights into the Secretome of Mesenchymal Stem Cells and Its Potential Applications. Int. J. Mol. Sci. 2019, 20, 4597. [Google Scholar] [CrossRef] [PubMed]
- Dubey, N.; Mishra, V.; Dubey, R.; Deng, Y.-H.; Tsai, F.-C.; Deng, W.-P. Revisiting the Advances in Isolation, Characterization and Secretome of Adipose-Derived Stromal/Stem Cells. Int. J. Mol. Sci. 2018, 19, 2200. [Google Scholar] [CrossRef]
- Rabani, V.; Shahsavani, M.; Gharavi, M.; Piryaei, A.; Azhdari, Z.; Baharvand, H. Mesenchymal stem cell infusion therapy in a carbon tetrachloride-induced liver fibrosis model affects matrix metalloproteinase expression. Cell Biol. Int. 2010, 34, 601–605. [Google Scholar] [CrossRef]
- Lee, E.J.; Hwang, I.; Lee, J.Y.; Park, J.N.; Kim, K.C.; Kim, G.-H.; Kang, C.-M.; Kim, I.; Lee, S.-Y.; Kim, H.-S. Hepatocyte Growth Factor Improves the Therapeutic Efficacy of Human Bone Marrow Mesenchymal Stem Cells via RAD51. Mol. Ther. 2018, 26, 845–859. [Google Scholar] [CrossRef]
- Meldolesi, J. Exosomes and Ectosomes in Intercellular Communication. Curr. Biol. 2018, 28, R435–R444. [Google Scholar] [CrossRef]
- Lombardi, F.; Palumbo, P.; Augello, F.R.; Cifone, M.G.; Cinque, B.; Giuliani, M. Secretome of Adipose Tissue-Derived Stem Cells (ASCs) as a Novel Trend in Chronic Non-Healing Wounds: An Overview of Experimental In Vitro and In Vivo Studies and Methodological Variables. Int. J. Mol. Sci. 2019, 20, 3721. [Google Scholar] [CrossRef] [PubMed]
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell. Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Psaraki, A.; Ntari, L.; Karakostas, C.; Korrou-Karava, D.; Roubelakis, M.G. Extracellular vesicles derived from Mesenchymal Stem/Stromal Cells: The regenerative impact in liver diseases. Hepatology 2021. [Google Scholar] [CrossRef]
- Brigstock, D.R. Extracellular Vesicles in Organ Fibrosis: Mechanisms, Therapies, and Diagnostics. Cells 2021, 10, 1596. [Google Scholar] [CrossRef] [PubMed]
- Rautiainen, S.; Laaksonen, T.; Koivuniemi, R. Angiogenic Effects and Crosstalk of Adipose-Derived Mesenchymal Stem/Stromal Cells and Their Extracellular Vesicles with Endothelial Cells. Int. J. Mol. Sci. 2021, 22, 10890. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Eom, Y.W.; Shim, K.Y.; Baik, S.K. Mesenchymal stem cell therapy for liver fibrosis. Korean J. Intern. Med. 2015, 30, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Afshari, A.; Shamdani, S.; Uzan, G.; Naserian, S.; Azarpira, N. Different approaches for transformation of mesenchymal stem cells into hepatocyte-like cells. Stem Cell Res. Ther. 2020, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Salehinejad, P.; Alitheen, N.B.; Mandegary, A.; Nematollahi-mahani, S.N.; Janzamin, E. Effect of EGF and FGF on the expansion properties of human umbilical cord mesenchymal cells. Vitr. Cell. Dev. Biol. Anim. 2013, 49, 515–523. [Google Scholar] [CrossRef]
- Neuss, S.; Becher, E.; Wöltje, M.; Tietze, L.; Jahnen-Dechent, W. Functional Expression of HGF and HGF Receptor/c-met in Adult Human Mesenchymal Stem Cells Suggests a Role in Cell Mobilization, Tissue Repair, and Wound Healing. Stem Cells 2004, 22, 405–414. [Google Scholar] [CrossRef]
- Hong, S.H.; Gang, E.J.; Jeong, J.A.; Ahn, C.; Hwang, S.H.; Yang, I.H.; Park, H.K.; Han, H.; Kim, H. In vitro differentiation of human umbilical cord blood-derived mesenchymal stem cells into hepatocyte-like cells. Biochem. Biophys. Res. Commun. 2005, 330, 1153–1161. [Google Scholar] [CrossRef]
- Miyajima, A.; Kinoshita, T.; Tanaka, M.; Kamiya, A.; Mukouyama, Y.; Hara, T. Role of Oncostatin M in hematopoiesis and liver development. Cytokine Growth Factor Rev. 2000, 11, 177–183. [Google Scholar] [CrossRef]
- Lange, C. Liver-specific gene expression in mesenchymal stem cells is induced by liver cells. World J. Gastroenterol. 2005, 11, 4497. [Google Scholar] [CrossRef] [PubMed]
- Meier, R.P.H.; Müller, Y.D.; Morel, P.; Gonelle-Gispert, C.; Bühler, L.H. Transplantation of mesenchymal stem cells for the treatment of liver diseases, is there enough evidence? Stem Cell Res. 2013, 11, 1348–1364. [Google Scholar] [CrossRef]
- Wang, P.; Xie, D.; Liang, X.-J.; Peng, L.; Zhang, G.; Ye, Y.; Xie, C.; Gao, Z. HGF and Direct Mesenchymal Stem Cells Contact Synergize to Inhibit Hepatic Stellate Cells Activation through TLR4/NF-kB Pathway. PLoS ONE 2012, 7, e43408. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Hu, K.; Chen, S.; Xie, S.; Tang, Z.; Lin, J.; Xu, R. Nerve growth factor-mediated paracrine regulation of hepatic stellate cells by multipotent mesenchymal stromal cells. Life Sci. 2009, 85, 291–295. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, S.; Lai, J.; Lu, H.; Sun, Y.; Guan, W. Therapeutic Potential of Bama Pig Adipose-Derived Mesenchymal Stem Cells for the Treatment of Carbon Tetrachloride-Induced Liver Fibrosis. Exp. Clin. Transplant. 2020, 18, 823–831. [Google Scholar] [CrossRef]
- Mehrabani, D.; Khajehahmadi, Z.; Tajik, P.; Tamadon, A.; Rahmanifar, F.; Ashraf, M.; Tanideh, N.; Zare, S. Regenerative Effect of Bone Marrow-derived Mesenchymal Stem Cells in Thioacetamide-induced Liver Fibrosis of Rats. Arch. Razi Inst. 2019, 74, 279–286. [Google Scholar] [CrossRef]
- Hao, T.; Chen, J.; Zhi, S.; Zhang, Q.; Chen, G.; Yu, F. Comparison of bone marrow-vs. adipose tissue-derived mesenchymal stem cells for attenuating liver fibrosis. Exp. Ther. Med. 2017, 14, 5956–5964. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.O.; Kim, Y.J.; Baik, S.K.; Kim, M.Y.; Eom, Y.W.; Cho, M.Y.; Park, H.J.; Park, S.Y.; Kim, B.R.; Kim, J.W.; et al. Histological improvement following administration of autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: A pilot study. Liver Int. 2014, 34, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.A.; Sabry, D.; Rashed, L.A.; Aref, W.M.; El-Ghobary, M.A.; Farhan, M.S.; Fouad, H.A.; Youssef, Y.A.-A. Short-term evaluation of autologous transplantation of bone marrow-derived mesenchymal stem cells in patients with cirrhosis: Egyptian study. Clin. Transplant. 2013, 27, 607–612. [Google Scholar] [CrossRef]
- Suk, K.T.; Yoon, J.-H.; Kim, M.Y.; Kim, C.W.; Kim, J.K.; Park, H.; Hwang, S.G.; Kim, D.J.; Lee, B.S.; Lee, S.H.; et al. Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: Phase 2 trial. Hepatology 2016, 64, 2185–2197. [Google Scholar] [CrossRef]
- Amer, M.-E.M.; El-Sayed, S.Z.; El-Kheir, W.A.; Gabr, H.; Gomaa, A.A.; El-Noomani, N.; Hegazy, M. Clinical and laboratory evaluation of patients with end-stage liver cell failure injected with bone marrow-derived hepatocyte-like cells. Eur. J. Gastroenterol. Hepatol. 2011, 23, 936–941. [Google Scholar] [CrossRef]
- Lanthier, N.; Lin-Marq, N.; Rubbia-Brandt, L.; Clément, S.; Goossens, N.; Spahr, L. Autologous bone marrow-derived cell transplantation in decompensated alcoholic liver disease: What is the impact on liver histology and gene expression patterns? Stem Cell Res. Ther. 2017, 8, 88. [Google Scholar] [CrossRef]
- Mohamadnejad, M.; Alimoghaddam, K.; Bagheri, M.; Ashrafi, M.; Abdollahzadeh, L.; Akhlaghpoor, S.; Bashtar, M.; Ghavamzadeh, A.; Malekzadeh, R. Randomized placebo-controlled trial of mesenchymal stem cell transplantation in decompensated cirrhosis. Liver Int. 2013, 33, 1490–1496. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.-J.; Li, H.Y.; Guan, L.-X.; Ritchie, G.; Zhou, J.X. The therapeutic potential of bone marrow-derived mesenchymal stem cells on hepatic cirrhosis. Stem Cell Res. 2009, 2, 16–25. [Google Scholar] [CrossRef] [PubMed]
- An, S.Y.; Jang, Y.J.; Lim, H.-J.; Han, J.; Lee, J.; Lee, G.; Park, J.Y.; Park, S.-Y.; Kim, J.H.; Do, B.-R.; et al. Milk Fat Globule-EGF Factor 8, Secreted by Mesenchymal Stem Cells, Protects Against Liver Fibrosis in Mice. Gastroenterology 2017, 152, 1174–1186. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, D.; Dias, I.; Freire, T.; Thole, A.A.; Stumbo, A.C.; Cortez, E.A.C.; de Carvalho, L.; de Carvalho, S.N. Effects of mesenchymal stem cells conditioned medium treatment in mice with cholestatic liver fibrosis. Life Sci. 2021, 281, 119768. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Cheng, X.; Wang, H.; Huang, W.; la Ga hu, Z.; Wang, D.; Zhang, K.; Zhang, H.; Xue, Z.; Da, Y.; et al. Mesenchymal stem cells and their secreted molecules predominantly ameliorate fulminant hepatic failure and chronic liver fibrosis in mice respectively. J. Transl. Med. 2016, 14, 45. [Google Scholar] [CrossRef]
- Liu, Y.; Dulchavsky, D.S.; Gao, X.; Kwon, D.; Chopp, M.; Dulchavsky, S.; Gautam, S.C. Wound Repair by Bone Marrow Stromal Cells through Growth Factor Production. J. Surg. Res. 2006, 136, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Mao, L.-C.; Cai, Q.-Q.; Jiang, W.-H. Effect of Hepatocyte Growth Factor-Transfected Human Umbilical Cord Mesenchymal Stem Cells on Hepatic Stellate Cells by Regulating Transforming Growth Factor-β1/Smads Signaling Pathway. Stem Cells Dev. 2021, 30, 1070–1081. [Google Scholar] [CrossRef]
- Yu, S.P.; Wei, Z.; Wei, L. Preconditioning Strategy in Stem Cell Transplantation Therapy. Transl. Stroke Res. 2013, 4, 76–88. [Google Scholar] [CrossRef]
- Feng, Y.; Huang, W.; Meng, W.; Jegga, A.G.; Wang, Y.; Cai, W.; Kim, H.W.; Pasha, Z.; Wen, Z.; Rao, F.; et al. Heat Shock Improves Sca-1 + Stem Cell Survival and Directs Ischemic Cardiomyocytes Toward a Prosurvival Phenotype Via Exosomal Transfer: A Critical Role for HSF1/miR-34a/HSP70 Pathway. Stem Cells 2014, 32, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, K.E.; Sharp, T.V.; McKay, T.R. The role of hypoxia in stem cell potency and differentiation. Regen. Med. 2013, 8, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Jahr, H.; van Osch, G.J.V.M.; Farrell, E. The Role of Hypoxia in Bone Marrow–Derived Mesenchymal Stem Cells: Considerations for Regenerative Medicine Approaches. Tissue Eng. Part B Rev. 2010, 16, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Bunnell, B.A.; Betancourt, A.M.; Sullivan, D.E. New concepts on the immune modulation mediated by mesenchymal stem cells. Stem Cell Res. Ther. 2010, 1, 34. [Google Scholar] [CrossRef]
- Ha, T.; Hua, F.; Liu, X.; Ma, J.; McMullen, J.R.; Shioi, T.; Izumo, S.; Kelley, J.; Gao, X.; Browder, W.; et al. Lipopolysaccharide-induced myocardial protection against ischaemia/reperfusion injury is mediated through a PI3K/Akt-dependent mechanism. Cardiovasc. Res. 2008, 78, 546–553. [Google Scholar] [CrossRef]
- Lee, S.C.; Jeong, H.J.; Lee, S.K.; Kim, S.-J. Lipopolysaccharide preconditioning of adipose-derived stem cells improves liver-regenerating activity of the secretome. Stem Cell Res. Ther. 2015, 6, 75. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yan, Y.; Wang, B.; Qian, H.; Zhang, X.; Shen, L.; Wang, M.; Zhou, Y.; Zhu, W.; Li, W.; et al. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Alleviate Liver Fibrosis. Stem Cells Dev. 2013, 22, 845–854. [Google Scholar] [CrossRef]
- Fiore, E.; Domínguez, L.M.; Bayo, J.; Malvicini, M.; Atorrasagasti, C.; Rodriguez, M.; Cantero, M.J.; García, M.; Yannarelli, G.; Mazzolini, G. Human umbilical cord perivascular cells-derived extracellular vesicles mediate the transfer of IGF-I to the liver and ameliorate hepatic fibrogenesis in mice. Gene Ther. 2020, 27, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Rong, X.; Liu, J.; Yao, X.; Jiang, T.; Wang, Y.; Xie, F. Human bone marrow mesenchymal stem cells-derived exosomes alleviate liver fibrosis through the Wnt/β-catenin pathway. Stem Cell Res. Ther. 2019, 10, 98. [Google Scholar] [CrossRef]
- Jun, J.H.; Kim, J.Y.; Choi, J.H.; Lim, J.-Y.; Kim, K.; Kim, G.J. Exosomes from Placenta-Derived Mesenchymal Stem Cells Are Involved in Liver Regeneration in Hepatic Failure Induced by Bile Duct Ligation. Stem Cells Int. 2020, 2020, 5485738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Gan, Z.; Tang, L.; Zhou, L.; Huang, X.; Wang, J. Exosomes from microRNA-145-5p-modified HUCB-MSCs attenuate CCl4-induced hepatic fibrosis via down-regulating FSCN1 expression. Life Sci. 2021, 119404. [Google Scholar] [CrossRef]
- Jiang, W.; Tan, Y.; Cai, M.; Zhao, T.; Mao, F.; Zhang, X.; Xu, W.; Yan, Z.; Qian, H.; Yan, Y. Human Umbilical Cord MSC-Derived Exosomes Suppress the Development of CCl 4 -Induced Liver Injury through Antioxidant Effect. Stem Cells Int. 2018, 2018, 6079642. [Google Scholar] [CrossRef] [PubMed]
- Rostom, D.M.; Attia, N.; Khalifa, H.M.; Abou Nazel, M.W.; El Sabaawy, E.A. The Therapeutic Potential of Extracellular Vesicles Versus Mesenchymal Stem Cells in Liver Damage. Tissue Eng. Regen. Med. 2020, 17, 537–552. [Google Scholar] [CrossRef]
- Qu, Y.; Zhang, Q.; Cai, X.; Li, F.; Ma, Z.; Xu, M.; Lu, L. Exosomes derived from miR-181-5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J. Cell. Mol. Med. 2017, 21, 2491–2502. [Google Scholar] [CrossRef] [PubMed]
- Ohara, M.; Ohnishi, S.; Hosono, H.; Yamamoto, K.; Yuyama, K.; Nakamura, H.; Fu, Q.; Maehara, O.; Suda, G.; Sakamoto, N. Extracellular Vesicles from Amnion-Derived Mesenchymal Stem Cells Ameliorate Hepatic Inflammation and Fibrosis in Rats. Stem Cells Int. 2018, 2018, 3212643. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Wang, J.; Li, H.; Gao, S.; Shi, R.; Yang, D.; Wang, X.; Wang, X.; Zhu, L.; Wang, X.; et al. Extracellular Vesicles Secreted by Human Adipose-derived Stem Cells (hASCs) Improve Survival Rate of Rats with Acute Liver Failure by Releasing lncRNA H19. EBioMedicine 2018, 34, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lou, G.; Li, A.; Zhang, T.; Qi, J.; Ye, D.; Zheng, M.; Chen, Z. AMSC-derived exosomes alleviate lipopolysaccharide/d-galactosamine-induced acute liver failure by miR-17-mediated reduction of TXNIP/NLRP3 inflammasome activation in macrophages. EBioMedicine 2018, 36, 140–150. [Google Scholar] [CrossRef]
- Dong, L.; Pu, Y.; Chen, X.; Qi, X.; Zhang, L.; Xu, L.; Li, W.; Ma, Y.; Zhou, S.; Zhu, J.; et al. hUCMSC-extracellular vesicles downregulated hepatic stellate cell activation and reduced liver injury in S. japonicum-infected mice. Stem Cell Res. Ther. 2020, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Lou, G.; Yang, Y.; Liu, F.; Ye, B.; Chen, Z.; Zheng, M.; Liu, Y. MiR-122 modification enhances the therapeutic efficacy of adipose tissue-derived mesenchymal stem cells against liver fibrosis. J. Cell. Mol. Med. 2017, 21, 2963–2973. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Liu, X.; Li, W.; Wang, L. Exosomes derived from mmu_circ_0000623-modified ADSCs prevent liver fibrosis via activating autophagy. Hum. Exp. Toxicol. 2020, 39, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.; Wang, B.; Kota, J.; Yu, J.; Costinean, S.; Kutay, H.; Yu, L.; Bai, S.; La Perle, K.; Chivukula, R.R.; et al. Essential metabolic, anti-inflammatory, and anti-tumorigenic functions of miR-122 in liver. J. Clin. Invest. 2012, 122, 2871–2883. [Google Scholar] [CrossRef]
- Bandiera, S.; Pfeffer, S.; Baumert, T.F.; Zeisel, M.B. miR-122—A key factor and therapeutic target in liver disease. J. Hepatol. 2015, 62, 448–457. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazarie, S.-R.; Gharbia, S.; Hermenean, A.; Dinescu, S.; Costache, M. Regenerative Potential of Mesenchymal Stem Cells’ (MSCs) Secretome for Liver Fibrosis Therapies. Int. J. Mol. Sci. 2021, 22, 13292. https://doi.org/10.3390/ijms222413292
Nazarie S-R, Gharbia S, Hermenean A, Dinescu S, Costache M. Regenerative Potential of Mesenchymal Stem Cells’ (MSCs) Secretome for Liver Fibrosis Therapies. International Journal of Molecular Sciences. 2021; 22(24):13292. https://doi.org/10.3390/ijms222413292
Chicago/Turabian StyleNazarie (Ignat), Simona-Rebeca, Sami Gharbia, Anca Hermenean, Sorina Dinescu, and Marieta Costache. 2021. "Regenerative Potential of Mesenchymal Stem Cells’ (MSCs) Secretome for Liver Fibrosis Therapies" International Journal of Molecular Sciences 22, no. 24: 13292. https://doi.org/10.3390/ijms222413292
APA StyleNazarie, S.-R., Gharbia, S., Hermenean, A., Dinescu, S., & Costache, M. (2021). Regenerative Potential of Mesenchymal Stem Cells’ (MSCs) Secretome for Liver Fibrosis Therapies. International Journal of Molecular Sciences, 22(24), 13292. https://doi.org/10.3390/ijms222413292









