Effect of Cobalt and Chromium Ions on the Chlorhexidine Digluconate as Seen by Intermolecular Diffusion
Abstract
:1. Introduction
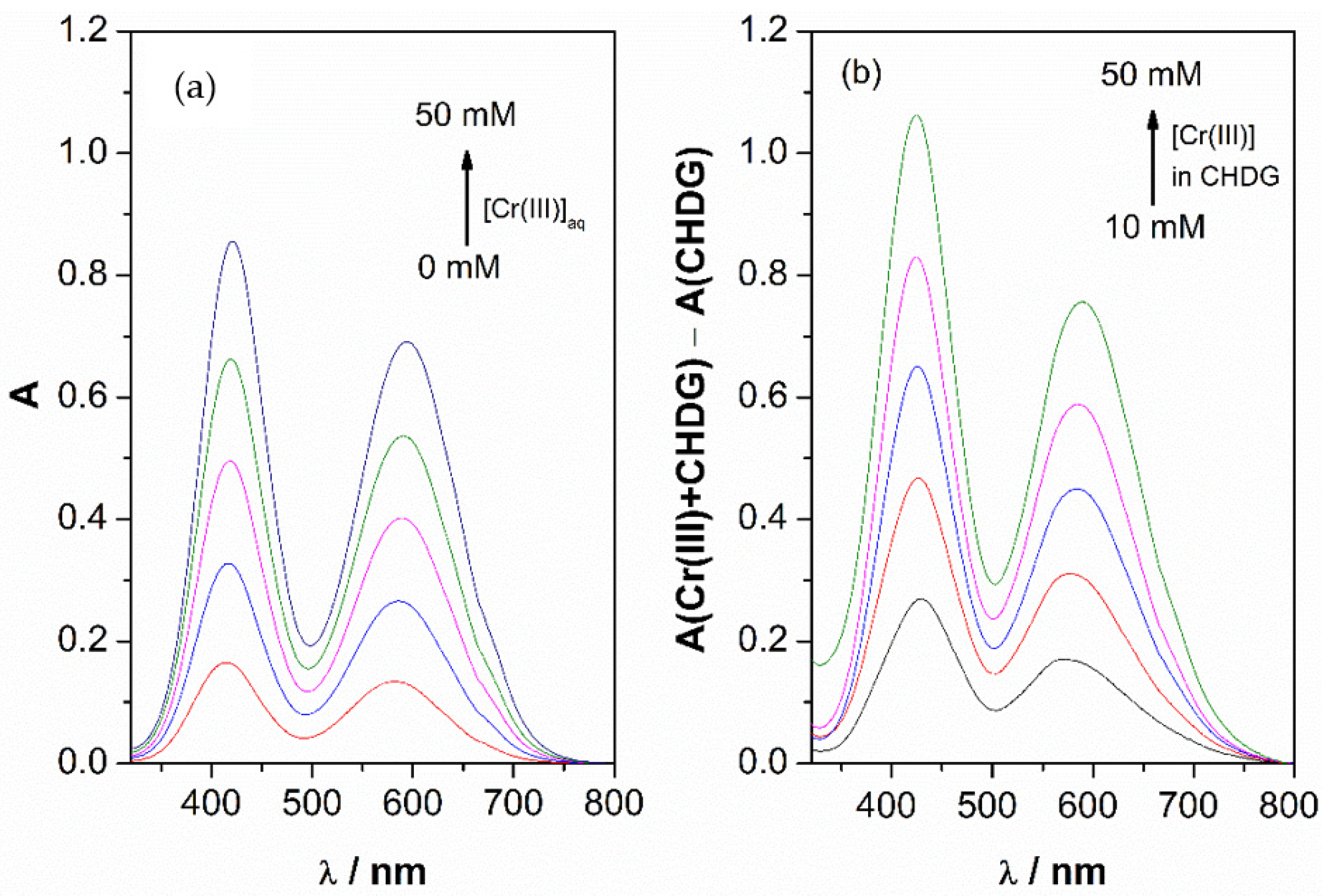
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Taylor Method
3.2.1. Tracer Diffusion Coefficients
3.2.2. Diffusion of Chlorhexidine Digluconate
3.3. UV-Vis Spectroscopy Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arregui, M.; Latour, F.; Gil, F.J.; Pérez, R.A.; Giner-Tarrida, L.; Delgado, L.M. Ion Release from Dental Implants, Prosthetic Abutments and Crowns under Physiological and Acidic Conditions. Coatings 2021, 11, 98. [Google Scholar] [CrossRef]
- Kassapidou, M.; Hjalmarsson, L.; Johansson, C.B.; Hammarström Johansson, P.; Morisbak, E.; Wennerberg, A.; Franke Stenport, V. Cobalt–chromium alloys fabricated with four different techniques: Ion release, toxicity of released elements and surface roughness. Dent. Mater. 2020, 36, e352–e363. [Google Scholar] [CrossRef]
- Wang, G.; Zreiqat, H. Functional Coatings or Films for Hard-Tissue Applications. Materials 2010, 3, 3994–4050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asri, R.I.M.; Harun, W.S.W.; Samykano, M.; Lah, N.A.C.; Ghani, S.A.C.; Tarlochan, F.; Raza, M.R. Corrosion and surface modification on biocompatible metals: A review. Mater. Sci. Eng. C 2017, 77, 1261–1274. [Google Scholar] [CrossRef] [Green Version]
- Hanawa, T. Titanium–Tissue Interface Reaction and Its Control with Surface Treatment. Front. Bioeng. Biotechnol. 2019, 7, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reclaru, L.; Cosmina Ardelean, L.; Florian Grecu, A.; Adrian Miu, C. Multicomponent Alloys for Biomedical Applications. In Engineering Steels and High Entropy-Alloys; IntechOpen: London, UK, 2020. [Google Scholar]
- Mulware, S.J. Trace elements and carcinogenicity: A subject in review. 3 Biotech 2013, 3, 85–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Castranova, V. Toxicology of Nanomaterials Used in Nanomedicine. J. Toxicol. Environ. Health Part B 2011, 14, 593–632. [Google Scholar] [CrossRef]
- Mihardjanti, M.; Ismah, N.; Purwanegara, M.K. Nickel and chromium ion release from stainless steel bracket on immersion various types of mouthwashes. J. Phys. Conf. Ser. 2017, 884, 012107. [Google Scholar] [CrossRef] [Green Version]
- Shruthi, D.; Patil, G.; Prithviraj, D. Comparative evaluation of ion release in bonded and nonbonded stainless steel brackets with use of different mouthwashes: An In vitro study. Contemp. Clin. Dent. 2020, 11, 15. [Google Scholar] [CrossRef]
- Noumbissi, S.; Scarano, A.; Gupta, S. A Literature Review Study on Atomic Ions Dissolution of Titanium and Its Alloys in Implant Dentistry. Materials 2019, 12, 368. [Google Scholar] [CrossRef] [Green Version]
- Mathew, M.T.; Srinivasa Pai, P.; Pourzal, R.; Fischer, A.; Wimmer, M.A. Significance of Tribocorrosion in Biomedical Applications: Overview and Current Status. Adv. Tribol. 2009, 2009, 250986. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.H.; Cai, Z.B.; Li, W.; Yu, H.Y.; Zhou, Z.R. Fretting in prosthetic devices related to human body. Tribol. Int. 2009, 42, 1360–1364. [Google Scholar] [CrossRef]
- Zheng, Y.; Bashandeh, K.; Shakil, A.; Jha, S.; Polycarpou, A.A. Review of dental tribology: Current status and challenges. Tribol. Int. 2022, 166, 107354. [Google Scholar] [CrossRef]
- Vaicelyte, A.; Janssen, C.; Le Borgne, M.; Grosgogeat, B. Cobalt–Chromium Dental Alloys: Metal Exposures, Toxicological Risks, CMR Classification, and EU Regulatory Framework. Crystals 2020, 10, 1151. [Google Scholar] [CrossRef]
- Taylor, G.I. Dispersion of soluble matter in solvent flowing slowly through a tube. Proc. R. Soc. London Ser. A Math. Phys. Sci. 1953, 219, 186–203. [Google Scholar] [CrossRef]
- Callendar, R.; Leaist, D.G. Diffusion Coefficients for Binary, Ternary, and Polydisperse Solutions from Peak-Width Analysis of Taylor Dispersion Profiles. J. Solut. Chem. 2006, 35, 353–379. [Google Scholar] [CrossRef]
- Loh, W. A técnica de dispersão de taylor para estudos de difusão em líquidos e suas aplicações. Quim. Nova 1997, 20, 541–545. [Google Scholar] [CrossRef] [Green Version]
- Suito, H.; Iwawaki, Y.; Goto, T.; Tomotake, Y.; Ichikawa, T. Oral Factors Affecting Titanium Elution and Corrosion: An In Vitro Study Using Simulated Body Fluid. PLoS ONE 2013, 8, e66052. [Google Scholar] [CrossRef]
- Edgar, W.M.; O’Mullane, D.M. Saliva and Oral Health, 2nd ed.; British Dental Association: London, UK, 1996; ISBN 0904588475. [Google Scholar]
- Puskar, T.; Jevremovic, D.; Williams, R.; Eggbeer, D.; Vukelic, D.; Budak, I. A Comparative Analysis of the Corrosive Effect of Artificial Saliva of Variable pH on DMLS and Cast Co-Cr-Mo Dental Alloy. Materials 2014, 7, 6486–6501. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, A.C.F.; Lobo, V.M.M.; Natividade, J.J.S. Diffusion Coefficients in Aqueous Solutions of Cobalt Chloride at 298.15 K. J. Chem. Eng. Data 2002, 47, 539–541. [Google Scholar] [CrossRef] [Green Version]
- Baes, C.F., Jr.; Mesmer, R.E. The Hydrolysis of Cations; John Wiley & Sons, Inc.: New York, NY, USA, 1976. [Google Scholar]
- Stuenzi, H.; Marty, W. Early stages of the hydrolysis of chromium(III) in aqueous solution. 1. Characterization of a tetrameric species. Inorg. Chem. 1983, 22, 2145–2150. [Google Scholar] [CrossRef]
- Jafari, K.; Rahimzadeh, S.; Hekmatfar, S. Nickel ion release from dental alloys in two different mouthwashes. J. Dent. Res. Dent. Clin. Dent. Prospects 2019, 13, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Rugg-Gunn, A. Fluoride toothpastes and fluoride mouthrinses. Acta Med. Acad. 2013, 42, 168–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mabilleau, G.; Bourdon, S.; Joly-Guillou, M.L.; Filmon, R.; Baslé, M.F.; Chappard, D. Influence of fluoride, hydrogen peroxide and lactic acid on the corrosion resistance of commercially pure titanium. Acta Biomater. 2006, 2, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, S.; Hattori, M.; Yoshinari, M.; Kawada, E.; Oda, Y. Corrosion behavior and surface characterization of titanium in solution containing fluoride and albumin. Biomaterials 2005, 26, 829–837. [Google Scholar] [CrossRef]
- Musilová, L.; Mráček, A.; Kašpárková, V.; Minařík, A.; Valente, A.J.M.; Azevedo, E.F.G.; Veríssimo, L.M.P.; Rodrigo, M.M.; Esteso, M.A.; Ribeiro, A.C.F. Effect of Hofmeister Ions on Transport Properties of Aqueous Solutions of Sodium Hyaluronate. Int. J. Mol. Sci. 2021, 22, 1932. [Google Scholar] [CrossRef]
- Melia Rodrigo, M.; Ribeiro, A.C.F.; Moço, G.; Cabral, A.M.T.D.P.V.; Valente, A.J.M.; Esteso, M.A.; Abreu, P.E.; Santos, J.R.C.; Marques, J.M.C. Effect of sodium chloride on the behaviour of the lactose in aqueous solution studied from diffusion experiments and molecular dynamics simulations. J. Chem. Thermodyn. 2021, 155, 106370. [Google Scholar] [CrossRef]
- Ribeiro, A.C.F.; Barros, M.C.F.; Veríssimo, L.M.P.; Santos, C.I.A.V.; Cabral, A.M.T.D.P.V.; Gaspar, G.D.; Esteso, M.A. Diffusion coefficients of paracetamol in aqueous solutions. J. Chem. Thermodyn. 2012, 54, 97–99. [Google Scholar] [CrossRef]
- Ribeiro, A.C.F.; Lobo, V.M.M.; Oliveira, L.R.C.; Burrows, H.D.; Azevedo, E.F.G.; Fangaia, S.I.G.; Nicolau, P.M.G.; Guerra, F.A.D.R.A. Diffusion Coefficients of Chromium Chloride in Aqueous Solutions at 298.15 K and 303.15 K. J. Chem. Eng. Data 2005, 50, 1014–1017. [Google Scholar] [CrossRef] [Green Version]
- Kittner, M.; Klapp, S.H.L. Screening effects on structure and diffusion in confined charged colloids. J. Chem. Phys. 2007, 126, 154902. [Google Scholar] [CrossRef] [PubMed]
- Scattergood, P.A. Recent Advances in Chromium Coordination Chemistry: Luminescent Materials and Photocatalysis. In Organometallic Chemistry; Patmore, N.J., Elliott, P.I.P., Eds.; Royal Society of Chemistry: London, UK, 2020; Volume 43, pp. 1–34. [Google Scholar] [CrossRef]
- Costa, D.; Pais, A.A.C.C.; Bastos, M.; Bai, G.; Miguel, M.d.G.; Ramos, M.L.; Valente, A.J.M.; Burrows, H.D.; Teixeira, M.H. Does cation dehydration drive the binding of metal ions to polyelectrolytes in water? What we can learn from the behaviour of aluminium(iii) and chromium(iii). Phys. Chem. Chem. Phys. 2012, 14, 7950. [Google Scholar] [CrossRef] [Green Version]
- Jiménez, J.-R.; Doistau, B.; Poncet, M.; Piguet, C. Heteroleptic trivalent chromium in coordination chemistry: Novel building blocks for addressing old challenges in multimetallic luminescent complexes. Coord. Chem. Rev. 2021, 434, 213750. [Google Scholar] [CrossRef]
- Sawyer, D.T. Metal-Gluconate Complexes. Chem. Rev. 1964, 64, 633–643. [Google Scholar] [CrossRef]
- Buckó, Á.; Kutus, B.; Peintler, G.; Kele, Z.; Pálinkó, I.; Sipos, P. Stability and structural aspects of complexes forming between aluminum(III) and D-heptagluconate in acidic to strongly alkaline media: An unexpected diversity. J. Mol. Liq. 2020, 314, 113645. [Google Scholar] [CrossRef]
- Marek, M. Dissolution of Mercury from Dental Amalgam at Different pH Values. J. Dent. Res. 1997, 76, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Horasawa, N.; Takahashi, S.; Marek, M. Galvanic interaction between titanium and gallium alloy or dental amalgam. Dent. Mater. 1999, 15, 318–322. [Google Scholar] [CrossRef]
- Barthel, J.; Gores, H.J.; Lohr, C.M.; Seidl, J.J. Taylor dispersion measurements at low electrolyte concentrations. I. Tetraalkylammonium perchlorate aqueous solutions. J. Solut. Chem. 1996, 25, 921–935. [Google Scholar] [CrossRef]
- Lopes, M.L.S.M.; Nieto de Castro, C.A.; Sengers, J.V. Mutual diffusivity of a mixture of normal-hexane and nitrobenzene near its consolute point. Int. J. Thermophys. 1992, 13, 283–294. [Google Scholar] [CrossRef]
- Alizadeh, A.; Nieto de Castro, C.A.; Wakeham, W.A. The theory of the Taylor dispersion technique for liquid diffusivity measurements. Int. J. Thermophys. 1980, 1, 243–284. [Google Scholar] [CrossRef]
- Price, W.E. Theory of the taylor dispersion technique for three-component-system diffusion measurements. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1988, 84, 2431. [Google Scholar] [CrossRef]
- Deng, Z.; Leaist, D.G. Ternary mutual diffusion coefficients of MgCl2 + MgSO4 + H2O and Na2SO4 + MgSO4 + H2O from Taylor dispersion profiles. Can. J. Chem. 1991, 69, 1548–1553. [Google Scholar] [CrossRef] [Green Version]
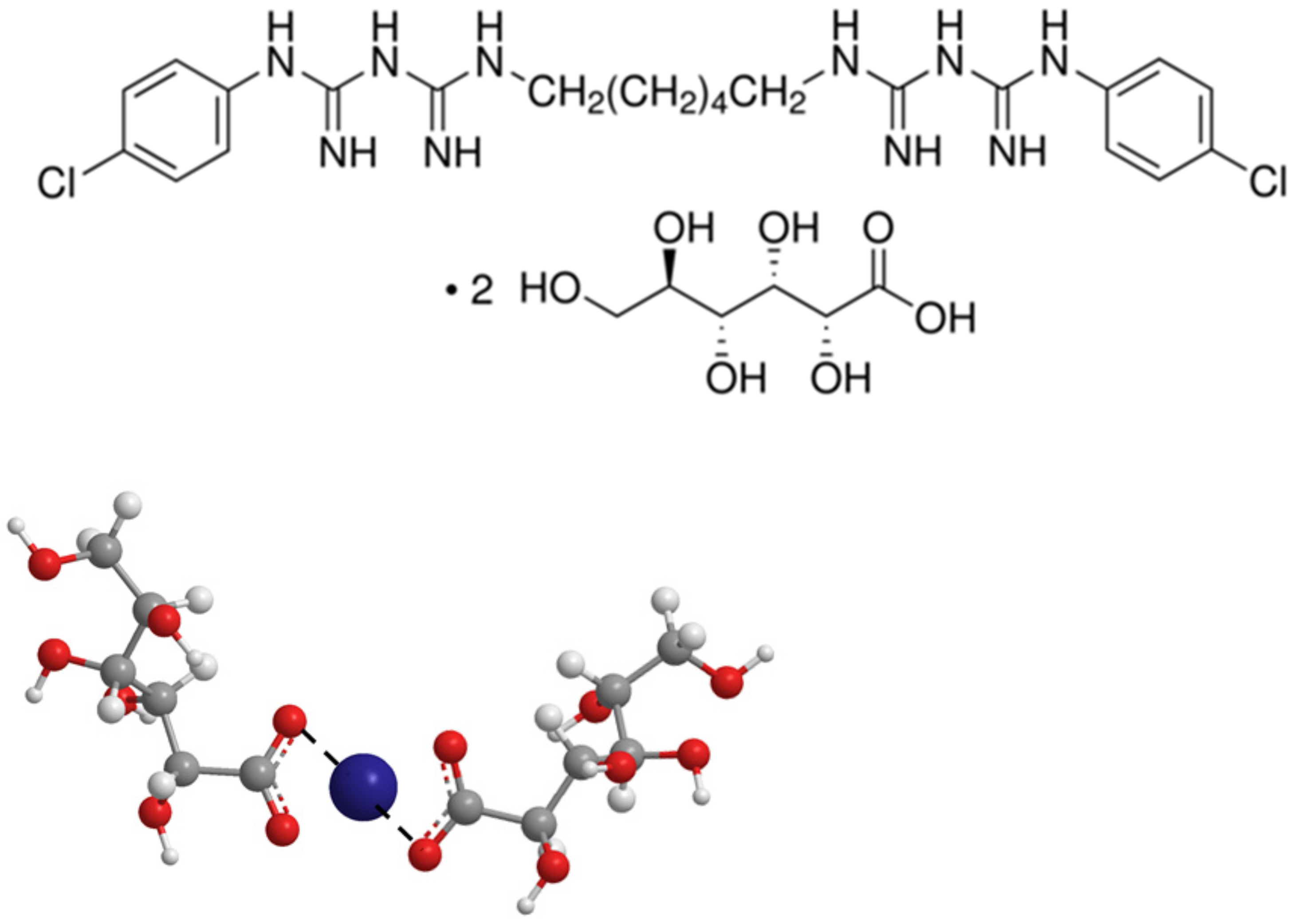

| D01 ± SD/(10−9 m2 s−1) | ||||
|---|---|---|---|---|
| pH | CoCl2 | CrCl3 | CoCl2/CrCl3 | |
| water | 6.4 | 1.295 ± 0.010 | 1.272 ± 0.014 | 1.189 ± 0.015 |
| 0.2% CHDG | 5.0 | 0.666 ± 0.015) | 0.656 ± 0.015 | - |
| CHDG commercial formulation | 5.7 | 0.709 ± 0.020) | 0.718 ± 0.018 | 0.915 ± 0.022 |
| D01 ± SD/(10−9 m2 s−1) (a) | ||||
|---|---|---|---|---|
| pH | CoCl2 | CrCl3 | CoCl2/CrCl3 | |
| AS | 8.3 | 1.823 ± 0.024 | 1.789 ± 0.040 | 1.890 ± 0.045 |
| AS | 7.0 | 1.860 ± 0.010 | 1.808 ± 0.020 | 1.909 ± 0.014 |
| AS + lactic acid | 2.3 | 0.921 ± 0.030 | 0.908 ± 0.012 | 0.917 ± 0.010 |
| AS + NaF | 7.0 | 1.701 ± 0.029 | 2.065 ± 0.019 | 2.315 ± 0.020 |
| AS + lactic acid + NaF | 2.3 | 0.899 ± 0.031 | 0.782 ± 0.030 | 0.826 ± 0.028 |
| C/(mol dm−3) | DCHDG/(10−9 m2 s−1) | DCHDG,cf/(10−9 m2 s−1) |
|---|---|---|
| 0.000 * | 0.635 ± 0.010 | 0.762 ± 0.016 |
| 0.001 | 0.617 ± 0.009 | 0.740 ± 0.012 |
| 0.004 | 0.602 ± 0.008 | 0.677 ± 0.013 |
| D11 ± SD | D12 ± SD | D21 ± SD | D22 ± SD | D12/D22 | |
|---|---|---|---|---|---|
| CoCl2 | 1.325 ± 0.018 | −0.105 ± 0.030 | −0.020 ± 0.020 | 0.780 ± 0.010 | −0.135 |
| CrCl3 | 1.310 ± 0.020 | −0.205 ± 0.030 | −0.050 ± 0.010 | 0.736 ± 0.010 | −0.279 |
| D11 ± SD | D12 ± SD | D21 ± SD | D22 ± SD | D12/D22 | D21/D11 | |
|---|---|---|---|---|---|---|
| CoCl2 | 1.193 ± 0.018 | −0.150 ± 0.030 | −0.003 ± 0.020 | 0.809 ± 0.010 | −0.060 | −0.003 |
| CrCl3 | 1.309 ± 0.020 | −1.080 ± 0.030 | −0.002 ± 0.010 | 0.819 ± 0.010 | −1.201 | −0.001 |
| Chemical | Source | CAS Number | Mass Fraction Purity |
|---|---|---|---|
| CoCl2·6H2O | Sigma-Aldrich | 7791-13-1 | >0.98 (a) |
| CrCl3·6H2O | Riedel-de-Haen, Seelze | 10060-12-5 | >0.98 (a) |
| NaF | Sigma-Aldrich | >0.99 (a) | |
| Lactic acid | Sigma-Aldrich | >0.85 wt.% (a) | |
| Artificial saliva (b) | |||
| Chlorhexidine digluconate (c) | Sigma-Aldrich | 18472-51-0 | 20% in water |
| H2O | Millipore-Q water (1.82 × 105 Ω m at 298.15 K) | 7732-18-5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fangaia, S.I.G.; Nicolau, P.M.G.; Guerra, F.A.D.R.A.; Rodrigo, M.M.; Utzeri, G.; Cabral, A.M.T.D.P.V.; Valente, A.J.M.; Esteso, M.A.; Ribeiro, A.C.F. Effect of Cobalt and Chromium Ions on the Chlorhexidine Digluconate as Seen by Intermolecular Diffusion. Int. J. Mol. Sci. 2021, 22, 13266. https://doi.org/10.3390/ijms222413266
Fangaia SIG, Nicolau PMG, Guerra FADRA, Rodrigo MM, Utzeri G, Cabral AMTDPV, Valente AJM, Esteso MA, Ribeiro ACF. Effect of Cobalt and Chromium Ions on the Chlorhexidine Digluconate as Seen by Intermolecular Diffusion. International Journal of Molecular Sciences. 2021; 22(24):13266. https://doi.org/10.3390/ijms222413266
Chicago/Turabian StyleFangaia, Sónia I. G., Pedro M. G. Nicolau, Fernando A. D. R. A. Guerra, M. Melia Rodrigo, Gianluca Utzeri, Ana M. T. D. P. V. Cabral, Artur J. M. Valente, Miguel A. Esteso, and Ana C. F. Ribeiro. 2021. "Effect of Cobalt and Chromium Ions on the Chlorhexidine Digluconate as Seen by Intermolecular Diffusion" International Journal of Molecular Sciences 22, no. 24: 13266. https://doi.org/10.3390/ijms222413266
APA StyleFangaia, S. I. G., Nicolau, P. M. G., Guerra, F. A. D. R. A., Rodrigo, M. M., Utzeri, G., Cabral, A. M. T. D. P. V., Valente, A. J. M., Esteso, M. A., & Ribeiro, A. C. F. (2021). Effect of Cobalt and Chromium Ions on the Chlorhexidine Digluconate as Seen by Intermolecular Diffusion. International Journal of Molecular Sciences, 22(24), 13266. https://doi.org/10.3390/ijms222413266











