Influence of Hydrogenation on Morphology, Chemical Structure and Photocatalytic Efficiency of Graphitic Carbon Nitride
Abstract
:1. Introduction
2. Results and Discussion
2.1. Transmission Electron Microscopy
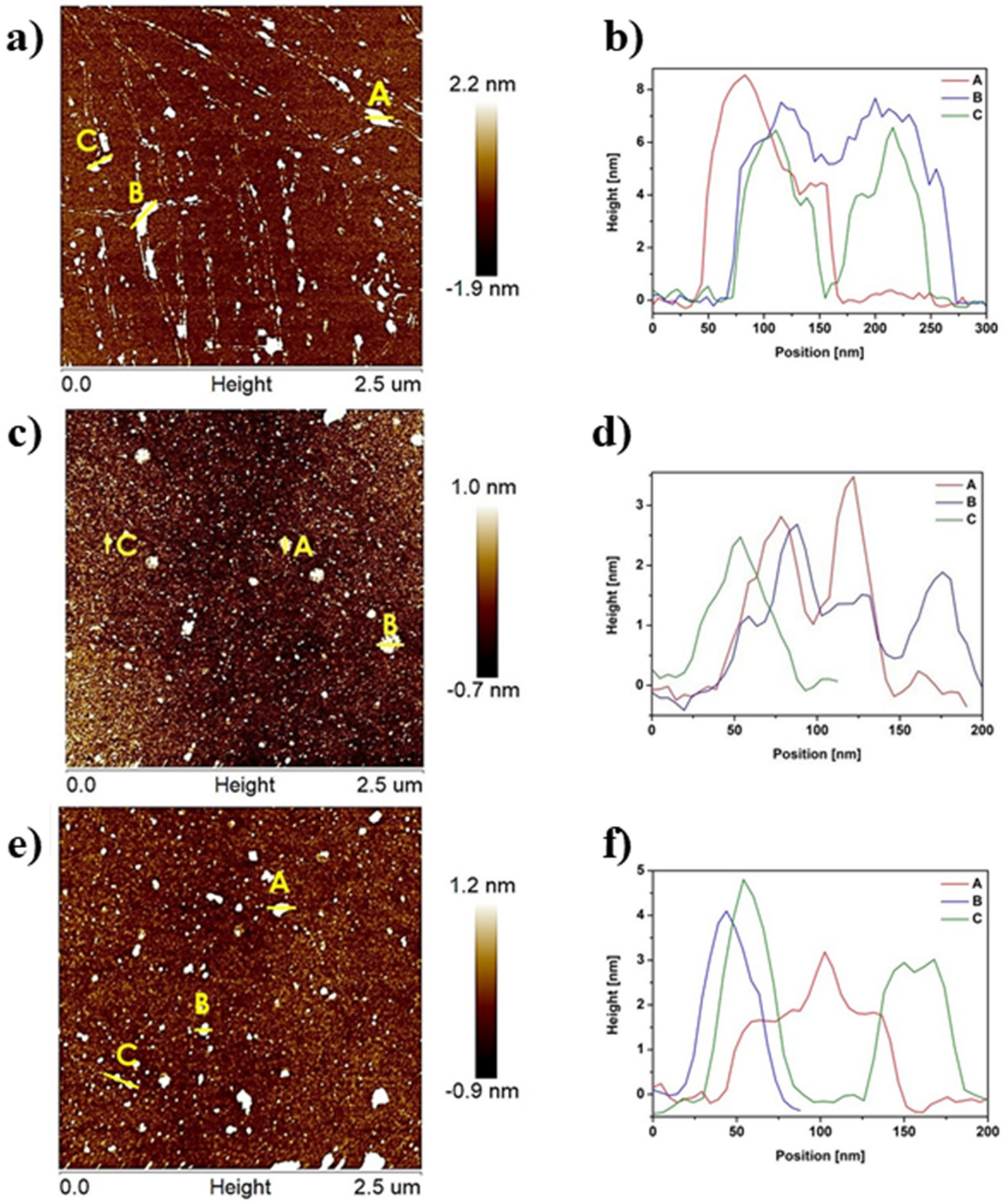
2.2. Atomic Force Microscopy
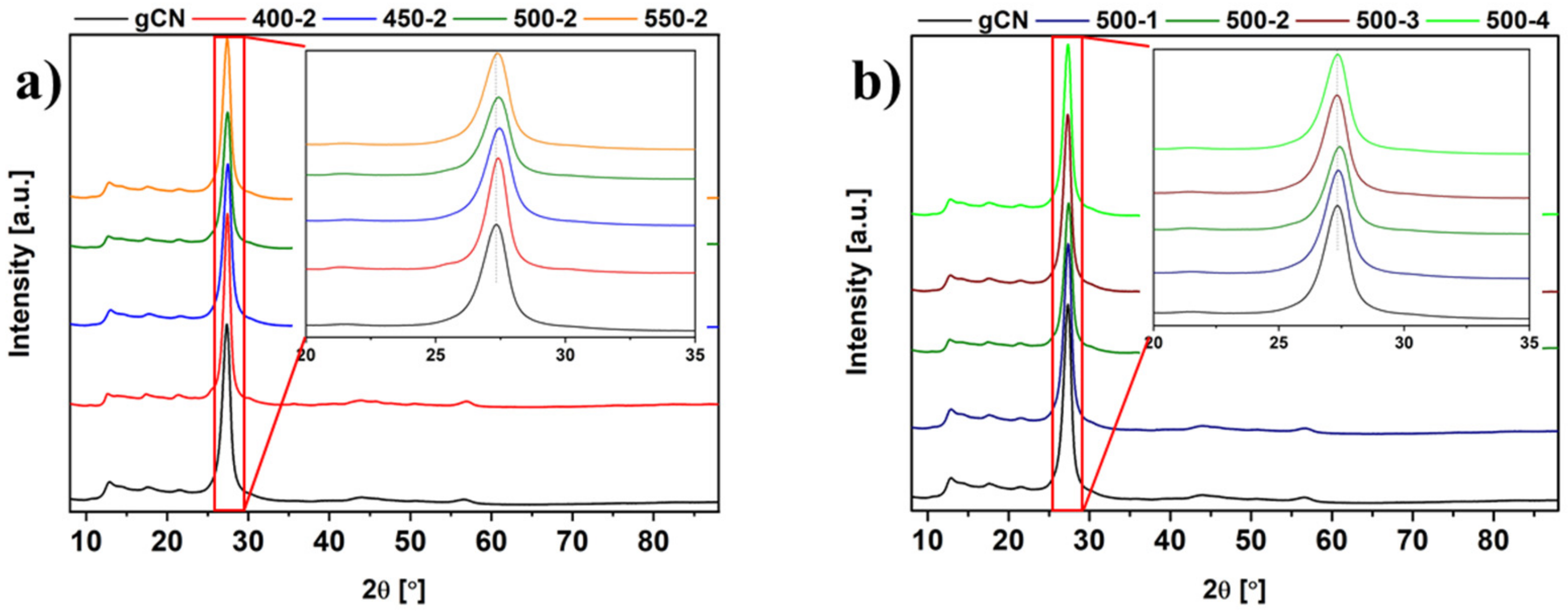
2.3. X-ray Powder Diffraction
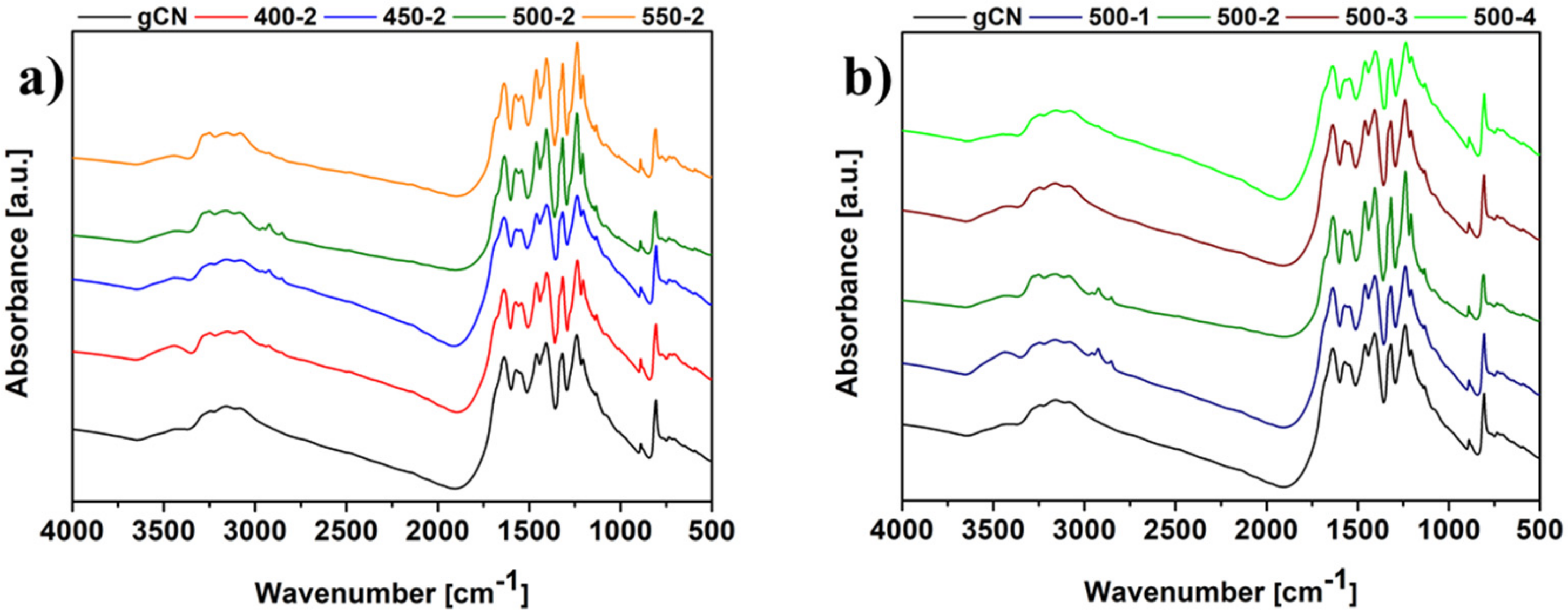
2.4. Fourier Transform Infrared Spectroscopy
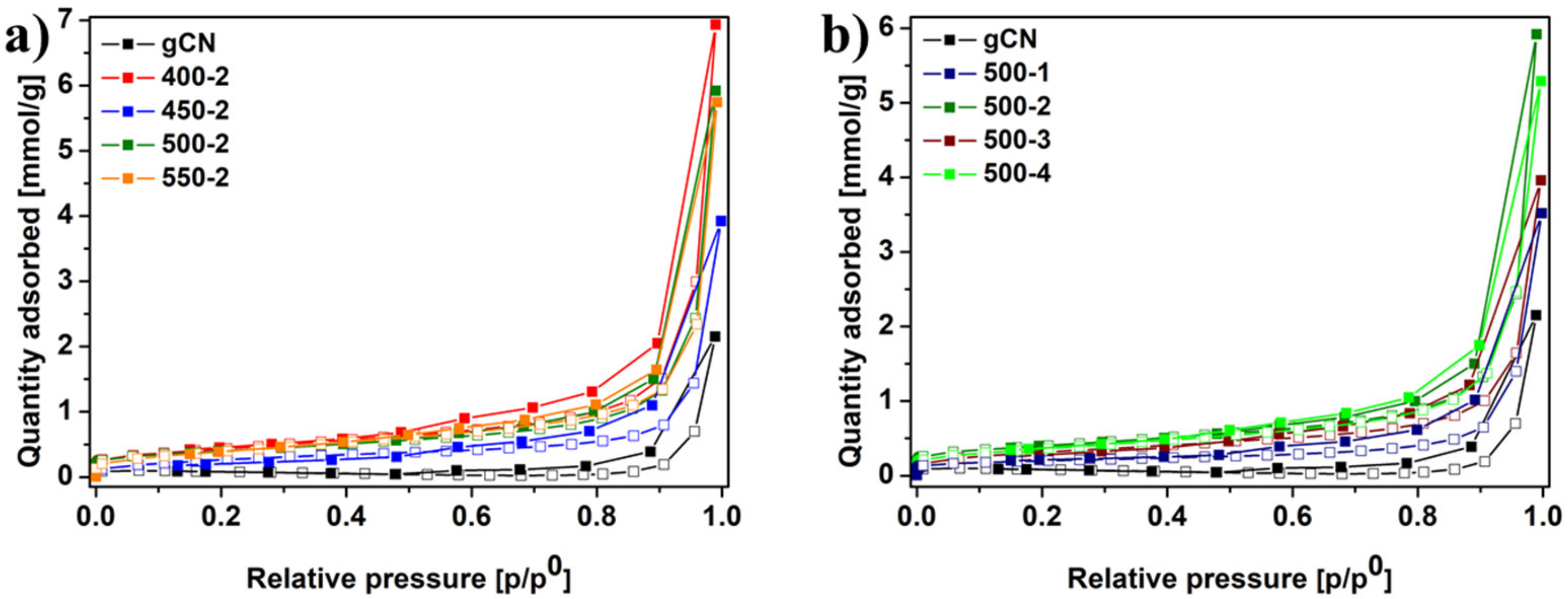
2.5. Specific Surface Area Measurement
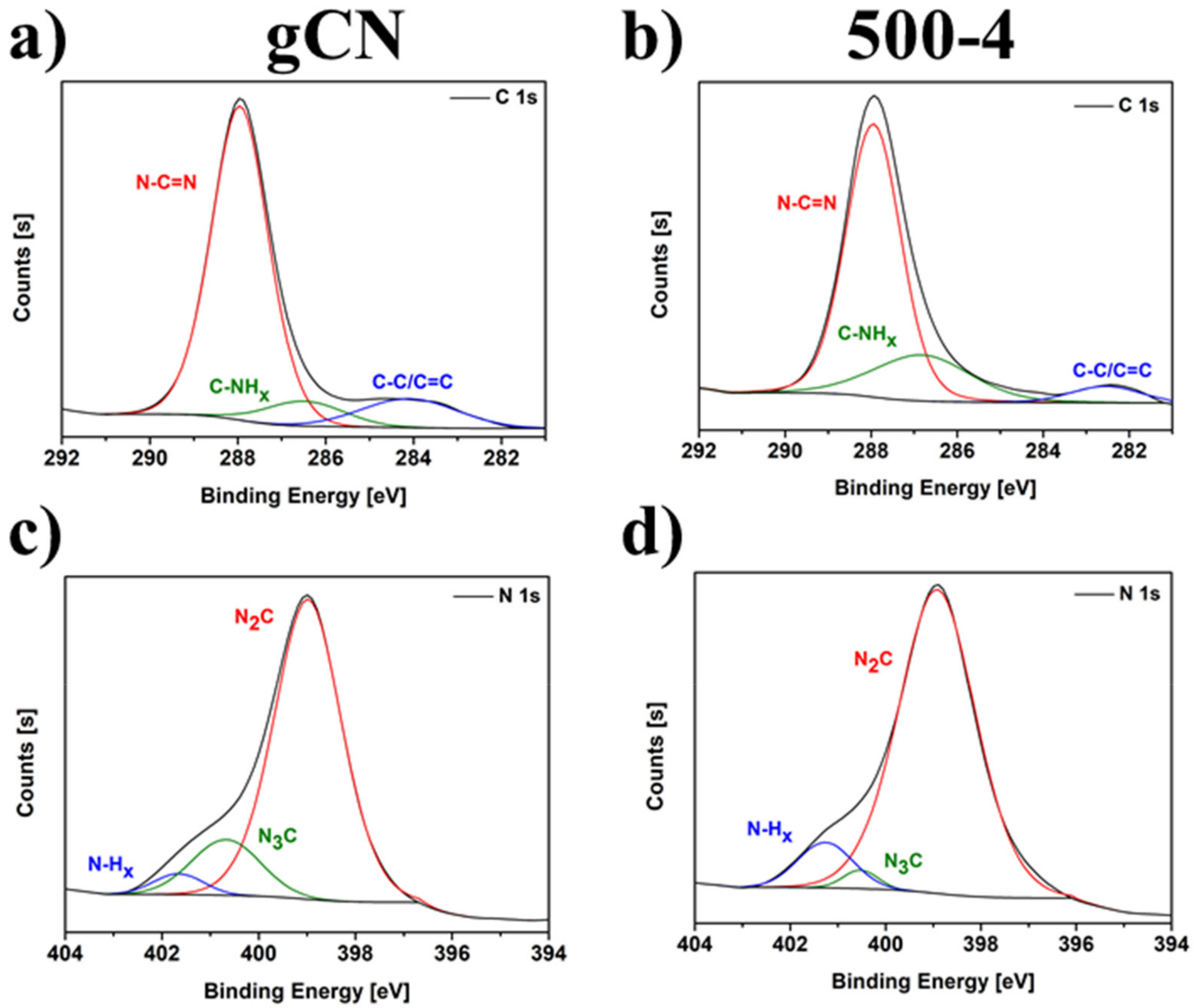
2.6. X-ray Photoelectron Spectroscopy
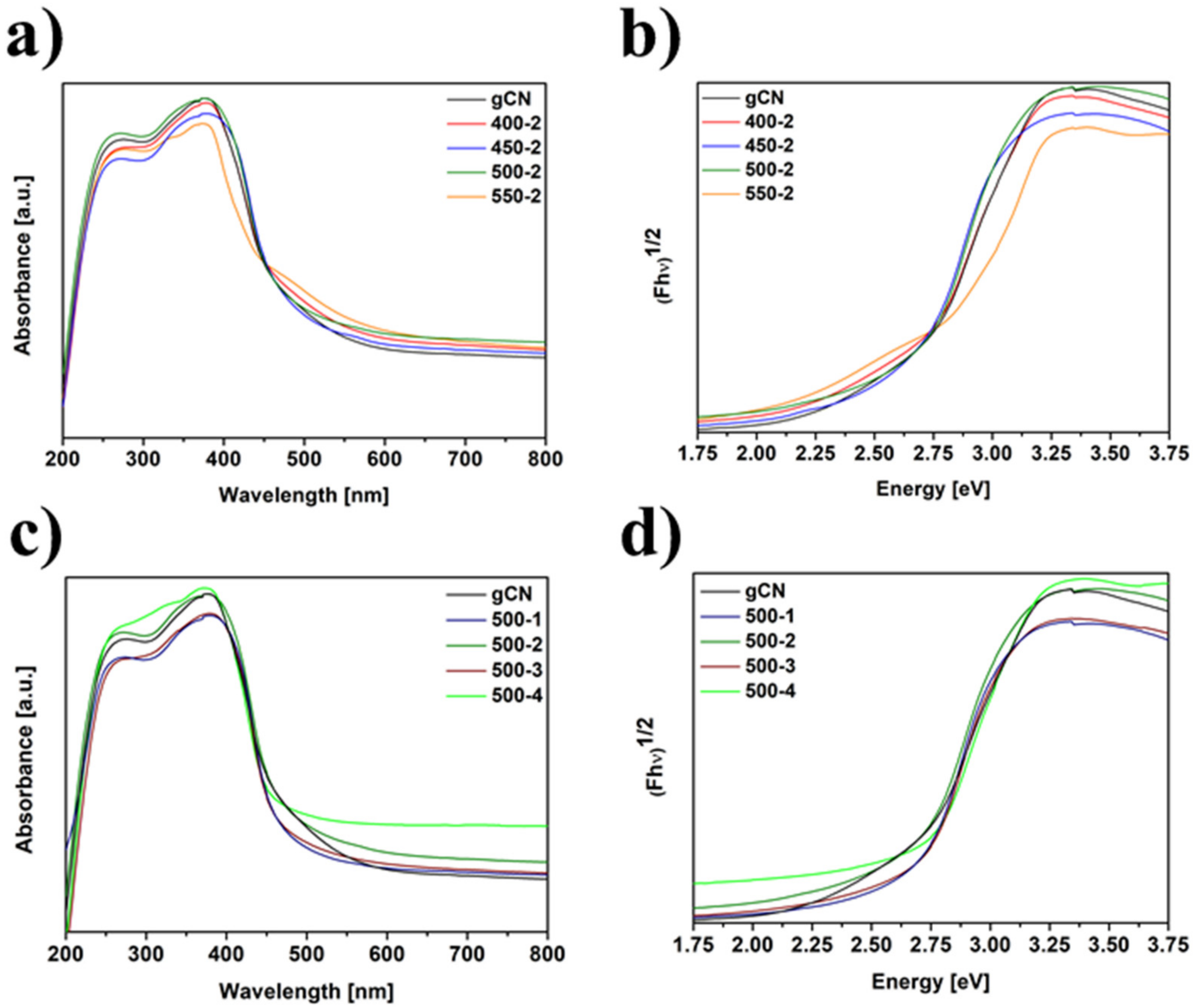
2.7. Diffuse Reflectance Spectroscopy
2.8. Photoluminescence
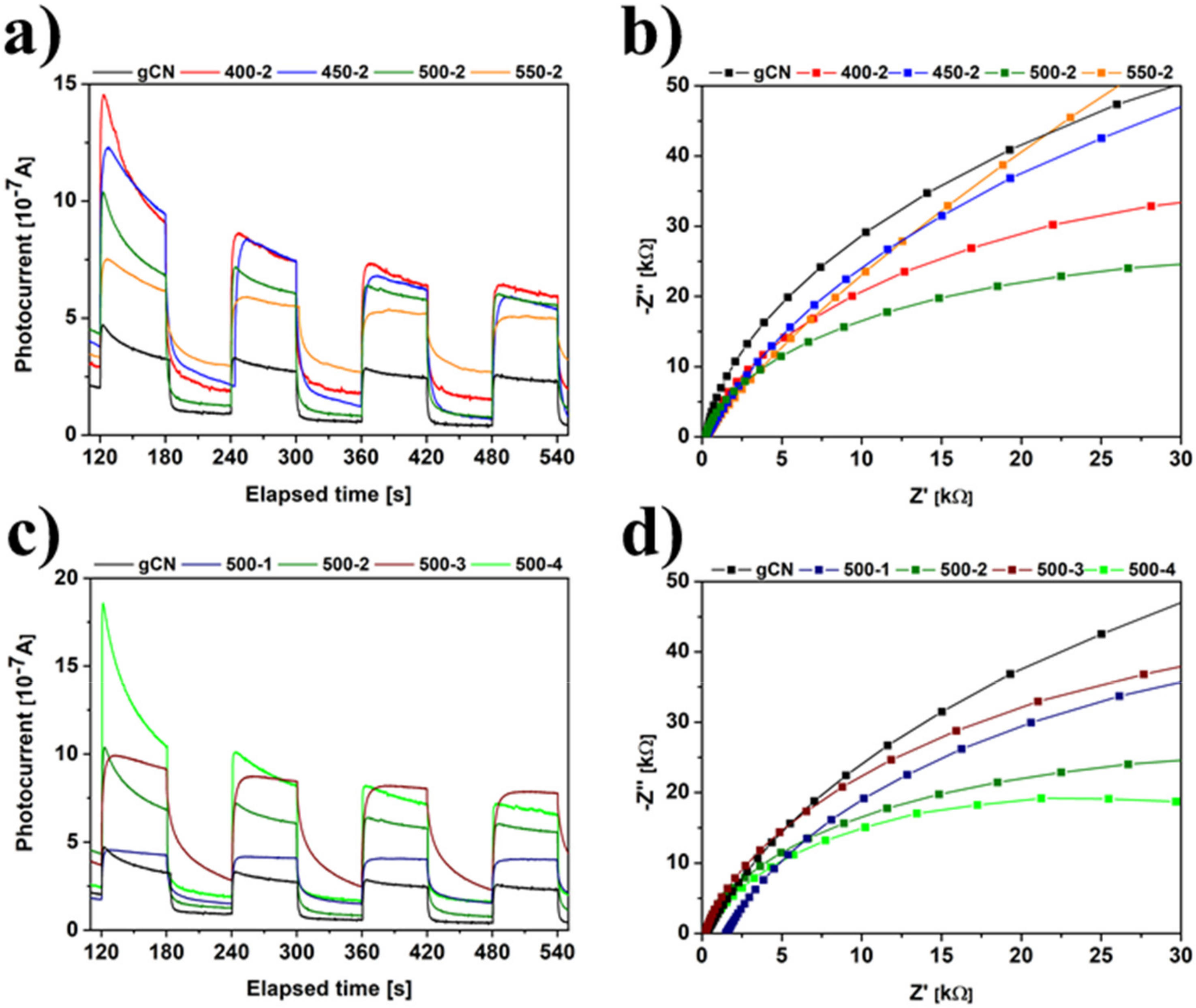
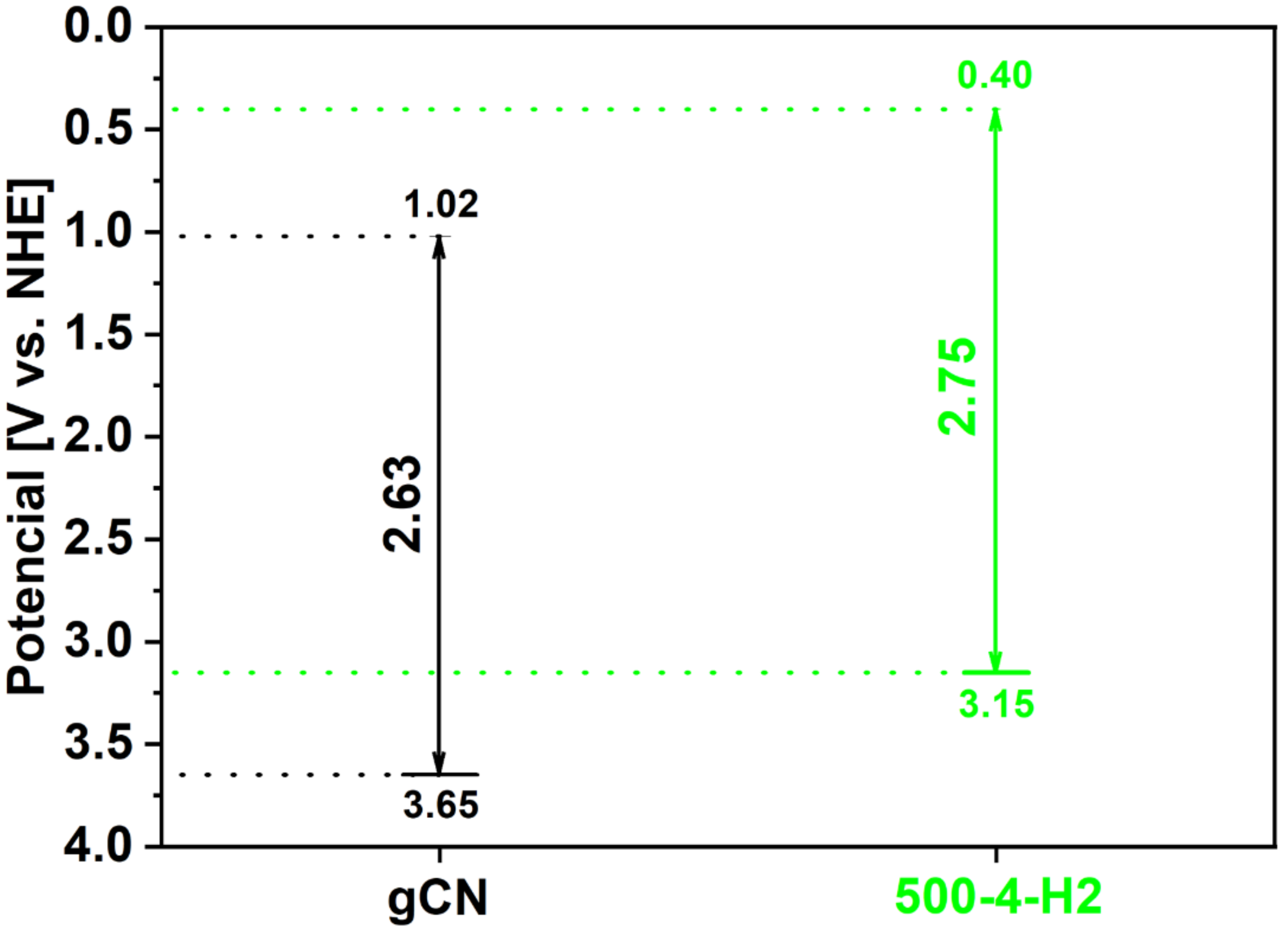
2.9. Electrochemistry
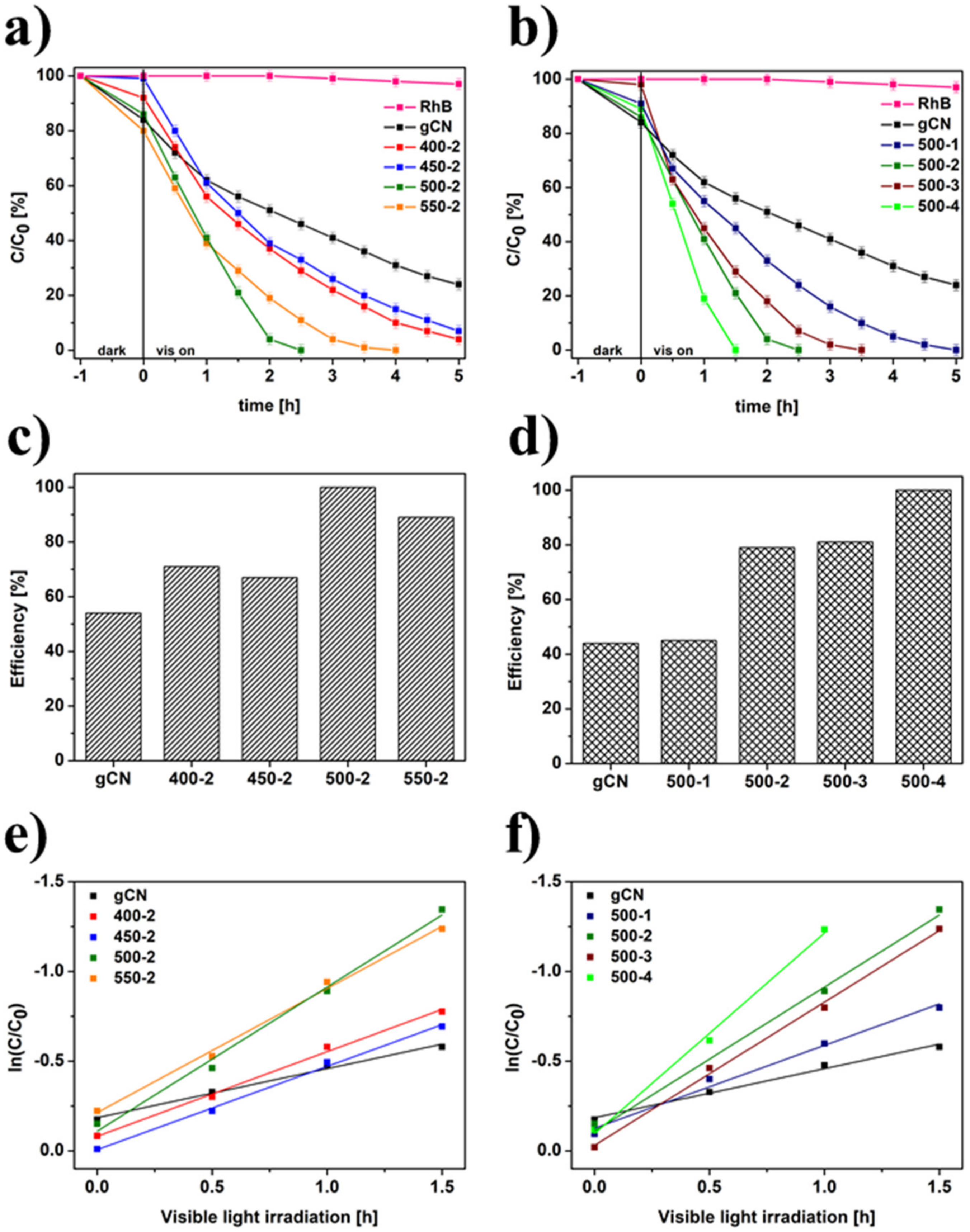
2.10. Photocatalytic Performance
3. Materials and Methods
3.1. Materials
3.1.1. Preparation of gCN
3.1.2. Preparation of Modified gCN by Annealing in H2 Atmosphere
3.2. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, A.; Wang, C.; Fu, L.; Wong, W.; Lan, Y. Recent Advances of Graphitic Carbon Nitride-Based Structures and Applications in Catalyst, Sensing, Imaging, and LEDs. Nano-Micro Lett. 2017, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Kroke, E.; Schwarz, M.; Horath-Bordon, E.; Kroll, P.; Noll, B.; Norman, A.D. Tri-s-triazine derivatives. Part I. From trichloro-tri-s-triazine to graphitic C3N4 structures. New J. Chem. 2002, 26, 508–512. [Google Scholar] [CrossRef]
- Teter, D.M.; Hemley, R.J. Low-compressibility carbon nitrides. Science 1996, 271, 53–55. [Google Scholar] [CrossRef]
- Goettmann, F.; Fischer, A.; Antonietti, M.; Thomas, A. Metal-free catalysis of sustainable Friedel–Crafts reactions: Direct activation of benzene by carbon nitrides to avoid the use of metal chlorides and halogenated compounds. Chem. Commun. 2006, 43, 4530–4532. [Google Scholar] [CrossRef]
- Li, X.; Hartley, G.; Ward, A.J.; Young, P.A.; Masters, A.F.; Maschmeyer, T. Hydrogenated Defects in Graphitic Carbon Nitride Nanosheets for Improved Photocatalytic Hydrogen Evolution. J. Phys. Chem. C 2015, 119, 14938–14946. [Google Scholar] [CrossRef]
- Kang, X.; Kang, Y.; Hong, X.; Sun, Z.; Zhen, C.; Hu, C.; Liu, G.; Cheng, H. Improving the photocatalytic activity of graphitic carbon nitride by thermal treatment in a high-pressure hydrogen atmosphere. Prog. Nat. Sci. Mater. Int. 2018, 28, 183–188. [Google Scholar] [CrossRef]
- Niu, P.; Yin, L.-C.; Yang, Y.-Q.; Liu, G.; Cheng, H.-M. Increasing the Visible Light Absorption of Graphitic Carbon Nitride (Melon) Photocatalysts by Homogeneous Self-Modification with Nitrogen Vacancies. Adv. Mater. 2014, 26, 8046–8052. [Google Scholar] [CrossRef]
- Nowotny, M.K.; Sheppard, L.R.; Bak, T.; Nowotny, J. Defect Chemistry of Titanium Dioxide. Application of Defect Engineering in Processing of TiO2-Based Photocatalysts. J. Phys. Chem. C 2008, 112, 5275–5300. [Google Scholar] [CrossRef]
- Niu, P.; Liu, G.; Cheng, H.M. Nitrogen Vacancy-Promoted Photocatalytic Activity of Graphitic Carbon Nitride. J. Phys. Chem. C 2012, 116, 11013–11018. [Google Scholar] [CrossRef]
- Tay, Q.; Kanhere, P.; Ng, C.F.; Chakraborty, S.; Huan, A.C.H.; Sum, T.C.; Ahuja, R.; Chen, Z. Defect Engineered g-C3N4 for Efficient Visible Light Photocatalytic Hydrogen Production. Chem. Mat. 2015, 27, 4930–4933. [Google Scholar] [CrossRef]
- Zhang, S.; Li, G.; Duan, L.; Wang, H.; Zhao, Y.; Zhang, Y. g-C3N4 synthesized from NH4SCN in a H2 atmosphere as a high performance photocatalyst for blue light-driven degradation of rhodamine B. RSC Adv. 2020, 10, 19669–19685. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Wang, X. Two-dimensional covalent carbon nitride nanosheets: Synthesis, functionalization, and applications. Energy Environ. Sci. 2015, 8, 3092–3108. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, L.; Shi, R.; Zhu, Y. Chemical exfoliation of graphitic carbon nitride for efficient heterogeneous photocatalysis. J. Mater. Chem. A 2013, 1, 14766–14772. [Google Scholar] [CrossRef]
- Thomas, A.; Fisher, A.; Goettmann, F.; Antonietti, M.; Muller, J.-O.; Schlogl, R.; Carlsson, J.M. Graphitic Carbon Nitride Materials: Variation of Structure and Morphology and Their Use as Metal-free Catalysts. J. Mater. Chem. 2008, 18, 4893–4908. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.Y.; Yu, A.C. Photophysics and Photocatalysis of Carbon Nitride Synthesized at Different Temperatures. J. Phys. Chem. C 2014, 118, 11628–11635. [Google Scholar] [CrossRef]
- Hong, J.; Wang, Y.; Wang, Y.; Zhang, W.; Xu, R. Noble-Metal-Free NiS/C3N4 for Efficient Photocatalytic Hydrogen Evolution from Water. ChemSusChem 2013, 6, 2263–2268. [Google Scholar] [CrossRef]
- Kang, Y.; Yang, Y.; Yin, L.-C.; Kang, X.; Liu, G.; Cheng, H.-M. An Amorphous Carbon Nitride Photocatalyst with Greatly Extended Visible-Light-Responsive Range for Photocatalytic Hydrogen Generation. Adv. Mater. 2015, 27, 4572. [Google Scholar] [CrossRef]
- Kang, Y.; Yang, Y.; Yin, L.-C.; Kang, X.; Wang, L.; Gang, L.; Cheng, H.-M. Selective Breaking of Hydrogen Bonds of Layered Carbon Nitride for Visible Light Photocatalysis. Adv. Mater. 2016, 28, 6471. [Google Scholar] [CrossRef]
- Ham, Y.; Maeda, K.; Cha, D.; Takanabe, K.; Domen, K. Synthesis and Photocatalytic Activity of Poly(triazine imide). Chem. Asian J. 2013, 8, 218–224. [Google Scholar] [CrossRef]
- Komatsu, T. Prototype Carbon Nitrides Similar to the Symmetric Triangular Form of Melon. J. Mater. Chem. 2001, 11, 802–803. [Google Scholar] [CrossRef]
- Khabashesku, V.N.; Zimmerman, J.L.; Margrave, J.L. Powder Synthesis and Characterization of Amorphous Carbon Nitride. Chem. Mater. 2000, 12, 3264–3270. [Google Scholar] [CrossRef]
- Heeger, A.J. Semiconducting and Metallic Polymers: The Fourth Generation of Polymeric Materials (Nobel lecture). Angew. Chem. Int. Ed. 2001, 40, 2591–2611. [Google Scholar] [CrossRef]
- Aleksandrzak, M.; Kijaczko, M.; Kukulka, W.; Baranowska, D.; Baca, M.; Zielinska, B.; Mijowska, E. Boosting of photocatalytic hydrogen evolution via chlorine doping of polymeric carbon nitride. Beilstein J. Nanotechnol. 2021, 12, 473–484. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Liu, Z.; Marcus, M.A.; Wang, W.-C.; Oyler, N.A.; Grass, M.E.; Mao, B.; Glans, P.-A.; Yu, P.Y.; et al. Properties of Disorder-Engineered Black Titanium Dioxide Nanoparticles through Hydrogenation. Sci. Rep. 2013, 3, 1510. [Google Scholar] [CrossRef]
- Hu, F.; Luo, W.; Hu, Y.; Dai, H.; Peng, X. Insight into the kinetics and mechanism of visible-light photocatalytic degradation of dyes onto the P doped mesoporous graphitic carbon nitride. J. Alloys Compd. 2019, 794, 594–605. [Google Scholar] [CrossRef]
- Baca, M.; Aleksandrzak, M.; Mijowska, E.; Kalenczuk, R.J.; Zielinska, B. Core/Shell Structure of Mesoporous Carbon Spheres and g-C3N4 for Acid Red 18 Decolorization. Catalysts 2019, 9, 1007. [Google Scholar] [CrossRef] [Green Version]
- Chuaicham, C.; Pawar, R.; Sasaki, K. Dye-sensitized Photocatalyst of Sepiolite for Organic Dye Degradation. Catalysts 2019, 9, 235. [Google Scholar] [CrossRef] [Green Version]

| Sample | Specific Surface Area [m2 g−1] | Total Pore Volume [cm3 g−1] | Average Pore Diameter [nm] | Energy Band Gap [eV] |
|---|---|---|---|---|
| gCN | 8.89 | 0.003 | 0.88 | 2.63 |
| 400-2 | 30.33 | 0.014 | 1.05 | 2.65 |
| 450-2 | 16.81 | 0.008 | 1.35 | 2.68 |
| 500-1 | 14.76 | 0.007 | 1.00 | 2.69 |
| 500-2 | 28.69 | 0.013 | 1.03 | 2.70 |
| 500-3 | 21.45 | 0.010 | 1.29 | 2.71 |
| 500-4 | 24.11 | 0.012 | 1.09 | 2.75 |
| 550-2 | 25.72 | 0.012 | 1.25 | 2.81 |
| Sample | Temperature [℃] | Duration [h] |
|---|---|---|
| 400-2 | 400 | 2 |
| 450-2 | 450 | 2 |
| 500-1 | 500 | 1 |
| 500-2 | 500 | 2 |
| 500-3 | 500 | 3 |
| 500-4 | 500 | 4 |
| 550-2 | 550 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baranowska, D.; Kędzierski, T.; Aleksandrzak, M.; Mijowska, E.; Zielińska, B. Influence of Hydrogenation on Morphology, Chemical Structure and Photocatalytic Efficiency of Graphitic Carbon Nitride. Int. J. Mol. Sci. 2021, 22, 13096. https://doi.org/10.3390/ijms222313096
Baranowska D, Kędzierski T, Aleksandrzak M, Mijowska E, Zielińska B. Influence of Hydrogenation on Morphology, Chemical Structure and Photocatalytic Efficiency of Graphitic Carbon Nitride. International Journal of Molecular Sciences. 2021; 22(23):13096. https://doi.org/10.3390/ijms222313096
Chicago/Turabian StyleBaranowska, Daria, Tomasz Kędzierski, Małgorzata Aleksandrzak, Ewa Mijowska, and Beata Zielińska. 2021. "Influence of Hydrogenation on Morphology, Chemical Structure and Photocatalytic Efficiency of Graphitic Carbon Nitride" International Journal of Molecular Sciences 22, no. 23: 13096. https://doi.org/10.3390/ijms222313096
APA StyleBaranowska, D., Kędzierski, T., Aleksandrzak, M., Mijowska, E., & Zielińska, B. (2021). Influence of Hydrogenation on Morphology, Chemical Structure and Photocatalytic Efficiency of Graphitic Carbon Nitride. International Journal of Molecular Sciences, 22(23), 13096. https://doi.org/10.3390/ijms222313096







