Mechanistic Insights into Potassium-Conferred Drought Stress Tolerance in Cultivated and Tibetan Wild Barley: Differential Osmoregulation, Nutrient Retention, Secondary Metabolism and Antioxidative Defense Capacity
Abstract
:1. Introduction
2. Results
2.1. Plant Growth, Chlorophyll and Photosynthetic Attributes
2.2. Plants K, Ca, Mg and Na Concentrations
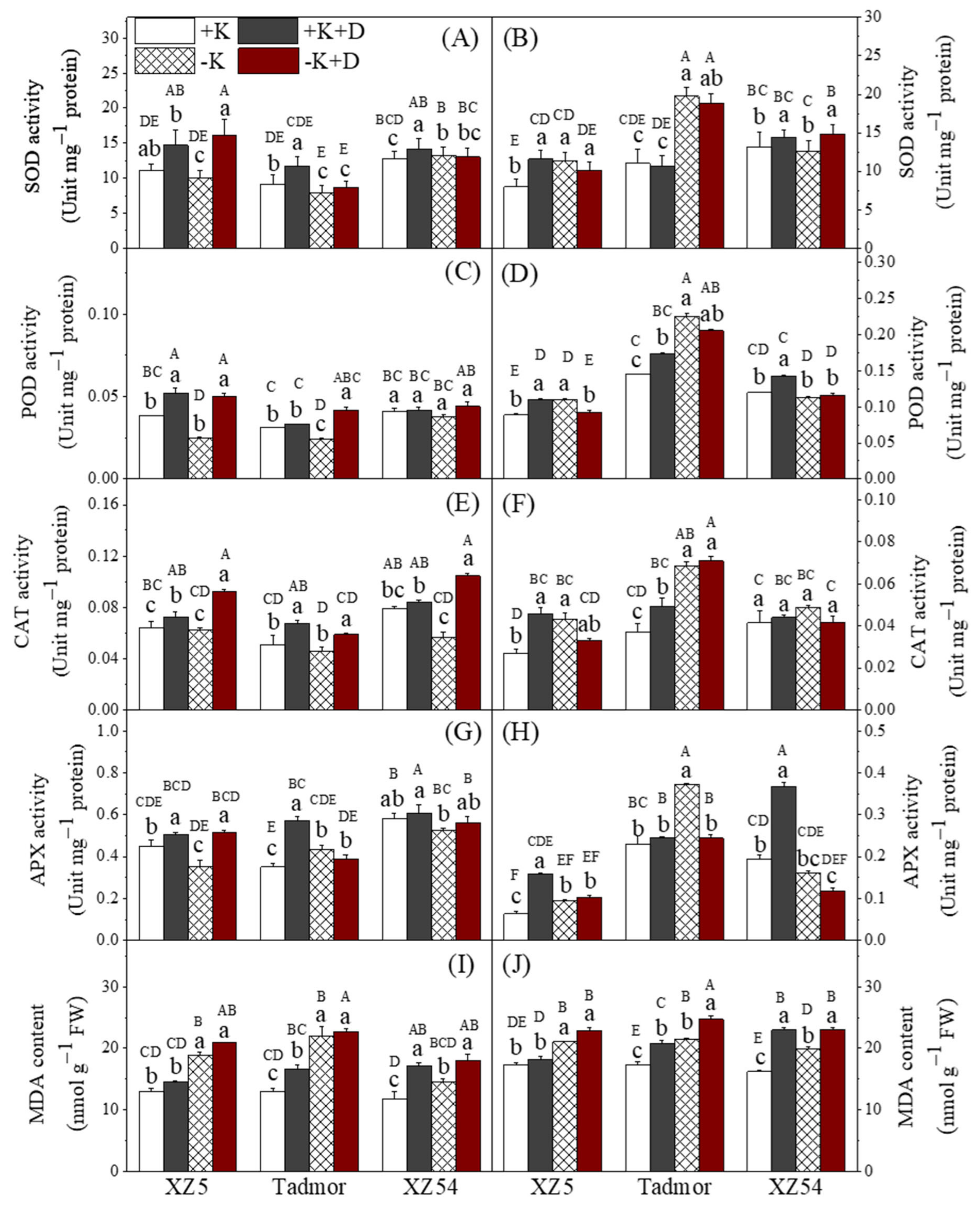
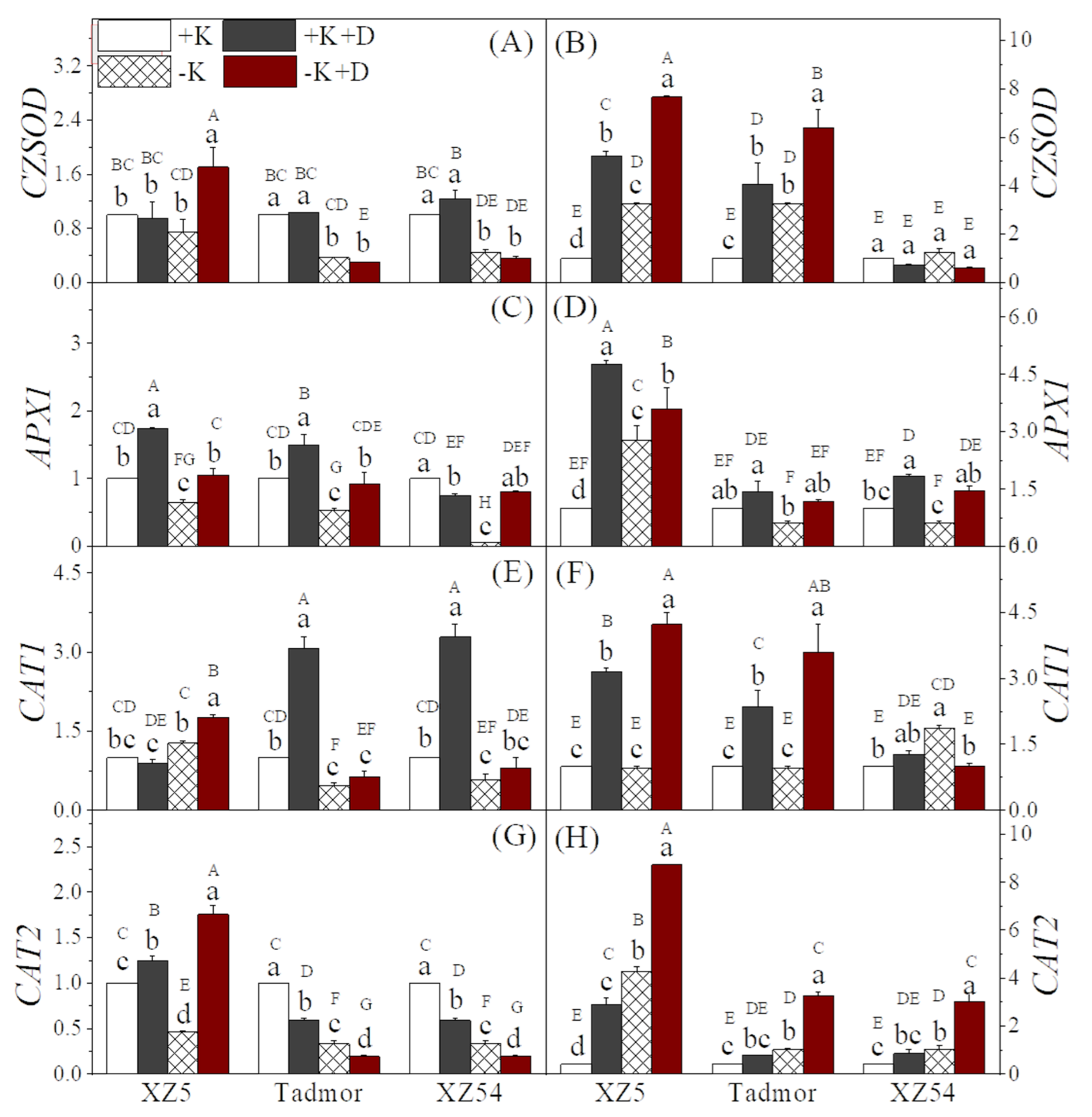
2.3. Antioxidative Enzyme Activities
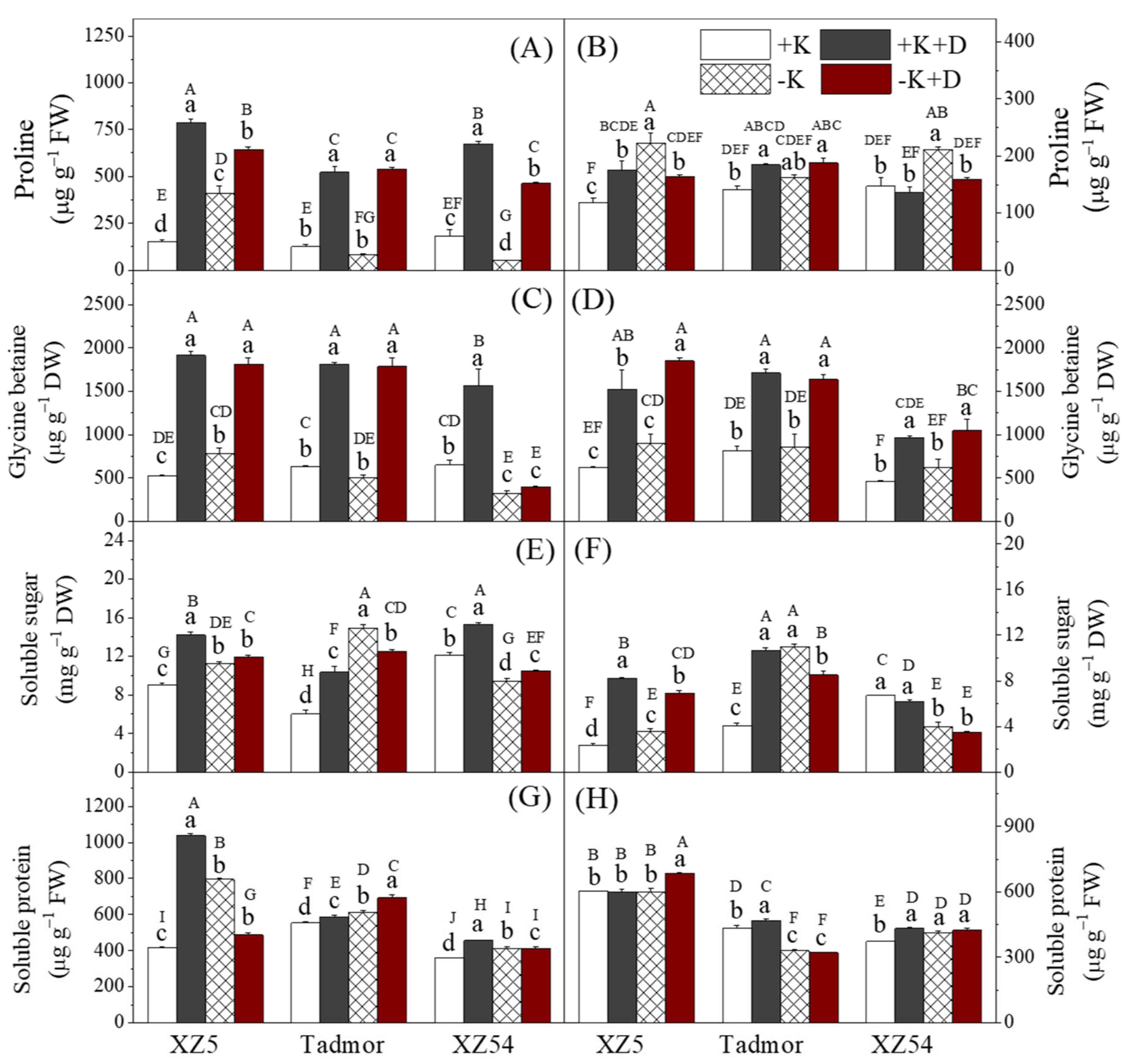
2.4. Proline, Glycine Betaine, Soluble Sugars and Proteins Contents
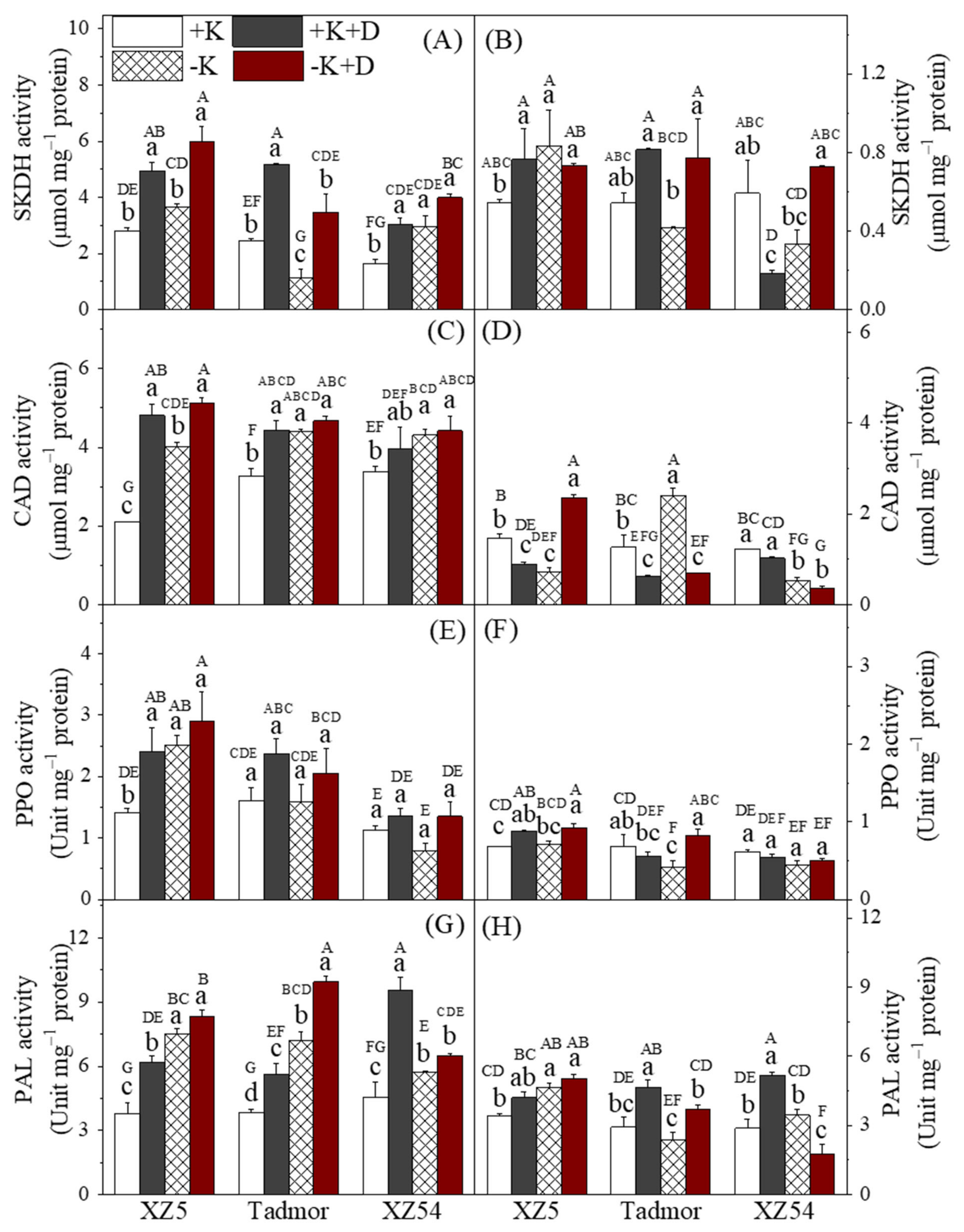
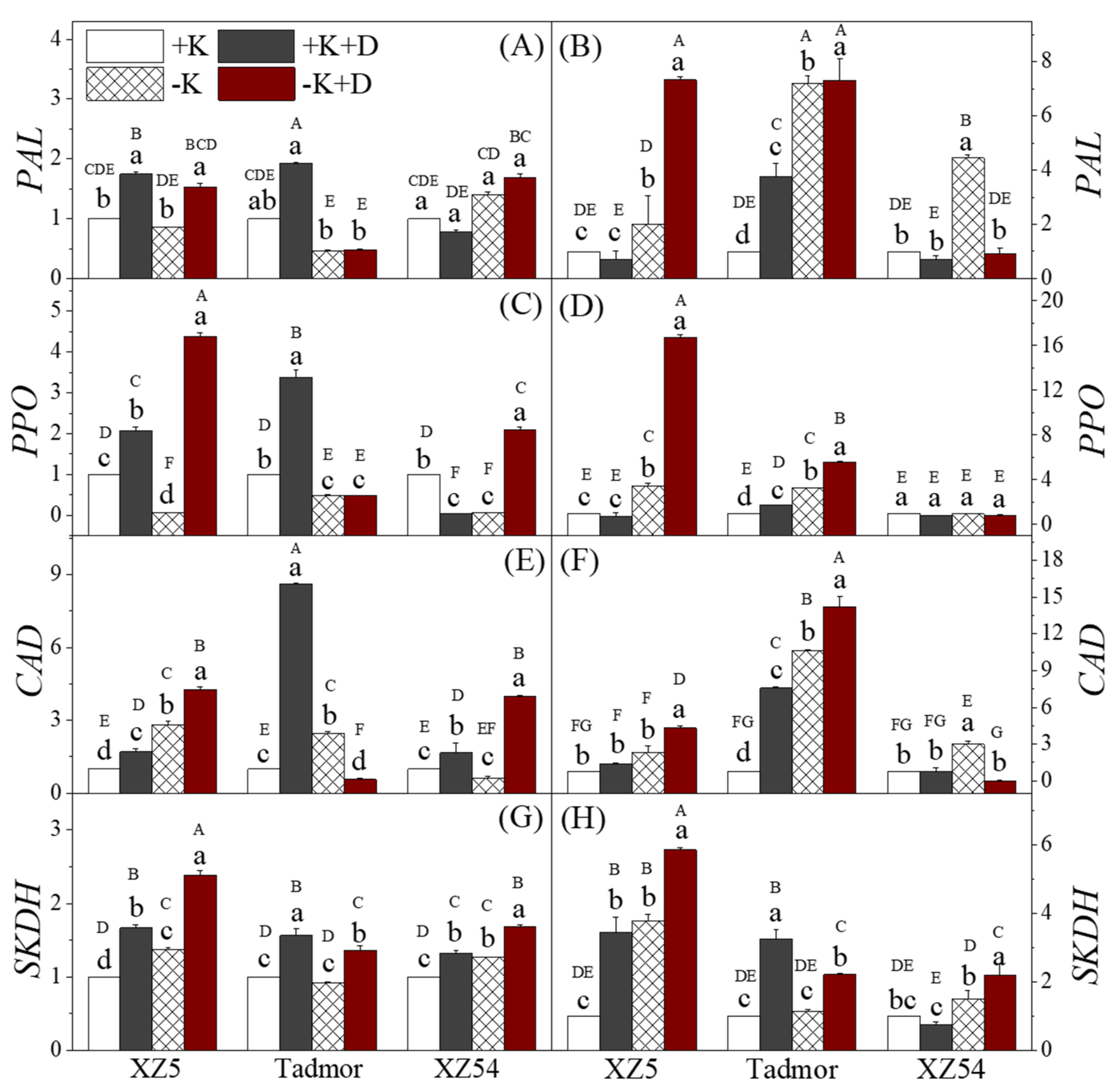
2.5. Secondary Metabolism-Related Enzyme Activities
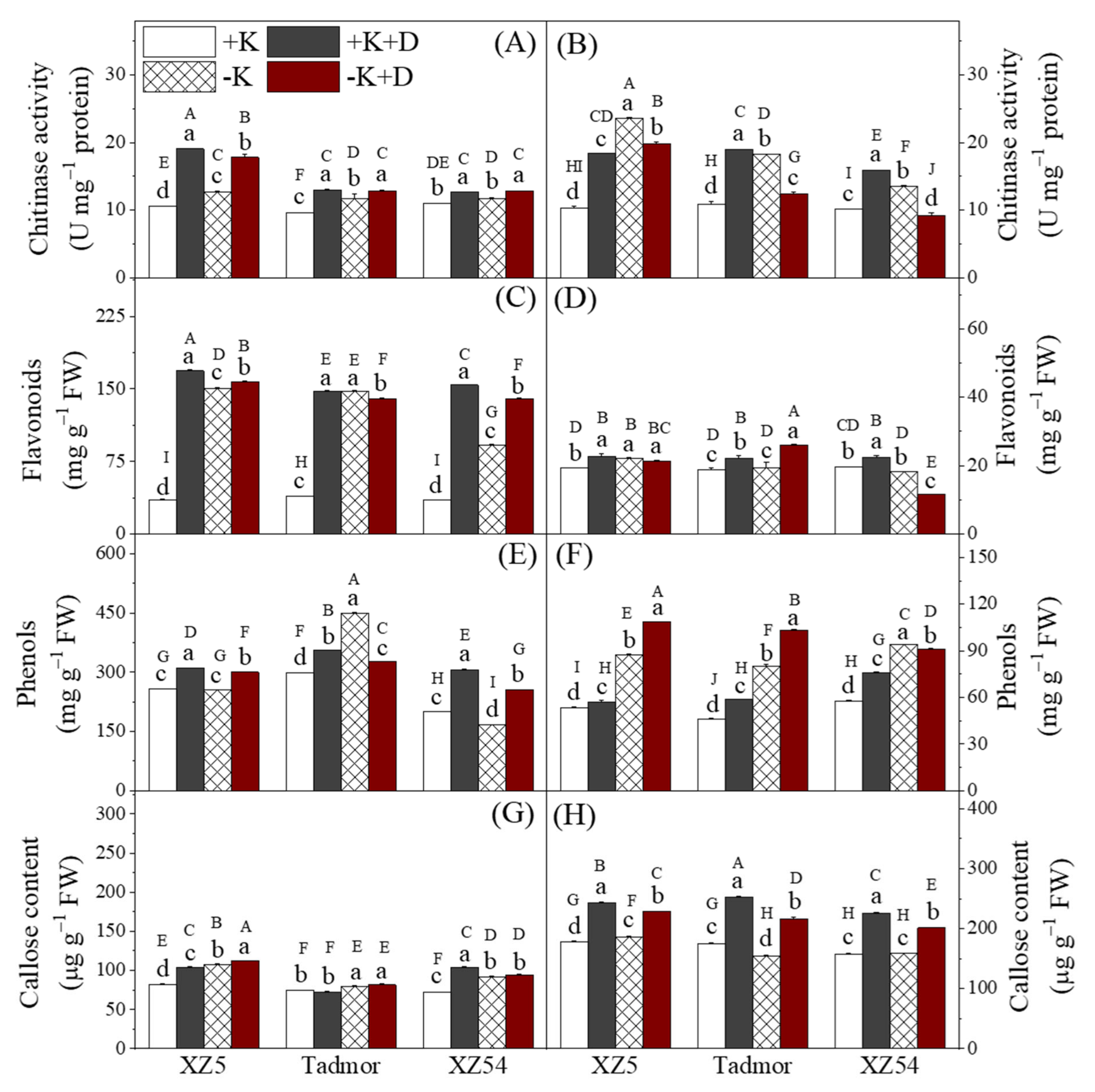
2.6. Secondary Metabolites
2.7. Gene Expression Associated with ROS Scavenging and Secondary Metabolism
3. Discussion
4. Materials and Methods
4.1. Plant Materials, Growth Conditions and Physiological Parameters
4.2. Chlorophyll and Photosynthetic Attributes
4.3. Measurement of Elemental Concentration
4.4. Determination of Antioxidant Enzyme Activities and Malondialdehyde Contents
4.5. Estimation of Osmoprotectants
4.6. Assessment of Phenol, Flavonoid, Callose Content and Chitinase Activity
4.7. Secondary Metabolism-Related Enzyme Activities
4.8. Total RNA Extraction, cDNA Synthesis and qRT-PCR Assay
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Etesami, H.; Maheshwari, D.K. Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246. [Google Scholar] [CrossRef]
- Rastegari, A.A.; Yadav, A.N.; Awasthi, A.A.; Yadav, N. Trends of Microbial Biotechnology for Sustainable Agriculture and Biomedicine Systems: Diversity and Functional Perspectives; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- He, X.; Zeng, J.; Cao, F.; Ahmed, I.M.; Zhang, G.P.; Vincze, E.; Wu, F.B. HvEXPB7, a novel β-expansin gene revealed by the root hair transcriptome of Tibetan wild barley, improves root hair growth under drought stress. J. Exp. Bot. 2015, 66, 7405–7419. [Google Scholar] [CrossRef] [Green Version]
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; SkZ, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Zandi, P.; Ali, S.; Mehmood, A.; Adnan, S.M. Impact of salicylic acid and PGPR on the drought tolerance and phytoremediation potential of Helianthus annus. Front. Microbiol. 2018, 9, 2507. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, I.M.; Cao, F.; Zhang, M.; Chen, X.; Zhang, G.; Wu, F.B. Difference in yield and physiological features in response to drought and salinity combined stress during anthesis in Tibetan wild and cultivated barleys. PLoS ONE 2013, 8, e77869. [Google Scholar]
- Kishori, K.; Polavarapu, B.; Sreenivasulu, N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell Environ. 2014, 37, 300–311. [Google Scholar] [CrossRef]
- Kaushal, M.; Wani, S.P. Plant-growth-promoting rhizobacteria: Drought stress alleviators to ameliorate crop production in drylands. Ann. Microbiol. 2016, 66, 35–42. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 1, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Faralli, M.; Lektemur, C.; Rosellini, D.; Gürel, F. Effects of heat shock and salinity on barley growth and stress-related gene transcription. Biol. Plant. 2015, 59, 537–546. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef] [Green Version]
- Guo, P.; Baum, M.; Grando, S.; Ceccarelli, S.; Bai, G.; Li, R.; Korff, M.V.; Varshney, R.K.; Graner, A.; Valkoun, J. Differentially expressed genes between drought-tolerant and drought-sensitive barley genotypes in response to drought stress during the reproductive stage. J. Exp. Bot. 2009, 60, 3531–3544. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Kronzucker, H.J. The physiology of channel-mediated K+ acquisition in roots of higher plants. Physiol. Plant. 2014, 151, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Blatt, M.R.; Chen, Z.H. Ion transport at the plant plasma membrane. eLS 2018, 1–6. [Google Scholar]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Sun, H.; Dai, H.; Zhang, G.P.; Wu, F.B. Difference in response to drought stress among Tibet wild barley genotypes. Euphytica 2010, 172, 395–403. [Google Scholar] [CrossRef]
- Dai, F.; Chen, Z.; Wang, X.; Li, Z.; Jin, G.; Wu, D.; Cai, S.; Wang, N.; Wu, F.; Nevo, E.; et al. Transcriptome profiling reveals mosaic genomic origins of modern cultivated barley. Proc. Natl. Acad. Sci. USA 2014, 111, 13403–13408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, I.M.; Cao, F.B.; Han, Y.; Nadira, U.A.; Zhang, G.P.; Wu, F.B. Differential changes in grain ultrastructure, amylase, protein and amino acid profiles between Tibetan wild and cultivated barleys under drought and salinity alone and combined stress. Food Chem. 2013, 141, 2743–2750. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Liu, J.; Su, Q.; Zhang, Q.; Gao, Y.; Yang, C. Study of the physiological mechanisms of two species of Spiraea during adaptation to drought treatment. Acta Physiol. Plant. 2017, 39, 186. [Google Scholar] [CrossRef]
- Su, L.; Dai, Z.; Li, S.; Xin, H. A novel system for evaluating drought-cold tolerance of grapevines using chlorophyll fluorescence. BMC Plant Biol. 2015, 15, 82. [Google Scholar] [CrossRef] [Green Version]
- Ye, Z.L.; Zeng, J.B.; Li, X.; Zeng, F.R.; Zhang, G.P. Physiological characterizations of three barley genotypes in response to low potassium stress. Acta Physiol. Plant. 2017, 39, 232. [Google Scholar] [CrossRef]
- James, R.A.; Munns, R.; Von Caemmerer, S.; Trejo, C.; Miller, C.; Condon, T. Photosynthetic capacity is related to the cellular and subcellular partitioning of Na+, K+ and Cl− in salt-affected barley and durum wheat. Plant Cell Environ. 2006, 29, 2185–2197. [Google Scholar] [CrossRef]
- Tuteja, N.; Mahajan, S. Calcium signaling network in plants: An overview. Plant Signal. Behav. 2007, 2, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Tadayyon, A.; Nikneshan, P.; Pessarakli, M. Effects of drought stress on concentration of macro- and micro-nutrients in Castor (Ricinus communis L.) plant. J. Plant Nutr. 2018, 41, 304–310. [Google Scholar] [CrossRef]
- Chen, F.; Wang, F.; Wu, F.; Mao, W.; Zhang, G.; Zhou, M. Modulation of exogenous glutathione in antioxidant defense system against Cd stress in the two barley genotypes differing in Cd tolerance. Plant Physiol. Biochem. 2010, 48, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.; Mateus, N.; de Freitas, V. Flavanols: Catechins and Proanthocyanidins. In Natural Products; Ramawat, K., Mérillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Hura, T.; Grzesiak, S.; Hura, K.; Thiemt, E.; Tokarz, K.; Wędzony, M. Physiological and biochemical tools useful in drought-tolerance detection in genotypes of winter triticale: Accumulation of ferulic acid correlates with drought tolerance. Ann. Bot. 2007, 100, 767–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Wang, Y.; Jia, L.; Yang, G.; Xu, X.; Zhai, H.; He, S.; Li, J.; Dai, X.; Qin, N.; et al. Involvement of an ABI-like protein and a Ca2+-ATPase in drought tolerance as revealed by transcript profiling of a sweet potato somatic hybrid and its parents Ipomoea batatas (L.) Lam. and I. triloba L. PLoS ONE 2018, 3, e0193193. [Google Scholar] [CrossRef] [PubMed]
- Morsomme, P.; Boutry, M. The plant plasma membrane H+-ATPase: Structure, function and regulation. Biochim Biophys Acta Biomembr. 2000, 1465, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Acosta-Motos, J.; Ortuño, M.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.; Hernandez, J. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Yamada, M.; Han, X.; Ohler, U.; Benfey, P.N. High-resolution expression map of the Arabidopsis root reveals alternative splicing and lincRNA regulation. Dev. Cell 2016, 39, 508–522. [Google Scholar] [CrossRef] [Green Version]
- Preisner, M.; Wojtasik, W.; Kostyn, K.; Boba, A.; Czuj, T.; Szopa, J.; Kulma, A. The cinnamyl alcohol dehydrogenase family in flax: Differentiation during plant growth and under stress conditions. J. Plant Physiol. 2018, 221, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Jin, Y.; Li, M.; Dong, L.; Guo, D.; Lu, C.; Qi, H. Analysis of CmCADs and three lignifying enzymes in oriental melon (‘CaiHong7′) seedlings in response to three abiotic stresses. Sci. Hortic. 2018, 237, 257–268. [Google Scholar] [CrossRef]
- Kumar, S.A.; Kumari, P.H.; Jawahar, G.; Prashanth, S.; Suravajhala, P.; Katam, R.; Sivan, P.; Rao, K.S.; Kirti, P.B.; Kishor, P.K. Beyond just being foot soldiers—Osmotin like protein (OLP) and chitinase (Chi11) genes act as sentinels to confront salt, drought, and fungal stress tolerance in tomato. Environ. Exp. Bot. 2016, 132, 53–65. [Google Scholar] [CrossRef]
- Nedukha, O.M. Callose: Localization, functions, and synthesis in plant cells. Cytol. Genet. 2015, 49, 49–57. [Google Scholar] [CrossRef]
- Wu, F.B.; Zhang, G.P.; Dominy, P. Four barley genotypes respond differently to cadmium: Lipid peroxidation and activities of antioxidant capacity. Environ. Exp. Bot. 2003, 50, 67–78. [Google Scholar] [CrossRef]
- Wu, F.B.; Wu, L.H.; Xu, F.H. Chlorophyll meter to predict nitrogen side dress requirements for short-season cotton (Gossypium hirsutum L.). Field Crops Res. 1998, 56, 309–314. [Google Scholar]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta Biomembr. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Chen, F.; Wu, F.B.; Dong, J.; Vincze, E.; Zhang, G.P.; Wang, F.; Huang, Y.Z.; Wei, K. Cadmium translocation and accumulation in developing barley grains. Planta 2007, 227, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Jiang, Y.N.; He, Z.Y.; Ma, M. Cadmium accumulation and oxidative stress burst in garlic (Allium sativum). J. Plant Physiol. 2005, 162, 977–984. [Google Scholar] [CrossRef]
- Shabnam, N.; Tripathi, I.; Sharmila, P.; Pardha-Saradhi, P. A rapid, ideal, and eco-friendlier protocol for quantifying proline. Protoplasma 2015, 253, 1577. [Google Scholar] [CrossRef] [PubMed]
- Greive, C.M.; Grattan, S.R. Rapid assay for determination of water-soluble quaternary-amino compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Irigoyen, J.J.; Emerich, D.W.; Sanches-Diaz, M. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol. Plant 1992, 84, 55–60. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram 1uantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Shedletzky, C.U.; Delmer, D.P. A microtiter-based fluorescence assay for (1,3)-β-glucan synthases. Anal. Biochem. 1997, 249, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.M.; Garcia, P.C.; Rivero, R.M.; Romero, L. Response of phenolic metabolism to the application of carbendazim plus boron in tobacco. Physiol. Plant. 1999, 106, 151–157. [Google Scholar] [CrossRef]
- Mitchell, H.J.; Hall, J.; Barber, M.S. Elicitor-induced cinnamyl alcohol dehydrogenase activity in lignifying wheat (Triticum aestivum L.) leaves. Plant Physiol. 1994, 104, 551–556. [Google Scholar] [CrossRef] [Green Version]
- Diaz, J.; Bernal, A.; Pomar, F.; Merino, F. Induction of shikimate dehydrogenase and peroxidase in pepper (Capsicum annuum L.) seedlings in response to copper stress and its relation to lignification. Plant Sci. 2001, 161, 179–188. [Google Scholar]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Tang, Q.Y.; Feng, M.G. Practical Statistics and Its DPS Statistical Software Package; China Agriculture Press: Bejing, China, 1997. [Google Scholar]
| Genotype | Treatment | Shoot DW (µg plant–1) | Root DW (µg plant–1) | Shoot Height (cm) | Root Length (cm) |
|---|---|---|---|---|---|
| XZ5 | +K | 143.4 ± 8.94 ABCa | 53 ± 0.86 ABa | 27.0 ± 0.41 Ca | 23.5 ± 1.21 Ba |
| +K+D | 139.0 ± 11.35 ABCa | 51.09 ± 1.03 Ba | 26.2 ± 1.06 Da | 23.5 ± 1.30 Ba | |
| –K | 123.8 ± 9.79 CDb | 49.97 ± 1.70 BCab | 24.9 ± 0.55 CDab | 23.3 ± 0.81 Ba | |
| –K+D | 119 ± 9.35 CDb | 45 ± 2.35 Cb | 23.0 ± 0.19 Eb | 19.7 ± 1.09 DEb | |
| Tadmor | +K | 150 ± 3.88 ABa | 43 ± 2.76 CDa | 22.6 ± 0.29 Ea | 23.7 ± 1.96 ABa |
| +K+D | 112.5 ± 10.97 DEb | 40 ± 2.46 Dab | 20.6 ± 0.99 Fb | 20.2 ± 0.72 Db | |
| –K | 133.4 ± 2.62 BCDab | 38 ± 2.27 DEb | 21.8 ± 0.39 EFa | 20.5 ± 0.41 Db | |
| –K+D | 108.8 ± 7.90 DEc | 33 ± 3.19 Ec | 20.1 ± 1.31 Fb | 19.2 ± 0.57 Ec | |
| XZ54 | +K | 161.6 ± 2.52 Aa | 56 ± 3.45 Aa | 29.1 ± 0.62 Aa | 24.7 ± 1.66 Aa |
| +K+D | 88.6 ± 8.02 Ec | 36 ± 1.52 DEbc | 23.6 ± 0.37 DEb | 22.9 ± 1.38 BCab | |
| –K | 131 ± 8.44 BCDb | 42 ± 0.8 CDb | 22.1 ± 0.53 Ebc | 21.5 ± 0.93 Cab | |
| –K+D | 89 ± 2.30 Ec | 34 ± 2.16 Ec | 21.4 ± 0.36 EFc | 20.0 ± 1.48 Db |
| Genotype | Treatment | Ca | K | Mg | Na | Na+/K+ Ratio |
|---|---|---|---|---|---|---|
| mg g–1 DW | ||||||
| Shoot mineral concentrations | ||||||
| XZ5 | +K | 0.61 ± 0.13 Cb | 39.16 ± 0.4 Aa | 0.81 ± 0.15 Cb | 1.30 ± 0.17 Ea | 0.03 Fa |
| +K+D | 1.30 ± 0.25 Aa | 36.83 ± 0.74 ABa | 0.79 ± 0.13 Cb | 1.23 ± 0.20 Ea | 0.03 Fa | |
| –K | 0.74 ± 0.04 Cb | 32.17 ± 1.94 BCab | 1.39 ± 0.09 Ba | 1.58 ± 0.31 DEa | 0.05 EFa | |
| –K+D | 1.19 ± 0.17 ABa | 28.03 ± 1.06 Cb | 1.22 ± 0.23 Ba | 1.58 ± 0.05 DEa | 0.06 EFa | |
| Tadmor | +K | 0.87 ± 0.20 BCb | 28.86 ± 4.37 Ca | 0.76 ± 0.13 CDb | 1.3 ± 0.24 Eb | 0.05 EFc |
| +K+D | 1.29 ± 0.15 Aa | 21.00 ± 1.57 Fb | 0.43 ± 0.02 DEbc | 1.72 ± 0.57 DEb | 0.08 Ebc | |
| –K | 0.77 ± 0.06 Cb | 14.57 ± 1.21 GHc | 1.44 ± 0.27 Ba | 2.51 ± 0.30 BCa | 0.17 Ca | |
| –K+D | 0.77 ± 0.21 Cb | 13.46 ± 1.09 Hc | 0.28 ± 0.07 Ec | 1.65 ± 0.33 DEb | 0.12 CDb | |
| XZ54 | +K | 0.80 ± 0.13 Cb | 24.52 ± 1.27 Ea | 0.59 ± 0.24 CDEbc | 1.81 ± 0.39 CDEc | 0.07 Ec |
| +K+D | 1.17 ± 0.27 Aba | 19.58 ± 2.72 FGb | 0.68 ± 0.31 CDbc | 2.10 ± 0.46 BCDbc | 0.11 CDc | |
| –K | 0.63 ± 0.04 Cb | 15.25 ± 1.43 Gbc | 1.84 ± 0.17 Aa | 2.55 ± 0.19 Bb | 0.17 Cb | |
| –K+D | 0.70 ± 0.11 Cb | 12.52 ± 0.97 Hc | 0.81 ± 0.06 Cb | 4.10 ± 0.25 Aa | 0.33 Aa | |
| Root mineral concentrations | ||||||
| XZ5 | +K | 2.40 ± 0.56 BCDab | 136.59 ± 17.66 Aa | 3.42 ± 0.56 ABa | 3.49 ± 0.67 CDb | 0.03 Db |
| +K+D | 1.34 ± 0.13 Db | 101.06 ± 24.51 Bb | 2.41 ± 0.39 CDb | 2.18 ± 0.38 Db | 0.02 Db | |
| –K | 2.53 ± 0.18 ABCa | 79.00 ± 5.69 CDbc | 3.79 ± 0.73 Aa | 8.05 ± 0.47 Ba | 0.1 Aa | |
| –K+D | 1.87 ± 0.13 Dab | 57.49 ± 4.02 DEc | 2.29 ± 0.09 Db | 3.53 ± 0.62 CDb | 0.06 Cab | |
| Tadmor | +K | 3.17 ± 0.98 ABab | 97.16 ± 21.53 Ba | 3.47 ± 1.09 ABa | 4.00 ± 1.25 CDb | 0.04 CDb |
| +K+D | 2.41 ± 0.77 BCDab | 86.55 ± 7.36 Cb | 2.69 ± 0.55 BCDa | 5.04 ± 0.96 Cb | 0.06 Cb | |
| –K | 3.33 ± 0.58 Aa | 75.08 ± 10.45 CDbc | 3.60 ± 0.71 ABa | 9.83 ± 0.43 ABa | 0.13 Aa | |
| –K+D | 2.11 ± 0.79 CDb | 47.34 ± 10.16 Ec | 2.81 ± 0.67 BCDa | 4.62 ± 0.75 Cb | 0.1 Aa | |
| XZ54 | +K | 2.55 ± 0.48 ABCDa | 101.02 ± 13.04 Ba | 3.37 ± 0.29 ABCab | 3.64 ± 0.59 CDb | 0.04 CDb |
| +K+D | 1.78 ± 0.11 Da | 82.96 ± 4.96 Cb | 2.25 ± 0.13 Dc | 3.01 ± 0.62 CDb | 0.04 CDb | |
| –K | 2.66 ± 0.09 ABCa | 78.71 ± 6.65 CDb | 4.02 ± 0.64 Aa | 10.62 ± 2.4 Aa | 0.13 Aa | |
| –K+D | 1.63 ± 0.19 Da | 53.08 ± 4.11 DEc | 2.74 ± 0.32 BCDbc | 4.15 ± 0.68 CDb | 0.08 BCb | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sehar, S.; Adil, M.F.; Zeeshan, M.; Holford, P.; Cao, F.; Wu, F.; Wang, Y. Mechanistic Insights into Potassium-Conferred Drought Stress Tolerance in Cultivated and Tibetan Wild Barley: Differential Osmoregulation, Nutrient Retention, Secondary Metabolism and Antioxidative Defense Capacity. Int. J. Mol. Sci. 2021, 22, 13100. https://doi.org/10.3390/ijms222313100
Sehar S, Adil MF, Zeeshan M, Holford P, Cao F, Wu F, Wang Y. Mechanistic Insights into Potassium-Conferred Drought Stress Tolerance in Cultivated and Tibetan Wild Barley: Differential Osmoregulation, Nutrient Retention, Secondary Metabolism and Antioxidative Defense Capacity. International Journal of Molecular Sciences. 2021; 22(23):13100. https://doi.org/10.3390/ijms222313100
Chicago/Turabian StyleSehar, Shafaque, Muhammad Faheem Adil, Muhammad Zeeshan, Paul Holford, Fangbin Cao, Feibo Wu, and Yizhou Wang. 2021. "Mechanistic Insights into Potassium-Conferred Drought Stress Tolerance in Cultivated and Tibetan Wild Barley: Differential Osmoregulation, Nutrient Retention, Secondary Metabolism and Antioxidative Defense Capacity" International Journal of Molecular Sciences 22, no. 23: 13100. https://doi.org/10.3390/ijms222313100
APA StyleSehar, S., Adil, M. F., Zeeshan, M., Holford, P., Cao, F., Wu, F., & Wang, Y. (2021). Mechanistic Insights into Potassium-Conferred Drought Stress Tolerance in Cultivated and Tibetan Wild Barley: Differential Osmoregulation, Nutrient Retention, Secondary Metabolism and Antioxidative Defense Capacity. International Journal of Molecular Sciences, 22(23), 13100. https://doi.org/10.3390/ijms222313100











